Abstract
Blunted anterior insula activation during interoceptive perturbations has been associated with stimulant (cocaine and amphetamine) use disorder (SUD) and is related to risk for and prognosis of SUD. However, little is known whether these interoceptive alterations extend to opioid use disorder (OUD). This exploratory study used the same experimental probe during functional magnetic resonance imaging (fMRI) to test the hypothesis that SUD and OUD exhibit interoceptive discrepancies characterized by subjective ratings and activation within the insula. Recently abstinent individuals diagnosed with current SUD (n=40) or current OUD (n=20) were compared to healthy individuals (CTL; n=30) on brain and self-report responses during an interoceptive attention task known to elicit insula activation. Participants selectively attended to interoceptive (heartbeat and stomach) and exteroceptive signals during blood-oxygen-level dependent fMRI recording. Groups and conditions were compared on: (1) activation within probabilistic cytoarchitectonic segmentations of the insula; and (2) self-reported stimulus intensity. First, SUD showed amplified ratings of heart-related sensations but attenuation of dorsal dysgranular insula activity relative to CTL. Amplified ratings were linked to drug use recency, while attenuation was normalized with greater past-year stimulant use. Second, SUD and OUD showed attenuation of dorsal dysgranular insula activity during attention to stomach sensations relative to CTL. Taken together, these results are consistent with altered neural processing of interoceptive signals in drug addiction, particularly as a function of SUD. Future studies will need to determine whether interoceptive metrics help to explain substance use disorder pathophysiology and are useful for predicting outcomes.
Keywords: stimulant use disorder, opioid use disorder, interoception, insula, cytoarchitecture
Interoceptive Attention in Stimulant and Opioid Use Disorder
Stimulant (amphetamine and cocaine) and opioid use disorders (SUD and OUD) are linked to millions of life years lost and early death due to overdose, suicide, and effects of comorbid physical disorders1–5. Approximately one third to half of chronic SUD experience persistent heart problems, and cardiovascular complications are the second-highest cause of death for these individuals after stimulant toxicity/overdose6–8. Comorbid opioid and stimulant use has also doubled within the past decade, with users consuming these substances simultaneously or sequentially to enhance drug highs, balance effects of stimulation and sedation, and/or ease opioid withdrawal symptoms9, the latter characterized by substantial gastrointestinal distress4. We have argued that addicted individuals consume drugs to correct perceived imbalances within the body, particularly those of an interoceptive nature; for example, starting to feel “bad” motivates drug use to avoid worsening negative emotional states and bodily symptoms forecasting withdrawal (i.e., negative reinforcement)10. Interoception covers the process of sensing, interpreting, and integrating internal bodily signals, such as those arising from cardiovascular and gastrointestinal systems, in order to maintain homeostasis11. Although interoception is believed to be disrupted as a result of substance use disorders10,12–15, the precise nature of this disruption is still unclear. Given that over 50% of individuals with SUD and/or OUD relapse within a year of treatment16–17, a nuanced understanding of interoceptive dysfunction within these disorders may facilitate targeted somatic interventions aimed at reducing drug craving, use, and relapse11,18.
Accurately assessing bodily imbalance and subsequently motivating action to restore balance are crucial components of an intact interoceptive system. Despite the hypothetical centrality of interoceptive processes in drug craving, intoxication, and withdrawal10,14, few studies outside of cue reactivity paradigms have examined whether individuals with SUD and/or OUD actually exhibit altered responses to bodily signals. Individuals with OUD may be less accurate in perceiving their own heartbeats than healthy comparisons19 and endorse greater symptoms of anxiety sensitivity, or fear of bodily sensations that resemble anxiety states, than individuals with SUD20. Similarly, users with SUD endorse greater frequency of cardiac and stomach sensations during imagined stress compared to imagined drug use scenarios21, but it is unclear whether they experience somatic symptoms within these contexts to a greater magnitude than other- or non-drug users.
With respect to brain function, numerous studies have demonstrated that the insular cortex is a central hub for numerous facets of interoceptive processing22–25. Although no functional magnetic resonance imaging (fMRI) research has specifically examined interoceptive processing in OUD, individuals with current SUD exhibit lower anterior/mid insular cortex fMRI blood-oxygen level dependent (BOLD) signal than healthy individuals (CTL) during appetitive (soft touch) and aversive (breathing load) perturbations of bodily sensation26–28. This blunted brain response suggests that SUD users may not adequately attend to externally manipulated changes involving respiration and touch. However, individuals with SUD and/or OUD may actually feel intensified bodily sensations within certain contexts. For instance, given sympathetic modulation occurring within the body during acute stimulant administration29–30, and prevalence of cardiac dysfunction following chronic stimulant abuse in SUD, it may be the case that heart sensations are perceived to be more intense by SUD users than OUD users. The converse may be true with respect to gastrointestinal symptoms, based on the antikinetic effects of acute opioid administration, and the prokinetic effects of opioid withdrawal31. Perceived amplification of these signals may not translate into corresponding amplifications in brain responses either. It may be that within the cycle of chronic addiction, allostatic changes proactively occur such that exaggerated intensity of certain body signals results in reduced allocation of brain resources to focus attention on these signals.
There is increasing recognition that different parts of the insula contribute to specific aspects of interoceptive processing. Anatomical insular subdivisions, carved out on the basis of primate granule cell geography32, map onto specific functions related to sensing, attending to, and registering bodily signals in humans33–34. Insular agranular, dysgranular, and granular subdivisions frequently studied in the animal literature correspond roughly to the anterior, mid, and posterior regions described in fMRI studies of addiction: (1) The granular subdivision, comprised of two distinct granule cell layers, maps onto posterior insula and appears to be functionally involved in pain, somatosensory, and somatomotor processes; (2) The agranular subdivision, absent of granule cell layers, maps onto anterior insula and has been linked to the experience of bodily feeling states as well as valuation of stimuli relevant to these states; and (3) The dysgranular subdivision, consisting of granule cells that are less organized/plentiful than the granular subdivision, appears to map onto regions of anterior/mid insula, activating in tasks requiring attentional control. Although we have argued that evaluation of internal signals as “bad” or “good” motivates a person to seek out or hold off from using drugs, respectively10, we do not know if insula signals involved in this process are limited to anterior insula or generalize across insular cortex. This knowledge is crucial for development of targeted brain-based interventions for addiction. And while it has been argued that integrating insula cytoarchitecture into human neuroimaging studies can bridge the gap between animal and human models of addiction35, few to date have attempted to do so. A recently available tool, the Brainnetome atlas36, allows for cytoarchitectonic segmentation of human insula on the basis of combined probabilistic tractography and connectivity-based parcellation, yielding a series of anatomically viable subregions. In the current study we apply this tool to further elucidate the role of insula function in addiction.
The present study explored the relationship between primary current drug of choice and interoception by comparing individuals with current SUD, current OUD, and CTL on activity within probabilistic cytoarchitectural subregions of the insular cortex during an fMRI interoception paradigm consistently shown to localize insula activity37–39. We predicted that there would be evidence of interoceptive alterations in the form of differences in insula activation and subjective experiences of heartbeat and stomach sensation in both SUD and OUD when compared to CTL.
Materials and Methods
Participants
Participants consisted of a subsample of the first 500 individuals recruited into the Tulsa 1000 (T1000) project, a naturalistic longitudinal study of 1000 individuals aged 18–65 comprised of treatment-seeking individuals with psychiatric symptoms and individuals without a history of psychiatric illness40. The T1000 study was approved by the Western Institutional Review Board and carried out in accordance with the Declaration of Helsinki. Participants provided informed written consent and subject confidentiality was ensured. Participants were recruited from radio, internet, and paper advertisements. Individuals with lifetime substance use disorders who were currently in treatment were referred from two local alcohol and drug treatment centers and screened for eligibility.
Individuals first orally consented to complete a telephone or in-person screen by trained staff to assess preliminary study eligibility. Eligible participants were then scheduled for a clinical interview session wherein trained staff administered the MINI International Neuropsychiatric Interview (version 6.0 or 7.0)41 to assess lifetime disorders in accordance with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition or 5th Edition42 criteria. Exclusion criteria for all groups were: (1) positive urine screen for alcohol and drugs of abuse at clinical interview/neuroimaging sessions; (2) bipolar, schizophrenia spectrum, and obsessive compulsive disorders; (3) active suicidal ideation with intent/plan; (4) moderate-to-severe traumatic brain injury; (5) significant or unstable medical disturbance not controlled by medication; and (6) fMRI contraindications (e.g., metal in body, pregnancy). See40 for detailed T1000 inclusion and exclusion criteria.
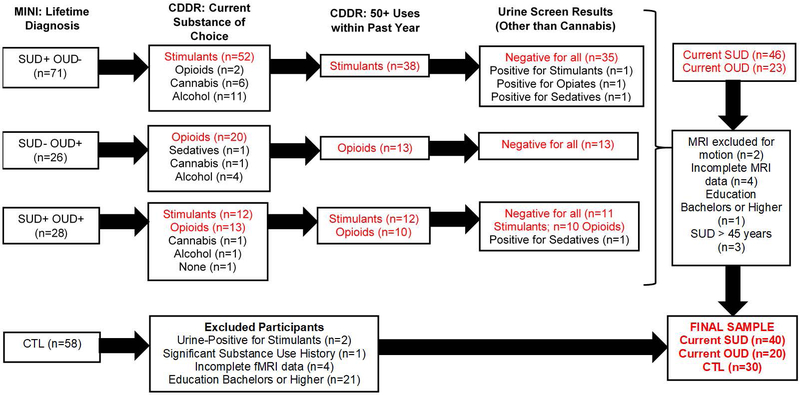
For the present analysis, participants were classified into three groups based on MINI diagnoses and Customary Drinking and Drug Use Record (CDDR)43 responses: (1) current SUD (n = 40): met criteria for current stimulant use disorder, endorsed a stimulant as their current substance of choice, and endorsed ≥ 50 uses of stimulants within the past year; (2) current OUD (n = 20): met criteria for current opioid use disorder, endorsed an opioid as their current substance of choice, and endorsed ≥ 50 uses of opioids within the past year; and (3) CTL (n = 30): did not meet any DSM disorder criteria. Figure 1 illustrates the group selection and elimination process. SUD and OUD were allowed to meet diagnostic criteria for other substance use, unipolar mood, and anxiety disorders. The remaining participants either did not endorse current SUD/OUD or were not healthy comparisons with comparable education/age and were therefore excluded from analyses.
Figure 1.
Diagram highlighting participant inclusion and exclusion into three groups for statistical analysis: Current stimulant use disorder (SUD; n = 40), current opioid use disorder (OUD, n = 20), and healthy comparison subjects (CTL, n = 30). MINI = MINI international neuropsychiatric interview. CDDR = Customary Drinking and Drug Use Record. MRI = magnetic resonance imaging.
Procedure
Participants completed baseline clinical interview, neuroimaging, biomarker, and behavioral sessions within a period of two weeks40. Details regarding clinical interview and neuroimaging sessions relevant to the current analysis are presented below.
Demographics and clinical characteristics.
At screening, participants completed the Patient Health Questionnaire 9 (PHQ-9)44 to index depression symptoms. During the clinical interview session, participants completed: (1) a demographics form querying age, education, race/ethnicity, and gender characteristics; (2) the Anxiety Sensitivity Index 3 (ASI-3)45 to assess fear of anxiety sensations; and (3) the Multidimensional Assessment of Interoceptive Awareness (MAIA)46 to evaluate self-reported experiences of interoceptive sensations. The ASI-3 scale produces a total score and three subscale scores evaluating concerns regarding cognitive, physical and social consequences. The MAIA scale produces eight subscale scores pertaining to various aspects of interoception: (1) noticing; (2) not-distracting; (3) not-worrying; (4) attention regulation; (5) emotional awareness; (6) self-regulation; (7) body-listening; and (8) body-trusting. CDDR interview responses43 were used to quantify recency of illicit substance use in days as well as lifetime stimulant, opioid, alcohol, cannabis, and nicotine uses.
Neuroimaging Session
Participants completed two runs of the Visceral Interoceptive Awareness (VIA) task37–39 during fMRI, wherein they were presented with three different attention modulation conditions cued by a word presented on a front-projection computer screen: (1) “heart” cued internal attention toward heartbeat sensations; (2) “stomach” cued internal attention toward stomach sensations; and (3) “target” cued external attention toward word color changes. Word cues were presented for the entire 10 s trial duration. 50% of trials were followed by prompts requesting participants to rate how intensely each stimulus was felt (0 = ‘no sensation’ to 6 = ‘extreme sensation’). Each run was comprised of 6 trials per condition, for a total of 36 trials for the entire task. Trials were spaced at an intertrial interval ranging from 2.5 to 12.5 s (fixation cross presented in the center of the screen). MRI data were acquired on two identical GE Discovery MR750 3T scanners operating identical pulse sequences for functional [repetition time (TR)/echo time (TE) = 2000/27 ms, field of view (FOV)/slice = 240/2.9 mm, 128 × 128 matrix, 39 axial slices, 180 TRs] and structural scans [magnetization prepared rapid acquisition gradient recalled echo (MP-RAGE) TR/TE = 5/2.012 ms, FOV/slice=240 × 192/0.9 mm, 186 axial slices].
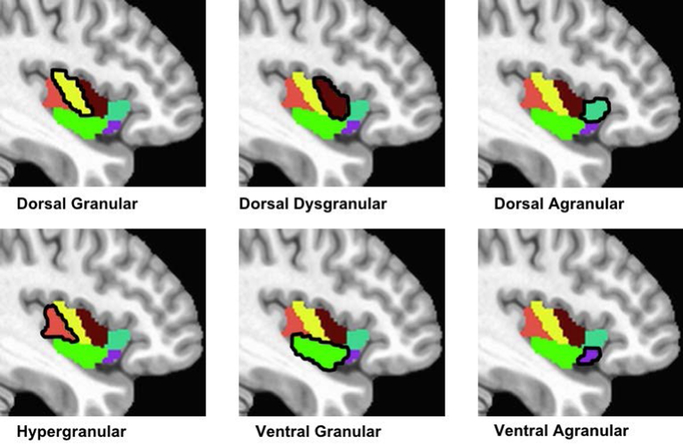
Neuroimaging Preprocessing
Single-subject preprocessing was completed using Analysis of Functional NeuroImages (AFNI) software47 and included despiking, slice-timing correction, motion correction, transformation to Montreal Neurological Institute space via an affine transformation, and a voxelwise general linear model (GLM) analysis. Block regressors were used for heart, stomach, and target conditions as well as rating prompts. Six motion parameters (three translations and three rotations) were included as nuisance regressors. Individual TRs with a Euclidian norm of the derivative of the motion parameters greater than .3 and one TR prior were censored. Regression coefficients estimated from the GLM were extracted from both hemispheres of all six probabilistic cytoarchitectonic segmentations of the insula insular regions defined by the Brainnetome atlas36 as illustrated in Figure 2: dorsal agranular, dorsal dysgranular, ventral agranular, hypergranular, ventral granular, and dorsal granular. For each region and hemisphere, the average beta values for heart, stomach, and target were extracted, only considering voxels with a temporal signal-to-noise ratio greater than 50. Beta values were multiplied by 100 to obtain percent BOLD signal change from baseline, which was used as the dependent variable in further analysis. The baseline condition used for comparison was comprised of fMRI data collected during the intertrial interval.
Figure 2.
Six insular cortex regions of interest (ROIs) extracted from the Brainnetome Atlas.
Statistical Analysis
Demographics and clinical characteristics.
Chi-square tests were computed to evaluate group (SUD, OUD, CTL) differences in gender, race/ethnicity (for the two most frequent subgroups, in this sample White and Native American), and education. For dimensional variables including age and questionnaire scores (PHQ-9, ASI-3, and MAIA), Levine’s test evaluated the homogeneity of variance assumption between groups; if this assumption was violated, a non-parametric Kruskal-Wallis (K-W) test was computed with group as the between subjects variable; otherwise, a univariate analysis of variance (ANOVA) was computed. A multivariate ANOVA (MANOVA) was first calculated for ASI-3 and MAIA with subscales as the repeated measure and group as the between-subjects factor. Overall Wilk’s λ significance (p < .05) justified inspection of ASI-3 and MAIA subscale tests.
Whereas DSM psychopathology was an exclusion criterion for CTL, psychiatric comorbidity was present in both user groups. Chi-squared tests evaluated frequency of lifetime disorders and current psychotropic medication between SUD and OUD. Due to non-normal distributions of drug use variables, nonparametric Mann-Whitney U tests compared lifetime substance uses as well as past year stimulant and opioid uses between SUD and OUD.
VIA intensity ratings.
A mixed ANOVA was computed with group (SUD, OUD, CTL) as the between subject factor and condition (heart, stomach, target) as the within subject factor. Average intensity rating was the dependent variable. Mauchly’s test evaluated potential sphericity violations and Greenhouse-Geisser/Huynh-Feldt corrections were applied where appropriate. Pairwise comparisons tested estimated marginal mean differences for interpretation of significant effects.
VIA insula ROIs.
A mixed ANOVA was computed with group (SUD, OUD, CTL) as the between subject factor, and condition (heart, stomach, target), insular region (dorsal agranular, dorsal dysgranular, ventral agranular, hypergranular, ventral granular, and dorsal granular), and hemisphere (left, right) as repeated factors. Percent BOLD signal change from baseline was the dependent variable. Pairwise comparisons tested estimated marginal mean differences for interpretation of significant effects.
Results
Demographic and Clinical Characteristics
The sample was 68% White, 19% Native American, 4% Other, 4% Black, 3% Hispanic, and 2% Asian. Table 1 illustrates group demographic, clinical symptoms, and user medication status. All three groups did not differ in gender, age, or education. SUD and OUD had similar rates of comorbid mood/anxiety disorders, lifetime alcohol/cannabis use disorders, lifetime cannabis/nicotine uses and current use of psychotropic medication; however, SUD reported greater lifetime alcohol uses and lower rates of lifetime sedative use disorder than OUD. Consistent with group categorizations, SUD had greater lifetime and past-year stimulant uses than OUD, whereas OUD had greater lifetime and past-year opioid uses than SUD. Questionnaire results shown in Table 2 indicate that user groups endorsed higher distress (depression and anxiety sensitivity) symptoms than CTL, paired with lower interoceptive awareness with respect to self-regulation, body-trusting, and not-worrying.
Table 1.
Group demographic and clinical characteristics.
| SUD (n = 40) | OUD (n = 20) | CTL (n = 30) | |||
|---|---|---|---|---|---|
| Demographics | % | % | % | Statistics | Three Group Differences |
| Female | 58 | 45 | 53 | χ2(2) = 0.66, p = .68 | None |
| Native American | 28 | 20 | 7 | χ2(2) = 4.88, p = .09 | None |
| White | 58 | 70 | 80 | χ2(2) = 4.03, p = .13 | None |
| Education | % | % | % | Statistics | Three Group Differences |
| Did not Finish HS | 23 | 20 | 0 | χ2(4) = 9.34, p = .06 | None |
| Finished HS/GED | 32 | 25 | 27 | ||
| Some College | 45 | 55 | 73 | ||
| M (SD) | M (SD) | M (SD) | Statistics | Three Group Differences | |
| Age (years) | 32.72 (6.56) | 31.06 (8.56) | 28.53 (9.58) | F(2, 87) = 2.28, p = .11 | None |
| CDDR1 | M (SD) | M (SD) | M (SD) | Statistics | User Group Differences |
| Recency Illicit Drug Use (days)# | 110.50 (74.29) | 77.67 (49.20) | N/A | Mann-Whitney U, p = .08 | None |
| Lifetime Alcohol Use (sessions) | 4105.57 (12885.05) | 2448.00 (3155.93) | N/A | Mann-Whitney U, p = .04 | SUD > OUD |
| Lifetime Cannabis Use (sessions) | 117929.78 (636402.60) | 12183.45 (30115.68) | N/A | Mann-Whitney U, p = .29 | None |
| Lifetime Nicotine Use (sessions) | 2917367.65 (15839605.62) | 56652.60 (77218.66) | N/A | Mann-Whitney U, p = .61 | None |
| Lifetime Stimulant Use (Sessions) | 9068.98 (8342.16) | 2158.70 (2796.54) | N/A | Mann-Whitney U, p < .001 | SUD > OUD |
| Lifetime Opioid Use (Sessions) | 1262.40 (2942.48) | 8462.95 (7875.82) | N/A | Mann-Whitney U, p < .001 | OUD > SUD |
| Past Year Stimulant Use (sessions)2 | 2251.03 (4410.02) | 312.90 (632.49) | N/A | Mann-Whitney U, p < .001 | SUD > OUD |
| Past Year Opioid Use (sessions)2 | 74.38 (221.09) | 959.25 (1694.03) | N/A | Mann-Whitney U, p < .001 | OUD > SUD |
| Lifetime DSM Disorders1 | % | % | % | Statistics | User Group Differences |
| Cannabis UD | 23 | 40 | N/A | χ2(1) = 2.01, p = .16 | None |
| Alcohol UD | 18 | 30 | N/A | χ2(1) = 1.23, p = .27 | None |
| Sedative UD | 15 | 45 | N/A | χ2(1) = 6.40, p = .01 | OUD > SUD |
| Stimulant UD | 100 | 45 | N/A | χ2(1) = 26.94, p < .01 | SUD > OUD |
| Opioid UD | 23 | 100 | N/A | χ2(1) = 32.07 p < .001 | OUD > SUD |
| Mood/Anxiety | 55 | 70 | N/A | χ2(1) = 1.25, p = .26 | None |
| Lifetime ≥1000 nicotine uses* | 53 | 55 | 10 | χ2(2) = 0.52, p = .77 | None |
| Medication | % | % | % | Statistics | User Group Differences |
| Any | 35 | 50 | N/A | χ2(1) = 1.25, p = .26 | None |
| Suboxone | 3 | 15 | N/A | ||
| Naltrexone | 3 | 0 | N/A | ||
| Antianxiety | 20 | 25 | N/A | ||
| Antipsychotic | 5 | 15 | N/A | ||
| SSRI/SNRI | 15 | 20 | N/A | ||
| Tricyclic Antidepressant | 5 | 5 | N/A | ||
| Atypical Antidepressant | 10 | 0 | N/A | ||
| Antihypertensive | 20 | 15 | N/A |
Note. M = mean. SD = standard deviation. SUD = current stimulant use disorder. OUD = current opioid use disorder. CTL = healthy comparisons.
This analysis was comprised of three values: 0 = no, 1 = yes, and 2 = missing data, as 15 SUD, 6 OUD, and 4 CTL were missing this information. #Missing data: 4 SUD and 5 OUD.
CTL excluded from this particular analysis.
One SUD participant was an extreme outlier on reported CDDR year stimulant and opioid uses (reporting 101,650 and 250,000 uses, respectively) and was therefore excluded from these estimates. HS = high school. GED = general education diploma. DSM = Diagnostic and Statistical Manual of Mental Disorders, 4th or 5th edition. UD = Use Disorder. CDDR = Customary Drinking and Drug Use Record. SSRI = selective serotonin reuptake inhibitor. SNRI = serotonin norepinephrine reuptake inhibitor. The Mood/Anxiety Disorder category includes social phobia, posttraumatic stress disorder, generalized anxiety disorder, panic disorder, and/or major depressive disorder. Antianxiety medications = gabapentin, buspirone, and hydroxyzine. Antipsychotic medications = quetiapine fumarate and paliperidone. Atypical antidepressants = trazodone and bupropion. Antihypertensives = clonidine, metoprolol and propranolol.
Table 2.
Questionnaire responses as a function of group membership.
| SUD (n = 40) | OUD (n = 20) | CTL (n = 30) | Statistics | Three Group Differences | |
|---|---|---|---|---|---|
| Clinical Ratings | M (SD) | M (SD) | M (SD) | ||
| PHQ-9 Depression1 | 5.52 (5.10) | 7.35 (6.28) | 0.70 (1.12) | K-W p < .001; p = .262 | Users > CTL |
| ASI-3 Subscales | M (SD) | M (SD) | M (SD) | Wilk’s λ(6, 170) = 4.01, p = .001 | |
| Cognitive1 | 2.93 (3.86) | 4.30 (5.43) | 0.27 (0.64) | K-W p < .001; p = .632 | Users > CTL |
| Physical1 | 3.55 (4.21) | 5.45 (5.85) | 1.00 (1.62) | K-W p < .01; p = .242 | Users > CTL |
| Social1 | 7.58 (5.42) | 9.70 (6.97) | 3.43 (3.14) | K-W p < .001; p = .282 | Users > CTL |
| MAIA Subscales | M (SD) | M (SD) | M (SD) | Wilk’s λ(16, 260) = 2.53, p < .001 | |
| Attention Regulation | 2.64 (0.97) | 3.00 (0.85) | 3.44 (0.74) | ANOVA p = .001 | CTL > SUD |
| Body Listening | 2.33 (1.11) | 2.03 (1.54) | 2.50 (1.54) | ANOVA p = .50 | None |
| Emotional Awareness | 3.44 (0.95) | 3.15 (1.17) | 3.29 (0.98) | ANOVA p = .57 | None |
| Not-Distracting | 2.03 (0.95) | 2.00 (0.91) | 2.12 (1.14) | ANOVA p = .90 | None |
| Noticing | 3.39 (0.92) | 3.19 (0.76) | 3.58 (0.93) | ANOVA p = .31 | None |
| Not-Worrying | 2.65 (1.07) | 2.90 (0.94) | 3.59 (0.93) | ANOVA p = .001 | CTL > Users |
| Self-Regulation | 2.84 (0.96) | 2.85 (1.36) | 3.48 (1.02) | ANOVA p = .04 | CTL > Users |
| Trusting | 3.11 (1.01) | 3.18 (1.42) | 4.00 (0.97) | ANOVA p < .01 | CTL > Users |
Note. M = mean. SD = standard deviation. SUD = current stimulant use disorder. OUD = current opioid use disorder. CTL = healthy comparisons.
sphericity assumption violated so non-parametric test results (Kruskal-Wallis; K-W) are reported.
healthy comparisons excluded from this particular analysis. PHQ-9 = Patient Health Questionnaire. ASI-3 = Anxiety Sensitivity Index. MAIA = Multidimensional Assessment of Interoceptive Awareness.
VIA Intensity Ratings
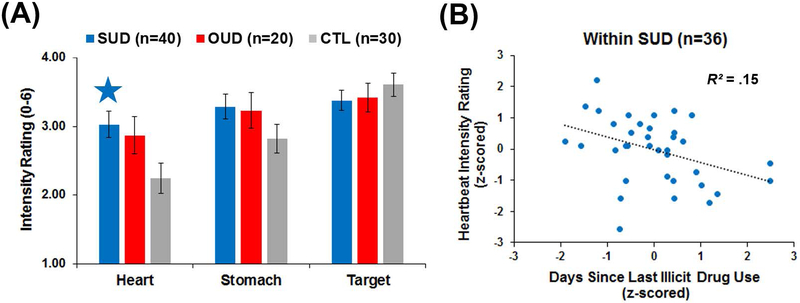
As the sphericity assumption was upheld for condition (p = .63), no corrections were warranted. Although no group main effect emerged (p = .17), a condition main effect (F(2, 174) = 2.76, p < .001, partial ƞ2 = .13) was qualified by a group*condition interaction (F(4, 174) = 2.76, p = .03, partial ƞ2 = .06). Figure 3a illustrates that SUD reported a higher intensity of heartbeat sensations than CTL (p < .01 and Cohen’s d = .66), whereas OUD did not differ from CTL (p = .07 and Cohen’s d = .52) or SUD (p = .61). No group differences emerged for stomach or target intensity ratings (all p ≥ .10).
Figure 3.
(A) Group*condition interaction results for stimulus intensity ratings. Intensity ratings could range from 0 = no sensation to 6 = extreme sensation. SUD = current stimulant use disorder. OUD = current opioid use disorder. CTL = healthy comparisons. The blue star indicates a significant difference for SUD versus CTL. Error bars reflect +/− 1 standard error. (B) Within SUD, more recent illicit drug use was associated with greater reported intensity of heartbeat sensations, sharing 15% of the variance.
To aid interpretation, Pearson correlations were computed within SUD between heartbeat intensity ratings and log-transformed: (1) days since illicit drug use; (2) past-year stimulant uses; and (3) lifetime stimulant uses. Variables were standardized before correlations were performed. Figure 3b illustrates that SUD with more recent illicit drug use reported more intense heartbeat sensations (r(36) = −.39, p = .02, R2 = .15). However, heartbeat intensity was not strongly related to past-year or lifetime stimulant use were not strongly related (p = .16 and .61, respectively).
VIA Insula ROIs
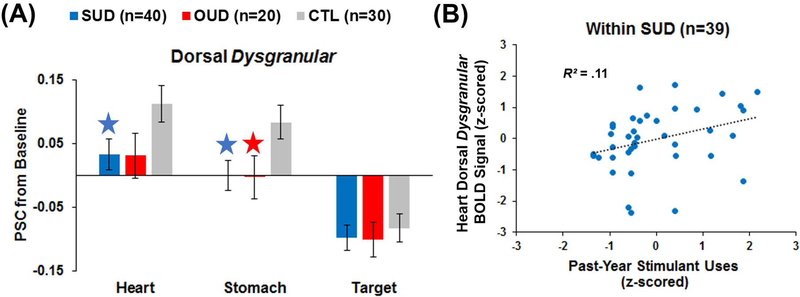
Non-group effects are detailed within Supplemental Material. Region, condition, and region*condition effects were qualified by a group*region*condition interaction (F(11, 459) = 1.94, p = .04, partial η2 = .04); Figure 4a illustrates that within dorsal dysgranular insula, SUD exhibited lower BOLD signal to heart sensations than CTL (p = .04, Cohen’s d = 0.51), whereas both SUD and OUD showed lower BOLD signal to stomach sensations than CTL (SUD-CTL: p = .02 and Cohen’s d = .57; OUD-CTL: p = .04 and Cohen’s d = 0.58). Furthermore, SUD exhibited lower hypergranular BOLD insula signal to heart sensations than OUD (p = .02 and Cohen’s d = 0.63).
Figure 4.
(A) Group*region*condition interaction results for insular blood-oxygen-level dependent (BOLD) percent signal change (PSC) from baseline. SUD = current stimulant use disorder. OUD = current opioid use disorder. CTL = healthy comparisons. The blue stars indicates a significant difference for SUD versus CTL, whereas the red star indicates a significant difference for OUD versus CTL. Error bars reflect +/− 1 standard error. (B) Within SUD, greater past-year stimulant use was associated with higher dorsal dysgranular insula BOLD signal during the heart condition, sharing 11% of the variance.
Drug use and dorsal dysgranular insula BOLD signal.
To aid interpretation, Pearson correlations were computed within SUD between heartbeat-related dorsal dysgranular insula and log-transformed: (1) days since illicit drug use; (2) past-year stimulant uses; and (3) lifetime stimulant uses. Variables were standardized before correlations were performed. Figure 4b shows that SUD with greater past-year stimulant uses exhibited higher heartbeat-related BOLD signal that was more similar to CTL (r(39) = .32, p < .05, R2 = .10), a relationship that remained significant when controlling for age (r(36) = .33, p < .05, R2 = .11). However, heartbeat signal was not strongly related to drug use recency or lifetime stimulant uses (p = .29 and .25, respectively). Similarly, Pearson correlations were computed across SUD and OUD between stomach-related dorsal dysgranular insula and log-transformed: (1) days since illicit drug use; (2) past-year opioid uses; and (3) lifetime opioid uses for all OUD as well as a subset of SUD who endorsed any opioid use. All three variables were standardized before correlations were performed. All correlations were non-significant (all p > .25).
Self-reported interoception metrics and dorsal dysgranular BOLD signal.
To examine whether subjective and brain metrics of interoception were measuring similar constructs, first we correlated standardized VIA intensity and dorsal dysgranular insula signal for: (1) heartbeat sensations within SUD; and (2) stomach sensations across SUD and OUD. Both correlations were non-significant (both p > .59). Second, we correlated MAIA interoception subscales differing between groups (attention regulation, self-regulation, not worrying, and body-trusting) with dorsal dysgranular signal for: (1) heartbeat sensations within SUD; and (2) stomach sensations across SUD and OUD. All correlations were non-significant (all p > .39).
Direct constrast of interoception versus exteroception VIA conditions.
A follow-up mixed ANOVA compared the BOLD signal contrast between the average of heart and stomach conditions (interoception) with the target condition (exteroception), the dependent variable in this analysis, between groups, hemispheres, and six insula regions. No group effects were significant (group main effect: p = .43, group*region interaction: p = .22).
Discussion
The present exploratory study compared SUD, OUD, and CTL on insula BOLD signal and intensity ratings during a task engaging attention to interoceptive (heartbeat and stomach) and exteroceptive (visual) signals. Consistent with our hypotheses, we observed evidence of interoceptive alterations in current SUD and OUD as manifested by blunted insula activation. Our study produced two main findings. First, SUD showed greater intensity ratings of heart-related interoception but lower dorsal dysgranular insula activation than CTL. Second, although SUD and OUD did not show heightened ratings of stomach sensations, both groups displayed lower dorsal dysgranular insula activation than CTL. Our findings replicate prior work demonstrating that CTL show greater dorsal dysgranular insula responses during attention to heart and stomach sensations than visual exteroceptive stimuli39 and we extend these findings to show that SUD and OUD do not show an identical pattern to CTL in this region. It is important to note, however, that a direct contrast of the average of VIA interoception conditions (heart and stomach) to the exteroception condition (target) did not replicate group differences as a function of insula region, suggesting that the type of interoceptive attention matters. More specifically, SUD showed a larger effect size difference for the heartbeat as opposed to the stomach condition. Additional research is warranted to replicate and extend this work to other interoception versus exteroception conditions.
How do our results fit into existing frameworks of interoception and addiction? Naqvi and Bechara14 propose that sensory effects associated with drug use play an important role in the rewarding aspect of the drug; they suggest that insula activity evoked by drug cues paired with a bodily response in the past will be positively associated with intensity of that bodily signal encoded in memory. To translate this model into a concrete example, given that stimulants cause increased heart rate and blood pressure, after repeated pairings of this drug and bodily state, we might expect SUD to show both heightened intensity ratings and insula responses to heart sensations in the absence of drug cues (or vice versa). Our findings support the heightened intensity postulate in that SUD with more recent illicit drug use experienced greater intensity of heartbeat sensations. Moreover, our results are also consistent with heightened insula responses, as SUD who reported greater past-year stimulant uses exhibited greater insula signal to heartbeat sensations. While Naqvi and Bechara14 focus on the rewarding aspects of bodily sensations and drug use, we do not know whether SUD found that paying attention to heart sensations was actually “good” or “bad”, i.e., that they experienced valence differences that may impact subjective and brain measures of interoceptive attention. Our findings also demonstrate that OUD and SUD (23% with a history of opioid use disorder, and 45% with past year opioid use), exhibit blunted insula responses to stomach sensations. As gastrointestinal distress is often associated with opioid withdrawal, perhaps stomach signals are perceived as negatively valenced and as a result of chronic use, brain responses are downregulated to reduce processing of this signal. Unlike our findings for heartbeat-related attention within SUD, drug use recency and past year opioid use were not related to stomach-related attention across SUD and OUD, warranting further investigation in future studies.
In our model of addiction10, we have argued that the bodily experience of drug use and the evaluation of a drug user’s predicted versus actual internal state (e.g., “do I feel better or worse than I thought I would?”), processes associated with insula signaling, determine whether or not that person experiences craving and seeks out drugs. We argue that addiction is characterized by impaired bodily prediction errors that are expressed by heightened insula responses to drug cues but reduced insula responses to non-drug stimuli. As VIA task stimuli were not explicitly linked to drug cues in any way, our insula results for interoceptive attention conditions could be consistent with blunted prediction errors to non-drug stimuli, particularly for SUD. However, the intensity results for heart and stomach sensations do not fit with this model. As we did not collect ratings to measure craving, positive and negative valence, or prediction error during or after the VIA task, we cannot determine whether focus on bodily sensations was perceived as an unexpected negative experience that prompted urges to use drugs.
What may be responsible for a mismatch between subjective intensity and insula responses to bodily stimuli? As VIA intensity ratings/MAIA interoceptive awareness and dorsal dysgranular insula signals were not strongly correlated, these metrics appear to be measuring distinct aspects of the interoceptive experience. With respect to heart sensations, cardiovascular strain caused by chronic stimulant use may recalibrate the internal scale users employ to evaluate heart changes; perhaps over time the insula becomes sensitized by downregulating resources devoted to attention of these sensations, resulting in users needing more drugs to maintain desired feeling states.
With respect to clinical ratings, our findings extend the literature on self-reported interoceptive awareness in substance use disorders, showing that SUD and OUD both endorsed greater difficulty self-regulating, trusting their bodies, and not worrying than CTL on the MAIA scale. Moreover, SUD reported more difficulty regulating attention to interoceptive cues than CTL. Additional research is needed to determine whether these interoceptive factors play a role in disorder course, prognosis, or treatment outcome.
Limitations
Although this study contributes to novel knowledge regarding the roles of insula and interoception in addiction, several limitations warrant consideration. First, our user groups were largely comprised of polysubstance users with significant psychiatric comorbidities, with 40% on current medications (including antihypertensives used to treat high blood pressure). The psychiatric comorbidity present in SUD and OUD are consistent with meta-analytic work demonstrating that illicit drug users are about four times more likely to meet criteria for a mood disorder and three times more likely to meet criteria for an anxiety disorder than non-users48. Furthermore the use of anti-hypertensive medications is consistent with cardiac dysfunction presenting in stimulant users6–8. Although in an ideal world, user groups and healthy comparisons could be matched on all demographic, clinical, and medical factors with the exception of the use of a particular drug of choice, the reality of comorbidity presents barriers to this type of matching but at the same time likely increases external validity and generalizability to samples of individuals presenting for addiction treatment. SUD and OUD groups were selected to emphasize the current use of one drug of choice (stimulants versus opioids) and evaluate this particular association with interoceptive attention. Second, the present analysis was based on a sample of convenience collected during the Tulsa 1000 study, and as such, warrants replication. As the initial aim of the Tulsa 1000 study did not focus on targeted differences between classes of substance use disorders, a second limitation of this analysis is that our OUD group is half the size of the SUD group; as a result, we are underpowered to detect OUD effects as well as reliable gender differences, despite the importance of this variable49. Third, beyond ratings of heartbeat and stomach sensations, it would be advantageous for future studies to include: (1) real-time valence ratings to determine whether users feel positively or negatively about these sensations in the moment; (2) behavioral indicators throughout the task indicating degree to which users feel drawn to approach or withdraw from these particular sensations; (3) drug craving ratings; and (4) continuous objective psychophysiological responses such as heart rate and skin conductance. Inclusion of these metrics may provide pertinent information regarding user interpretation of bodily cues. Lastly, although whole-brain fMRI data is available for the VIA task, we chose to focus on insular subdivisions due to hypotheses regarding interoceptive attention previously linked to dorsal agranular and dysgranular insula elicited by this task. As prior work also shows attenuated frontocingulate and thalamic processing in SUD during touch and respiratory perturbations26–28, incorporating these regions will be important to examine in future analyses.
Conclusions
The present study demonstrates that current SUD and OUD are both characterized by interoceptive alterations, with cardiac impairments strongly linked to SUD. SUD show an exaggerated perception of heart sensations, paired with attenuated brain resources devoted to processing of heart sensations; these patterns differ as a function of drug use recency and past year stimulant use frequency. Future work can build upon this knowledge to identify whether: (1) attention to these bodily signals serve to trigger craving and relapse; and (2) this brain-behavior mismatch is a consequence of, as opposed to a risk factor for, addiction.
Supplementary Material
Acknowledgements
We wish to thank Brad Collins from 12&12 and Mimi Tarrasch from Women in Recovery for assistance with participant recruitment. This research was supported by NIGMS grant number P20GM121312 (MPP, SSK), NIMH grant number K23MH112949 (SSK), and The William K. Warren Foundation. Authors have no conflicts of interest to report.
References
- 1.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Network Open 2018;1:e180217–e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health 2010;31:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oquendo MA, Volkow ND. Suicide: a silent contributor to opioid-overdose deaths. N Engl J Med 2018;378:1567–1569. [DOI] [PubMed] [Google Scholar]
- 4.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med 2016;375:357–368. [DOI] [PubMed] [Google Scholar]
- 5.Turner C, Chandrakumar D, Rowe C, Santos GM, Riley ED, Coffin PO. Cross-sectional cause of death comparisons for stimulant and opioid mortality in San Francisco, 2005–2015. Drug Alcohol Depend 2018;185:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darke S, Duflou J, Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine-related death: A national study. Drug Alcohol Depend 2017;179:174–179. [DOI] [PubMed] [Google Scholar]
- 7.Stankowski RV, Kloner RA, Rezkalla SH. Cardiovascular consequences of cocaine use. Trends Cardiovasc Med 2015;25:517–526. [DOI] [PubMed] [Google Scholar]
- 8.Paratz ED, Cunningham NJ, MacIsaac AI. The cardiac complications of methamphetamines. Heart Lung Circ 2016;25:325–332. [DOI] [PubMed] [Google Scholar]
- 9.Ellis MS, Kasper ZA, Cicero TJ. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend 2018;193:14–20. [DOI] [PubMed] [Google Scholar]
- 10.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology 2014;76:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, Meuret AE, Nemeroff CB, Oppenheimer S, Petzschner FH, Pollatos O, Rhudy JL, Schramm LP, Simmons WK, Stein MB, Stephan KE, Van den Bergh O, Van Diest I, von Leupoldt A, Paulus MP, Interoception Summit 2016 Participants. Interoception and mental health: A roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein RZ, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci 2009;13:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naqvi NH, Bechara A. The hidden island of addiction: The insula. Trends Neurosci 2009;32: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct 2010;214:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev 2012;36:1857–1869. [DOI] [PubMed] [Google Scholar]
- 16.Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend 2014;139:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Jong CA, Roozen HG, Van Rossum LG, Krabbe PF, Kerkhof AJ. High abstinence rates in heroin addicts by a new comprehensive treatment approach. Am J Addict 2007;16:124–130. [DOI] [PubMed] [Google Scholar]
- 18.Price CJ, Thompson EA, Crowell SE, Pike K, Cheng SC, Parent S, Hooven C. Immediate effects of interoceptive awareness training through Mindful Awareness in Body-oriented Therapy (MABT) for women in substance use disorder treatment. Subst Abus 2018;20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sönmez MB, Kahyacı Kılıç E, Ateş Çöl I, Görgülü Y, Köse Çınar R. Decreased interoceptive awareness in patients with substance use disorders. J Subst Use 2017;22:60–65. [Google Scholar]
- 20.Lejuez CW, Paulson A, Daughters SB, Bornovalova MA, Zvolensky MJ. The association between heroin use and anxiety sensitivity among inner-city individuals in residential drug use treatment. Behav Res Ther 2006;44:667–677. [DOI] [PubMed] [Google Scholar]
- 21.Bergquist KL, Fox HC, Sinha R. Self-reports of interoceptive responses during stress and drug cue-related experiences in cocaine-and alcohol-dependent individuals. Exp Clin Psychopharmacol 2010;18:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avery JA, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK. A common gustatory and interoceptive representation in the human mid‐insula. Hum Brain Mapp 2015;36:2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Critchley HD, Garfinkel SN. Interactions between visceral afferent signaling and stimulus processing. Front Neurosci 2015;9:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, Bodurka J, Paulus MP, Khalsa, SS. The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology 2018;43:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz SM. Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Philos Trans Royal Soc B Biol Sci 2016;371: 20160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Depend 2013;131:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart JL, Juavinett AL, May AC, Davenport PW, Paulus MP. Do you feel alright? Attenuated neural processing of aversive interoceptive stimuli in current stimulant users. Psychophysiology 2015;52:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. You are the danger: attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend 2014;142:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De La Garza R, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend 2010;106:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PloS One 2010;5:e15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leppert W Emerging therapies for patients with symptoms of opioid-induced bowel dysfunction. Drug Design, Development and Therapy, 2015;9:2215–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 1982;212:38–52. [DOI] [PubMed] [Google Scholar]
- 33.Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol 2017;34:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wager TD, Barrett LF. From affect to control: Functional specialization of the insula in motivation and regulation. bioRxiv 2017;102368. [Google Scholar]
- 35.Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci 2015;19:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T. The Human Brainnetome Atlas: A new brain atlas based on connectional architecture. Cereb Cortex 2016;26:3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 2014;76:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology 2016;41:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 2013;34:2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victor TA, Khalsa SS, Simmons WK, Feinstein JS, Savitz J, Aupperle RL, Yeh HW, Bodurka J, Paulus MP. Tulsa 1000: A naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 2018;8:e016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan D, Janavs J, Baker R, Sheehan KH, Knapp E, Sheehan M. MINI international neuropsychiatric interview–version 7.0. Jacksonville, FL: Medical Outcomes System Inc.; 2015. [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington DC: American Psychiatric Publications; 2013. [Google Scholar]
- 43.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol 1998;59:427–438. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Abramowitz JS, Holaway RM, Sandin B, Stewart SH, Coles M, Eng W, Daily ES, Arrindell WA, Bouvard M, Cardenas SJ. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess 2007;19:176–188. [DOI] [PubMed] [Google Scholar]
- 46.Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The multidimensional assessment of interoceptive awareness (MAIA). PloS One 2012;7:e48230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox RW. AFNI: What a long strange trip it’s been. NeuroImage 2012;62:743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai HMX, Cleary M, Sitharthan T, Hunt, GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Depend 2015;154:1–13. [DOI] [PubMed] [Google Scholar]
- 49.Zakiniaeiz Y, Potenza MN. Gender-related differences in addiction: A review of human studies. Curr Opin Behav Sci 2018;23:171–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.