Abstract
Pancreatic cancer (PC) is a highly lethal malignancy with a dismal five-year survival rate. This is due to its asymptomatic nature, lack of reliable biomarkers, poor resectability, early metastasis, and high recurrence rate. Limited efficacies of current treatment modalities and treatment-associated toxicity underscore the need for the development of immunotherapy-based approaches. For non-resectable, locally advanced metastatic PC, immunotherapy-based approaches including vaccines, antibody-targeted, checkpoint inhibition, CAR-T-cells, and adoptive T-cell transfer could be valuable additions to existing treatment modalities. Thus far, the vaccine candidates in PC have demonstrated modest immunological responses in different treatment modalities. The identification of tumor-associated antigens (TAA) and their successful implication in PC treatment is still a challenge. MUC4, a high molecular weight glycoprotein that functionally contributes to PC pathogenesis, is an attractive TAA. It is not detected in the normal pancreas; however, it is overexpressed in mouse and human pancreatic tumors. The recombinant MUC4 domain, as well as predicted immunogenic T-cell epitopes, showed efficacy as a PC vaccine in vitro as well as in vivo in preclinical models, suggesting its importance as a vaccine candidate in PC. Existence of PC associated MUC4 splice variants, autoantibodies against overexpressed and aberrantly glycosylated MUC4 and presence of T-cell clones against the mutations present in MUC4 further reinforce its significance as a tumor antigen for vaccine development. Herein, we review the significance of MUC4 as a tumor antigen in PC immunotherapy and discuss both the development and challenges associated with MUC4 based immunotherapy. Lastly, we will present our perspective on MUC4 antigenicity for the future development of MUC4-based PC immunotherapy.
Keywords: Pancreatic cancer, MUC4, Neoantigen, Vaccine, Immunotherapy
1. Introduction:
Pancreatic cancer (PC) is a highly lethal malignancy with almost equal incidences in both males and females. Based on its five-year survival rate of ~9% and annually increasing incidence rates, PC is projected to become the second leading cause of cancer related deaths in the United States by 2030 [1]. PC is largely asymptomatic and there is no clinically approved biomarker for early detection of the disease. Consequently, PC is diagnosed late, and the majority of the patients are positive for distant metastasis by the time of diagnosis. The early dissemination of PC cells to distant sites disqualifies more than 80% of patients from surgical resection, which is considered the most effective treatment approach to date since it correlates with better survival of PC patients [2, 3]. Therefore, a majority of PC patients are treated with chemotherapy with or without radiation [4]. Unfortunately, PC patients with non-resectable tumors show a lower survival rate compared to those undergoing resection, despite their treatment with standard therapeutic approaches. Among other challenges, PC patients have a high recurrence rate, develop drug resistance, and are refractory to systemic therapies due to the desmoplastic and immunosuppressive tumor microenvironment (TME) [5–7]. Ongoing endeavors to develop more effective and safer treatment modalities for PC are focused on developing and testing immunotherapy-based approaches, including anticancer vaccines, checkpoint inhibitors, adoptive T-cell transfer, and antibody targeted therapies [7]. Unlike vascular tumors, like melanoma, where immunotherapy has changed the treatment paradigm and improved patients’ survival significantly [8], immunotherapy-based approaches have been met with limited success in treating PC. However, several clinical trials have investigated the utility of vaccine formulations and checkpoint inhibition therapies [9]. Despite the inherently poor immunogenic nature and TME-associated challenges in PC, which cause poor immune infiltration as well as suboptimal response to systemic therapies, recent trials support that combinational approaches, including vaccines and checkpoint inhibitors, might be useful additions to standard therapeutic approaches in PC treatment. In these trials, the vaccine candidates, including whole cell vaccines, peptide vaccines developed from predicted T-cell epitopes of overexpressed tumor associated antigens (TAAs), and DNA vaccines are still in the early stages of development in PC [9, 10].
Among various overexpressed proteins, mucins have been thoroughly investigated for their functional roles in PC progression and metastasis. However, except MUC1, the immunological significance of mucins is still not clear. Previous studies have shown limited efficacies of MUC1 vaccine formulations in PC patients [11], which further warrants more research into immunogenic neoantigen(s) for PC immunotherapy. MUC4 expression is exclusively associated with PC and is absent in the normal pancreas. Its aberrant glycosylation, existence of splice variants and PC specific mutations, and MUC4 specific autoantibodies in PC patients suggests that MUC4 has the potential to serve as a vaccine candidate. It is pertinent to highlight some of the major challenges in developing MUC4-based PC immunotherapy and to note where we currently stand with MUC4 vaccine development. In line with existing evidence from previous reports, we will discuss in detail the characteristics of MUC4 as a candidate for a PC vaccine and discuss its immunogenic potential with the help of computational tools that show how the MUC4 protein, its splice variants, and PC-associated mutation could be targeted for developing PC immunotherapy.
2. Challenges in PC immunotherapies
The architecture of PC tumors is highly desmoplastic and hypovascular, leading to poor uptake and intratumoral distribution of chemotherapeutic agents, which causes suboptimal therapy response. Therefore, better therapeutic approaches are warranted that could overcome the ongoing challenges associated with clinical management of PC. Immunotherapy has changed the treatment paradigm in some cancers, like melanoma, and presents hope for several other difficult to treat malignancies like PC. Despite the poor immunogenic nature of PC, different arms of immunotherapy alone or in combination with standard therapies are under investigation for both locally invasive and metastatic PC [9, 10]. Immunologically, PC is considered cold and non-inflamed with a lower mutation burden as compared to other malignancies [12, 13]. Although intratumoral T-cell infiltration correlates with a better survival in PC patients [14, 15], the majority of PC tumors are poorly infiltrated and are either immune excluded or completely immune desert, suggesting that the immune infiltration in these tumors is clinically non-significant [13]. Overall, the low neoantigen burden, desmoplastic barrier in PC tumors, and immunosuppressive TME have contributed to the limited success of immunotherapy in PC. Hence, to overcome these challenges, the major focus of PC immunotherapy includes; 1) selection of a neoantigen that is capable of inducing an effector T-cell response; 2) enhancing the immune infiltration within desmoplastic PC tumors; 3) retention of memory response and, 4) countering the immunosuppressive mechanisms in the TME.
The TME in PC is immunosuppressive and mainly comprised of different subtypes of cancer associated fibroblasts (CAFs) and immune cell populations like regulatory T-cells, tumor associated macrophages (TAMs), and myeloid derived suppressor cells (MDSCs), which synergize with each other to shape the TME to favor proliferation and metastasis of PC cells (Figure 1) [7, 16]. CAF-secrete extracellular matrix (ECM) proteins, like collagen(s) and fibronectin (FN), and cancer cell/immune cell-secreted cytokines and interleukins, like IL-10, transforming growth factor-β (TGF-β), and IL-6. Together, these provide a niche to PC cells to thrive and undergo the epithelial-mesenchymal-transition (EMT) for metastasis [17–19]. The secreted ECM proteins also create a physical barrier for activated immune cells (effector T-cells), leading to their poor infiltration within pancreatic tumors [20]. Studies have shown that effector T-cell infiltration directly correlated with a better prognosis in PC patients [21]. Therefore, increased infiltration of activated T-cells is important for effective immunotherapy. Next, the activated immune cells that can breach the ECM-barricade are trapped inside the suppressive TME. These sparsely distributed T cells are under the influence of the immunosuppressive TME, and have been reported to undergo exhaustion, anergy, or the senescence pathway as seen by expression of markers for immune suppression [22, 23]. For instance, upregulated checkpoint molecules, programmed cell death-1 (PD-1) and cytotoxic T lymphocyte activation-4 (CTLA-4), have been extensively targeted using monoclonal antibodies (mAbs) alone or in combination with standard therapy [9, 24–27]. However, in early stages, recent investigations related to other checkpoint inhibitors like T-cell immunoglobulin and mucin domain-3 (TIM-3), lymphocyte activation gene-3 (LAG-3), T cell immunoreceptor with Ig and ITIM domains (TIGIT), and V-domain immunoglobulin suppressor of T-cell activation (VISTA), also suggests their role in immunosuppression and T-cell dysfunction. Recently, higher expression of VISTA on CD68+ macrophages in PC have been demonstrated to negatively regulate the T-cell infiltration in PC tumors [12]. Higher expressions of VISTA in PC tumors compared to melanoma suggest its potential in checkpoint inhibition therapy. Altogether, targeting tumor-stroma, suppressive immune cells, and immune-checkpoints are indispensable approaches to achieve successful immunotherapy in PC. However, the challenge for poorly immunogenic PC is to select an appropriate antigen that could elicit a robust and long-lasting immune response in both preventive and therapeutic treatment modalities.
Figure 1. Pancreatic tumor microenvironment (TME) and immunosuppression.
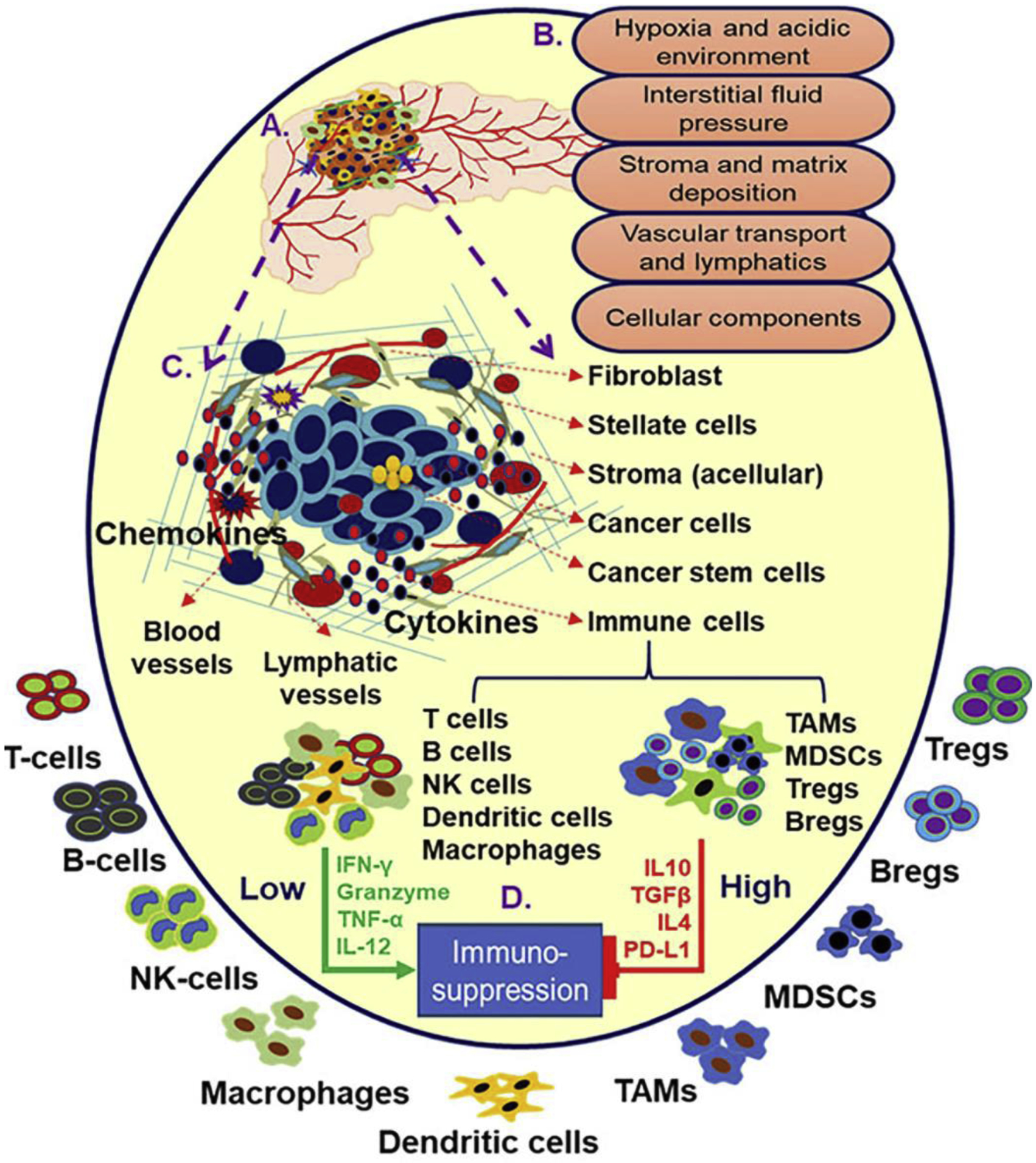
Pancreatic tumor microenvironment, in addition to disrupted transport system, is comprised of physical and biochemical barriers including high interstitial fluid pressure (IEF), hypoxia, acidic pH along with cellular infiltrates including fibroblasts, cancer stem cells tumor associated macrophages (TAMs), and other immune cells (A-C). Both cellular and acellular components in pancreatic tumor including regulatory T and B cells, myeloid derived suppressive cells (MDSCs), TAMs, other suppressive immune cells, and their secreted cytokines contribute to the immunosuppression (D). The immunosuppression and high desmoplasia are major challenges to PC immunotherapy.
3. Tumor antigen pipeline for PC immunotherapy
Besides the whole cell vaccine GVAX, only few antigens have been investigated as vaccine candidates for PC immunotherapy [9]. Most of these antigens have been analyzed in combination with either standard chemotherapy or with checkpoint inhibitors [28]. For instance, the irradiated and genetically modified whole cell vaccine GVAX that secretes granulocyte-macrophage colony stimulating factor (GM-CSF) has demonstrated that it elicits anti-tumor immunity in both locally invasive non-resectable (active clinical trial) and metastatic PC. Moreover, when GVAX was administered in combination with standard chemotherapy, radiation, and checkpoint inhibitors, or when boosted with CRS-207 vaccine, a live, attenuated, and double deleted Listeria monocytogenes expressing mesothelin, more profound therapeutic response was observed in PC patients [29–31]. In the case of non-resectable locally invasive and metastatic PC, it is pertinent to evaluate novel vaccine regimens. Recently, Kinkead et al., used whole exome sequencing, RNA-Seq, and an in silico prediction algorithm to identify a neoantigen vaccine, PancVAX, and when administered with STING-adjuvant in a PC xenograft mouse model, it enhanced the neo-epitope-specific T-cell repertoire [32]. Interestingly, when the PancVAX formulation was administered with anti-PD-1 and anti-OX-40 antibodies, the response was more robust and durable, suggesting that the vaccine could induce the anti-tumor memory response. Combination treatment with anti-OX-40 decreased the exhaustion markers LAG3 and PD-1 from the T-cell population, further suggesting the potential role of the targeted immunotherapy in maintaining effector T-cell function. Recently, based on a clinical trial (NCT03586869) Seery et al. emphasized the coordinated approach using NANT-cancer vaccine, chemotherapy, radiation therapy, and immunotherapy [33]. As the mutation load is low in PC, the majority of the PC vaccine candidates that have been clinically tested for anti-tumor immune response are TAAs. It has been evident from the previous studies that tolerance against overexpressed TAAs is compromised and therefore, these antigens have been demonstrated to elicit scalable and clinically significant antitumor immune response [10]. To deliver these TAAs in patients, DCs have been widely investigated [34] For instance, Wilm’s tumor-1 (WT-1) is highly expressed in PC pathogenesis and WT-1 loaded DCs have been investigated in PC patients in different treatment modalities [34, 35]. When multiple doses of dendritic cells (DCs) loaded with MHCI restricted WT-1 peptides were administered alone or in combination with standard therapies, both OS and PFS were improved in PC patients with recurrence or existing metastasis. Importantly, the cytotoxic T-lymphocytes (CTLs) mediated immune response was specific WT-1 expressing cells. Interestingly, the combination treatment reduced the myeloid derived suppressor cells (MDSCs) and vascular endothelial cells from the TME, suggesting that the therapy is able to target the immunosuppressive TME in the patients [36]. Similarly, when combined with anti-CTLA4 treatment, the DC vaccine showed an IL3 dependent increase in effector T-cell infiltration [37]. Additionally, p53 expressing vaccinia virus-based formulation p53MVA, when administered with anti-PD-1 antibody, pembrolizumab, showed antigen specific infiltration in borderline unresectable PC patients, suggesting that a virus-based approach might be useful in designing a PC vaccine [38].
In the case of poorly immunogenic PC, the limited efficacy of the available vaccine candidates underscores the need for identification of neoantigens for PC immunotherapy. The selection of tumor antigens is mainly based on their mutational burden, cancer-specific aberrant processing, and expression profile during PC pathogenesis. The ideal tumor antigen is assumed to have a high mutation load, high antigenicity score, and should exhibit cancer specific processing and expression. The advent of advanced genome analysis techniques and computational tools have provided a significant push to PC immunotherapy. Recently, Chen et al. highlighted the importance of genomic and computational approaches in designing a personalized immunotherapy for refractory solid tumors. Based on sequencing of pancreatic tumors, the mutant peptides showing high variant allele frequency and high-predicted human leukocyte antigen (HLA)-binding affinity were synthesized and characterized for memory recall under in vitro conditions. The neoantigen loaded DC vaccine showed a partial response in metastatic PC patients [39]. This study demonstrated the feasibility of tailoring neoantigen based personalized vaccines using bioinformatic and genomic technologies.
4. Mucins as PC vaccine candidates
Mucins are high molecular weight glycoproteins that have been demonstrated to play a critical role in PC pathogenesis [40, 41]. Based on their differential overexpression and functional involvement during PC pathogenesis, mucins have emerged as ideal biomarkers and therapeutic targets [42–44]. For instance, we have previously demonstrated the utility of the secretory mucin MUC5AC for early detection of PC. Moreover, we found that the combined detection of secretory mucin MUC5AC and CA-19–9 antigen in PC patient’s sera could be used as biomarkers for PC diagnosis to further increase the diagnostic sensitivity and specificity [44]. Similarly, transmembrane mucins MUC1 and MUC4 were detected in the PC patient’s sera [45, 46], suggesting their potential implication as biomarkers. Nevertheless, both MUC1 and MUC4 contain targetable functional domains that have been reported to contribute to PC progression and metastasis [47–51]. Another transmembrane mucin, MUC16, also overexpresses during PC pathogenesis with a functional role in disease progression and metastasis [52–54]. We have also observed that MUC16, like other transmembrane mucins, has pathobiological implications in PC [55]. In addition, a recent study also highlighted MUC16 as a target for PC immunotherapy [56]. Overall, mucins’ expression, aberrant glycosylation, and their role in PC pathogenesis make them suitable targets for biomarkers and therapeutic development in PC.
In the perspective of PC immunotherapy, mucins have not been extensively explored, with the exception of MUC1, which has been evaluated in clinical trials as a vaccine for several cancers, including hematological malignancies, breast cancer, lung cancer, and unresectable, locally advanced, and metastatic PC [57]. In PC, MUC1 neo-epitopes derived from signal peptides or from the tandem repeats elicited enhanced activated T-cells infiltration and correlated with better survival [57]. In another study, adoptive administration of three doses of MUC1 specific cytotoxic T lymphocytes along with standard gemcitabine regiment improved the disease-free survival from 15.8 months to 24.7 months in resected PC patients. Importantly, the therapy significantly prevented liver metastasis and local recurrence [58]. Earlier, retrospective analysis in a cohort of 313 patients having 36 PC patients, the efficacy of DC-based vaccine pulsed with dual antigens, WT1 and MUC1, was evaluated. Based on the Response Evaluation Criteria in Solid Tumors (RECIST) analysis, patients showed clinical benefits with an increase in survival and a response rate and disease control rate of 22% and 64% respectively [59]. Further, MUC1 specific antibody has been demonstrated in priming the T-cells to elicit antigen-specific cellular immune response through opsonization of MUC1 antigen. Briefly, when MUC1 specific antibody mAbAR20.5administered in combination with TLR3 ligand polyICLC, and anti-PD-L1 in hMUC1-Tg mouse model, it induced significant increases in activated T-cells [60]. The multitier targeting approach using antigen specific antibodies and checkpoint inhibitors has provided a mechanism to boost the tumor specific immune response. Ongoing clinical trials for MUC1 efficacy in different treatment modalities will pave the path for a future development of MUC1-based immunotherapy. Mucin-16 (MUC16) is another TAA that has been investigated as a target for PC immunotherapy. Balachandran et al., showed high MUC16 neoantigen frequency in PC and highlighted that long-term survival in PC patients is associated with the quality of neoantigens as described in their fitness model [61]. These patients showed circulatory as well as intratumoral neoantigen MUC16 specific T-cells; however, this study also found that because of neoantigen-immuno-editing, there was a selective loss of effector neoantigenic T cell clones during progression [61]. Like other transmembrane mucins, MUC16 is also highly glycosylated and contains different functional domains that might be the hotspots for targeted immunotherapies. Future studies are warranted to explore in depth the potential of MUC16 as TAA for PC immunotherapy.
5. MUC4: A PC tumor antigen
While its functional role is known in PC progression, MUC4 as a tumor antigen is not thoroughly explored. However, there is ample evidence to show MUC4 as a potential tumor-antigen for PC immunotherapy. First, MUC4 is not expressed in the normal pancreas and its expression exclusively upregulates during PC progression [42, 62–64]. Second, MUC4 interacts with different proteins involved in PC pathogenesis and promotes PC progression and metastasis [50, 65–67]; therefore, targeting the functional domains will reduce the tumor progression. Third, the altered glycosylation and mutations in pathogenesis associated with the MUC4 protein suggest its potential as a neoantigen in PC [68]. In the last two decades, MUC4 has been studied extensively for its structural and functional significance in PC pathogenesis. A positive correlation of MUC4 expression with the PC progression and with the patient’s survival rate has been reported previously [69], which emphasized its therapeutic relevance in PC. MUC4 also interacts with key oncogenic signaling pathways to promote aggressiveness and chemoresistance in PC [70–72]. Previously, MUC4 has been investigated as a therapeutic target and the treatment with small molecule inhibitors, natural products, micro-RNAs, si-RNA, and sh-RNAs have demonstrated decreased proliferation, invasion, and metastasis in different PC models [62]. In contrast, our knowledge is limited about the antigenic profile of the MUC4 protein to establish it as a tumor antigen in PC. Nevertheless, the existing evidence and our recent findings support that MUC4 could be a potential tumor antigen for PC immunotherapy. Wei et al., first reported that predicted human leukocyte antigen A1 and A2 (HLA-A1 and HLA-A2) specific MUC4 epitopes, when transduced in dendritic cells with universal DR-restricted T-helper epitope (PADRE), showed upregulation of DC activation markers like HLA-DR, CD80, CD86, and induced a potent MUC4 specific cytotoxic T-cell response [73]. This was the first report that highlighted that computationally predicted major histocompatibility complex (MHC)-restricted epitopes of MUC4 could potentially induce the antitumor response in MUC4-positive cancers. Similarly, Wu et al., used SYFPEITHI and ProPred in silico prediction tools to identify high affinity peptide epitopes in the MUC4 protein, and showed their efficacies in vitro using an ELISPOT assay for IFN-γ production and a 51Chromium (51Cr) release assay for measuring the T-cell induced cytotoxicity. Among five predicted epitopes, alpha-domain peptide P01204 (1126LLGVGTFVV1134) induced proliferation of IFN-γ producing T-cells, which showed CTL-response in T2-cells as well as MUC4 expressing cells in a HLA-A2 restricted manner [74]. Later, Chen et al., demonstrated the utility of MUC4 and Survivin RNA transfected DCs for generation of an anti-tumor immune response. Interestingly, DCs co-transfected with both MUC4 and survivin transcripts induced a stronger CTL response than single-RNA transfection. The immune response elicited by co-transfection was restricted to MHC class I, suggesting that the multiple epitopes derived from MUC4 and other TAAs could be used for further clinical investigation as DC vaccines using this RNA transfection approach [75]. Based on previous studies, we have dissected the MUC4 sequence for its immunological potential in PC (Figure 2 A & B). Recently, mice immunized with a glycopeptide from the MUC4-tandem repeat region showed a potent antigen-specific immune response. Results from the antibody-titer and specificity analysis suggested the potential role of glycosylation in mounting the antigen-specific immune response [76].
Figure 2: Immunological significance of MUC4 in pancreatic cancer.

(A) MUC4 expression is absent in the normal pancreas, whereas the expression begins as early as neoplastic changes (PanINs) occur following oncogenic mutation. MUC4 expression gradually increases from PanINs to PDAC (B) Different studies showing MUC4 specific immune response in PC preclinical settings. The predicted T-cell epitopes as well as recombinant subunit of MUC4 have been evaluated in different settings to test the utility of MUC4 as a vaccine candidate.
Though the computational approaches contributed significantly in predicting the immuno-dominant epitopes in difficult to purify antigens, and helped in their immunological evaluation, analysis of gross immunogenicity of these antigens needs to be tested to understand the overall immunological significance. In this direction, we recently have demonstrated that the recombinant beta-domain of MUC4 (MUC4β) encapsulated in polyanhydride nanoparticle-based adjuvant system (MUC4β-Nanovaccine) activated the mouse bone marrow derived DC (BMDCs) as suggested by upregulation of activation markers MHCI, MHCII, and costimulatory markers CD80, CD86, and CD40 [77]. Polyanhydride nanoparticles, in addition to their role as an adjuvant system, also help in sustained antigen release, which is important for a robust and long-lasting immune response. Analysis of cytokines in the culture supernatant derived from stimulated DCs suggested that the nanovaccine induced a predominantly Th1 type immune response, which was further supported by the cytokine analysis of sera derived from the immunized mice [77]. The MUC4 nanovaccine elicited a higher titer of anti-MUC4 antibodies as compared to immunization with protein alone and other control formulations. More efforts are warranted to purify the other domains of MUC4 to evaluate their immunogenicity. Recent technical advances, like whole exome sequencing and RNA-Seq, are useful approaches to discover actionable mutations which might be more clinically relevant in designing the vaccine. Recently, using similar approaches as Cafri et al., a study showed that memory T-cells isolated from the peripheral blood of metastatic cancer patients recognize the mutation in SMAD4 and MUC4 antigens, suggesting the existence of somatic mutation(s) in these antigens during cancer progression [78]. Overall, due to its role in oncogenic progression, high expression, aberrant glycosylation, and existence of actionable mutations, the MUC4 antigen could serve as potential vaccine candidate in PC patients.
6. Computational insight for MUC4 antigenicity
Computational tools have emerged as valuable resources for cancer immunotherapy, mainly in the development of peptide based anti-cancer vaccines. For example, the immune epitope database (IEDB) has been used to predict various aspects of the immune response, like prediction of MHCI and MHCII epitopes and their affinities, B-cell epitope prediction, peptide antigenicity prediction, and linear and conformation epitope prediction. Interestingly, the predicted MHCI epitopes substantially demonstrated anti-tumor immunity in various malignancies, including PC [79–83]. Moreover, the high molecular weight proteins like mucins are difficult to purify for their immunological evaluations and, therefore, for such bulky proteins, epitope prediction methods significantly accelerate the development of a vaccine. Considering both MUC1 and MUC4 as examples, thus far, most of the immunological evaluations have used the T-cell epitope prediction methods to identify the immunogenic epitopes. Earlier studies have demonstrated that the predicted T-cell epitopes induced MUC4-specific cytotoxicity and high IFN-γ release in a HLA-restricted manner, suggesting the significance of computational tools in the development of anti-cancer vaccines.
There is paucity of neoantigens in PC and very few patients carry mutations that are clinically relevant for PC immunotherapy. In previous studies, mutant KRAS and TP53 peptides were investigated for antitumor immune response in PC [9, 84]. However, up to now there has been no systematic study on MUC4 mutational profile and its immunogenic potential. Therefore, we extracted all somatic mutations present in the MUC4 sequence of PC patient datasets available in The Cancer Genome Atlas (TCGA) [85]. Analysis of PC patient datasets from TCGA suggests that there are somatic mutations present in the MUC4 sequence. To study the possible immunogenicity of these mutations, we generated all possible peptide sequences of nine amino acids (9-mers) containing these missense mutations. Then, we predicted the HLA-I binding affinity of mutated peptides and corresponding wildtype peptides using IEDB and NetCTL [86]. We selected mutated peptides for which both tools predicted a binding affinity equal to or smaller than 500nM but whose corresponding wildtype peptides had low affinity for the HLA. Based on this analysis, we could predict the potential immunogenicity of MUC4 peptides carrying mutations. One of these mutations (V868I) is present in the N-terminus of MUC4 (subunit α) and another mutation (G4932R) is present in the VWD domain (subunit β) (Figure 3A). Such mutations, if tested, might be immunogenic and could warrant further investigation, as these peptides could elicit MUC4 specific immune response in PC patients. Similarly, we also generated possible 9-mers sequences for each of the four MUC4 protein coding splice variants found in Ensembl [87], and extracted putative immunogenic peptides with a predicted binding affinity to HLA-I <= 500 nM. The location of each of the predicted immunogenic peptides was then plotted onto the genomic structure of the MUC4 gene (Figure 3B). The presence of mutations in PC patients’ MUC4 and existence of different splice variants suggest that MUC4 is an immunologically important target for PC immunotherapy.
Figure 3. Analysis of MUC4 immunogenicity.

(A) The mutational profile of MUC4 antigen in PDA patients was analyzed in TCGA dataset and observed somatic mutations are highlighted in red. Somatic mutations V868I and G4932R are present in N-terminus (subunit-α) and C-terminus (subunit-β) respectively (B) Multiple putative 9-mer immunogenic peptides on chromosome 3 were predicted by using NetCTL and IEDB prediction methods (binding affinity ≤500nM). The red and blue lines show the exon boundaries and the figure shows both exons and introns that are present in MUC4 sequence.
Currently, there are different prediction methods that are available for predicting B-cell epitopes, T-cell epitopes with MHC restriction, epitope affinity for MHCI, antigen processing, as well as the post-translational modifications like glycosylation. It is important to analyze the glycosylation on the epitopes because there is a possibility that the synthetic peptides that exist naturally glycosylated show no expected immunological response. Further, if the extent of glycosylation is accurately predicted on immunogenic peptides, synthetic glycopeptides could also be examined for immunogenicity. Nevertheless, the validation of these predicted peptides in activating the antigen presenting cells, evaluation of their antigen-specific cytotoxic effect in cell based colorimetric assays and measuring the IFN-γ release are gold standards before moving to in vivo testing. Interestingly, considering all these parameters, MUC4 peptide epitopes demonstrated immunological significance as vaccine candidates for PC immunotherapy.
7. Conclusions
The field of immunotherapy is still striving for novel tumor antigens that can be evaluated for sustainable antitumor immunity against poorly immunogenic PC. Poor immunogenicity together with immunosuppression in PC patients induce insufficient anti-tumor immunity and enhance the disease aggressiveness. Within immunosuppressive TME, the tumor cells escape the immune surveillance and adopt different immune evasion mechanisms. Mechanistically, immune evasion in PC has been reported mainly due to MHC downregulation, the suppressive TME, and checkpoint upregulation [9, 10, 28]. Different tumor specific and TAAs have been evaluated for their potentials to elicit clinically significant anti-tumor immune response in various combination approaches so that immune evasion and suppression could be inhibited in PC. In addition to tumor antigen, there are several other parameters including the adjuvant system, checkpoint regulation, dosing, and delivery route that cumulatively decide the fate of vaccine candidates. Under optimum conditions, immune response generated by vaccines is defined by generation of antigen specific CTL response in terms of both quality and quantity. Low mutation rates in PC created a dearth of private and tumor specific antigens; therefore, major focus is shifted towards harnessing the potential of TAAs for PC immunotherapy. Despite being self to the PC patients, tolerance has been reported compromised against majority of TAAs and therefore, these antigens have been shown to elicit clinically significant immune response. The compromised immunological tolerance against TAAs has been associated with their higher expressions, organ specific expression, presence of splice variants, and altered post-translational modifications.
MUC4 holds potential as a tumor antigen not only for vaccine development, but also as a target for armed/unarmed anti-MUC4 monoclonal antibodies [88, 89]. Importantly, MUC4 overexpression, its aberrant glycosylation, and its role in PC pathogenesis justify it as a potential target. Recent data reporting the somatic mutations in cancer are associated with MUC4 expression, which further suggests that MUC4 could be used as a tumor antigen for PC immunotherapy. Furthermore, our recent work also provided evidence that recombinant MUC4 domain(s) could be used to generate a MUC4 specific antitumor immune response [90]. More importantly, the polyanhydride nanoparticles used for delivery of recombinant MUC4β protein not only served as an excellent adjuvant system but also allowed the sustained release of immunogen, which is important for a robust and long-lasting immune response. This nanoparticle-based delivery system allowed us to evaluate the larger protein fragments (with a diverse epitope repertoire) in an unbiased manner rather than “cherry-picked” immuno-dominant peptide epitopes. Another important parameter in designing the vaccine is to add universal T-helper epitopes or other immuno-dominant epitopes to enhance the immune response, as it has been reported previously that when MUC4 immunogenic epitope was transduced with PADRE, it showed a potent Th1 type immune response. Lack of animal models has been a major impediment in determining if MUC4-targeted immunotherapy can overcome the immune tolerance to mount a potent anti-tumor response. A transgenic mouse model expressing human-MUC4 can serve as a valuable tool to evaluate a MUC4 vaccine. Unlike melanoma and hematological malignancies, optimizing immunotherapeutic approaches in PC is challenging due to both the immunosuppressive TME and high desmoplasia. Therefore, adding anti-stromal therapies to deplete stroma and checkpoint inhibitors to retain the effector function of activated T-cells will be important while designing the strategies to test MUC4-based immunotherapy.
8. Perspectives
The advances in genome sequencing and RNA-Seq approaches have generated more evidence of the high mutational burden and presence of cancer specific splice variants in various malignancies, including PC, which have been correlated with high antigenicity of these cancer associated proteins. Similarly, the cancer specific glycosylation and other post-translational modifications (PTMs) of target proteins can alter their immunogenicity. Therefore, PC tumor antigens need to be critically evaluated for the existence of immunologically important mutations, splice variants, and the PTMs to evaluate their immunological potential as vaccine candidates. As the predicted epitopes, as well as recombinant MUC4 protein, have been investigated for their potentials as a vaccine candidate, it would be of interest to further investigate the role of the altered mutational profile, splice variants, and PTMs on MUC4 immunogenicity. These parameters would help in developing the MUC4-specific personalized vaccine for PC patients. While optimizing the MUC4 peptide or polypeptide-based formulations, it would be important to consider other parameters like the adjuvant system, formulation-dose, combinations with checkpoint inhibitors, and anti-stromal therapies to address multiple challenges associated with PC immunotherapy. Nonetheless, the development of the huMUC4 transgenic mouse model will help in future preclinical evaluations of MUC4 tumor antigen in PC immunotherapy.
Highlights.
Tumor antigen MUC4 is absent in normal pancreas but overexpresses during PC pathogenesis. Previous work from our lab and others demonstrated multifaceted role of MUC4 in tumor growth, metastasis, and drug resistance. However, MUC4 has not been explored much as a tumor-antigen to develop vaccine-based immunotherapy against PC.
Predicted immunodominant T cell epitopes and recombinant subunitβ protein of MUC4 activate APCs and elicit antigen specific cytotoxic immune response, suggesting the potential of MUC4 antigen as a vaccine candidate for PC immunotherapy. It would be noteworthy to optimize the stability of formulation and adjuvant system to get sustainable and profound anti-tumor immune response.
PC is poorly immunogenic; however, presence of somatic mutations within MUC4 sequence and existence of MUC4 splice variants in PDA patients’ samples available in TCGA suggest that MUC4 might be an important neoantigen for PC immunotherapy.
Advanced computational tools are useful in dissecting the bulky proteins like MUC4 to find the immunologically important sequences/epitopes and domains as hot spots for developing vaccine against PC.
As pancreatic tumors and highly desmoplastic and immunosuppressive, MUC4 vaccine must be investigated in combination with multiple checkpoint inhibitors, anti-stromal and anti-vascular therapies to get a better therapeutic response.
Acknowledgements –
We would like to thank Mr. Jeffrey M. Patterson for editing this manuscript.
Funding – The authors on this manuscript are supported, in parts, by the following grants from the National Institutes of Health (P01 CA217798, U01 CA200466, U01 CA210240, and R01 CA195586)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate – Not applicable
Consent for publication – Not applicable
Availability of data and material – Not applicable
Competing interest - SB is a co-founder of Sanguine Diagnostics and Therapeutics. All other authors declare that they have no conflict of interest with the information presented in this manuscript.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA: a cancer journal for clinicians 69(1) (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Stathis A, Moore MJ, Advanced pancreatic carcinoma: current treatment and future challenges, Nature Reviews Clinical Oncology 7 (2010) 163. [DOI] [PubMed] [Google Scholar]
- [3].Werner J, Combs SE, Springfeld C, Hartwig W, Hackert T, Büchler MW, Advanced-stage pancreatic cancer: therapy options, Nature Reviews Clinical Oncology 10 (2013) 323. [DOI] [PubMed] [Google Scholar]
- [4].Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH, Therapeutic developments in pancreatic cancer: current and future perspectives, Nature Reviews Gastroenterology & Hepatology 15(6) (2018) 333–348. [DOI] [PubMed] [Google Scholar]
- [5].Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH, Pancreatic cancer: understanding and overcoming chemoresistance, Nature Reviews Gastroenterology &Amp; Hepatology 8 (2010) 27. [DOI] [PubMed] [Google Scholar]
- [6].Gbolahan OB, Tong Y, Sehdev A, O’Neil B, Shahda S, Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study, BMC Cancer 19(1) (2019) 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Looi C-K, Chung FF-L, Leong C-O, Wong S-F, Rosli R, Mai C-W, Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment, Journal of Experimental & Clinical Cancer Research 38(1) (2019) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Glitza Oliva IC, Alqusairi R, Immunotherapy for Melanoma, Adv Exp Med Biol 995 (2018) 43–63. [DOI] [PubMed] [Google Scholar]
- [9].Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ, Narasimhan B, Solheim JC, Batra SK, Jain M, Emerging trends in the immunotherapy of pancreatic cancer, Cancer Lett 417 (2018) 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu AA, Jaffee E, Lee V, Current Status of Immunotherapies for Treating Pancreatic Cancer, Curr Oncol Rep 21(7) (2019) 60. [DOI] [PubMed] [Google Scholar]
- [11].Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R, Latest developments in MUC1 immunotherapy, Biochemical Society transactions 46(3) (2018) 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blando J, Sharma A, Higa MG, Zhao H, Vence L, Yadav SS, Kim J, Sepulveda AM, Sharp M, Maitra A, Wargo J, Tetzlaff M, Broaddus R, Katz MHG, Varadhachary GR, Overman M, Wang H, Yee C, Bernatchez C, Iacobuzio-Donahue C, Basu S, Allison JP, Sharma P, Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer, Proceedings of the National Academy of Sciences of the United States of America 116(5) (2019) 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hilmi M, Bartholin L, Neuzillet C, Immune therapies in pancreatic ductal adenocarcinoma: Where are we now?, World J Gastroenterol 24(20) (2018) 2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Protti MP, De Monte L, Immune infiltrates as predictive markers of survival in pancreatic cancer patients, Frontiers in Physiology 4(210) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H, CD8+ Tumor-Infiltrating Lymphocytes Together with CD4+ Tumor-Infiltrating Lymphocytes and Dendritic Cells Improve the Prognosis of Patients with Pancreatic Adenocarcinoma, Pancreas 28(1) (2004) e26–e31. [DOI] [PubMed] [Google Scholar]
- [16].Murakami T, Hiroshima Y, Matsuyama R, Homma Y, Hoffman RM, Endo I, Role of the tumor microenvironment in pancreatic cancer, Ann Gastroenterol Surg 3(2) (2019) 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu T, Zhou L, Li D, Andl T, Zhang Y, Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment, Front Cell Dev Biol 7 (2019) 60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S, Lin Q, Liu Y, Li Z, Chen R, Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer, Cell Death & Disease 9(11) (2018) 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, Hwang C-I, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA, Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer, J Exp Med 214(3) (2017) 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harper J, Sainson RCA, Regulation of the anti-tumour immune response by cancer-associated fibroblasts, Seminars in Cancer Biology 25 (2014) 69–77. [DOI] [PubMed] [Google Scholar]
- [21].Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H, CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma, Pancreas 28(1) (2004) e26–31. [DOI] [PubMed] [Google Scholar]
- [22].Crespo J, Sun H, Welling TH, Tian Z, Zou W, T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment, Current opinion in immunology 25(2) (2013) 214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bauer C, Kuhnemuth B, Duewell P, Ormanns S, Gress T, Schnurr M, Prevailing over T cell exhaustion: New developments in the immunotherapy of pancreatic cancer, Cancer Lett 381(1) (2016) 259–68. [DOI] [PubMed] [Google Scholar]
- [24].Kabacaoglu D, Ciecielski KJ, Ruess DA, Algul H, Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options, Front Immunol 9 (2018) 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, Yao S, Anders RA, Laheru D, Wolfgang CL, Edil BH, Schulick RD, Jaffee EM, Zheng L, PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors, J Immunother 38(1) (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH, Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma, Cancer Immunol Res 3(4) (2015) 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gao M, Lin M, Moffitt RA, Salazar MA, Park J, Vacirca J, Huang C, Shroyer KR, Choi M, Georgakis GV, Sasson AR, Talamini MA, Kim J, Direct therapeutic targeting of immune checkpoint PD-1 in pancreatic cancer, British journal of cancer 120(1) (2019) 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balachandran VP, Beatty GL, Dougan SK, Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities, Gastroenterology 156(7) (2019) 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matsui H, Hazama S, Shindo Y, Nagano H, Combination treatment of advanced pancreatic cancer using novel vaccine and traditional therapies, Expert review of anticancer therapy 18(12) (2018) 1205–1217. [DOI] [PubMed] [Google Scholar]
- [30].Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L, Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation, Cancer Immunol Res 2(7) (2014) 616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T Jr., Brockstedt DG, Jaffee EM, Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33(12) (2015) 1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, Woolman S, Vithayathil T, Glickman LH, Ndubaku CO, McWhirter SM, Dubensky TW Jr., Armstrong TD, Jaffee EM, Zaidi N, Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer, JCI Insight 3(20) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seery TE, Lee JH, Sender LS, Jones FR, Shinde AM, Annamalai A, Kistler M, Jafari O, Soon-Shiong P, NANT cancer vaccine an orchestration of immunogenic cell death by overcoming immune suppression and activating NK and T cell therapy in patients with third line or greater metastatic pancreatic cancer, Journal of Clinical Oncology 37(4_suppl) (2019) TPS463–TPS463. [Google Scholar]
- [34].Cannon MJ, Block MS, Morehead LC, Knutson KL, The evolving clinical landscape for dendritic cell vaccines and cancer immunotherapy, Immunotherapy 11(2) (2019) 75–79. [DOI] [PubMed] [Google Scholar]
- [35].Koido S, Okamoto M, Kobayashi M, Shimodaira S, Sugiyama H, Significance of Wilms’ tumor 1 antigen as a cancer vaccine for pancreatic cancer, Discov Med 24(130) (2017) 41–49. [PubMed] [Google Scholar]
- [36].Hanada S, Tsuruta T, Haraguchi K, Okamoto M, Sugiyama H, Koido S, Long-term survival of pancreatic cancer patients treated with multimodal therapy combined with WT1-targeted dendritic cell vaccines, Hum Vaccin Immunother 15(2) (2019) 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zaidi N, Quezada SA, Kuroiwa JMY, Zhang L, Jaffee EM, Steinman RM, Wang B, Anti-CTLA-4 synergizes with dendritic cell-targeted vaccine to promote IL-3-dependent CD4(+) effector T cell infiltration into murine pancreatic tumors, Ann N Y Acad Sci 1445(1) (2019) 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hardwick NR, Carroll M, Kaltcheva T, Qian D, Lim D, Leong L, Chu P, Kim J, Chao J, Fakih M, Yen Y, Espenschied J, Ellenhorn JD, Diamond DJ, Chung V, p53MVA therapy in patients with refractory gastrointestinal malignancies elevates p53-specific CD8+ T-cell responses, Clin Cancer Res 20(17) (2014) 4459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen F, Zou Z, Du J, Su S, Shao J, Meng F, Yang J, Xu Q, Ding N, Yang Y, Liu Q, Wang Q, Sun Z, Zhou S, Du S, Wei J, Liu B, Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors, The Journal of clinical investigation 130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kaur S, Kumar S, Momi N, Sasson AR, Batra SK, Mucins in pancreatic cancer and its microenvironment, Nat Rev Gastroenterol Hepatol 10(10) (2013) 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jonckheere N, Skrypek N, Van Seuningen I, Mucins and pancreatic cancer, Cancers 2(4) (2010) 1794–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rachagani S, Torres MP, Kumar S, Haridas D, Baine M, Macha MA, Kaur S, Ponnusamy MP, Dey P, Seshacharyulu P, Johansson SL, Jain M, Wagner KU, Batra SK, Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy, J Hematol Oncol 5 (2012) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kufe DW, Mucins in cancer: function, prognosis and therapy, Nature reviews. Cancer 9(12) (2009) 874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kaur S, Smith LM, Patel A, Menning M, Watley DC, Malik SS, Krishn SR, Mallya K, Aithal A, Sasson AR, Johansson SL, Jain M, Singh S, Guha S, Are C, Raimondo M, Hollingsworth MA, Brand RE, Batra SK, A Combination of MUC5AC and CA19–9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study, Am J Gastroenterol 112(1) (2017) 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang G, Lipert RJ, Jain M, Kaur S, Chakraboty S, Torres MP, Batra SK, Brand RE, Porter MD, Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering, Anal Chem 83(7) (2011) 2554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gold DV, Modrak DE, Ying Z, Cardillo TM, Sharkey RM, Goldenberg DM, New MUC1 serum immunoassay differentiates pancreatic cancer from pancreatitis, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24(2) (2006) 252–8. [DOI] [PubMed] [Google Scholar]
- [47].Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, Kelly DL, Caffrey TC, Hollingsworth MA, Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line, Cancer research 63(16) (2003) 5011–20. [PubMed] [Google Scholar]
- [48].Satoh S, Hinoda Y, Hayashi T, Burdick MD, Imai K, Hollingsworth MA, Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule, International journal of cancer 88(4) (2000) 507–18. [DOI] [PubMed] [Google Scholar]
- [49].Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL, Bajaj E, Shanmugam K, Lee YY, Hwang SI, Gendler SJ, Mukherjee P, MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition, Oncogene 30(12) (2011) 1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK, MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells, Cancer research 68(7) (2008) 2065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Moniaux N, Chaturvedi P, Varshney GC, Meza JL, Rodriguez-Sierra JF, Aubert JP, Batra SK, Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells, British journal of cancer 97(3) (2007) 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Das S, Batra SK, Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy, Cancer Research 75(22) (2015) 4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen S-H, Hung W-C, Wang P, Paul C, Konstantopoulos K, Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation, Scientific Reports 3 (2013) 1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Muniyan S, Haridas D, Chugh S, Rachagani S, Lakshmanan I, Gupta S, Seshacharyulu P, Smith LM, Ponnusamy MP, Batra SK, MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism, Genes & cancer 7(3–4) (2016) 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM, Anderson JM, Wittel UA, Hollingsworth MA, Batra SK, Pathobiological Implications of MUC16 Expression in Pancreatic Cancer, PLOS ONE 6(10) (2011) e26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O, I. Australian Pancreatic Cancer Genome, R. Garvan Institute of Medical, H. Prince of Wales, H. Royal North Shore, G. University of, H. St Vincent’s, Q.B.M.R. Institute, C.f.C.R. University of Melbourne, I.f.M.B. University of Queensland, Bankstown H, Liverpool H, C.O.B.L. Royal Prince Alfred Hospital, Westmead H, Fremantle H, H. St John of God, H. Royal Adelaide, C. Flinders Medical, Envoi P, Princess Alexandria H, Austin H, I. Johns Hopkins Medical, A.R.-N.C.f.A.R.o. Cancer, Gönen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD, Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer, Nature 551(7681) (2017) 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R, Latest developments in MUC1 immunotherapy, Biochem Soc Trans 46(3) (2018) 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Matsui H, Hazama S, Sakamoto K, Shindo Y, Kanekiyo S, Nakashima M, Matsukuma S, Tokuhisa Y, Iida M, Suzuki N, Yoshimura K, Takeda S, Ueno T, Yoshino S, Oka M, Nagano H, Postoperative Adjuvant Therapy for Resectable Pancreatic Cancer With Gemcitabine and Adoptive Immunotherapy, Pancreas 46(8) (2017) 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kato Y, [Efficacy of WT1 peptide-/MUC-1 peptide-pulsed dendritic cell therapy in 313 patients with a wide range of cancers], Gan to kagaku ryoho. Cancer & chemotherapy 41(10) (2014) 1280–2. [PubMed] [Google Scholar]
- [60].Mehla K, Tremayne J, Grunkemeyer JA, O’Connell KA, Steele MM, Caffrey TC, Zhu X, Yu F, Singh PK, Schultes BC, Madiyalakan R, Nicodemus CF, Hollingsworth MA, Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors, Cancer Immunol Immunother 67(3) (2018) 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O, I. Australian Pancreatic Cancer Genome, R. Garvan Institute of Medical, H. Prince of Wales, H. Royal North Shore, G. University of, H. St Vincent’s, Q.B.M.R. Institute, C.f.C.R. University of Melbourne, I.f.M.B. University of Queensland, Bankstown H, Liverpool H, C.O.B.L. Royal Prince Alfred Hospital, Westmead H, Fremantle H, H. St John of God, H. Royal Adelaide, C. Flinders Medical, Envoi P, Princess Alexandria H, Austin H, I. Johns Hopkins Medical, A.R.-N.C.f.A.R.o. Cancer, Gonen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD, Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer, Nature 551(7681) (2017) 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gautam SK, Kumar S, Cannon A, Hall B, Bhatia R, Nasser MW, Mahapatra S, Batra SK, Jain M, MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma, Expert Opin Ther Targets 21(7) (2017) 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, Osako M, Takao S, Batra SK, Aikou T, Imai K, Yonezawa S, MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas, J Clin Pathol 58(8) (2005) 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P, MUC4 expression increases progressively in pancreatic intraepithelial neoplasia, Am J Clin Pathol 117(5) (2002) 791–6. [DOI] [PubMed] [Google Scholar]
- [65].Jahan R, Macha MA, Rachagani S, Das S, Smith LM, Kaur S, Batra SK, Axed MUC4 (MUC4/X) aggravates pancreatic malignant phenotype by activating integrin-beta1/FAK/ERK pathway, Biochim Biophys Acta Mol Basis Dis 1864(8) (2018) 2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pai P, Rachagani S, Lakshmanan I, Macha MA, Sheinin Y, Smith LM, Ponnusamy MP, Batra SK, The canonical Wnt pathway regulates the metastasis-promoting mucin MUC4 in pancreatic ductal adenocarcinoma, Mol Oncol 10(2) (2016) 224–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK, Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer, Clin Cancer Res 17(2) (2011) 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kumar S, Cruz E, Joshi S, Patel A, Jahan R, Batra SK, Jain M, Genetic variants of mucins: unexplored conundrum, Carcinogenesis 38(7) (2017) 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Urey C, Andersson B, Ansari D, Sasor A, Said-Hilmersson K, Nilsson J, Andersson R, Low MUC4 expression is associated with survival benefit in patients with resectable pancreatic cancer receiving adjuvant gemcitabine, Scand J Gastroenterol 52(5) (2017) 595–600. [DOI] [PubMed] [Google Scholar]
- [70].Ponnusamy MP, Seshacharyulu P, Vaz A, Dey P, Batra SK, MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells, Journal of Ovarian Research 4(1) (2011) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK, Inhibition of MUC4 Expression Suppresses Pancreatic Tumor Cell Growth and Metastasis, Cancer Research 64(2) (2004) 622–630. [DOI] [PubMed] [Google Scholar]
- [72].Bafna S, Kaur S, Momi N, Batra SK, Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin, Br J Cancer 101(7) (2009) 1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wei J, Gao W, Wu J, Meng K, Zhang J, Chen J, Miao Y, Dendritic cells expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine induce multiple cytotoxic T-cell responses, Cancer Biother Radiopharm 23(1) (2008) 121–8. [DOI] [PubMed] [Google Scholar]
- [74].Wu J, Wei J, Meng K, Chen J, Gao W, Zhang J, Xu Z, Miao Y, Identification of an HLA-A*0201-restrictive CTL epitope from MUC4 for applicable vaccine therapy, Immunopharmacol Immunotoxicol 31(3) (2009) 468–76. [DOI] [PubMed] [Google Scholar]
- [75].Chen J, Guo XZ, Li HY, Liu X, Ren LN, Wang D, Zhao JJ, Generation of CTL responses against pancreatic cancer in vitro using dendritic cells co-transfected with MUC4 and survivin RNA, Vaccine 31(41) (2013) 4585–90. [DOI] [PubMed] [Google Scholar]
- [76].Cai H, Palitzsch B, Hartmann S, Stergiou N, Kunz H, Schmitt E, Westerlind U, Antibody induction directed against the tumor-associated MUC4 glycoprotein, Chembiochem 16(6) (2015) 959–67. [DOI] [PubMed] [Google Scholar]
- [77].Banerjee K, Gautam SK, Kshirsagar P, Ross KA, Spagnol G, Sorgen P, Wannemuehler MJ, Narasimhan B, Solheim JC, Kumar S, Batra SK, Jain M, Amphiphilic polyanhydride-based recombinant MUC4βnanovaccine activates dendritic cells Genes and Cancer 10(3–4) (2019) 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cafri G, Yossef R, Pasetto A, Deniger DC, Lu YC, Parkhurst M, Gartner JJ, Jia L, Ray S, Ngo LT, Jafferji M, Sachs A, Prickett T, Robbins PF, Rosenberg SA, Memory T cells targeting oncogenic mutations detected in peripheral blood of epithelial cancer patients, Nature communications 10(1) (2019) 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, Woolman S, Vithayathil T, Glickman LH, Ndubaku CO, McWhirter SM, Dubensky TW Jr., Armstrong TD, Jaffee EM, Zaidi N, Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer, JCI insight 3(20) (2018) e122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C, Tumor neoantigens: from basic research to clinical applications, Journal of Hematology & Oncology 12(1) (2019) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leao IC, Ganesan P, Armstrong TD, Jaffee EM, Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma, Clin Transl Sci 1(3) (2008) 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Koşaloğlu-Yalçın Z, Lanka M, Frentzen A, Logandha Ramamoorthy Premlal A, Sidney J, Vaughan K, Greenbaum J, Robbins P, Gartner J, Sette A, Peters B, Predicting T cell recognition of MHC class I restricted neoepitopes, Oncoimmunology 7(11) (2018) e1492508–e1492508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Smith CC, Chai S, Washington AR, Lee SJ, Landoni E, Field K, Garness J, Bixby LM, Selitsky SR, Parker JS, Savoldo B, Serody JS, Vincent BG, Machine-Learning Prediction of Tumor Antigen Immunogenicity in the Selection of Therapeutic Epitopes, Cancer Immunology Research (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Quandt J, Schlude C, Bartoschek M, Will R, Cid-Arregui A, Schölch S, Reissfelder C, Weitz J, Schneider M, Wiemann S, Momburg F, Beckhove P, Long-peptide vaccination with driver gene mutations in p53 and Kras induces cancer mutation-specific effector as well as regulatory T cell responses, Oncoimmunology 7(12) (2018) e1500671–e1500671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Raphael BJ, Hruban RH, Aguirre AJ, Moffitt RA, Yeh JJ, Stewart C, Robertson AG, Cherniack AD, Gupta M, Getz G, Gabriel SB, Meyerson M, Cibulskis C, Fei SS, Hinoue T, Shen H, Laird PW, Ling S, Lu Y, Mills GB, Akbani R, Loher P, Londin ER, Rigoutsos I, Telonis AG, Gibb EA, Goldenberg A, Mezlini AM, Hoadley KA, Collisson E, Lander E, Murray BA, Hess J, Rosenberg M, Bergelson L, Zhang H, Cho J, Tiao G, Kim J, Livitz D, Leshchiner I, Reardon B, Allen EV, Kamburov A, Beroukhim R, Saksena G, Schumacher SE, Noble MS, Heiman DI, Gehlenborg N, Kim J, Lawrence MS, Adsay V, Petersen G, Klimstra D, Bardeesy N, Leiserson MDM, Bowlby R, Kasaian K, Birol I, Mungall KL, Sadeghi S, Weinstein JN, Spellman PT, Liu Y, Amundadottir LT, Tepper J, Singhi AD, Dhir R, Paul D, Smyrk T, Zhang L, Kim P, Bowen J, Frick J, Gastier-Foster JM, Gerken M, Lau K, Leraas KM, Lichtenberg TM, Ramirez NC, Renkel J, Sherman M, Wise L, Yena P, Zmuda E, Shih J, Ally A, Balasundaram M, Carlsen R, Chu A, Chuah E, Clarke A, Dhalla N, Holt RA, Jones SJM, Lee D, Ma Y, Marra MA, Mayo M, Moore RA, Mungall AJ, Schein JE, Sipahimalani P, Tam A, Thiessen N, Tse K, Wong T, Brooks D, Auman JT, Balu S, Bodenheimer T, Hayes DN, Hoyle AP, Jefferys SR, Jones CD, Meng S, Mieczkowski PA, Mose LE, Perou CM, Perou AH, Roach J, Shi Y, Simons JV, Skelly T, Soloway MG, Tan D, Veluvolu U, Parker JS, Wilkerson MD, Korkut A, Senbabaoglu Y, Burch P, McWilliams R, Chaffee K, Oberg A, Zhang W, Gingras M-C, Wheeler DA, Xi L, Albert M, Bartlett J, Sekhon H, Stephen Y, Howard Z, Judy M, Breggia A, Shroff RT, Chudamani S, Liu J, Lolla L, Naresh R, Pihl T, Sun Q, Wan Y, Wu Y, Jennifer S, Roggin K, Becker K-F, Behera M, Bennett J, Boice L, Burks E, Junior CGC, Chabot J, Tirapelli D.P.d.C., Santos J.S.d., Dubina M, Eschbacher J, Huang M, Huelsenbeck-Dill L, Jenkins R, Karpov A, Kemp R, Lyadov V, Maithel S, Manikhas G, Montgomery E, Noushmehr H, Osunkoya A, Owonikoko T, Paklina O, Potapova O, Ramalingam S, Rathmell WK, Rieger-Christ K, Saller C, Setdikova G, Shabunin A, Sica G, Su T, Sullivan T, Swanson P, Tarvin K, Tavobilov M, Thorne LB, Urbanski S, Voronina O, Wang T, Crain D, Curley E, Gardner J, Mallery D, Morris S, Paulauskis J, Penny R, Shelton C, Shelton T, Janssen K-P, Bathe O, Bahary N, Slotta-Huspenina J, Johns A, Hibshoosh H, Hwang RF, Sepulveda A, Radenbaugh A, Baylin SB, Berrios M, Bootwalla MS, Holbrook A, Lai PH, Maglinte DT, Mahurkar S, T.J.T. Jr., Berg DJVD, Weisenberger DJ, Chin L, Kucherlapati R, Kucherlapati M, Pantazi A, Park P, Saksena G, Voet D, Lin P, Frazer S, Defreitas T, Meier S, Chin L, Kwon SY, Kim YH, Park S-J, Han S-S, Kim SH, Kim H, Furth E, Tempero M, Sander C, Biankin A, Chang D, Bailey P, Gill A, Kench J, Grimmond S, Johns A, APGI APCGI, Postier R, Zuna R, Sicotte H, Demchok JA, Ferguson ML, Hutter CM, Shaw KRM, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zhang JJ, Felau I, Zenklusen JC, Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma, Cancer Cell 32(2) (2017) 185–203.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O, Nielsen M, Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction, BMC Bioinformatics 8 (2007) 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Giron CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P, Ensembl 2018, Nucleic Acids Res 46(D1) (2018) D754–d761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jain M, Venkatraman G, Moniaux N, Kaur S, Kumar S, Chakraborty S, Varshney GC, Batra SK, Monoclonal antibodies recognizing the non-tandem repeat regions of the human mucin MUC4 in pancreatic cancer, PLoS One 6(8) (2011) e23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK, Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans, J Histochem Cytochem 52(2) (2004) 253–61. [DOI] [PubMed] [Google Scholar]
- [90].Banerjee K, Gautam SK, Kshirsagar P, Ross KA, Spagnol G, Sorgen P, Wannemuehler MJ, Narasimhan B, Solheim JC, Kumar S, Batra SK, Jain M, Amphiphilic polyanhydride-based recombinant MUC4beta-nanovaccine activates dendritic cells, Genes & cancer 10(3–4) (2019) 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


