Abstract
Background
In 2014, the U.S. Drug Enforcement Agency (DEA) reclassified hydrocodone from Schedule III to Schedule II of the Controlled Substances Act, resulting in new restrictions on refills. We hypothesized that hydrocodone rescheduling led to in decreases in total opioid dispensing within 30 days of surgery and reduced new long-term opioid dispensing among surgical patients.
Methods
We studied privately-insured, opioid-naïve adults undergoing 10 general or orthopedic surgeries between 2011 and 2015. We conducted a differences-in-differences analysis that compared overall opioid dispensing before versus after the rescheduling rule for patients treated by surgeons who frequently prescribed hydrocodone before rescheduling (i.e. patients who were functionally “exposed” to rescheduling’s impact) while adjusting for secular trends via a comparison group of patients treated by surgeons who rarely prescribed hydrocodone (i.e. “unexposed” patients). The primary outcome was any filled opioid prescription between 90 and 180 days after surgery; secondary outcomes included the 30-day refill rate and the amount of opioids dispensed initially and at 30 days postoperatively.
Results
The sample included 65,136 patients. The percentage of patients filling a prescription beyond 90 days was similar after versus before rescheduling (absolute risk difference, −1.1%; 95% CI −2.3%, 0.1%, P=0.084).We estimated the rescheduling rule to be associated with a 45.4 mg oral morphine equivalent increase (difference-in-differences estimate; 95% CI: 34.2 mg, 56.7 mg, P < 0.001) in initial opioid dispensing; a 4.1% absolute decrease (95% CI: −5.5%, −2.7%, P < 0.001) in refills within 30 days; and a 37.7mg oral morphine equivalent increase (95% CI: 20.6 mg, 54.8 mg, P = 0.008) in opioids dispensed within 30 days.
Conclusions
Among patients treated by surgeons who frequently prescribed hydrocodone before the DEA 2014 hydrocodone rescheduling rule, rescheduling did not impact long-term opioid receipt, although it was associated with an increase in opioid dispensing within 30 days of surgery.
INTRODUCTION
In October 2014, the US Drug Enforcement Agency (DEA) reclassified hydrocodone from Schedule III to Schedule II of the Controlled Substances Act,1 prohibiting refills from being written in individual hydrocodone prescriptions. At a population level, rescheduling was associated with decreases in dispensing of hydrocodone2,3 specifically and opioids overall.4 However, its impact on postoperative opioid dispensing remains unclear; one analysis found rescheduling to have unintentionally increased opioid dispensing immediately after surgery,5 while another observed no difference in opioid dispensing after versus before rescheduling.6 Both analyses were limited by not having accounted for secular trends in opioid dispensing and neither examined rescheduling’s impact on distal outcomes such as new long-term opioid receipt among previously opioid-naïve individuals.
Understanding rescheduling’s impact on postoperative opioid dispensing has importance for health policy. Multiple policy interventions have targeted excess opioid dispensing for acute indications,7,8 and limiting acute prescribing has been theorized to prevent development of new long-term opioid use.9–14 Hydrocodone rescheduling may have altered short-term postoperative opioid dispensing by limiting refills or by unintentionally encouraging larger initial prescriptions; therefore, rescheduling provides an opportunity to examine the impact of changes in short-term postoperative opioid dispensing on new long-term opioid receipt.
We tested the impact of hydrocodone rescheduling on overall opioid dispensing (i.e. dispensing of hydrocodone or another opioid analgesic) in a sample of commercially insured US adults undergoing 10 general or orthopedic surgeries. To account for secular prescribing trends, we used a “difference-in-differences”15 approach that compared dispensing outcomes after versus before rescheduling across groups of patients who were more versus less likely to have been impacted by the policy change based on their surgeon’s tendency to prescribe hydrocodone prior to the schedule change. We hypothesized that rescheduling was associated with a decrease in opioid dispensing within 30 days after surgery (due to a decrease in refills) and, consequently, with a decrease in the rate of opioid dispensing beyond 90 days after surgery.
MATERIALS AND METHODS
Policy context
The DEA’s final rule regarding hydrocodone rescheduling was published on August 21, 2014 and took effect on October 6, 2014.1 After this date, initial hydrocodone prescriptions could no longer include refills and could not be “called in” by phone to pharmacies, aligning with rules applicable to most other opioids.
Overview of study design
A data analysis and statistical plan was written and posted on a publically accessible server (arxiv.org) after the data were accessed.16 Briefly, we designed a difference-in-differences analysis that divided patients into “exposed” and “unexposed” groups based on the relative impact that we anticipated rescheduling would have on their care. Since medication selection tends to be stable over time within prescribers,17–19 we reasoned that opioid dispensing would be unlikely to vary as a direct consequence of the rescheduling rule for patients treated by surgeons who rarely prescribed hydrocodone before rescheduling. Conversely, we reasoned that hydrocodone rescheduling could impact care received by patients whose surgeons frequently prescribed hydrocodone before rescheduling. We estimated the impact of hydrocodone rescheduling by comparing opioid prescribing patterns after versus before rescheduling among patients treated by clinicians who frequently prescribed hydrocodone prior to rescheduling (i.e. those functionally “exposed” to the policy effect of the rule) versus those treated by clinicians who rarely prescribed hydrocodone (“unexposed” patients).
Data
We used data from the 2004–2016 Optum® de-identifed Clinformatics® Data Mart Database, a US health insurance database that includes approximately 17–19 million annual covered lives and comprises both commercial and Medicare Advantage health plan data. The population is geographically diverse, spanning all 50 states, and includes medical and pharmacy claims and tables with member eligibility and inpatient confinement data. Information on filled opioid prescriptions was obtained from pharmacy claims files within the Optum database; the database did not include information on prescriptions that were issued but not filled.
Characterizing provider prescribing before rescheduling
Using uniform provider identifiers in the study database, we identified all individual surgeons or medical group practices submitting 5 or more claims between August 22, 2011 and August 21, 2014 for any of 10 common ambulatory or short-stay orthopedic or general surgeries among patients who filled an opioid prescription within 7 days after the procedure. Relevant opioids included oral analgesic formulations of codeine, hydrocodone, hydromorphone, levorphanol, meperidine, morphine, oxycodone, oxymorphone, pentazocine, tramadol, fentanyl, and tapentadol. Eligible procedures included: laparoscopic cholecystectomy; open cholecystectomy; inguinal hernia repair; laparoscopic appendectomy; open appendectomy; breast excision; carpal tunnel release; knee arthroscopy; total knee replacement; and total hip replacement, as identified by Current Procedural Terminology codes (Appendix 1). For each provider, we calculated the proportion of filled initial postoperative opioid prescriptions accounted for by hydrocodone products. Providers for whom hydrocodone products represented at least 75% of initial filled prescriptions were classified as “hydrocodone prescribers;” providers for whom hydrocodone accounted for 25% or fewer of initial filled prescriptions were classified as “hydrocodone non-prescribers.”
Defining the study sample
In order to permit a sufficient window of observation to confirm the absence of a notable trend in key outcome measures prior to the policy change,20,21 we defined the 3 years before the final rule date (August 22, 2011 through August 21, 2014) as the “pre-implementation” period. We defined the year after the effective date (October 6, 2014 through October 5, 2015) as the “post-implementation” period. We included patients aged 18 years or older who had any of the above surgical procedures during the pre- or post-implementation period based on the procedure or hospital discharge date, whichever came later. We restricted our sample to patients treated by hydrocodone prescribers and hydrocodone non-prescribers as defined above. Patients treated by hydrocodone prescribers were classified as “exposed;” patients treated by hydrocodone non-prescribers were classified as “unexposed.” Patients treated by clinicians prescribing hydrocodone products for between 25% and 75% of cases were excluded because we could not attribute changes in dispensing for these patients to the impact of rescheduling versus other factors.
For patients with more than one eligible surgery, we used the first available claim. To permit uniform windows for assessment of patient characteristics and outcomes, we restricted the sample to patients who had at least 90 days of continuous enrollment before the procedure or admission date (whichever came first) and at least 180 days of enrollment after the procedure or discharge date (whichever came last). Since we aimed to examine the impact of rescheduling on the incidence of new long-term opioid dispensing, we restricted the sample to individuals with no filled opioid prescriptions in the 90 days prior to surgery (i.e. “opioid-naïve” individuals). Finally, after confirming in preliminary analyses that the rate of any filled opioid prescription within 7 days after surgery was similar after versus before rescheduling for exposed versus unexposed patients, we restricted our sample to patients who filled at least one opioid prescription within 7 days of their procedure.
Outcome
Our primary outcome was a filled prescription for any of the 12 above-listed opioids between 90 and 180 days after surgery.12,22,23 In separate work, our group evaluated the sensitivity and specificity of 24 measures of long-term opioid dispensing for predicting opioid-related adverse events in the year after surgery, we found this measure to have similarly high sensitivity (sensitivity: 95%) with a higher degree of specificity than most other measures (sensitivity: 12%).24 Secondary outcomes included (1) the total amount of opioid dispensed in the first postoperative prescription filled within 7 days of surgery or discharge as measured in milligram (mg) oral morphine equivalents (OME);25 (2) filling of a refill prescription for any opioid refill in the first 30 days after surgery; and (3) the total amount of opioids dispensed across all filled prescriptions within the first 30 days after surgery or discharge in mg OME.
Covariates
We obtained demographic data from registration files. We defined baseline comorbidities using pharmacy claims and International Classification of Disease-9-Clinical Modification diagnosis codes listed from inpatient and outpatient encounters during the 90 days prior to surgery.26,27 We created variables for surgery type, length of hospital stay, and whether the provider submitting the claim for the index procedure was an individual practitioner or a group practice.
Statistical Analysis
Initial analyses compared baseline characteristics and outcomes of exposed versus unexposed patients using chi-squared tests and two-sample t-tests. We explored changes in outcomes before versus after rescheduling by plotting each outcome for exposed versus unexposed patients in the pre- and post-implementation periods.
We next carried out our difference-in-differences analysis. This analysis estimated changes in opioid dispensing among exposed patients (those treated by hydrocodone prescribers) between the post-implementation and pre-implementation periods, and quantified outcome differences between exposed and unexposed patients (those treated by hydrocodone non-prescribers). This approach allowed us to account for other contemporaneous influences on opioid dispensing, which would be reflected in trends among unexposed patients.15,28
Specifically, we fit multivariable linear regression models to predict each study outcome; analyses of binary outcomes were confirmed using logistic regression. Robust standard errors were used to account for clustering of observations within providers.29 As we anticipated low rates of missing data for key outcomes or covariates, our models handled missing data via complete case analysis (i.e. individuals with missing data on any covariate were excluded from study models). All models included an interaction term between exposure status (exposed versus unexposed) and period (pre- versus post-implementation), which allowed us to estimate how adjusted outcomes varied between exposed and unexposed patients after versus before rescheduling. In order to adjust for confounding due to patent, procedure, and provider characteristics that could differ between exposed patients, all models also adjusted for demographics, comorbidities, surgery type, length of stay, and provider type (individual versus group practice). Age and length of stay were entered into the model as continuous variables without transformation. The difference-in-differences estimate represents the effect of hydrocodone rescheduling on exposed patients, accounting for secular trends and the above-named covariates.15,28 No formal statistical power calculations were conducted; all analyses were based on the available data.
Supplementary analyses
Since postoperative opioid selection could differ for ambulatory surgery patients versus inpatients, we conducted subgroup analyses restricted to ambulatory surgery patients. As reported in the technical preprint,16 we assessed whether patients in the exposed versus unexposed groups had parallel pre-implementation outcome trends using standard methods.15 These analyses found no evidence for violations of the parallel trends assumption for 3 of 4 study outcomes but did find a small but statistically significant difference across study groups in trends over time for the total amount of opioid dispensed in the initial filled postoperative prescription. As such, we confirmed all findings in supplemental differences-in-differences regressions that formally modeled differential trends between exposed and unexposed groups over time (see additional methods in Appendix 2).30 Finally, to assess the robustness of our findings to alternate definitions of exposed versus unexposed groups, we repeated our analyses using more restrictive and more inclusive thresholds for categorizing surgeons as hydrocodone prescribers or non-prescribers.
Analysis of the complete study database began only after publication of our technical preprint on June 10, 2019 and used SAS version 9.4 (SAS Institute, Cary, NC). All hypothesis tests were two-tailed; we considered P<0.05 to indicate statistical significance.
RESULTS
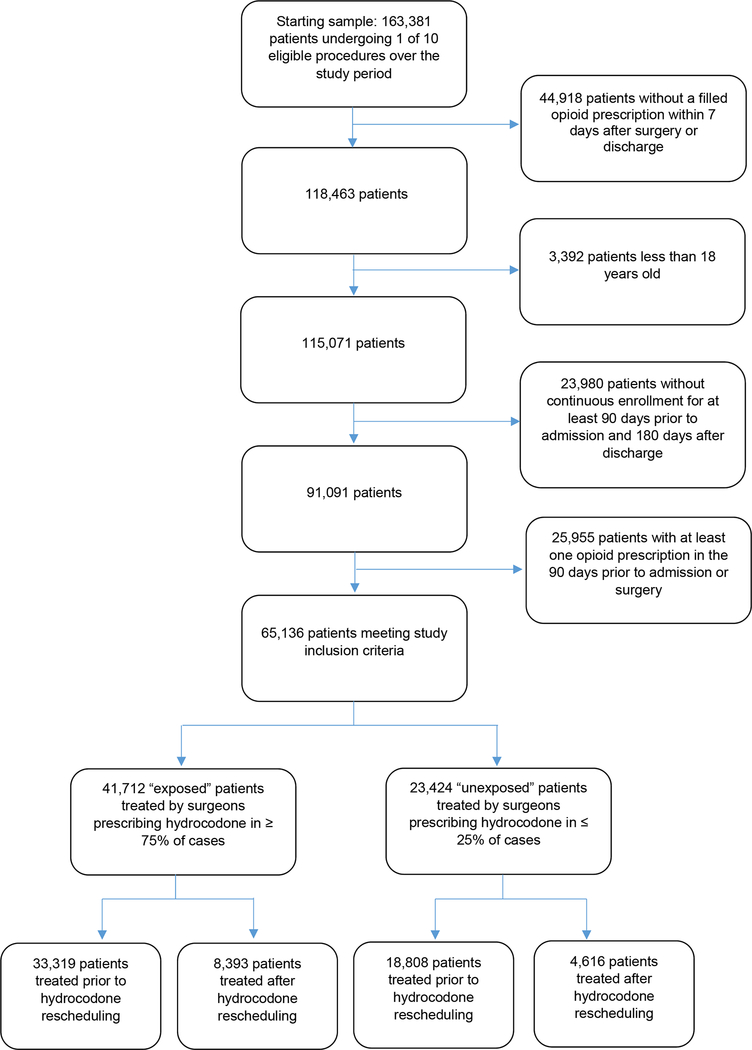
65,136 patients met study inclusion criteria, including 41,712 “exposed” patients (33,319 pre-implementation; 8,393 post-implementation) treated by 4,620 hydrocodone prescribers and 23,424 “unexposed” patients (18,808 pre-implementation; 4,616 post-implementation; Figure 1) treated by 2,798 hydrocodone non-prescribers. By design, all patients included in the sample filled at least one opioid prescription within 7 days after surgery; over the full period, the first filled opioid prescription was for hydrocodone in 35,746 of 41,712 (85.7%) exposed patients versus 2,941 of 23,424 (12.6%) unexposed patients. Compared to unexposed patients, exposed patients were more often treated on an outpatient basis and more often underwent carpal tunnel release and knee arthroscopy. Total joint replacement occurred more commonly among unexposed versus exposed patients (Table 1; additional data available in Appendix 3).
Figure 1:
Development of the study sample
Table 1. Characteristics of patients included in the study sample.
“Exposed” patients are those treated by surgeons prescribing hydrocodone in ≥ 75% of cases prior to rescheduling; “Unexposed” patients are those treated by surgeons prescribing hydrocodone in ≤ 25% of cases prior to rescheduling.
| Variable | Exposed patients | Unexposed patients | P |
|---|---|---|---|
| (N=41,712) | (N=23,424) | ||
| Age, Median (IQR) | 52 (40–62) | 54 (42–64) | <0.001 |
| Sex, N (%) | 0.222 | ||
| Male | 19,120 (45.8) | 10,852 (46.3) | |
| Female | 22,587 (54.2) | 12,566 (53.7) | |
| Provider type, N (%) | <0.001 | ||
| Individual | 26,526 (63.6) | 15,717 (67.1) | |
| Group practice | 15,186 (36.4) | 7,707 (32.9) | |
| Length of stay, N (%) | <0.001 | ||
| 0 days | 33,511 (80.3) | 15,686 (67.0) | |
| 1or 2 days | 3,995 (9.6) | 3,980 (17.0) | |
| 3 or more days | 4,206 (10.1) | 3,758 (16.0) | |
| Procedure type, N (%) | |||
| Laparoscopic cholecystectomy | 10,506 (25.2) | 5,366 (22.9) | <0.001 |
| Open cholecystectomy | 219 (0.5) | 127 (0.5) | 0.773 |
| Laparoscopic appendectomy | 3,630 (8.7) | 2,058 (8.8) | 0.718 |
| Open appendectomy | 276 (0.7) | 203 (0.9) | 0.003 |
| Inguinal hernia repair | 4,643 (11.1) | 2,846 (12.1) | <0.001 |
| Carpal tunnel release | 4,686 (11.2) | 1,344 (5.7) | <0.001 |
| Knee arthroscopy | 9,318 (22.3) | 3,918 (16.7) | <0.001 |
| Total knee replacement | 3,268 (7.8) | 3,688 (15.7) | <0.001 |
| Total hip replacement | 1,430 (3.4) | 2,009 (8.6) | <0.001 |
| Breast excision | 3,736 (9.0) | 1,865 (8.0) | <0.001 |
| Comorbidities, N(%) | |||
| Congestive heart failure | 749 (1.8) | 504 (2.2) | 0.002 |
| Cardiac arrhythmia | 3,503 (8.4) | 2,179 (9.3) | <0.001 |
| Cardiac valve disease | 1,403 (3.4) | 1,090 (4.7) | <0.001 |
| Peripheral vascular disorders | 1,175 (2.8) | 730 (3.1) | 0.030 |
| Hypertension, uncomplicated | 15,255 (36.6) | 9,302 (39.7) | <0.001 |
| Hypertension, complicated | 1,111 (2.7) | 771 (3.3) | <0.001 |
| Other neurological disorders | 614 (1.5) | 373 (1.6) | 0.227 |
| Chronic pulmonary disease | 4,515 (10.8) | 2,919 (12.5) | <0.001 |
| Diabetes, uncomplicated | 4,987 (12.0) | 2,905 (12.4) | 0.094 |
| Diabetes, complicated | 1,070 (2.6) | 594 (2.5) | 0.820 |
| Hypothyroidism | 4,986 (12.0) | 3,013 (12.9) | <0.001 |
| Renal Failure | 1,012 (2.4) | 653 (2.8) | 0.005 |
| Liver disease | 2,956 (7.1) | 1,572 (6.7) | 0.071 |
| Solid tumor without metastasis | 3,334 (8.0) | 1,932 (8.2) | 0.252 |
| Rheumatoid arthritis | 1,136 (2.7) | 731 (3.1) | 0.004 |
| Coagulopathy | 512 (1.2) | 411 (1.8) | <0.001 |
| Obesity | 5,497 (13.2) | 3,651 (15.6) | <0.001 |
| Fluid and electrolyte disorders | 1,979 (4.7) | 1,342 (5.7) | <0.001 |
| Iron deficiency anemia | 882 (2.1) | 631 (2.7) | <0.001 |
| Depression | 4,200 (10.1) | 2,599 (11.1) | <0.001 |
| Antidepressant receipt in last 90 days | 6,424 (15.4) | 3,398 (14.5) | 0.002 |
Figure 2 depicts outcome trends for exposed versus unexposed patients. Rates of filled opioid prescriptions beyond 90 days were similar over time in both groups (exposed: 12.0% pre-implementation (3,982 of 33,319) versus 10.5% post-implementation (883 of 8,393), P<0.001; unexposed: 11.1% pre-implementation (2,088 of 18,808) versus 10.8% post-implementation (498 of 4,616), P=0.548). The mean OME dispensed to exposed patients in the initial filled postoperative prescription was 259 mg (SD 200.4 mg) pre-implementation versus 295 mg (SD 250 mg, P < 0.001) post-implementation compared to 382 mg (SD 310.7 mg) versus 366 mg (SD 280.9 mg, P=0.026) for unexposed patients. The rate of refills within 30 days after surgery among exposed patients was 20.5% (6,830 of 33,319) before implementation versus 15.8% 1,323 of 8,393) after implementation (P < 0.001), compared to 22.7% (4,266 of 18,808) versus 21.2% (977 of 4,616) among unexposed patients (P=0.062). The total OME dispensed within 30 days increased between the pre-implementation to post-implementation period for exposed patients from 329 mg (SD 370.1 mg) to 355 mg (SD 391.5 mg, P < 0.001) while decreasing among control patients from 492 mg (537.7 mg) to 468 mg (504.6 mg, P = 0.049). Among exposed patients, the percentage receiving hydrocodone as the first filled opioid prescription after surgery decreased from 89.9% (29,931 of 33,319) to 69.3% (5,815 of 8,393) before vs after rescheduling (P< 0.001); among unexposed patients, the percentage receiving hydrocodone increased from 11.5% (2,158 of 18,808) to 17.0% (783 of 4,616) across periods (P<0.001).
Figure 2: Pre- and post-implementation study outcomes by exposure group.
(A) Percentage filling any opioid prescription between 90 and 180 days; (B) Total amount of opioid dispensed in Oral Morphine Equivalents (OME) in milligrams (mg) at 7 days; (C) Percentage obtaining an opioid refill within 30 days of surgery; (D) Total OME dispensed at 30 days, in mg. Blue lines correspond to patients treated by surgeons prescribing hydrocodone in ≤ 25% of cases (“unexposed” patients); red lines correspond to patients treated by surgeons prescribing hydrocodone in ≥ 75% of cases (“exposed” patients).
We included 65,125 patients with complete study data (>99.9% of the full sample) in our adjusted difference-in-differences analysis (Table 2); 11 patients were excluded due to missing data on sex. The incidence of filled opioid prescriptions between 90 and 180 days was similar after versus before rescheduling for exposed versus unexposed patients (difference-in-differences estimate: −1.1%; 95% CI −2.3%, 0.1%, P = 0.084). Rescheduling was associated with a 45.4 mg (95% CI: 34.2 mg, 56.7 mg, P < 0.001) adjusted increase in OME dispensed at 7 days after surgery among exposed versus unexposed patients; a 4.1% percentage point decrease (95% CI: −5.5%, −2.7%, P < 0.001) in refills within 30 days of surgery; and a net increase of 37.7 mg (95% CI: 20.6 mg, 54.8 mg, P = 0.008) in total OME dispensed within 30 days. We observed similar results using logistic models for binary endpoints (Appendix 4); in a subgroup analysis restricted to ambulatory surgery patients (Appendix 5); in models with controls for pre-implementation trends (Appendix 6), and in models using alternate thresholds for categorizing surgeons as hydrocodone prescribers or non-prescribers (Appendix 7.)
Table 2:
Adjusted study outcomes
| Outcome | Adjusted change, after vs. before hydrocodone rescheduling | Difference-in-differences estimate (95% CI)* | |
|---|---|---|---|
| Exposed patients (95% CI)* | Unexposed patients (95% CI)* | ||
| Oral Morphine Equivalents | |||
| Oral morphine equivalents dispensed in initial postoperative prescription, up to day 7 | 26.9 mg (20.8 mg, 33.0 mg) | −18.5 mg (−27.9 mg, −9.1 mg) | 45.4 mg (34.2 mg, 56.7 mg) |
| Oral morphine equivalents dispensed within first 30 days after surgery | 10.3 mg (2.8 mg, 17.9 mg) | −27.4 mg (−42.7 mg, −12.0 mg) | 37.7 mg (20.6 mg, 54.8 mg) |
| Percentage points | |||
| Percent with any opioid refill within first 30 days after surgery | −5.6% (−6.5%, −4.8%) | −1.6% (−2.7%, −0.4%) | −4.1% (−5.5%, −2.7%) |
| Percent with any opioid prescription between 90 and 180 days after surgery | −1.6% (−2.4%, −0.9%) | −0.5% (−1.5%, 0.5%) | −1.1% (−2.3%, 0.1%) |
Results obtained from linear models that included an interaction term between patient exposure status (exposed vs unexposed) and period (pre- vs post-implementation), and were adjusted for sex; individual provider vs group practice; age; length of stay; surgery type; peripheral vascular disorders, hypertension (uncomplicated); hypertension (complicated); neurological disorders; chronic pulmonary disease; diabetes (uncomplicated); diabetes (complicated); hypothyroidism; renal failure; liver disease; solid tumor without metastasis; rheumatoid arthritis; coagulopathy; obesity; fluid and electrolyte disorders; iron deficiency anemia; depression; antidepressant receipt in last 90 days; congestive heart failure; cardiac arrhythmia; and cardiac valve disease.
DISCUSSION
Among 65,136 opioid-naïve individuals undergoing 10 general or orthopedic surgeries, we estimated the DEA’s 2014 hydrocodone rescheduling to have resulted in a 4% absolute decrease in opioid refills within 30 days of surgery among patients treated by clinicians who frequently prescribed hydrocodone before rescheduling (i.e. those functionally “exposed” to the policy effect of the rule) versus those treated by clinicians who rarely prescribed hydrocodone (“unexposed” patients); this change was in accordance with the specific goals of the rescheduling rule, which explicitly prevented prescribers from issuing refill prescriptions at the time of an initial opioid prescription. However, we also found rescheduling to have been associated with a 38 mg increase in OME dispensed within 30 days of surgery, likely due to larger initial prescriptions written in response to new restrictions on refills that came with rescheduling. We found no evidence that these changes in short-term opioid dispensing impacted long-term opioid receipt after surgery as measured between 90 and 180 days either in our full sample or in a subgroup of ambulatory surgery patients.
This work extends prior evaluations of hydrocodone rescheduling’s impact on postoperative opioid dispensing. Using data from one US academic center, Tan and colleagues used interrupted time series analysis and found no difference in the average amount of opioid initially dispensed after surgery after versus before rescheduling.6 Using data from 75 Michigan hospitals, Habbouche and colleagues applied similar methods and observed a 5% decrease in refills and an increase in the amount of opioid initially dispensed after surgery, but no change in total OME dispensed at 30 days after surgery after versus before rescheduling.5
Neither of these prior studies accounted for secular changes in opioid dispensing that could confound estimates of rescheduling’s impact. In contrast, our difference-in-differences design allows us to separate the impact of the rescheduling rule from other secular changes in opioid dispensing.15 Moreover, we extend the generalizability of prior analyses through use of a national claims database and go beyond prior work in examining rescheduling’s impact on filled opioid prescriptions beyond 90 days after surgery. As an incidental finding, we observed a modest decrease in OME dispensed within 30 days after surgery among patients treated by hydrocodone non-prescribers over the 12 months after rescheduling. We observe this change occurring at an earlier date than most changes in postoperative opioid dispensing have been described, highlighting opportunities for future research to more broadly characterize trends in postoperative opioid dispensing over time.
This work has limitations. Although our statistical models adjusted for a variety of potential confounders, our results could have been affected by residual confounding if the study database failed to capture important differences between patients treated by hydrocodone prescribers versus non-prescribers, or between patients treated after versus before rescheduling. Because of limitations of the study dataset, we were unable to control for provider-level differences in experience or training that may have influenced opioid prescribing habits and responses to hydrocodone rescheduling. As the rate of hydrocodone prescribing was greater than zero in our unexposed (control) group prior to rescheduling, it is possible that the rule change may have had some effects on opioid dispensing outcomes for this group; therefore, our findings may underestimate the true impact of rescheduling. Finally, our observation of differences in 7 day OME dispensing trends for exposed versus non-exposed groups over the 3 years prior to rescheduling could raise concern that our findings may not be solely attributable to the impact of hydrocodone rescheduling. This concern is mitigated by our confirmation of our main findings in regression models that adjusted for differences in pre-implementation trends; the fact that the identified difference in pre-implementation trends was small in magnitude and likely to be clinically insignificant; and our finding of no difference in pre-implementation trends for our 3 other study outcomes. As we focus exclusively on opioid dispensing outcomes among surgical patients, our analysis is limited in its ability to assess the net benefit or harm to public health attributable to hydrocodone rescheduling.
Despite these limitations work has important implications for clinical practice and health policy. We find a modest increase in postoperative opioid dispensing attributable to rescheduling. As the stated intent of the hydrocodone rescheduling rule was to limit, rather than to encourage, additional opioid dispensing, this finding highlights the potential for unintended consequences to arise from interventions that affect one aspect of postoperative prescribing, such as the ability to issue refills in the initial postoperative prescription, in isolation. While we cannot comment based on this analysis on the specific benefits or harms of hydrocodone rescheduling at the level of the individual patient, the increase we observe in 30-day opioid dispensing may have had negative consequences at the level of the population if it increased the volume of unused opioids available for diversion or misuse. At the same time, we find that, despite an increase in 30-day opioid dispensing, patients experienced no consequent increase in the risk of new long-term opioid receipt, arguing against negative effects of the hydrocodone rescheduling act with regard to population-level patterns of new long-term opioid use. While further work is required to fully understand the association between the extent of short-term opioid dispensing, refill rates, and the development of new long-term use,13,31 our findings argue against a link between modest variations in the amount of opioids dispensed in the first 30 days after surgery and greater rates of new opioid receipt beyond 90 days.
In conclusion, among patients treated by surgeons who frequently prescribed hydrocodone prior to the DEA’s 2014 hydrocodone schedule change, rescheduling was associated with a modest net increase in opioids dispensed within 30 days of surgery, but was not associated with changes in the incidence of new opioid receipt beyond 90 days. These findings suggest that hydrocodone rescheduling may have had limited unintended consequences if it increased the volume of unused opioids available for diversion or misuse, but was unlikely to have impacted patterns of new long-term opioid use after surgery.
Supplementary Material
Summary statement.
Among 65,136 adults undergoing general or orthopedic surgery, the US DEA’s 2014 rescheduling of hydrocodone was associated with an increase in opioid dispensing within 30 days of surgery but did not impact long-term opioid receipt.
Acknowledgments
Funding statement: This study was supported by a grant from the National Institute on Drug Abuse (#1R01DA042299-01A1).
Conflicts of interest: BTB is an investigator on grants to his institution from Baxalta, Lilly, GSK, Pfizer, and Pacira for unrelated studies and is a consultant to the Alosa Foundation and Aetion, Inc.
SH has consulted for Braeburn Pharmaceuticals, Inc; Daiichi Sankyo, Inc; Egalet Corporation; Esteve Pharmaceuticals LLC; Indivior, Inc; Inspiron Delivery Sciences, LLC; Nektar Therapeutics Inc; and Purdue Pharma, LP.
Footnotes
The remaining authors declare no competing interests.
Clinical trial number/registry URL: Not applicable.
Prior presentations: This work was presented as a research poster at the 2019 FAER Mentored Research Training Grant meeting in Washington, DC (August 1–2, 2019).
REFERENCES
- 1.US Drug Enforcement Administration: Final Rule: Schedules of Controlled Substances: Rescheduling of Hydrocodone Combination Products From Schedule III to Schedule II.. Federal Register Volume 79, Number 163, 2014, pp 49661–49682 [PubMed] [Google Scholar]
- 2.Jones CM, Lurie PG, Throckmorton DC: Effect of US Drug Enforcement Administration’s Rescheduling of Hydrocodone Combination Analgesic Products on Opioid Analgesic Prescribing. JAMA Intern Med 2016; 176: 399–402 [DOI] [PubMed] [Google Scholar]
- 3.Kuo YF, Raji MA, Liaw V, Baillargeon J, Goodwin JS: Opioid Prescriptions in Older Medicare Beneficiaries After the 2014 Federal Rescheduling of Hydrocodone Products. J Am Geriatr Soc 2018; 66: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raji MA, Kuo YF, Adhikari D, Baillargeon J, Goodwin JS: Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol Drug Saf 2018; 27: 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habbouche J, Lee J, Steiger R, Dupree JM, Khalsa C, Englesbe M, Brummett C, Waljee J: Association of Hydrocodone Schedule Change With Opioid Prescriptions Following Surgery. JAMA Surg 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan WH, Feaman S, Milam L, Garber V, McAllister J, Blatnik JA, Brunt LM: Postoperative opioid prescribing practices and the impact of the hydrocodone schedule change. Surgery 2018; 164: 879–886 [DOI] [PubMed] [Google Scholar]
- 7.Baker-White A: A Look at State Legislation Limiting Opioid Prescriptions. Arlington, VA, US: Association of State and Territorial Health Officials, 2017 [Google Scholar]
- 8.Lowenstein M, Grande D, Delgado MK: Opioid Prescribing Limits for Acute Pain - Striking the Right Balance. N Engl J Med 2018; 379: 504–506 [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, O’Kane N, Van Otterloo J, Wright DA, Leichtling G, Millet LM: Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med 2017; 32: 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah A, Hayes CJ, Martin BC: Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66: 265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell D, Haegerich TM, Chou R: CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 2016; 315: 1624–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert ASB, Kheterpal S, Nallamothu BK: New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 2017; 152: e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooten WM, Brummett CM, Sullivan MD, Goesling J, Tilburt JC, Merlin JS, St Sauver JL, Wasan AD, Clauw DJ, Warner DO: A Conceptual Framework for Understanding Unintended Prolonged Opioid Use. Mayo Clin Proc 2017; 92: 1822–1830 [DOI] [PubMed] [Google Scholar]
- 14.Barnett ML, Olenski AR, Jena AB: Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use. N Engl J Med 2017; 376: 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimick JB, Ryan AM: Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA 2014; 312: 2401–2 [DOI] [PubMed] [Google Scholar]
- 16.Neuman MD, Hennessy S, Small D, Newcomb C, Gaskins L, Wijeysundera D, Bateman BT, Wunsch H: Technical Preprint: Rationale and Design of a Planned Observational Study to Evaluate the Impact of Hydrocodone Rescheduling on Opioid Prescribing After Surgery, 2019. https://arxiv.org/abs/1906.04246v2. Accessed December 9, 2019. [Google Scholar]
- 17.Brookhart MA, Rassen JA, Wang PS, Dormuth C, Mogun H, Schneeweiss S: Evaluating the validity of an instrumental variable study of neuroleptics: can between-physician differences in prescribing patterns be used to estimate treatment effects? Med Care 2007; 45: S116–22 [DOI] [PubMed] [Google Scholar]
- 18.Brookhart MA, Schneeweiss S: Preference-based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat 2007; 3: Article 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S: Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology 2006; 17: 268–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wing C, Simon K, Bello-Gomez RA: Designing Difference in Difference Studies: Best Practices for Public Health Policy Research. Annual Review of Public Health, Vol 39 2018; 39: 453–469 [DOI] [PubMed] [Google Scholar]
- 21.Angrist JD, Pischke J-S: The credibility revolution in empirical economics: how better research design is taking the con out of econometrics., NBER Working Papers,. Washington, D.C., National Bureau of Economic Research, 2010 [Google Scholar]
- 22.Johnson SP, Chung KC, Zhong L, Shauver MJ, Engelsbe MJ, Brummett C, Waljee JF: Risk of Prolonged Opioid Use Among Opioid-Naive Patients Following Common Hand Surgery Procedures. J Hand Surg Am 2016; 41: 947–957 e3 [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Hu HM, Edelman AL, Brummett CM, Englesbe MJ, Waljee JF, Smerage JB, Griggs JJ, Nathan H, Jeruss JS, Dossett LA: New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 2017; 35: 4042–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jivraj N, Raghavji F, Bethell J, Neuman M, Wunsch H: Defining persistent opioid use among opioid naive patients after surgery: a systematic review and population-based study [unpublished manuscript]. Toronto, Sunnybrook Health Sciences Centre, 2019 [Google Scholar]
- 25.US Centers for Medicare and Medicaid Services: Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors.. Baltimore, MD, US Centers for Medicare and Medicaid Services,, 2017 [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–9 [DOI] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care 1998; 36: 8–27 [DOI] [PubMed] [Google Scholar]
- 28.Borza T, Oerline MK, Skolarus TA, Norton EC, Dimick JB, Jacobs BL, Herrel LA, Ellimoottil C, Hollingsworth JM, Ryan AM, Miller DC, Shahinian VB, Hollenbeck BK: Association Between Hospital Participation in Medicare Shared Savings Program Accountable Care Organizations and Readmission Following Major Surgery. Ann Surg 2019; 269: 873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White H: A Heteroskedasticity-Consistent Covariance-Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980; 48: 817–838 [Google Scholar]
- 30.Ryan AM, Kontopantelis E, Linden A, Burgess JF Jr, : Now trending: Coping with non-parallel trends in difference-in-differences analysis. Stat Methods Med Res 2018: 962280218814570. [DOI] [PubMed] [Google Scholar]
- 31.Neuman MD, Bateman BT, Wunsch H: Inappropriate opioid prescription after surgery. Lancet 2019; 393: 1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.