Abstract
Background:
Pre-existing factors such as age and cognitive performance can influence the electroencephalogram (EEG) during general anesthesia. Specifically, spectral EEG power is lower in the elderly as compared to younger subjects. Here we investigate age-related changes in EEG architecture in patients undergoing general anesthesia through a detailed examination of spectral and entropic measures.
Methods:
We retrospectively studied 180 frontal EEG recordings from patients undergoing general anesthesia, induced with propofol/fentanyl and maintained by sevoflurane at the Waikato Hospital in Hamilton, New Zealand. We calculated power spectral density and normalized power spectral density, the entropic measures approximate and permutation entropy, as well as the beta ratio and spectral entropy as exemplary parameters used in current monitoring systems from segments of EEG obtained prior to the onset of surgery; i.e. with no noxious stimulation.
Results:
The oldest quartile of patients had significantly lower 1/f characteristics (p<0.001; AUC= 0.84 [0.76 0.92]), indicative of a more uniform distribution of spectral power. Analysis of the normalized power spectral density revealed no significant impact of age on relative alpha (p=0.693; AUC=0.52 [0.41 0.63]) and a significant but weak effect on relative beta power (p=0.041; AUC= 0.62 [0.52 0.73]). Using entropic parameters, we found a significant age-related change towards a more irregular and unpredictable EEG (permutation entropy: p<0.001, AUC=0.81 [0.71 0.90]; approximate entropy: p<0.001, AUC=0.76 [0.66 0.85]). With approximate entropy, we could also detect an age-induced change in alpha-band activity (p=0.002, AUC=0.69 [0.60 78]).
Conclusions:
Like the sleep literature, spectral and entropic EEG features under general anesthesia change with age revealing a shift towards a faster, more irregular, oscillatory composition of the EEG in older patients. Age-related changes in neurophysiological activity may underlie these findings however the contribution of age-related changes in filtering properties or the signal to noise ratio must also be considered. Regardless, most current EEG technology used to guide anesthetic management focus on spectral features, and improvements to these devices might involve integration of entropic features of the raw EEG.
Keywords: Age, Anesthesia, General, Electroencephalography
Introduction
We are experiencing a strong shift in population demographics towards an aging society.1 This shift is going to result in an increased number of surgeries in geriatric patients.2 Older patients are at higher risk of developing adverse outcomes like delirious episodes after surgery with general anesthesia.3,4 Electroencephalographic (EEG) monitoring devices may help to estimate the patients’ level of neurophysiologic activity and to prevent episodes of excessively high administered doses of anesthesia as characterized by EEG burst suppression. The presence of these episodes seem to represent an independent risk factor for cognitive impairments after anesthesia,5,6 however some controversy exists regarding strategies designed to reduce the duration of burst suppression.3,7
Despite these possible advantages, the current generation of monitoring devices does not account for age-related changes in EEG characteristics. In general, EEG characteristics during general anesthesia vary greatly among patients of different age and cognitive performance.8–10 Older patients exhibit lower EEG amplitudes (and consequently, lower power) during wakefulness,11 sleep,12 and general anesthesia.8,9 Previous publications have described age-related changes in power spectral density under general anesthesia to some degree,8,9 but a detailed description of age-related differences in other aspects of quantitative EEG analysis is still missing. We investigated age-related changes in the EEG recorded from patients from 18 to 90 years under general anesthesia with the goal to (i) characterize the EEG of older patients in more detail to further understand the neurophysiological changes that occur with advanced age and to (ii) estimate the influence of these changes on current EEG -based monitoring systems. We analyzed power spectral density, normalized power spectral density, the 1/f characteristics of the power spectrum, as well as the entropic measures permutation entropy13 and approximate entropy14 to investigate age-related changes in the EEG activity. The 1/f characteristic and information extracted from power spectral density and normalized power spectral density analysis help to get a good (more broad-band) overview of age-related changes. The entropic measures can help to identify subtler changes in the EEG. These analytical parameters were originally developed to characterize the complexity of a time-series signal and are reported as good measures to estimate the anesthetic level of a patient.15–17 We also used two parameters beta-ratio18 and spectral entropy,19 that are incorporated in current monitoring systems to estimate possible impact of age on the index these systems generate to reflect the (hypnotic) level of anesthesia.
Methods
We used frontal EEG records from 180 patients during general anesthesia, collected at the Waikato District Health Board Hospital in Hamilton, New Zealand. These patients gave written informed consent, and had contributed to an earlier observational study.20 The ethical approval was specifically for the establishment of an anonymous EEG database that could be used for various post-hoc analyses. We selected those patients who had received propofol for induction, and sevoflurane for maintenance of anesthesia. For each patient, we selected ten seconds of artifact-free, non-burst-suppression EEG, recorded five to two minutes prior to the onset of surgery, which represent a clinical level of general anesthesia without any surgical stimulation. We recorded the EEG with either the BIS (Medtronic, Dublin, Ireland) or the Entropy Module (GE Healthcare, Helsinki, Finland) monitors at 128 and 100 Hz respectively. Raw EEG from the BIS was then resampled to 100 Hz for ease of comparison.
We estimated effect-site concentrations of sevoflurane, opioid, and propofol using standard pharmacokinetic models. We calculated the effect-site sevoflurane concentration (in minimum alveolar concentration (MAC), CeMAC) using a simple end-tidal to brain delay model with a diffusion half-time constant (Keo) of 144 seconds.21 Based on these values, we calculated age-adjusted MAC values (referenced to 1 MAC in a 40 year old, i.e. MAC40) as described by Mapleson.22 Opioid concentration (in fentanyl-equivalents; 1 ng/ml of fentanyl equals 20 ng/ml of morphine) was calculated using the two-compartment model parameters in Mazoit, Butscher et al. 200723 for morphine, and in Shafer and Varve24 for fentanyl. We estimated effect-site propofol concentrations according to the model and parameters described by Wiczling et al.25
EEG analysis
Spectral analysis
We calculated the power spectral density using Thompson’s multitaper power spectral density estimate. We used the MATLAB R2015a (The MathWorks Inc., Natick, MA) pmtm function (default settings and NFFT=256). Based on power spectral density, we calculated the power in the 0.5–30 Hz range, the alpha-band power (7.8–12.5 Hz), and the beta-band power (12.5–25 Hz) as well as the lower frequency delta-band (0.4–3.9 Hz) and theta-band (3.9–7.8 Hz). We also computed a normalized power spectral density by dividing the power spectral density by the sum from 0.4 to 30.5 Hz. We used the Python based fitting oscillations & one over f toolbox using the provided MATLAB-wrapper26 to identify periodic activity as well as the aperiodic component of the EEG. The fitting oscillations & one over f algorithm decomposes the power spectral density into periodic components as well as an aperiodic component that reflects 1/f like characteristics. The aperiodic component is fitted according to L=b-log(Fa) with b being the (broadband) offset, F being the frequency vector, and a being the slope. We did not consider a “knee” parameter and hence used the “fixed model” as described in the original publication.26 We defined the range to detect possible oscillatory components from 1 to 30 Hz and focused on the detection of these peaks in the alpha range.
Furthermore, we obtained the relative alpha- and beta-band power by dividing the sum of power spectral density in the 8–12 Hz (alpha) or 12–25 Hz range (beta) by the sum of power spectral density in the 0.4–30 Hz range.
Entropy analysis
Entropic measures constitute a straightforward, time-domain approach to evaluate EEG features. We individually calculated approximate entropy and permutation entropy for the EEG 0.5–30 Hz range, the EEG alpha-band, and the EEG beta-band. We applied an adaptive filtering routine (Butterworth filter, order 3–5) using the MATLAB filtfilt functions that preserves the phase of the signal. So as not to include edge effects caused by filtering, we applied the filter to a 30 s EEG segment and used the central 10 s to calculate the entropies for the different frequency ranges.
For approximate entropy we used a custom routine, for permutation entropy, we adapted the my_permutation_entropy function from MATLAB Central. We chose an embedding dimension m=3 and a time delay τ=1 for permutation entropy15 and m=2 / τ=1 together with tolerance r=0.2SD for approximate entropy.27 These parameter settings are commonly used for EEG analyses.15,16,27,28 A detailed description of how to calculate the parameters can be found in the papers initially presenting the methods by Steven M Pincus for approximate entropy14 and Bandt and Pompe for permutation entropy.13 Approximate entropy searches for similar amplitude patterns (of length m) in the EEG and calculates the probability of the patterns remaining similar if it is extended to a length of m+1. Similar in this context means that the amplitude values between the patterns do not differ by more than the defined tolerance r. permutation entropy as an ordinal measure codes small segments of length m according to their ranks, with the highest amplitude in the segment having the highest rank. permutation entropy presents the Shannon entropy29 of the probability distribution of the possible patterns (here 6, if m=3). A graphical explanation for approximate entropy and permutation entropy can be found here30.
Phase-randomized surrogate analysis
In order to clearly delineate the specific contribution of extracting information from the entropic measures vs the spectral measures of a signal we used phase-randomized surrogate data. We calculated 200 phase-randomized surrogates for each of the 180 EEG episodes and compared the entropic measures to the spectral EEG band powers. For surrogate generation we used a modified version of the surrogate function for phase randomization of the PhysioNet Toolkit31. We modified this function, so that no amplitude transformation, but only a phase randomization was performed. We then calculated the approximate entropy and permutation entropy for the alpha and beta range as well as the relative alpha- ad beta band power for the surrogates.
Parameters for comparison to available monitors
In order to estimate the influence of age on available monitoring systems like the BIS and Entropy module we calculated the beta ratio=log(sum(power spectral density30–47Hz)/sum(power spectral density11–20Hz)) as proxy for the sub-parameter BetaRatio of the BIS.32 We further calculated the spectral entropy of the normalized power spectral density for settings mimicking the state entropy (to 32 Hz) and response entropy (47 Hz) for different lower band limits of 0.8 and 1.1 Hz.19 For 168 of the 180 patients we also had BIS indices available. In order to evaluate the influence of age on BIS, we used the last index value displayed within the 10 s analysis window we used for spectral and entropic analysis.
Statistical analysis
Because of the retrospective nature of our investigation, no statistical power calculation was conducted prior to the study and the sample size was based on the available number of patient EEG. Our spectral analyses (except the spectral entropy with the 1.1 Hz lower limit were a priori and the entropic analyses (approximate entropy, permutation entropy) were post hoc analyses after evaluating different parameter settings.
Regression analyses
We generated models using the least squares method for linear regression analysis for each dependent variable with respect to age. For each linear model, we generated the regression curve and performed a one-sample t-test comparing the slope coefficient against a slope of zero. Additionally, we determined the strength of the correlation, or rather the fit of the model as an R2 value.
Evaluation of interaction between sevoflurane concentration and EEG parameters
In order to evaluate if the EEG parameters (i.e., permutation entropy and approximate entropy, differ significantly based on an interaction between age and age-adjusted MAC at a 5% significance level, we calculated the linear model interaction terms using the MATLAB fitlm function.
Comparison of the youngest versus the oldest quartiles
For each parameter, we compared the youngest 25% (n=46, 1st quartile, Y25) and the oldest 25% (n=46, 4th quartile, O25) of patients using a Mann-Whitney U test at a confidence level of 95% together with the area under the receiver operator characteristics curve (AUC) and 10000-fold bootstrapped 95% confidence intervals (CI) as effect size. We used the MATLAB-based MES toolbox for AUC and 95% CI calculation.33 By including all subjects of a certain age our youngest and oldest quartiles each contained 46 subjects (not 45). Our excluded middle age range (44 to 72 years old) contained 88 instead of the expected 90 subjects. According to the traditional academic point system, AUC values can be interpreted as excellent: 1≥AUC≥0.9; good: 0.9>AUC≥0.8; fair: 0.8>AUC≥0.7; poor: 0.7>AUC≥0.6; or fail: AUC<0.6. For the (normalized) power spectral density comparison, we only defined significant results if at least two neighboring frequencies showed significant differences between the young and old group. This procedure has been applied for similar studies , by other groups.34
All tests applied were two-tailed tests and we considered p<0.05 being significant.
Results
Of 234 patients undergoing surgical intervention with propofol induction and sevoflurane maintenance, 54 patients were excluded from analysis due to missing EEG or incomplete volatile anesthetic concentrations data in the period prior to surgery onset, resulting in 180 patients being included in the final analysis. The subject ages ranged from 18 to 90 years (mean (SD) = 56.7(18.4) years). The age range for the youngest 25% (Y25) was from 18 to 43 years and for the oldest 25% (O25) from 73 to 90 years. Figure 1 presents a flow chart of patient and group selection. The results of all linear regressions as well as all the comparisons between the youngest 25% and the oldest 25% are presented in Table 1.
Figure 1:
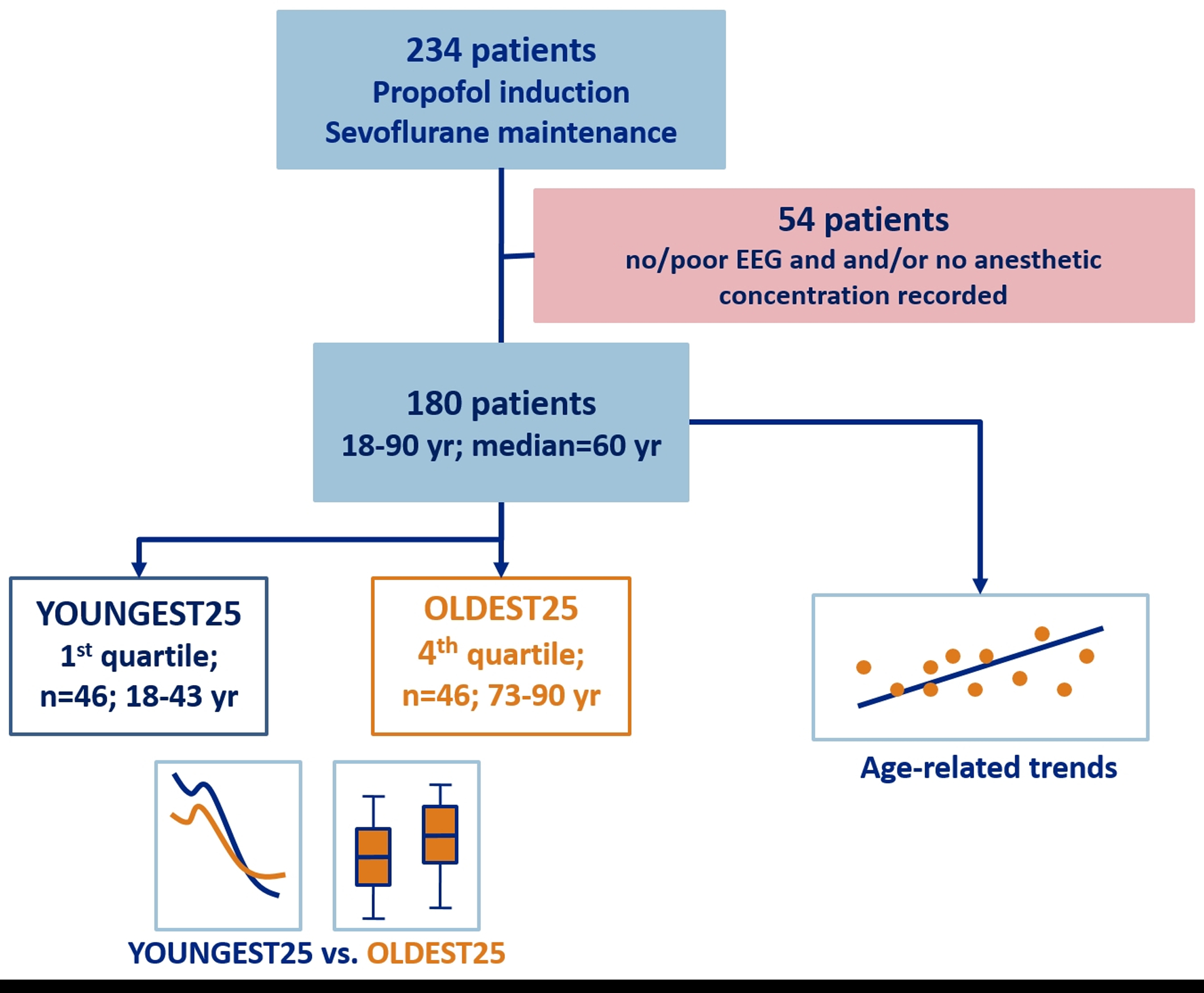
Flow chart of the excluded patients and groups defined for analysis
| Parameter | Intercept | Slope | 95% CI Slope | t-Stat | p Slope | R2 | Youngest 25% | Oldest 25% | p (Ranksum) | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| EEG Band Power | ||||||||||
| Absolute Delta | 37.66 | −0.15 | [−0.18 −0.13] | −10.99 | <0.001 | 0.40 | 32.72 [30.80 35.87] | 5.98 [23.90 28.36] | <0.001 | 0.94 [0.89 0.98] |
| Absolute Theta | 29.82 | −0.17 | [−0.20 −0.15] | −13.13 | <0.001 | 0.49 | 24.13 [22.33 26.93] | 16.02 [13.85 19.27] | <0.001 | 0.93 [0.86 0.98] |
| Absolute Alpha | 30.71 | −0.18 | [−0.20 −0.15] | −12.25 | <0.001 | 0.46 | 24.81 [21.85 28.19] | 17.77 [15.31 19.72] | <0.001 | 0.92 [0.86 0.98] |
| Absolute Beta | 23.02 | −0.13 | [−0.16 −0.10] | −9.74 | <0.001 | 0.35 | 18.30 [16.29 21.04] | 13.75 [10.02 15.24] | <0.001 | 0.90 [0.83 0.96] |
| Relative Delta | 0.69 | 0.000 | [−0.001 0.002] | 0.53 | 0.599 | 0.00 | 0.76 [0.69 0.82] | 0.75 [0.63 0.83] | 0.806 | 0.52 [0.40 0.63] |
| Relative Theta | 0.11 | 0.000 | [−0.001 0.000] | −0.77 | 0.445 | 0.00 | 0.10 [0.07 0.12] | 0.09 [0.06 0.13] | 0.375 | 0.55 [0.43 0.68] |
| Relative Alpha | 0.17 | −0.001 | [−0.002 0.000] | −1.36 | 0.176 | 0.01 | 0.10 [0.08 0.17] | 0.10 [0.07 0.17] | 0.693 | 0.52 [0.42 0.63] |
| Relative Beta | 0.02 | 0.000 | [−0.000 0.001] | 1.78 | 0.077 | 0.02 | 0.03 [0.02 0.04] | 0.04 [0.02 0.06] | 0.041 | 0.62 [0.52 0.73] |
| Fitting Oscillations & One Over f | ||||||||||
| Exponent | 2.65 | −0.009 | [−0.011 −0.007] | −8.14 | <0.001 | 0.27 | 2.36 [2.19 2.60] | 2.00 [1.89 2.16] | <0.001 | 0.84 [0.76 0.92] |
| Offset | −0.43 | −0.008 | [−0.009 −0.006] | −8.27 | <0.001 | 0.29 | −0.59 [−0.90 −0.48] | −0.96 [−1.15 −0.85] | <0.001 | 0.81 [0.71 0.89] |
| Entropic Measures | ||||||||||
| Permutation Entropy 0.5–30 Hz | 1.97 | 0.002 | [0.001 0.003] | 7.04 | <0.001 | 0.22 | 2.02 [1.98 2.07] | 2.11 [2.06 2.15] | <0.001 | 0.81 [0.71 0.90] |
| Permutation Entropy Delta | 1.34 | 0.000 | [−0.000 0.001] | 1.67 | 0.097 | 0.02 | 1.35 [1.32 1.37] | 1.36 [1.34 1.38] | 0.129 | 0.59 [0.48 0.70] |
| Permutation Entropy Theta | 1.72 | 0.000 | [−0.000 0.000] | 1.12 | 0.263 | 0.01 | 1.72 [1.70 1.75] | 1.73 [1.71 1.75] | 0.219 | 0.57 [0.44 0.71] |
| Permutation Entropy Alpha | 1.92 | 0.000 | [−0.000 0.000] | 0.69 | 0.489 | 0.00 | 1.92 [1.91 1.95] | 1.93 [1.91 1.95] | 0.384 | 0.55 [0.43 0.67] |
| Permutation Entropy Beta | 2.21 | 0.001 | [0.001 0.001] | 4.95 | <0.001 | 0.12 | 2.24 [2.20 2.27] | 2.27 [2.24 2.29] | <0.001 | 0.71 [0.61 0.80] |
| Approximate Entropy 0.5–30 Hz | 0.76 | 0.002 | [0.001 0.003] | 4.87 | <0.001 | 0.12 | 0.83 [0.77 0.89] | 0.93 [0.84 0.99] | <0.001 | 0.76 [0.66 0.85] |
| Approximate Entropy Delta | 0.41 | −0.001 | [−0.001 −0.001] | −2.20 | 0.029 | 0.03 | 0.40 [0.34 0.44] | 0.37 [0.33 0.41] | 0.088 | 0.60 [0.50 0.70] |
| Approximate Entropy Theta | 0.62 | 0.000 | [−0.000 0.000] | −1.95 | 0.052 | 0.02 | 0.61 [0.61 0.62] | 0.61 [0.60 0.62] | 0.143 | 0.59 [0.46 0.71] |
| Approximate Entropy Alpha | 0.55 | 0.001 | [0.000 0.001] | 4.18 | <0.001 | 0.09 | 0.57 [0.56 0.59] | 0.60 [0.57 0.62] | 0.002 | 0.69 [0.60 0.78] |
| Approximate Entropy Beta | 1.01 | 0.001 | [0.001 0.001] | 2.44 | 0.016 | 0.03 | 1.05 [1.00 1.08] | 1.08[1.03 1.12] | 0.007 | 0.66 [0.55 0.77] |
| Monitoring Parameter | ||||||||||
| BIS | 33.96 | 0.16 | [0.078 0.24] | 3.84 | <0.001 | 0.08 | 42 [32 44] | 45 [40 51] | 0.026 | 0.65 [0.52 0.76] |
| Beta Ratio | −4.74 | 0.02 | [0.01 0.02] | 5.00 | <0.001 | 0.12 | −4.20 [−4.63 −3.71] | −3.61 [−4.02 −3.27] | <0.001 | 0.73 [0.63 0.82] |
| Spectral Entropy (1.1–32 Hz) | 2.73 | 0.007 | [0.005 0.01] | 5.81 | <0.001 | 0.16 | 3.02 [2.78 3.17] | 3.25 [3.13 3.46] | <0.001 | 0.79 [0.70 0.87] |
| Spectral Entropy (1.1–47 Hz) | 2.73 | 0.007 | [0.005 0.010] | 6.08 | <0.001 | 0.17 | 3.02 [2.79 3.18] | 3.29 [3.18 3.47] | <0.001 | 0.80 [0.71 0.88] |
| Spectral Entropy (0.8–32 Hz) | 2.64 | 0.002 | [−0.002 0.005] | 0.79 | 0.433 | 0.00 | 2.72 [2.31 2.91] | 2.78 [2.50 3.16] | 0.202 | 0.58 [0.47 0.68] |
| Spectral Entropy (0.8–47 Hz) | 2.64 | 0.002 | [−0.002 0.006] | 0.37 | 0.372 | 0.00 | 2.73 [2.31 2.91] | 2.79 [2.51 3.16] | 0.161 | 0.58 [0.47 0.69] |
Abbreviations:; BIS: bispectral index; CI: confidence interval;
Medications
Despite the lack of any prescribed anesthetic protocol, the delivered sevoflurane concentration was lower in the older patients. We could eliminate this trend by age-adjusting the MAC according to Mapleson.22 Similarly, the estimated propofol concentration decreased with age. By contrast, our data did not reveal any age-related difference in the opioid concentrations, measured in fentanyl equivalents. Supplemental Figure S1 presents the details and corresponding plots for describing the drug dose to age relationships. While the relationships for propofol and sevoflurane and age were statistically significant, the R2-values were rather low (R2≤0.06), indicating substantial contribution by other unmeasured factors. These results may reflect that the providers in our study consider age in their titration of dosages of propofol and sevoflurane but other nuanced factors go into decisions on opioid administration (e.g., surgery type, hemodynamic changes).
Older patients exhibit a more uniform distribution of relative spectral power
We obtained very similar age to power spectral density relationships as presented in a previous study9 and provide the results and the corresponding plots as supplemental data in Figure S2 and Figure S3. The normalized power spectral density showed significant differences only in the low (0.5–5 Hz) and high (>21 Hz) frequency ranges when comparing the youngest 25% versus the oldest 25% patients (Figure 2A). Figure 2B presents exemplary traces from the youngest 25% and the oldest 25% group.
Figure 2: normalized power spectral density exemplary raw EEG traces, and the aperiodic (1/f) component from young and old patients.
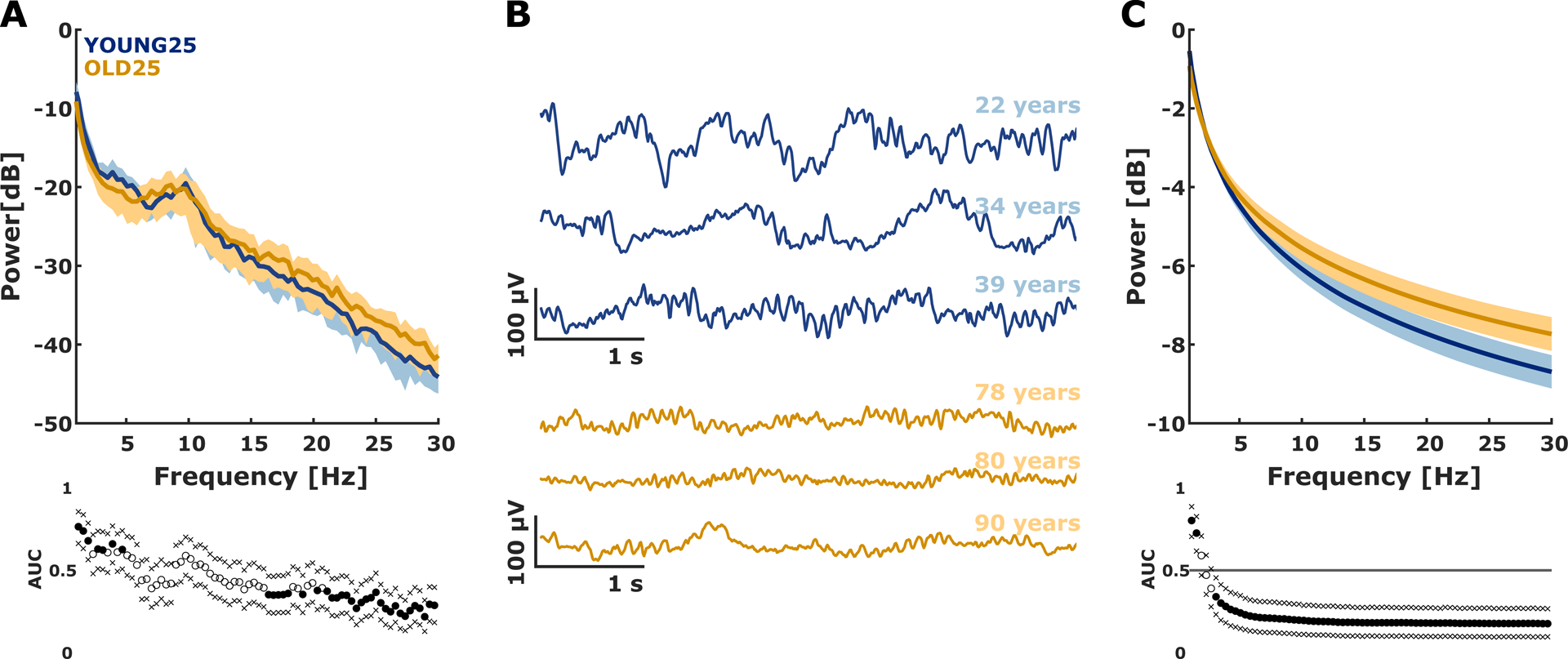
A) Median (±median absolute deviation) normalized power spectral density plots of EEG derived from the Y25 (blue) and O25 (orange) patients of the data set. power spectral density is presented with corresponding AUC values and bootstrapped 95% confidence intervals. The relative power spectral density indicated a more uniform distribution of the EEG from the old group with lower relative power at low frequencies (0.5–5 Hz) and higher relative power at high frequencies (>21 Hz).
B) Exemplary raw EEG traces from patients in the Y25 group (blue) and O25 group (orange). These traces highlight the age-induced differences on the EEG, especially fewer slow oscillations and an increased amount of high frequent activity.
C) Median (±median absolute deviation) of the exponential fit of the aperiodic (background) 1/f component between the Y25 (blue) and O25 (orange) patients. In addition, the AUC values and 95% bootstrapped confidence intervals are presented. In general, the aperiodic component of the power spectral density was more uniformly distributed in the old patients.
Filled circles indicate a significant difference, between Y25 and O25 evaluated by AUC confidence intervals excluding 0.5. The areas of light colors indicate the median absolute deviation. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis. Y25: youngest 25% O25: oldest 25%
We did not find significant differences between the youngest 25% and the oldest 25% in normalized power spectral density in the EEG alpha range (p=0.693; AUC=0.52 [0.42 0.63]) (Figure 3A) but a “poor” and significant effect (p=0.041; AUC= 0.62 [0.52 0.73]) in the EEG beta range (Figure 3B) as we did not observe a linear relationship of age with relative alpha and beta power and the difference in relative beta power. We take these results as evidence that age induces a change in the EEG, but that these changes may not be reliably detected by using the power in the classical frequency ranges. The evaluation of the relative power in the lower frequency delta and theta band did not show any age induced effects as well (Figure S4).
Figure 3: Linear regression and box plots of the youngest vs. the oldest quartile for (A) the relative (normalized) EEG alpha power, (B) the relative EEG beta power, and (C) the slope of the aperiodic 1/f component with corresponding box plots.
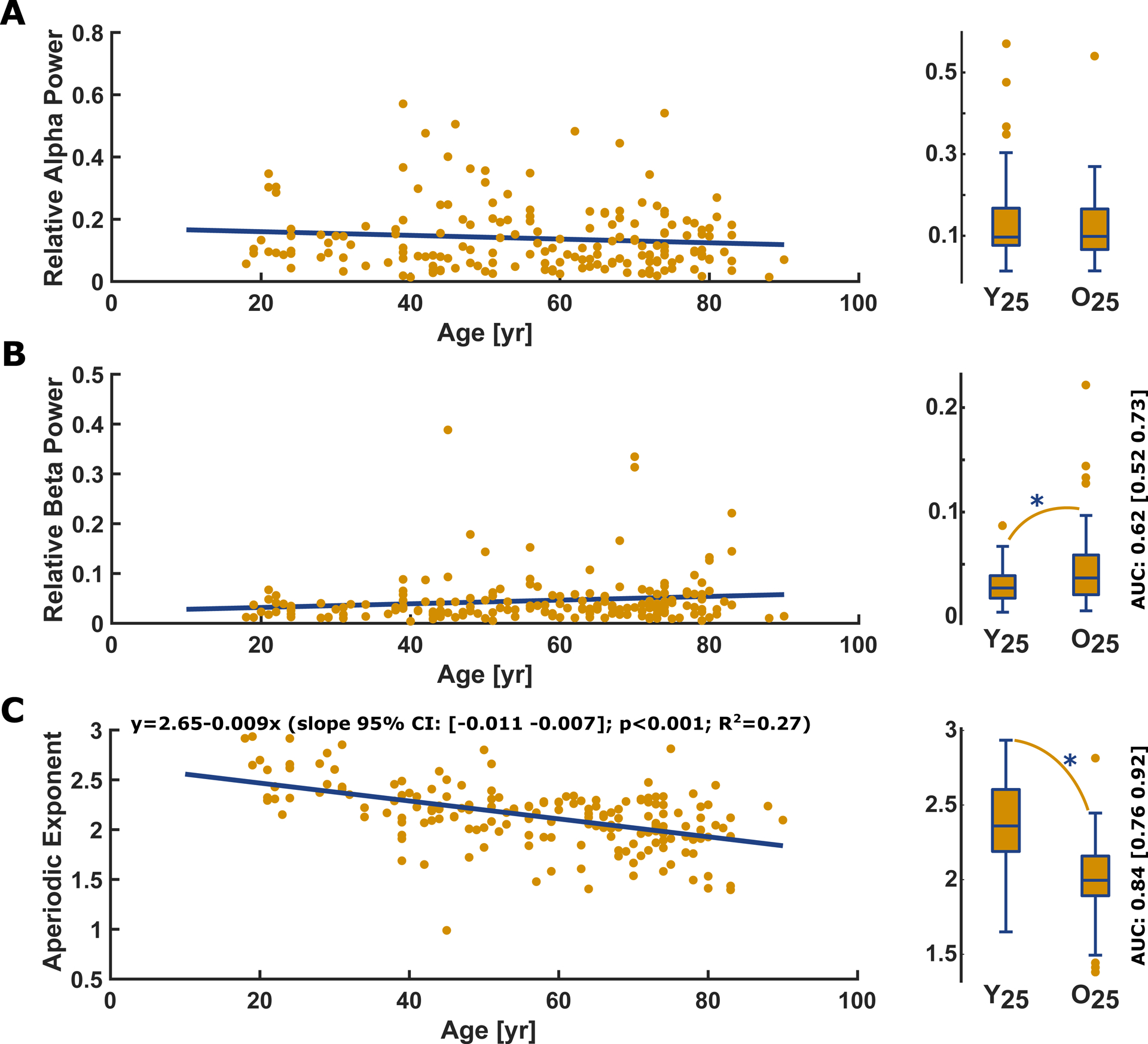
A) Relative power in the alpha-band EEG did not significantly (p= 0.176, t-statistic: −1.36) change with age. There was no significant difference (p= 0.693, AUC= 0.52 [0.42 0.63]) in relative alpha power between Y25 (0.10 [0.08 0.17]) and O25 (0.10 [0.07 0.17]).
B) Relative EEG beta power did not significantly (p=0.077, t-statistic: 1.78) change with age, but there was a significant difference (p= 0.041) in relative beta power between Y25 (0.03 [0.02 0.04]) and O25 (0.04 [0.02 0.06]). The AUC=0.62 [0.52 0.73] as effect site indicated a “poor” effect
C) The slope of the aperiodic 1/f component derived by the fitting oscillations & one over f algorithm significantly decreased with age (p<0.001, t-statistic: −8.14). The box plot indicates a significant flatter (p<0.001) slope in O25 patients (median [1st 3rd quartile]: 2.00 [1.89 2.16]) compared to the Y25 (2.36 [2.19 2.60]). The AUC=0.84 [0.76 0.92] as effect site indicated a “good” effect.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year
The fitting oscillations & one over f analysis revealed that in 174/180 patients (97%) at least one oscillatory component in the 8–12 Hz alpha range could be observed. Because the six patients without such a periodic component were distributed over the age range, we decided to keep these patients included. The parameters of the aperiodic component of the normalized power spectral density changed with age (Figure 3C). For the comparison between the youngest 25% and the oldest 25% the exponent was affected significantly and strongly (p<0.001, AUC= 0.84 [0.76 0.92]) by age as was the offset (p<0.001, AUC=0.81 [0.71 0.89]. Figure 2C shows the more uniform distribution of the aperiodic 1/f component of the power spectral density in the old patients.
Age related changes can be observed using entropy-based analyses
permutation entropy increased with age in the 0.5–30 Hz range as well as in the EEG beta range, but not in the EEG alpha range EEG (Figure 4A–C). Comparing the youngest 25% and the oldest 25%, we found a strong and significant (p<0.001, AUC=0.81 [0.71 0.90]) effect of age on the (0.5–30 Hz) filtered EEG and a fair and significant (p=0.0006, AUC=0.71 [0.61 0.81]) effect on the beta band EEG. We found no significant difference for the alpha band EEG (AUC=0.55 [0.43 0.67], p=0.384). These results signify that permutation entropy tracks the shift towards higher-frequency EEG activity with age.
Figure 4: Permutation entropy (m=3, τ=1): Linear regression and box plots of the youngest vs. the oldest quartile for the (A) 0.5–30 Hz range, (B) the alpha range, (C) and the EEG beta range.
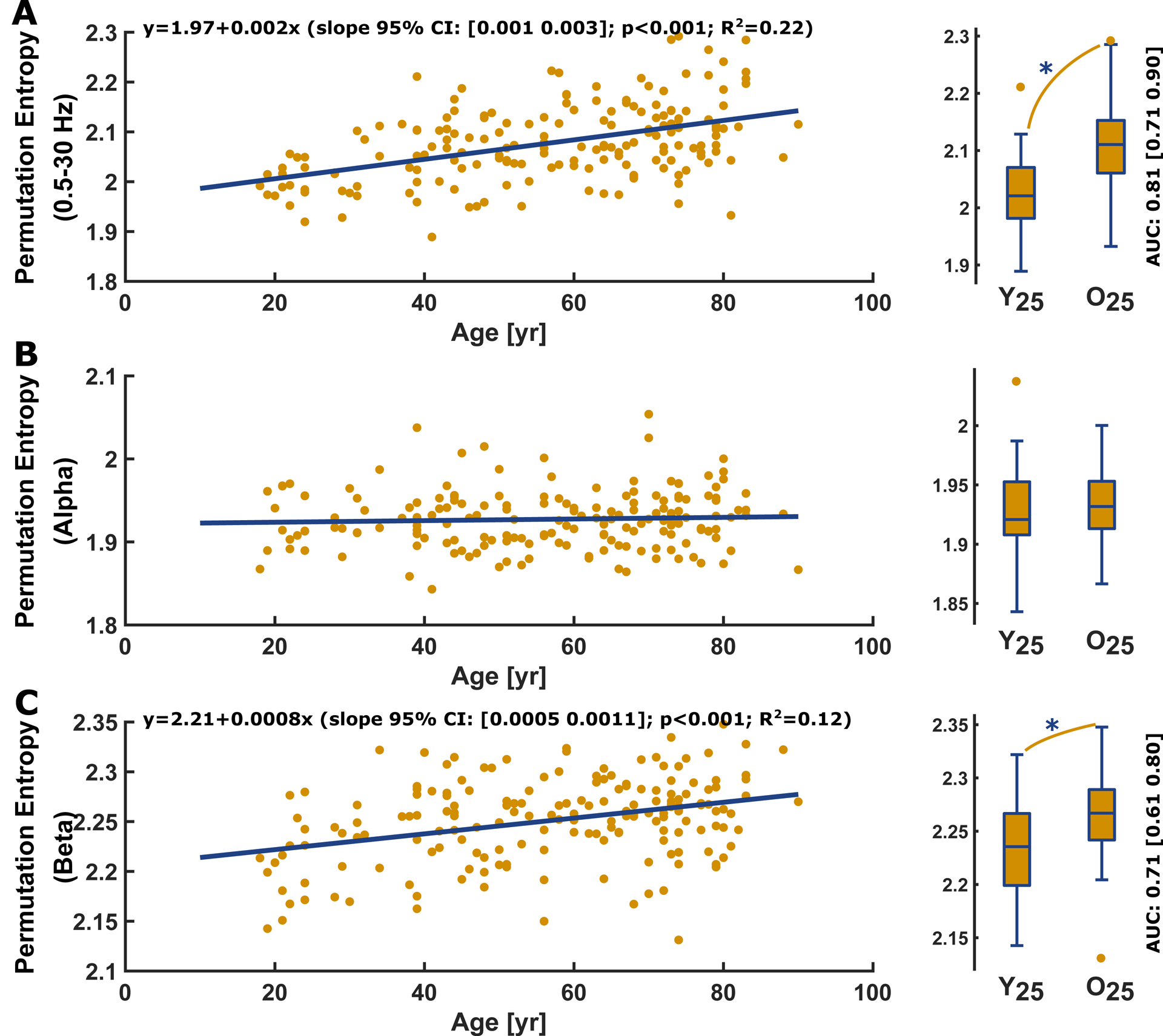
A) Permutation entropy of the 0.5–30 Hz filtered EEG significantly increased (p<0.001, t-statistic: 7.04) with age. Age had a “good” and significant (p<0.001; AUC=0.81 [0.71 0.90]) effect on permutation entropy as depicted in the comparison between Y25 (2.02 [1.98 2.07]) and O25 (2.11 [2.06 2.15]).
B) Permutation entropy of the alpha-band EEG showed no significant age-related effect (p=0.489, t-statistic: 0.69) and the AUC for the comparison between Y25 and O25 indicated no effect (p=0.384; AUC=0.55 [0.43 0.67]).
C) Permutation entropy of the beta-band EEG significantly (p>0.001, t-statistic: 4.95) increased with age. Age had a “fair” and significant (p<0.001; AUC= 0.71 [0.61 0.80]) effect on permutation entropy as depicted in the comparison between Y25 (2.24 [2.20 2.27]) and O25 (2.27 [2.24 2.29]).
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis. Y25: youngest 25% O25: oldest 25%; yr: year
approximate entropy of all three frequency ranges increased with age (Figure 5A–C). The comparisons of approximate entropy for the youngest 25% and the oldest 25% patients revealed a significant and moderate to strong effect of age in the 0.5–30 Hz range (p<0.001, AUC=0.76 [0.66 0.85]), in the EEG alpha range (p=0.002, AUC=0.69 [0.60 0.78]), as well as in the EEG beta range (p=0.007, AUC=0.66 [0.55 0.77]). The fact that approximate entropy, in contrast to permutation entropy, revealed an effect on the alpha-band possibly indicates a higher sensitivity of approximate entropy to lower frequencies.
Figure 5: Approximate entropy (m=2, r=0.2SD, τ=1) vs. age and corresponding youngest vs. oldest quartile box plot for the (A) 0.5–30 Hz EEG range, (B) the EEG alpha range, (C) and the EEG beta range.
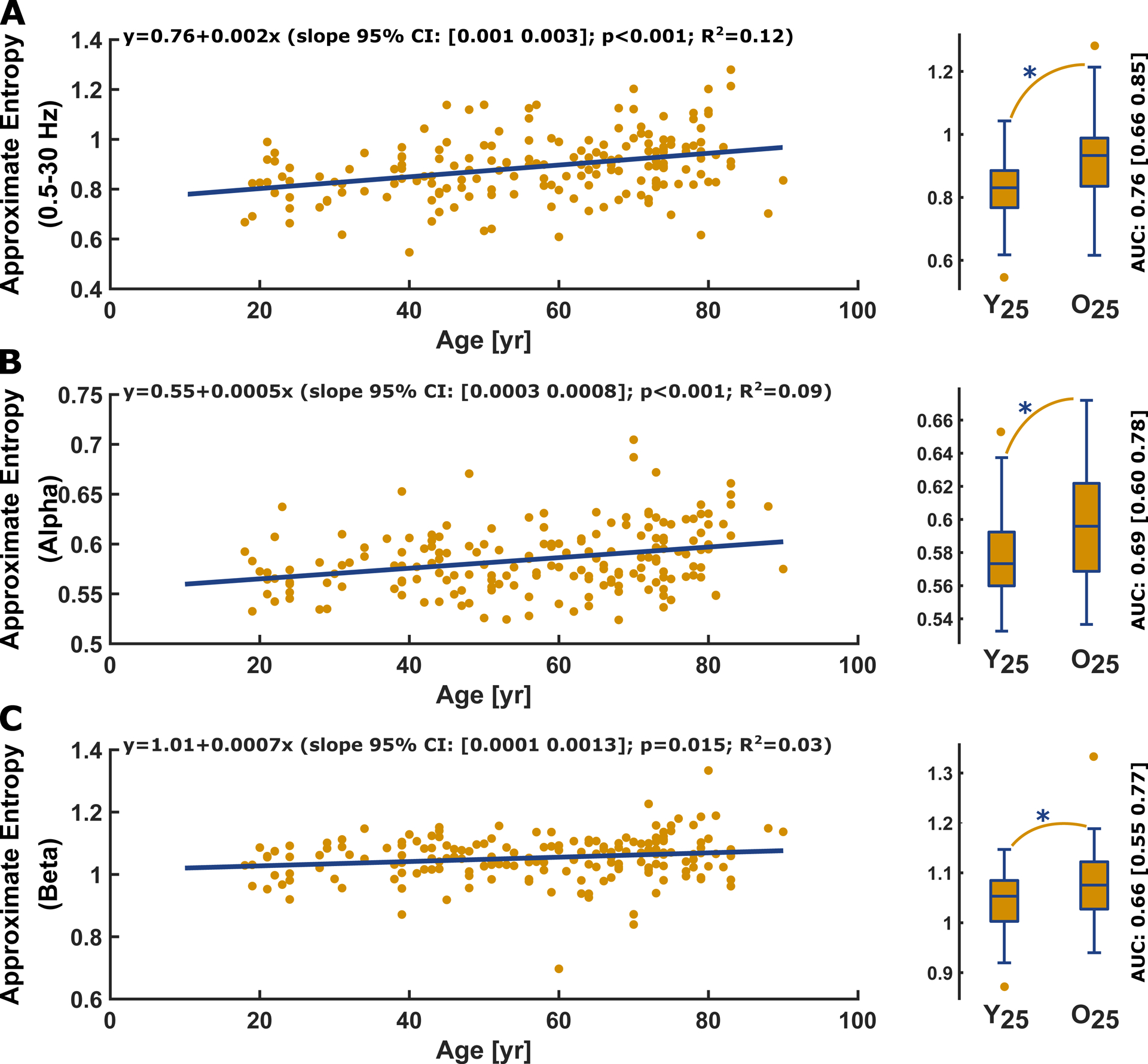
A) Approximate entropy of the 0.5–30 Hz filtered EEG significantly (p<0.001, t-statistic: 4.87) increased with age. Age had a “fair” and significant (p<0.001; AUC= 0.76 [0.66 0.85]) effect on approximate entropy as depicted in the comparison between Y25 (0.83 [0.77 0.89]) and O25 (0.93 [0.84 0.99]).
B) Approximate entropy of the alpha-band EEG significantly (p<0.001, t-statistic 4.18) increased with age. Age had a “poor”/”fair” and significant (p=0.002; AUC= 0.69 [0.60 0.78]) effect on approximate entropy as depicted in the comparison between Y25 (0.57 [0.56 0.59]) and O25 (0.60 [0.57 0.62])
C) Approximate entropy of the beta-band EEG significantly increased with age (p=0.015; AUC=0.66 [0.55 0.77]). Age had a “fair” and significant effect on approximate entropy as depicted in the comparison between O25 (1.08[1.03 1.12]) and Y25 (1.05 [1.00 1.08]) of the data set.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis. Y25: youngest 25% O25: oldest 25%; yr: year
The entropic parameters did not undergo an age-related change in the slower dynamics, i.e., when applied to the EEG filtered to the delta and theta range. We present the detailed statistical parameters in Table 1 and the corresponding regression and box plots in the supplemental Figure S5.
Surrogates
The surrogate analysis revealed a lower regression line for approximate entropy in the alpha and beta band as well as for permutation entropy in the beta band for the original signals. The phase randomization had no influence on the relative alpha- and beta band power. The supplemental Figure S6 shows the corresponding plots.
Monitoring parameters show age-related changes
We used the beta ratio and spectral entropy to estimate a possible influence of age on neurophysiological measures as implemented in commonly used monitoring systems. The BIS revealed a strong dependence on age as did the spectral entropy for the 1.1 to 32 Hz and 1.1 to 47 Hz range (Table 1 and Figure S7). The comparison of the youngest 25% and the oldest 25% revealed significant and fair effects of age on beta ratio (p<0.001, AUC=0.73 [0.63 0.82]) and spectral entropy (1.1–32 Hz: p<0.001, AUC=0.79 [0.70 0.87]; 1.1–47 Hz: p<0.001, AUC=0.80 [0.71 0.88]. For the 0–8 to 32 Hz (p=0.202; AUC=0.58 [0.47 0.68]) or 47 Hz (p=0.161; AUC=0.58 [0.47 0.69]), we could not observe a significant difference with age. These results indicate an influence of age on the (sub-) parameters that are used to track neurophysiological changes in EEG-based monitoring systems which seems strongly dependent on the frequency range. For the sample of 168 patients we could observe an increase of the recorded BIS with age (linear regression: p>0.001, t-statistic: 3.84; youngest 25% vs. the oldest 25%: P=0.026, AUC=0.65 [0.52 0.76])
Discussion
Our results show that age-dependent changes in EEG characteristics during general anesthesia extend beyond a mere decrease in EEG amplitude. Our demonstrable changes in power spectral density of the EEG recorded under general anesthesia have been reported by other groups.8,9 The absolute power decreases with age in every frequency range. After normalization, we found that delta oscillations contributed less to total power with age, while (high) beta oscillations contributed more. The change in the 1/f characteristics as revealed by the fitting oscillations & one over f analysis confirm this finding. We did not observe a significant change in the relative power in the specific bands, but the additional usage of entropic parameters revealed that these parameters are capable of tracking subtler changes in the oscillatory composition of the EEG that are not detected by power spectral density based approaches, also in the alpha- and beta band. The entropic parameters seem to analyze additional content in the signal as shown by surrogate analysis. The higher entropies in the surrogates point towards a loss in deterministic signal properties, as has been also reported earlier.35 The monitoring parameters BIS, BetaRatio, and spectral entropy were also affected by age, a finding highlighting that age adjustments should be considered for monitoring.
Influence of age on EEG amplitude and power spectral density
Reductions in grey matter, including cortical thinning,36–40 or a decrease in skull conductance41 with age cause a decrease in EEG amplitude, and hence lower power spectral density. Furthermore, the EEG amplitude also depends somewhat on neuronal synchrony, but it is unknown at present to what extent this is altered by aging.42
Age influences EEG spectral power
Our power spectral density analyses are in line with previously published findings, i.e., power spectral density decreases with age9. The body of knowledge we can add to these results is the more uniformly distributed normalized power spectral density that is reflected by a flatter (aperiodic) 1/f slope. Schultz et al.8 reported changes in relative band power for propofol anesthesia and other groups for non-rapid eye movement sleep.12,43 Age-related cortical activation during non-rapid eye movement sleep seems to increase relative beta power,12 a scenario that sounds plausible for our findings under general anesthesia as well. Further, aged women had lower relative EEG delta-band and higher beta-band power during wakefulness and rapid eye movement sleep compared to a middle-aged group.44 In general, there may be a number of potential explanations that cause the observed shift in the relative power spectrum. An increase of neural noise may be one of them. Older test subjects had a flatter 1/f slope during visual tasks, due to increased neural noise.42,45 This increase does not have to represent a more aroused brain state since recent research found increased higher beta-frequencies to be associated with poorer memory test outcome in geriatric women.46 Volunteers with eyes closed exhibited higher beta-coherence with age, indicative of higher synchrony in this frequency range.47 But, besides a possible increase in neural noise, the changes in spatiotemporal filtering properties may be due to a change of age-related, physiological changes affecting the cortex40 for instance the extracellular space, which can act as 1/f filter.48 Further, age, and the decline in EEG power also reduces the signal to noise ratio, as e.g. shown in experiments with event-related potentials.49 Our findings may either reflect increased cortical neural noise, i.e., spiking not correlated to oscillatory activity in the elderly brain, the age-induced change of physiological 1/f filtering properties, or changes in the signal to noise ratio of the EEG with age, or a combination of these factors. Our observational study was not designed to closely investigate the cause for the flatter slope. The results further showed that the relative alpha and beta-band power was not affected by age. This information could become important for the design of future monitoring devices but does also question the use of this approach to investigate age-related changes. Entropic measures in the time domain, like approximate entropy and permutation entropy, provide information separate from spectral features.15–17,27 An understanding of both spectral and entropic features may broaden our clinical model of estimations regarding consciousness in patients under general anesthesia
Older patients express higher signal entropy
Our findings showed increasing approximate entropy and permutation entropy (except in the alpha-band) values with age. The results of approximate entropy and permutation entropy differ to some degree, because both parameters may target different EEG characteristics.50 permutation entropy is regarded as superior to approximate entropy in distinguishing conscious from unconscious EEG,16,17,51 while approximate entropy performs better than permutation entropy in tracking different levels of anesthesia.17 These differences are in accordance with the strong effect of age on permutation entropy in the high frequencies (beta-band), as well as the ability of approximate entropy to identify differences in the alpha band – where permutation entropy showed no contrast. The age-related increase in entropic measures seem to apply to other vigilance states and encephalographic modalities as well: in a magnetoencephalography study, permutation entropy increased with age in volunteers that were awake with their eyes closed.52 In general, the increase of entropic measures with age probably reflects the effect on the 1/f slope by indicating a more uniform distribution of ordinal EEG patterns (permutation entropy), and a decreased signal predictability (approximate entropy) in the elderly. In this regard, an association between permutation entropy (for m=3) and the spectral centroid of the (weighted) power spectral density was recently described.53 This proposition may eventually add a general link between spectral analytical approaches and permutation entropy, such that ordinal irregularity may become usable as a proxy for changes in the oscillatory EEG composition. Admittedly, this link is still missing for approximate entropy, though. But these measures seem to track deterministic properties in the signal, in contrast to power spectral density measures. Although other settings of permutation entropy could have tracked age-related changes with higher precision, we do not know the underlying cause for that and hence we refrained from presenting the results in this manuscript. Since using lags of τ>1 could lead to unintended distortions in the signal,53 we chose to apply permutation entropy with τ=1 to EEG filtered to the different frequency bands. In any case, our analyses demonstrate the sensitivity of entropic measures to subtle changes in the EEG.
Reasons for altered EEG characteristics
There is evidence that the aged brain reacts to general (sevoflurane) anesthesia differently than the young brain. In young brains, usually, a peak in the EEG alpha range develops under general anesthesia34 as a marker of adequate anesthesia. This peak in the EEG alpha-band, as well as strong interhemispheric EEG alpha-band coherence34 most probably is associated with thalamocortical pacemaker cells and their activity spreading to the cortex.54 Older and cognitively impaired patients express lower alpha power and alpha coherence during general anesthesia.9,10,55 We did not observe an influence of age using the relative alpha-band power, a finding that is in line with Schultz et al., who found age related differences in relative alpha power only at very profound levels of propofol anesthesia.8
Hence, the described decrease in alpha power may be due to the general decrease in EEG amplitude with age.
Although we did not see an influence of age in relative alpha-band power in our results, approximate entropy of the alpha-band revealed a significant change. Because strong and synchronous (i.e., low approximate entropy) alpha oscillations may correlate with good cognitive function and better outcomes after general anesthesia,10,56,57 this parameter may be useful to identify patients with a ‘frail’ brain using EEG recordings during general anesthesia in the future. For both entropic parameters we observed changes in the EEG beta range. This frequency range seems associated with an activated cortex and intracortical as well as corticocortical information processing.54,58 Hence our findings of a flatter 1/f slope may reflect a state of higher cortical activation in the elderly, or a higher influence of noise. During visual tasks the flatter 1/f slope may represent a decoupling of (cortical) population spiking activity from an oscillatory regimen.42 Furthermore, findings from sleep research indicate that the EEG of older subjects during sleep may be closer to the wake state than in middle-aged to young subjects.44 At the same time, age seems to affect thalamocortical regulatory mechanisms during sleep as expressed by lower sleep spindle density, duration, and amplitude.59 In general, the EEG of older patients may have a smaller dynamic range. During the awake state, the EEG is slower in the older population8 and it shows increased relative beta power during general anesthesia. Hence, the aged brain may not be capable of expressing activated or synchronized activity to the same degree that the young adult brain is capable of. In conclusion, a difference between chronologic vs. functional brain age should be considered to reveal functional-age-related differences in the EEG in more detail. Young patients with potential for having a frail brain can express EEG activity typical for an old patient.55 Furthermore, (mild) cognitive impairments like early-stage Alzheimer’s seem to change the EEG architecture in a similar fashion as aging.60
Implications for titration of anesthesia
We utilized BIS, beta ratio and spectral entropy to estimate the presumed behavior of existing monitoring systems. In general, these parameters exhibited an increase with age. Consequently, our results hint at a possible influence of age on the indices of commonly used EEG monitors (BIS and GE Entropy) towards a lower dose. But the presented BIS values may not correlate with our analyzed EEG segments because of a considerable time delay of up to 60 s.61,62 Still, recent findings from Ni et al. show higher BIS in older adults, hence emphasizing our results.63 At least some of the commercially available monitors were developed using data from rather young adult subjects.64 A study found that at the propofol-induced loss of consciousness, older patients expressed higher BIS and state entropy values, projecting a “more awake” EEG by means of the indices.65 These and our own findings imply that future EEG -based “depth of anesthesia” monitoring systems should account for patient age, or use parameters not affected by age. In our data limited to EEG during unstimulated unconsciousness, relative alpha and beta power did not change with age but showed considerable variability. Hence, a possible use for monitoring purposes has to be investigated more thoroughly.
Limitations
General anesthesia was not conducted by any strict protocol but navigated by best clinical practice. For sevoflurane, we could overcome a possible limitation of age and drug requirement by using age-adjusted MAC estimates.22 While we did find a decrease in residual propofol concentration with age, lower propofol requirements with age have been reported previously.66 Some patients also received opioids, but these concentrations did not show any age-related trend. We did not evaluate the EEG characteristics during general anesthesia with surgical stimulation. We also cannot make a statement regarding age-related EEG changes for other anesthetic drugs triggering different EEG patterns and having different receptor targets – like ketamine or dexmedetomidine. Although, we did not observe any consistent age-related differences in the sevoflurane MAC and the opioid concentration, we cannot completely exclude a complex confounding relationship between age and anesthetics or opioids. To tease out these relationships would require some, specific, tightly controlled, prospective interventional studies. Another limitation is that we only recorded single channel EEG. We could thus not evaluate the influence of age on multivariate parameters, and cannot add information to reported changes in spectral coherence with age.9 And ultimately, the EEG is a signal originating from a large number of (mainly) cortical neurons and transmitted through layers of cerebrospinal fluid, bone, skin, and hair.67 Hence, we refrain from drawing mechanistic conclusions on the receptor level. Still, an age-related influence on inhibitory network activity is highly likely.
In conclusion, we could show that the EEG under general anesthesia changes with age towards activity patterns of higher frequencies that cause a flatter 1/f slope of power spectral density as well as an increase of entropic measures. These changes may be due to changes in neurophysiological filtering properties or the signal to noise ratio. But in general, patient age should be taken into account when using the EEG. Current, EEG -based monitoring approaches do not seem to correct for it.
Supplementary Material
Figure S1: Different drug concentrations versus age; yr: year
A) There was a significant linear trend between sevoflurane MAC and age, before age adjustment.
B) After age adjustment according to Mapleson19 there was no significant trend for sevoflurane MAC and age.
C) There was a significant trend with residual propofol and age.
D) There was no significant trend between opioids delivered and age.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis; yr: year
Figure S2: Power spectral density of the youngest and oldest quartile and the entire data set
A) Median (±median absolute deviation) power spectral density plots of EEG derived from the 46 youngest (blue) and 46 oldest (yellow) patients of the data set. power spectral density is presented with corresponding AUC values and bootstrapped 95% confidence intervals. Solid dots indicate a significant difference between old and young.
B) Absolute power spectral density for each single patient, sorted by age displayed as heat map or spectral array. With age the colors tend to become colder in all frequencies, reflecting the age-dependent decrease in spectral power. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis; yr: year
Figure S3: Power spectral density measures vs. age: Power vs. age and corresponding youngest vs. oldest quartile box plot for the delta power(A), the theta power (B), EEG alpha power (C) and the EEG beta power (D).
A) Delta power decreased with age (p<0.001, t-statistic −10.99) and age had an excellent and significant (p<0.001, (AUC=0.94 [0.89 0.98])) effect as depicted in the Y25 vs. O25 boxplot.
B) Theta power decreased with age (p<0.001, t-statistic −13.13) and age had an excellent and significant (p<0.001, (AUC=0.93 [0.86 0.98])) effect as depicted in Y25 vs. O25 boxplot.
C) Power in the alpha-band significantly (p<0.001, t-statistic −12.25) decreased with age. Age had had an excellent and significant (p<0.001, AUC=0.92 [0.86 0.98]) effect on absolute alpha-band power as depicted in Y25 vs. O25 boxplot
D) EEG beta power significantly (p<0.001, t-statistic −9.74) decreased with age. had an excellent and significant (AUC=0.90 [0.83 0.96]) and significant (p<0.001) effect as depicted in Y25 vs. O25 boxplot.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year
Figure S4: Linear regression and box plots of the youngest vs. the oldest quartile for the (A) relative EEG delta power and the (B) relative EEG theta power. Neither of them showed an age-related trend.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year
Figure S5: Age-induced change of approximate entropy and permutation entropy in the slow dynamics, i.e., the delta and theta range of the EEG.
A) Approximate entropy decreased with age (p=0.029, t-statistic: −2.20), but the fit was quite poor with R2=0.03. The comparison of Y25 vs. O25 did not reveal a significant difference
B) Approximate entropy did not show a n aged-induced trend in the EEG theta range
C) Age did not influence permutation entropy, when applied to the EEG filtered to the delta range.
D) Age did not influence permutation entropy, when applied to the EEG filtered to the theta range.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit.
In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis. Y25: youngest 25% O25: oldest 25%; yr: year
Figure S6: Results from the phase-randomized surrogate analysis of the parameter settings showing significant changes with age, i.e., for the approximate entropy in the alpha- (A) and beta-band (B), the permutation entropy in the beta-band (C), as well as for the relative alpha- (D) and beta-band power (E). yr: year
Figure S7: Age induced-change of (A) bispectral index (BIS), (B) BetaRatio, and spectral entropy for (C) the 0.8–32 Hz and (D) the 1.1–32 Hz range.
A) BIS values increased with age (p<0.001, t-statistic 3.84) and age had a “poor” but significant (p=0.026, AUC=0.65 [0.52 0.76]) effect as depicted in Y25 vs. O25 boxplot.
A) The beta ratio as proxy for the bispectral index increased (p<0.001, t-statistic 5.00) with age. Age had a “fair” (AUC=0.73 [0.63 0.82]) and significant (p<0.001) effect on beta ratio as depicted in Y25 vs. the O25 boxplot.
B) The beta ratio as subparameter of the BIS increased (p<0.001, t-statistic: 5.00) with age. Age had a “fair” and significant (p<0.001, AUC=0.73 [0.63 0.82]) effect on beta ratio as depicted in Y25 vs. O25 boxplot.
C) Spectral entropy for the 1.1–32 Hz range as proxy for the state entropy index from the Entropy Module increased (p<0.001, t-statistic: 5.81) with age. Age had a “fair” to “good” and significant (p<0.001, AUC=0.79 [0.70 0.87]) effect as depicted in the youngest 25% Y25 vs. the oldest 25% O25 boxplot.
D) Spectral entropy for the 0.8–32 Hz range as proxy for the state entropy index from the did not show a significant influence of age on spectral entropy (p=0.433, t-statistic: 0.79) with age. Age no effect (p=0.201, AUC=0.58 [0.47 0.68]) effect as depicted in Y25 vs. O25 boxplot.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year
Funding Statement:
The research effort of Dr. García is supported in part by a Career Development Award #BX00167 (PI: PS García, MD, PhD) from the United States Department of Veteran Affairs, by the Biomedical Laboratory Research and Development Service and the James S. McDonnell Foundation Grant #220023046 (PI: PS García, MD, PhD).
Footnotes
Conflicts of Interest: The authors declare no competing interests
References
- 1.Delafuente JC: The silver tsunami is coming: will pharmacy be swept away with the tide? Am. J. Pharm. Educ 2009; 73: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etzioni DA, Liu JH, Maggard MA, Ko CY: The aging population and its impact on the surgery workforce. Ann. Surg 2003; 238: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, Escallier KE, Ben Abdallah A, Lin N, Avidan MS: Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth. Analg 2016; 122: 234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radtke F, Franck M, Hagemann L, Seeling M, Wernecke K, Spies C: Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. Minerva Anestesiol. 2010; 76: 394–403 [PubMed] [Google Scholar]
- 5.Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW: Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit. Care Med 2008; 36: 3171–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radtke F, Franck M, Lendner J, Krüger S, Wernecke K, Spies C: Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110: i98–i105 [DOI] [PubMed] [Google Scholar]
- 7.Wildes TS, Mickle AM, Ben Abdallah A, Maybrier HR, Oberhaus J, Budelier TP, Kronzer A, McKinnon SL, Park D, Torres BA, Graetz TJ, Emmert DA, Palanca BJ, Goswami S, Jordan K, Lin N, Fritz BA, Stevens TW, Jacobsohn E, Schmitt EM, Inouye SK, Stark S, Lenze EJ, Avidan MS: Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. JAMA 2019; 321: 473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz A, Grouven U, Zander I, Beger FA, Siedenberg M, Schultz B: Age-related effects in the EEG during propofol anaesthesia. Acta Anaesthesiol Scand 2004; 48: 27–34 [DOI] [PubMed] [Google Scholar]
- 9.Purdon P, Pavone K, Akeju O, Smith A, Sampson A, Lee J, Zhou D, Solt K, Brown E: The Ageing Brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth 2015; 115: i46–i57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giattino C, Gardner J, Sbahi F, Roberts K, Cooter M, Moretti E, Browndyke J, Mathew J, Woldorff M, Berger M: Intraoperative Frontal Alpha-Band Power Correlates with Preoperative Neurocognitive Function in Older Adults. Front. Syst. Neurosci 2017; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polich J: EEG and ERP assessment of normal aging. Electroencephalography and Clinical Neurophysiology/ Evoked Potentials Section 1997; 104: 244–256 [DOI] [PubMed] [Google Scholar]
- 12.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH: The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 2001; 38: 232–242 [PubMed] [Google Scholar]
- 13.Bandt C, Pompe B: Permutation Entropy: A Natural Complexity Measure for Time Series. Phys. Rev. Lett 2002; 88: 174102. [DOI] [PubMed] [Google Scholar]
- 14.Pincus SM: Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. U. S. A 1991; 88: 2297–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olofsen E, Sleigh JW, Dahan A: Permutation entropy of the electroencephalogram: a measure of anaesthetic drug effect. Br J Anaesth 2008; 101: 810–21 [DOI] [PubMed] [Google Scholar]
- 16.Jordan D, Stockmanns G, Kochs EF, Pilge S, Schneider G: Electroencephalographic Order Pattern Analysis for the Separation of Consciousness and Unconsciousness: An Analysis of Approximate Entropy, Permutation Entropy, Recurrence Rate, and Phase Coupling of Order Recurrence Plots. Anesthesiology 2008; 109: 1014–1022 [DOI] [PubMed] [Google Scholar]
- 17.Schneider G, Jordan D, Schwarz G, Bischoff P, Kalkman CJ, Kuppe H, Rundshagen I, Omerovic A, Kreuzer M, Stockmanns G, Kochs EF: Monitoring depth of anesthesia utilizing a combination of electroencephalographic and standard measures. Anesthesiology 2014; 120: 819–28 [DOI] [PubMed] [Google Scholar]
- 18.Rampil IJ: A Primer for EEG Signal Processing in Anesthesia. Anesthesiology 1998; 89: 980–1002 [DOI] [PubMed] [Google Scholar]
- 19.Viertio-Oja H, Maja V, Sarkela M, Talja P, Tenkanen N, Tolvanen-Laakso H, Paloheimo M, Vakkuri A, Yli-Hankala A, Merilainen P: Description of the Entropy algorithm as applied in the Datex-Ohmeda S/5 Entropy Module. Acta Anaesthesiol Scand 2004; 48: 154–61 [DOI] [PubMed] [Google Scholar]
- 20.Hight D, Voss LJ, Garcia PS, Sleigh J: Changes in Alpha Frequency and Power of the Electroencephalogram during Volatile-Based General Anesthesia. Front. Syst. Neurosci 2017; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKay ID, Voss LJ, Sleigh JW, Barnard JP, Johannsen EK: Pharmacokinetic-pharmacodynamic modeling the hypnotic effect of sevoflurane using the spectral entropy of the electroencephalogram. Anesth. Analg 2006; 102: 91–97 [DOI] [PubMed] [Google Scholar]
- 22.Mapleson W: Effect of age on MAC in humans: a meta-analysis. Br J Anaesth 1996; 76: 179–185 [DOI] [PubMed] [Google Scholar]
- 23.Mazoit JX, Butscher K, Samii K: Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth. Analg 2007; 105: 70–78 [DOI] [PubMed] [Google Scholar]
- 24.Shafer SL, Varvel JR: Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology 1991; 74: 53–63 [DOI] [PubMed] [Google Scholar]
- 25.Wiczling P, Bienert A, Sobczyński P, Hartmann-Sobczyńska R, Bieda K, Marcinkowska A, Malatyńska M, Kaliszan R, Grześkowiak E: Pharmacokinetics and pharmacodynamics of propofol in patients undergoing abdominal aortic surgery. Pharmacol. Rep 2012; 64: 113–122 [DOI] [PubMed] [Google Scholar]
- 26.Haller M, Donoghue T, Peterson E, Varma P, Sebastian P, Gao R, Noto T, Knight RT, Shestyuk A, Voytek B: Parameterizing neural power spectra. bioRxiv 2018: 299859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruhn J, Ropcke H, Hoeft A: Approximate Entropy as an Electroencephalographic Measure of Anesthetic Drug Effect during Desflurane Anesthesia. Anesthesiology 2000; 92: 715–726 [DOI] [PubMed] [Google Scholar]
- 28.Nicolaou N, Houris S, Alexandrou P, Georgiou J: Entropy measures for discrimination of ‘awake’ Vs ‘anaesthetized’ state in recovery from general anesthesia. Conf Proc IEEE Eng Med Biol Soc 2011; 2011: 2598–601 [DOI] [PubMed] [Google Scholar]
- 29.Shannon C: A Mathematical Theory of Communication. Bell Syst. Tech. J 1948; 27: (379–423):623–56 [Google Scholar]
- 30.Kreuzer M: EEG Based Monitoring of General Anesthesia: Taking the Next Steps. Front. Comput. Neurosci 2017; 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberger A, Amaral L, Glass L, Hausdorff J, Ivanov P, Mark R, Mietus J, Moody G, Peng C, Stanley H: PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000; 101: e215–e220 [DOI] [PubMed] [Google Scholar]
- 32.Schneider G, Schöniger S, Kochs E: Does bispectral analysis add anything but complexity? BIS sub-components may be superior to BIS for detection of awareness. Br J Anaesth 2004; 93: 596–597 [DOI] [PubMed] [Google Scholar]
- 33.Hentschke H, Stüttgen MC: Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci 2011; 34: 1887–1894 [DOI] [PubMed] [Google Scholar]
- 34.Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, Purdon PL: Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 2014; 121: 990–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan D, Stockmanns G, Kochs EF, Schneider G: Is Detection of Different Anesthetic Levels Related to Nonlinearity of the Electroencephalogram?, 4th European Conference of the International Federation for Medical and Biological Engineering. Edited by Sloten J, Verdonck P, Nyssen M, Haueisen J, Springer Berlin Heidelberg, 2009, pp 335–339 [Google Scholar]
- 36.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD: Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 2005; 15: 1676–1689 [DOI] [PubMed] [Google Scholar]
- 37.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B: Thinning of the cerebral cortex in aging. Cereb. Cortex 2004; 14: 721–730 [DOI] [PubMed] [Google Scholar]
- 38.Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM: One-Year Brain Atrophy Evident in Healthy Aging. J. Neurosci 2009; 29: 15223–15231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B: High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex 2009: bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson PM, Hayashi KM, Dutton RA, CHIANG MC, Leow AD, Sowell ER, De Zubicaray G, Becker JT, Lopez OL, Aizenstein HJ: Tracking Alzheimer’s disease. Ann. N. Y. Acad. Sci 2007; 1097: 183–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoekema R, Wieneke GH, Leijten FSS, van Veelen CWM, van Rijen PC, Huiskamp GJM, Ansems J, van Huffelen AC: Measurement of the conductivity of skull, temporarily removed during epilepsy surgery. Brain Topogr. 2003; 16: 29–38 [DOI] [PubMed] [Google Scholar]
- 42.Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, Gazzaley A: Age-Related Changes in 1/f Neural Electrophysiological Noise. J Neurosci 2015; 35: 13257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinoy ED, Frey DJ, Kaslovsky DN, Meyer FG, Wright KP: Age-related changes in slow wave activity rise time and NREM sleep EEG with and without zolpidem in healthy young and older adults. Sleep Med. 2014; 15: 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce EN, Bruce MC, Vennelaganti S: Sample entropy tracks changes in EEG power spectrum with sleep state and aging. J. Clin. Neurophysiol 2009; 26: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dave S, Brothers TA, Swaab TY: 1/f neural noise and electrophysiological indices of contextual prediction in aging. Brain Research 2018; 1691: 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiser AK, Doppelmayr M, Iglseder B: EEG beta 2 power as surrogate marker for memory impairment: a pilot study. Int. Psychogeriatr 2017; 29: 1515–1523 [DOI] [PubMed] [Google Scholar]
- 47.Vysata O, Kukal J, Prochazka A, Pazdera L, Simko J, Valis M: Age-related changes in EEG coherence. Neurol. Neurochir. Pol 2014; 48: 35–38 [DOI] [PubMed] [Google Scholar]
- 48.Bedard C, Kroger H, Destexhe A: Does the 1/f frequency scaling of brain signals reflect self-organized critical states? Phys. Rev. Lett 2006; 97: 118102. [DOI] [PubMed] [Google Scholar]
- 49.Hammerer D, Li SC, Volkle M, Muller V, Lindenberger U: A lifespan comparison of the reliability, test-retest stability, and signal-to-noise ratio of event-related potentials assessed during performance monitoring. Psychophysiology 2013; 50: 111–23 [DOI] [PubMed] [Google Scholar]
- 50.Keller K, Mangold T, Stolz I, Werner J: Permutation Entropy: New Ideas and Challenges. Entropy 2017; 19: 134 [Google Scholar]
- 51.Brown EN, Lydic R, Schiff ND: General Anesthesia, Sleep, and Coma. N. Engl. J. Med 2010; 363: 2638–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shumbayawonda E, Fernández A, Hughes MP, Abásolo D: Permutation Entropy for the Characterisation of Brain Activity Recorded with Magnetoencephalograms in Healthy Ageing. Entropy 2017; 19: 141 [Google Scholar]
- 53.Berger S, Schneider G, Kochs EF, Jordan D: Permutation Entropy: Too Complex a Measure for EEG Time Series? Entropy 2017; 19: 692 [Google Scholar]
- 54.John ER, Prichep LS: The Anesthetic Cascade: A Theory of How Anesthesia Suppresses Consciousness. Anesthesiology 2005; 102: 447–471 [DOI] [PubMed] [Google Scholar]
- 55.Kreuzer M, Whalin MK, Hesse SD, Riso MA, García PS: Anesthetic Management of a Patient With Multiple Previous Episodes of Postanesthesia Care Unit Delirium: A Case Report. A&A Case Reports 2017; 8: 311–315 [DOI] [PubMed] [Google Scholar]
- 56.Chander D, Garcia PS, MacColl JN, Illing S, Sleigh JW: Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PloS one 2014; 9: e106291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hesse S, Kreuzer M, Hight D, Gaskell A, Devari P, Singh D, Taylor N, Whalin M, Lee S, Sleigh J: Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the post-anaesthesia care unit: an early sign of postoperative complications. Br J Anaesth 2019; 122: 622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R: Cognitive fitness of cost-efficient brain functional networks. Proc. Natl. Acad. Sci. U. S. A 2009; 106: 11747–11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM: The effects of normal aging on sleep spindle and K-complex production. Clin. Neurophysiol 2002; 113: 1615–1622 [DOI] [PubMed] [Google Scholar]
- 60.Frantzidis CA, Ladas A, Diamantoudi MD, Semertzidou A, Grigoriadou E, Tsolaki A, Liapi D, Papadopoulou A, Kounti F, Vivas AB: What are the symbols of Alzheimer? A permutation entropy based symbolic analysis for the detection of early changes of the electroencephalographic complexity due to mild Alzheimer, Bioinformatics & Bioengineering (BIBE), 2012 IEEE 12th International Conference on, IEEE, 2012, pp 282–287 [Google Scholar]
- 61.Pilge S, Zanner R, Schneider G, Blum J, Kreuzer M, Kochs E: Time Delay of Index Calculation: Analysis of Cerebral State, Bispectral, and Narcotrend Indices. Anesthesiology 2006; 104: 488–494 [DOI] [PubMed] [Google Scholar]
- 62.Zanner R, Pilge S, Kochs EF, Kreuzer M, Schneider G: Time delay of electroencephalogram index calculation: analysis of cerebral state, bispectral, and Narcotrend indices using perioperatively recorded electroencephalographic signals. Br J Anaesth 2009; 103: 394–399 [DOI] [PubMed] [Google Scholar]
- 63.Ni K, Cooter M, Gupta DK, Thomas J, Hopkins TJ, Miller TE, James ML, Kertai MD, Berger M: Paradox of age: older patients receive higher age-adjusted minimum alveolar concentration fractions of volatile anaesthetics yet display higher bispectral index values. Br J Anaesth 2019; 123: 288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prichep L, Gugino L, John E, Chabot R, Howard B, Merkin H, Tom M, Wolter S, Rausch L, Kox W: The Patient State Index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth 2004; 92: 393–399 [DOI] [PubMed] [Google Scholar]
- 65.Lysakowski C, Elia N, Czarnetzki C, Dumont L, Haller G, Combescure C, Tramer MR: Bispectral and spectral entropy indices at propofol-induced loss of consciousness in young and elderly patients. Br J Anaesth 2009; 103: 387–93 [DOI] [PubMed] [Google Scholar]
- 66.Dundee J, Robinson FP, McCollum J, Patterson C: Sensitivity to propofol in the elderly. Anaesthesia 1986; 41: 482–485 [DOI] [PubMed] [Google Scholar]
- 67.Teplan M: Fundamentals of EEG measurement. Meas. Sci. Rev 2002; 2: 1–11 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Different drug concentrations versus age; yr: year
A) There was a significant linear trend between sevoflurane MAC and age, before age adjustment.
B) After age adjustment according to Mapleson19 there was no significant trend for sevoflurane MAC and age.
C) There was a significant trend with residual propofol and age.
D) There was no significant trend between opioids delivered and age.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis; yr: year
Figure S2: Power spectral density of the youngest and oldest quartile and the entire data set
A) Median (±median absolute deviation) power spectral density plots of EEG derived from the 46 youngest (blue) and 46 oldest (yellow) patients of the data set. power spectral density is presented with corresponding AUC values and bootstrapped 95% confidence intervals. Solid dots indicate a significant difference between old and young.
B) Absolute power spectral density for each single patient, sorted by age displayed as heat map or spectral array. With age the colors tend to become colder in all frequencies, reflecting the age-dependent decrease in spectral power. In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis; yr: year
Figure S3: Power spectral density measures vs. age: Power vs. age and corresponding youngest vs. oldest quartile box plot for the delta power(A), the theta power (B), EEG alpha power (C) and the EEG beta power (D).
A) Delta power decreased with age (p<0.001, t-statistic −10.99) and age had an excellent and significant (p<0.001, (AUC=0.94 [0.89 0.98])) effect as depicted in the Y25 vs. O25 boxplot.
B) Theta power decreased with age (p<0.001, t-statistic −13.13) and age had an excellent and significant (p<0.001, (AUC=0.93 [0.86 0.98])) effect as depicted in Y25 vs. O25 boxplot.
C) Power in the alpha-band significantly (p<0.001, t-statistic −12.25) decreased with age. Age had had an excellent and significant (p<0.001, AUC=0.92 [0.86 0.98]) effect on absolute alpha-band power as depicted in Y25 vs. O25 boxplot
D) EEG beta power significantly (p<0.001, t-statistic −9.74) decreased with age. had an excellent and significant (AUC=0.90 [0.83 0.96]) and significant (p<0.001) effect as depicted in Y25 vs. O25 boxplot.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year
Figure S4: Linear regression and box plots of the youngest vs. the oldest quartile for the (A) relative EEG delta power and the (B) relative EEG theta power. Neither of them showed an age-related trend.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year
Figure S5: Age-induced change of approximate entropy and permutation entropy in the slow dynamics, i.e., the delta and theta range of the EEG.
A) Approximate entropy decreased with age (p=0.029, t-statistic: −2.20), but the fit was quite poor with R2=0.03. The comparison of Y25 vs. O25 did not reveal a significant difference
B) Approximate entropy did not show a n aged-induced trend in the EEG theta range
C) Age did not influence permutation entropy, when applied to the EEG filtered to the delta range.
D) Age did not influence permutation entropy, when applied to the EEG filtered to the theta range.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit.
In the boxplots, the circles indicate outliers as defined by the MATLAB plotting routine. They were not excluded from analysis. Y25: youngest 25% O25: oldest 25%; yr: year
Figure S6: Results from the phase-randomized surrogate analysis of the parameter settings showing significant changes with age, i.e., for the approximate entropy in the alpha- (A) and beta-band (B), the permutation entropy in the beta-band (C), as well as for the relative alpha- (D) and beta-band power (E). yr: year
Figure S7: Age induced-change of (A) bispectral index (BIS), (B) BetaRatio, and spectral entropy for (C) the 0.8–32 Hz and (D) the 1.1–32 Hz range.
A) BIS values increased with age (p<0.001, t-statistic 3.84) and age had a “poor” but significant (p=0.026, AUC=0.65 [0.52 0.76]) effect as depicted in Y25 vs. O25 boxplot.
A) The beta ratio as proxy for the bispectral index increased (p<0.001, t-statistic 5.00) with age. Age had a “fair” (AUC=0.73 [0.63 0.82]) and significant (p<0.001) effect on beta ratio as depicted in Y25 vs. the O25 boxplot.
B) The beta ratio as subparameter of the BIS increased (p<0.001, t-statistic: 5.00) with age. Age had a “fair” and significant (p<0.001, AUC=0.73 [0.63 0.82]) effect on beta ratio as depicted in Y25 vs. O25 boxplot.
C) Spectral entropy for the 1.1–32 Hz range as proxy for the state entropy index from the Entropy Module increased (p<0.001, t-statistic: 5.81) with age. Age had a “fair” to “good” and significant (p<0.001, AUC=0.79 [0.70 0.87]) effect as depicted in the youngest 25% Y25 vs. the oldest 25% O25 boxplot.
D) Spectral entropy for the 0.8–32 Hz range as proxy for the state entropy index from the did not show a significant influence of age on spectral entropy (p=0.433, t-statistic: 0.79) with age. Age no effect (p=0.201, AUC=0.58 [0.47 0.68]) effect as depicted in Y25 vs. O25 boxplot.
In the regression plots, the yellow dots present the single patients and the blue line the linear fit. Y25: youngest 25% O25: oldest 25%; yr: year


