Abstract
The transformation from normal to malignant phenotype in human cancers is associated with aberrant cell-surface glycosylation. It has frequently been reported that MUC1, the heavily glycosylated cell-surface mucin, is altered in both, expression and glycosylation pattern, in human carcinomas of the epithelium. The presence of incomplete or truncated glycan structures, often capped by sialic acid, commonly known as tumor-associated carbohydrate antigens (TACAs), play a key role in tumor initiation, progression, and metastasis. Accumulating evidence suggests that expression of TACAs is associated with tumor escape from immune defenses. In this report, we will give an overview of the oncogenic functions of MUC1 that are exerted through. TACA interactions with endogenous carbohydrate-binding proteins (lectins). These interactions often lead to creation of a protumor microenvironment, favoring tumor progression and metastasis, and tumor evasion. In addition, we will describe current efforts in the design of cancer vaccines with special emphasis on the synthetic MUC1 glycopeptide vaccines. Analysis of key factors that govern structure-based design of immunogenic MUC1 glycopeptide epitopes are described. The role of TACA type, position, and density on observed humoral and cellular immune responses is evaluated.
Keywords: MUC1, Tumor-associated carbohydrate antigens, Lectins, Vaccines, Immune response
1. MUC1 structure and function in normal cells
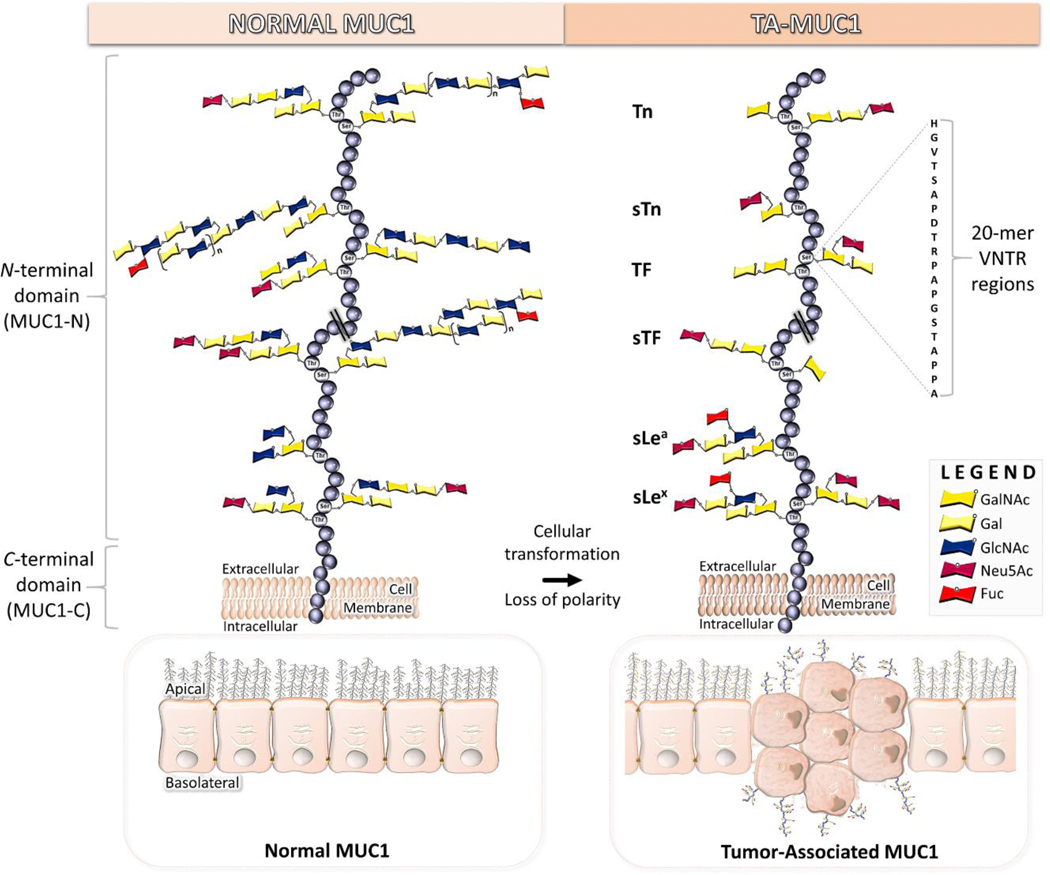
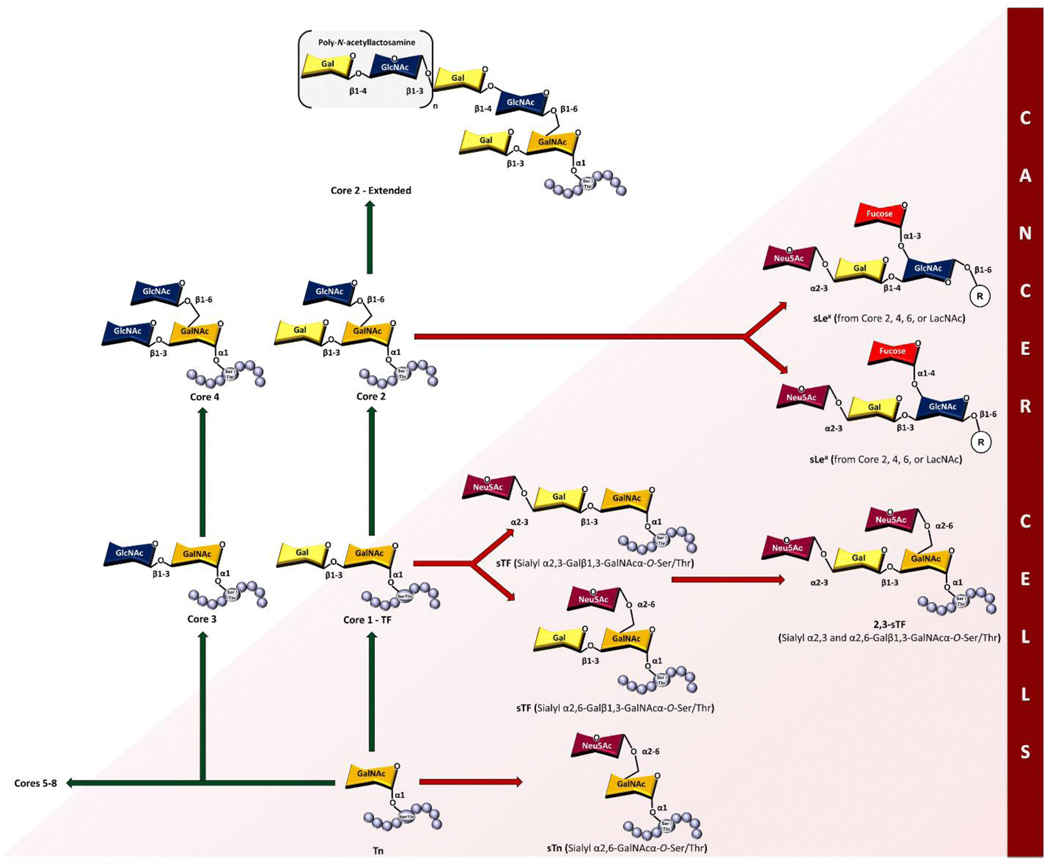
Mucin 1 (MUC1, CD227) is a type I transmembrane glycoprotein consisting of two subunits (Fig. 1). A large, heavily glycosylated extracellular N-terminal domain (MUC1-N) that protrudes from the cell surface up to 200 – 500 nm and an intracellular C-terminal domain (MUC1-C) that is non-covalently interlinked through a degenerative sequence [1–3]. It is expressed in the apical and basolateral surfaces of most secretory glandular epithelium [4] but is occluded from mesenchymal cells and skin epithelium [5]. The extracellular mucin-like domain of each allele contains a variable number of identical 20-amino acid tandem repeats (VNTR, HGVTSAPDTRPAPGSTAPPA) that can range from 20 to 120 repeats with five potential sites for post translational O-linked glycosylation at serine (Ser) and threonine (Thr) residues [5, 6]. Various monosaccharides comprise the extensively branched O-glycan chains, such as N-acetylgalactosamine (GalNAc), galactose (Gal), N-acetylglucosamine (GlcNAc), N-acetylneuraminic acid (sialic acid, Neu5Ac), and fucose (Fuc) (Fig. 1). Alpha O-linked GalNAc is always the first glycan attached to Ser or Thr residues of MUC1 tandem repeats, and in normal cells GalNAc is supplemented by additional sugar moieties to form eight main core structures (Fig. 2)[7]. These are further modified, giving rise to extended dense clusters of MUC1 with variable termini. T-synthase enzyme (core 1 β3-galactosyltransferase or β3GalT), chaperoned with oligomeric endoplasmic reticulum-localized Cosmc protein, facilitates synthesis of the core I structure (Galβ1,3-GalNAcα-O-Ser/Thr) [8]. Core 2 GlcNAc transferases (C2GnTs) extends the core I structure by adding GlcNAc in a β1–6 linkage to the existing GalNAc of core I structure [9]. Core 1 and 2 structures are acceptors for a wide range of glycosyltransferase, and elongation of the C6-branch of core 2 is the most frequently found form of MUC1 [5]. Lengthening of the GalNAc moiety by addition of GlcNAc in β1,3-linkage, followed by addition of a second GlcNAc in β1,6-linkage results in formation of core 3 and 4 structures, respectively [10]. Core 1–4 structures comprise the primary glycan structures observed in humans. The extensive and diverse array of glycan permutations from the MUC1 polypeptide backbone (apomucin) serve as a protective barrier for epithelium, and in addition plays a functional role in monitoring the extracellular environment and signal transduction into the cell [11].
Fig. 1.
Structural differences between normal and tumor-associated MUC1.
Fig 2.
Core O-glycan and TACA structures.
Alternatively, co-translational N-linked glycosylation links the amidic nitrogen of asparagine to GlcNAc at five possible sites [12]. Four of these sites are in the degenerative region of MUC1-N, whereas one site is located in the extracellular region of MUC1-C [12]. The biantennary chain composition builds and branches with sugar variations inclusive of mannose, xylose, and 12 others common glycans. N-linked glycosylation plays a role in secretion, protein folding, and trafficking of MUC1 [12].
2. Tumor-associated MUC1
In cancer, tumor-associated MUC1 (TA MUC1) becomes redistributed over the entire surface of the cell due to a loss in apical-basal polarity [3, 11]. Additionally, the glycosylation pattern of the extracellular N-terminal domain of TA MUC1 differs from that of MUC1 expressed on normal cells. The long-branched glycan chains are truncated and mostly exhibit core 1 O-glycans, that reveal the underlying tandem repeat region(s) (Fig. 1) [3, 13, 14]. Cessation of core 2 structures stems from a loss of core 2 β6-GlcNAc transferase activity [15] and/or mutations in Cosmc chaperone [7, 16, 17]. Additional implications regarding cell homeostasis and development of an alkaline pH of the Golgi lumen are proposed in creation of the core 1 structures [17, 18]. Some truncated glycans of TA MUC1 are capped by sialic acid, mostly due to the overexpression of α2,6- and α2,3-sialyl transferases [19, 20]. Consequently, the most common TACAs formed from incomplete synthesis are GalNAcα-O-Ser/Thr (Tn, Thomsen Nouveau, CD175), Neu5Acα2,6-GalNAcα-O-Ser/Thr (sTn, sialyl Tn, CD175s), Galβ1,3-GalNAcα-O-Ser/Thr (TF, Thomsen-Friedenreich, CD176, T antigen) and Neu5Acα2,6- and Neu5Acα2,3-Galβ1,3-GalNAcα-O-Ser/Thr (2,6-sTF, 2,3-sTF) (Fig. 1 and 2). Their expression levels have been used as biomarkers of poor prognosis [21]. While oncofetal TF antigen is virtually absent from healthy tissue, most TACAs are found expressed in low amounts in normal tissue but increased amounts in premalignant and malignant tissue [3, 22]. Tn and sTn antigens are found in breast, prostate, colon, respiratory, pancreas, ovarian [23, 24], and gastric cancers [7, 25]. The Tn antigen is expressed in >90% of breast cancers [7, 26], 10–90% of other forms of cancers and is prevalent in 25–70% of premalignant tissues in the colon [7]. Many epithelial cancers (>80%) display sTn antigen and its expression is associated with decreased overall survival of patients [27]. TF and sTF are both expressed on breast cancer, while TF is more prevalent in gastric, colon, pancreas, ovary, prostate, and stomach cancers and is also found in 90% of cancer types [28, 29]. Tn, sTn, and TF antigens are found co-expressed on tumor cells and their expression correlates to disease progression from their involvement in tumor invasion, metastasis, and evasion of the immune system [19]. Sialylated forms of Tn and TF glycans have been found to play key roles in immune suppression [24, 30].
In addition to truncated O-glycans, the expression of specific sialyl and fucosyl transferases generates sialyl-Lewis antigens, NeuAcα2,3-Galβ1,3-(Fucα1,4)-GlcNAc-R (sLea, sialyl-Lewisa) and NeuAcα2,3-Galβ1,3-(Fucα1,3)-GlcNAc-R (sLex, sialyl-Lewisx) (Fig. 1 and 2) [7, 28]. Sialyl-Lewisa structures are found in >50% of colon, stomach, and pancreas cancers, along with lung, liver, breast, and mesothelioma cancers [7]. Whereas, sialyl-Lewisx structures are found in >90% of pancreas and stomach cancers, along with colon, esophagus, ovary, and breast cancers [7].
Broad distribution of TA MUC1 on both primary tumors and metastasis, including cancer stem cells, has rendered MUC1 as a widely explored target in many diagnostic and (immuno)therapeutic approaches [31–33]. Based on certain criteria, such as therapeutic function, immunogenicity, and cancer cell specificity, MUC1 was listed by the National Cancer Institute Translational Research Working Group as the second most promising target in cancer research from a list of 75 tumor-associated antigens [34].
3. Cell-surface TACA-lectin interactions: Role in tumor progression, metastasis and immune evasion
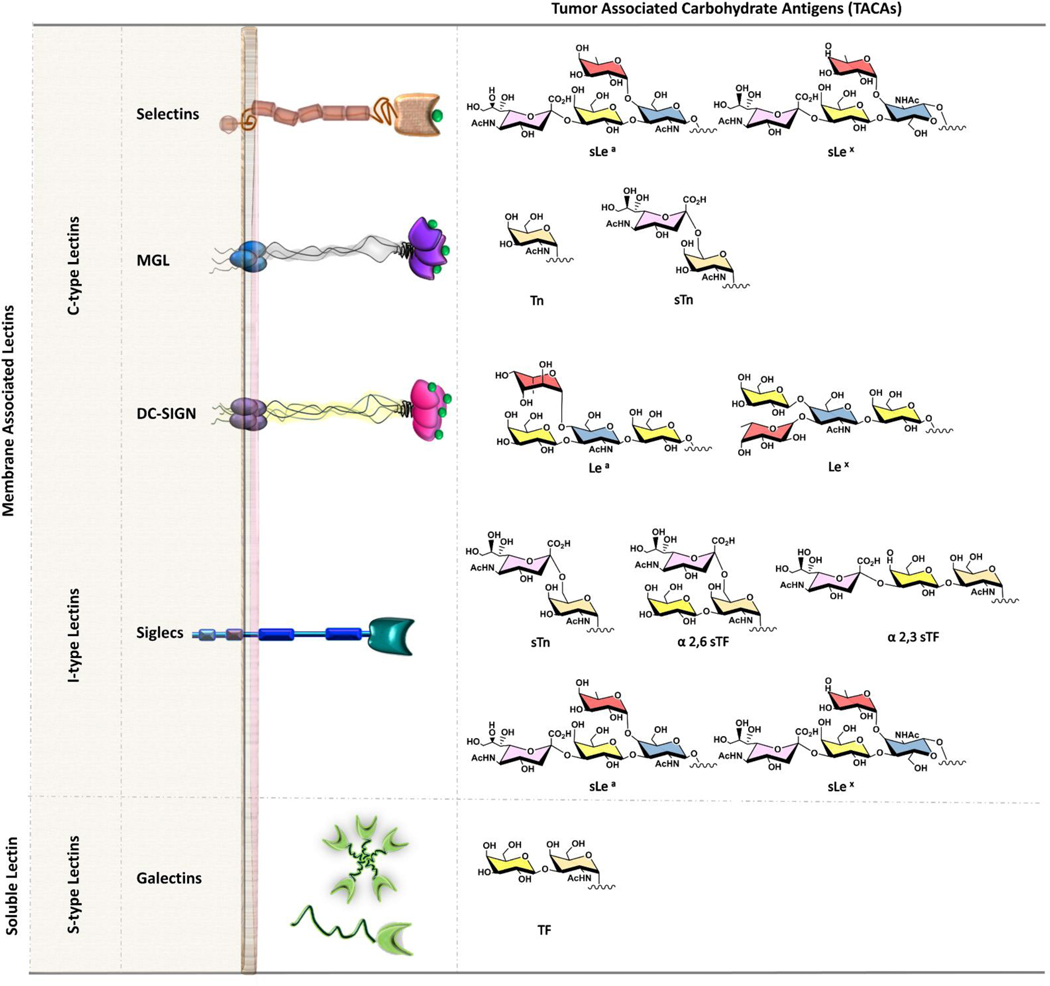
Carbohydrate-binding proteins, lectins, are the main binding partners of TACAs on the surface of the cell [35, 36]. These interactions often lead to creation of a pro-tumor microenvironment, favoring tumor progression and metastasis [37]. In addition, glycan-protein recognition may contribute to tumor progression through evasion of antitumor immune-responses [38–40]. It has recently been proposed that the tumor glyco-code may be considered as novel immune checkpoints [41]. Common human cell surface lectins that bind to TACAs of MUC1 belong to three main groups: C-, S- and I-type lectins [42] (Fig. 3).
Fig. 3.
Ligands for TA MUC1 and the TACAs that ligate them.
3.1. C-type lectins
C-type lectins, the largest and most diverse of the lectin families, share a carbohydrate recognition domain (CRD) signature motif that binds glycans in a calcium dependent manner [43]. Selectins, macrophage galactose lectin (MGL), and dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) are examples of common human C-type cell surface lectins that bind to TACAs of MUC1. Most members of this family function as adhesion molecules, and all are considered to engage as signaling receptors and contribute to different steps of the metastatic spread of cancer.
Selectins are perhaps the best characterized molecules among the C-type lectins [44]. They are single-chain transmembrane glycoproteins that are found on the surface of endothelial cells (E-and P-selectins), platelets (P-selectins), and leukocytes (L-selectins), and are known to participate in lymphocyte and leukocyte rolling, an early step in the extravasation process [45]. Carbohydrate ligands on tumor cells exploit the selectin-dependent mechanism mediating cell tethering and rolling interactions that progress distant organ metastasis. Sialylated and fucosylated ligands, such as sialyl-Lewis antigens (sLea and sLex), are recognized by selectin CRDs. The increased levels of these antigens on TA MUC1 lead to enhanced interactions with selectins on endothelial cells, thus contributing to metastatic progression [19, 28, 46–48].
Macrophage galactose lectin (MGL) is the best studied of the multiple C-type lectins expressed exclusively on macrophages and dendritic cells (DCs) [49]. It is a homotrimer cluster protein, and is thought to have a similar structure to the asialoglycoprotein receptor (ASGPR) [50]. However, the carbohydrate-recognition profile specificity of MGL differs significantly from that of ASGPR. ASGPR recognizes tri- or tetra-antennary glycans containing both Gal and GalNAc, whereas MGL recognizes only GalNAc structures [51]. The specificity of MGL for GalNAc residues with exposed C3- and C4-hydroxyl groups [50] explains the ability of the receptor to bind to tumor-associated forms of MUC1 bearing Tn [52] and sialyl-Tn antigens [53], but not to more complex and branched O-glycan structures of MUC1 found on normal cells. It has been shown that MGL is expressed by M2-like tumor-associated macrophages (TAMs) which are known to promote tumor growth and metastasis [54]. Thus, interaction of MGL with MUC1-Tn and MUC1-sTn is likely to modulate the TAM phenotype and/or activity, leading to metastatic extravasation. MGLs preferential expression on tolerogenic APCs found at carcinoma tumor sites [55, 56], further indicates their role as an immunomodulator within the tumor microenvironment contributing to tumor-induced immune tolerance [57, 58]. Yet, MGL is able to internalize epitopes that positively instruct dendritic cell differentiation and subsequent T cell responses. MGL targeting a MUC1-Tn glycopeptide consisting of three tandem repeats, lead to modulation of DC maturation and initiation of strong CD8+ T cell immune response [59]. These findings demonstrate that MGL activation can be used for anticancer vaccination strategies. Furthermore, it has been shown that the nature of the MGL receptor dictates intracellular processing, and depending on the structure of the ligand and its affinity for MGL, the resulting immune responses can differ significantly [60–63]. Thus, glycan-based CLR targeting offers he ability to manipulate the glycan density and their presentation, allowing the design of effective multivalent binding systems. Considering the crucial role of tumor-associated forms of MUC1 with MGL in tumor immunology, an in-depth understanding of this interaction is essential for it to be exploited for cancer vaccine strategies.
DC-SIGN is predominantly expressed on DCs where it functions as an adhesion receptor and mediates binding and internalization of pathogens through interaction with N-linked high mannose oligosaccharides [64]. However, DC-SIGN participates in cell-cell interactions through binding with endogenous ligands, such as Lewis antigens [65]. Indeed, glycan-modified (Lex or Leb)-coated liposomes were shown to boost T cell responses by targeting DC-SIGN receptors on DCs [66]. Consequently, DC-SIGN interaction with TA MUC1 plays a role in the adhesion of tumor cells to endothelial cell. Correlation of antitumor response failure has been linked to suppression of DCs functional maturation caused by DC-SIGN bridging interactions between DCs and the Lewis glycans present on some colorectal carcinoma cells [67]. In addition, it has been found that interaction of TA MUC1 with DC-SIGN leads to induction of regulatory T cells (Tregs) [68]. This functional suppression induces immune evasion, that favors metastasis of colon cancer [65].
3.2. S-type lectins (Galectins)
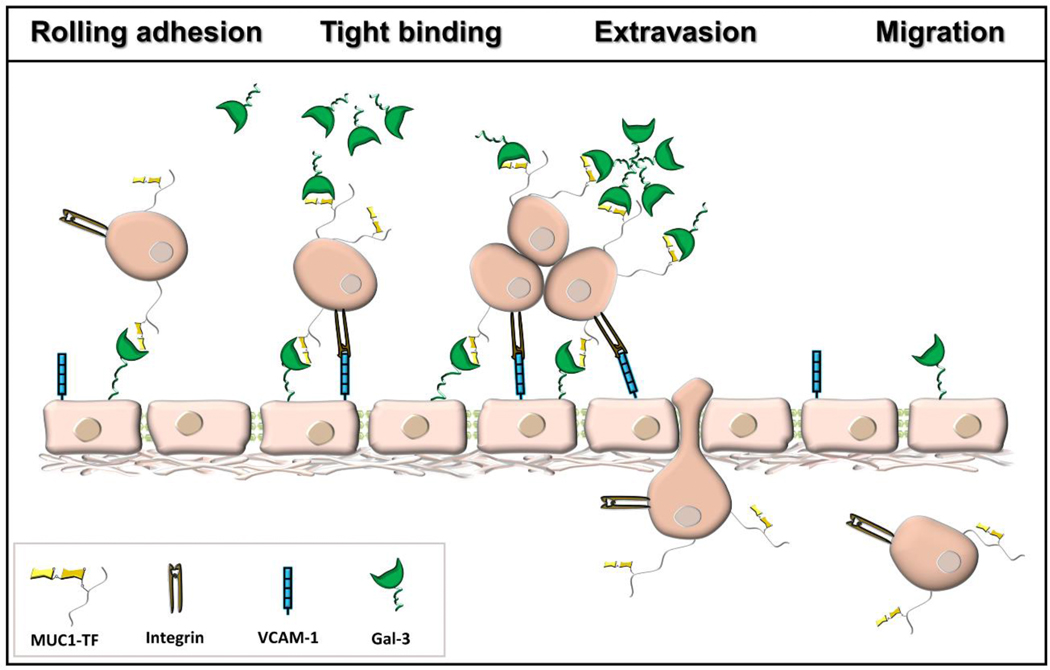
Galectins are a growing class of ß-galactoside-specific animal lectins [69, 70]. Of the 15 members of this family found in mammals, galectin-1 and galectin-3 are the most ubiquitously expressed types. In some tissues, only galectin-1 or galectin-3 is expressed, while both galectins are often co-expressed in immune cells [71]. Galectins are defined by a common CRD, and their capacity for bivalent or multivalent binding, which permits formation of galectin-glycoprotein networks called “lattices” that regulate cell-surface glycoprotein organization and signaling. Galectins are differentially expressed in cancer [72]. A number of important roles in cancer initiation and progression [73–75], as well as in tumor-immune escape [76, 77] have been associated with these two galectins. TF antigen of TA MUC1 has been shown to be actively involved in tumor metastasis, promoting several key cell-cell interactions via association with galectin-3 (Fig. 4) [29, 78, 79]. The interaction between TF antigen and galectin-3 represents an important early step in heterotypic cancerendothelial adhesion and the formation of intravascular metastatic deposits [80, 81]. Furthermore, binding of galectin-3 to TA MUC1, predominantly its extracellular domain, induces MUC1 cell surface polarization, and increases MUC1– epidermal growth factor receptor (EGFR) interaction [82]. This interaction leads to EGFR activation and likely makes an important contribution to EGFR associated tumorigenesis and cancer progression. Furthermore, galectin-3-MUC1-induced cancer cell homotypic aggregation increases cancer cell survival by preventing the initiation of cellular anoikis [83]. Analysis of the molecular recognition features of galectin-3 binding to TF antigen revealed enhancement in affinity for the TF antigen linked to MUC1, as it exists in its natural cellular context [84]. The dissociation constants for interaction of galectin-3 and the glycosylated MUC1 fragments measured by isothermal titration calorimetry decreased up to 10 times in comparison to that of the free TF disaccharide [84]. The most notable feature of the binding of MUC1 glycopeptides to galectin-3 was a shift from a favorable enthalpy to an entropy-driven binding process. Similarly, structural analysis of binding of avian galectin-3 to TF-threonine conjugate showed transient interaction between galectin-3 and amino acid threonine [85]. These additional lectin-peptide scaffold contacts may contribute to the high selectivity of endogenous lectins for their natural counter-receptors.
Fig. 4.
Galectin-3 interaction with TF antigen of TA MUC1 promote heterotypic cancer-endothelial adhesion and cancer cell homotypic aggregation. Integrins and VCAM-1 are additional mediators in the adhesion process.
Galectins are also heavily involved in regulation of immune functions [86]. Galectin-1 and galectin-3 bind to the discrete sets of glycoproteins on the surface of T cells [87] and trigger T cell death [88, 89]. It was suggested that galectin-3 may serve as a ligand for MUC1 expressed by activated T cells, and this interaction could potentially modulate immune effector functions [90]. Galectins expressed by tumor cells exhibit tolerogenic effects, that facilitate cytokine imbalance, and induction of anergy, deletion of antigen-reactive T cells, and stimulation of suppressive Tregs [19, 90]. Targeted inhibition of galectin-1 and galectin-3 has demonstrated a strong immunosuppressive effect on T cells [91, 92].
3.3. I-type lectins
Siglecs (sialic acid binding Ig-like lectins) are a family of lectins that are mostly expressed by cells of the immune system [93, 94]. They play a key role in mediating cell-cell interactions via recognition of different sialylated glycoconjugates which can lead to the activation or inhibition of the immune response [95]. Signaling through cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM)-bearing siglecs usually results in cell activation, while engagement of intracellular immunoreceptor tyrosine-based inhibition motif (ITIM)-bearing receptors is usually inhibitory. Interaction of sTn of TA MUC1 with siglecs via ITIM motifs reduces anti-tumor immunity and thus is thought that these interactions play a central role in tumor evasion [96]. For example, recognition of sialylated glycans on cancer cells as non- or altered-self, by natural killer (NK) cells and their mediated cytotoxicity as part of an initial immune response to the tumor, is blocked when Siglecs-7 and Siglec-9 are recruited to the heavily sialylated tumor cells [97]. MUC1-sTF binding to Siglec-9 on monocytes and macrophages induced the release of factors that can promote tumor growth and modulate the microenvironment [96]. These macrophages are also characterized by increased expression of programmed cell death-1 (PD-L1) ligand, an immune check point inhibitor [41]. While the specific mechanistic role of MUC1 sLex epitopes on the immune system remains unclear, they are known to bind with Siglec-9 [98]. However, research thus far seems to have established that siglecs interaction with sialylated epitopes on MUC1 likely inhibit the immune response.
4. Cell-surface TACAs: Targets for cancer immunotherapy
TACAs of MUC1 are antigenic due to the lack of their expression on healthy cells, making them ideal targets for anti-tumor immune prevention and therapy. Indeed, a broad spectrum of natural anti-TACA antibodies are present in human serum [99–102]. These anti-glycan antibodies are IgM type, however, recently the IgG type cancer-associated auto-antibodies have been detected toward distinct O-glycopeptide epitopes [103]. The presence of circulating naturally occurring anti-MUC1 antibodies have been correlated with better disease prognosis in cancer patients [104–106].
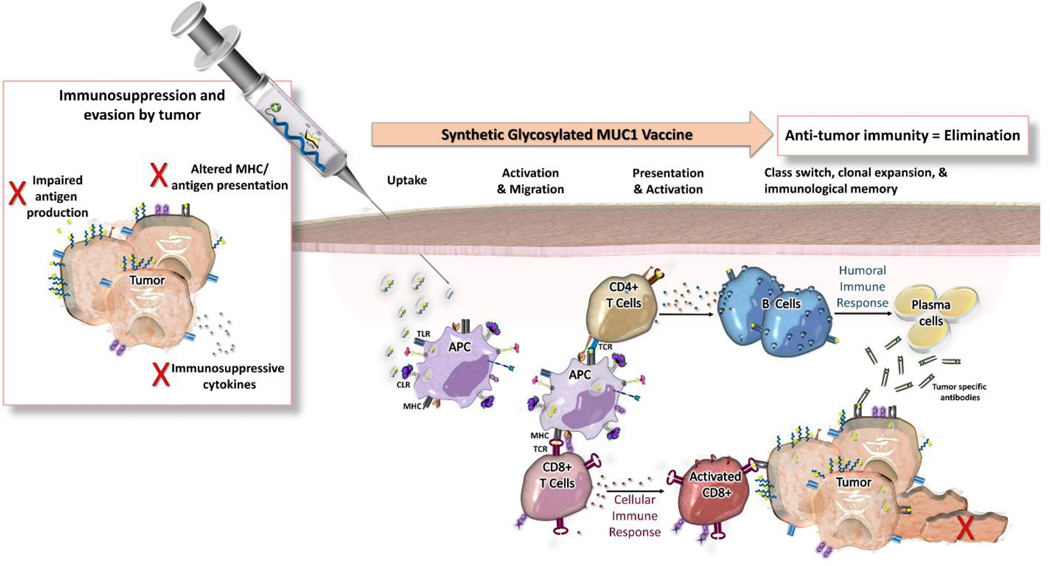
Envisioned vaccine design strategies based on TA MUC1 need to consider features such as prophylactic or therapeutic approaches. The overall goal in preventative cancer vaccine design is to intervene before natural immunosurveillance is sequestered and prevent precancerous tumors from shifting into a microenvironment that favors regulatory and immunosuppressive mechanisms [107–109] (Fig. 5). This is based on the hypothesis of cancer immune surveillance or “tumor editing,” that incorporates three different outcomes for cancer progression: tumor elimination, equilibrium with the immune system, and escape from immune control [110]. However, the general objective in therapeutic cancer vaccine design differs, as established tumors have already escaped immune surveillance. These vaccine concepts aim to induce strong antigen-specific T cell responses. Therapeutic vaccines and checkpoint inhibitors are designed to restore immunosurveillance [31, 105, 111, 112]. The future of immunotherapy lies in various combinations of drugs that modulate the tumor microenvironment and strengthen natural cancer immunosurveillance (and induce regression of tumors). Regardless of the approach, the vaccine candidate should trigger both humoral and cellular immunity in order to generate an effective MUC1 anti-cancer immune response [113]. In addition to an antigen (target for the immune response), and an adjuvant (antigen immune enhancers), current vaccines incorporate novel carrier formulations for more efficient delivery and additional stimulus, initializing a more potent immune response[114].
Fig. 5.
Cancer vaccines either aim to prevent precancerous tumors from shifting into a microenvironment that favors regulatory and immunosuppressive mechanisms (preventive) or to restore immunosurveillance (therapeutic).
Earlier attempts at immunization with nonglycosylated MUC1 were not successful as mice failed to produce high levels of anti-tumor cytotoxic T lymphocytes (CTL) and IgG antibodies due to the relatively low immunogenicity and immune tolerance of MUC1 [115–117]. The immune tolerance of non-glycosylated MUC1 can be overcome by use of MUC1 glycopeptide epitopes and/or specialized adjuvants and novel formulations in vaccine constructs. Glycopeptides bearing TACAs appear to be recognized as foreign or “abnormal-self,” while non-glycosylated MUC1 peptides, are perceived as “self” [118]. These data also point out that MUC1 glycopeptides bearing TACAs could be considered as safe and effective cancer vaccines. However, we must bear in mind that tumor cells display a collective array of TA MUC1 epitopes, while that is not mirrored in the current synthetic TACA-MUC1 peptide vaccine designs. Many current vaccine constructs include a single type of TACA-MUC1 epitope that may elicit specific anti-MUC1 antibodies, however, the lack of natural glycan variety, may result in the inability of the vaccine to generate an extended adaptive immune response. Nevertheless, the increased immune stimulation has been attributed to the glycan(s) type, and site(s) of attachment, and/or glycosylation induced conformational changes within the three immunodominant peptide epitopes (PDTRP, GSTA, and GVTS) [119–123]. However, rules that govern the immunogenicity of the three O-glycosylated peptide motifs within the MUC1 tandem repeat sequence are still poorly defined [124–127].
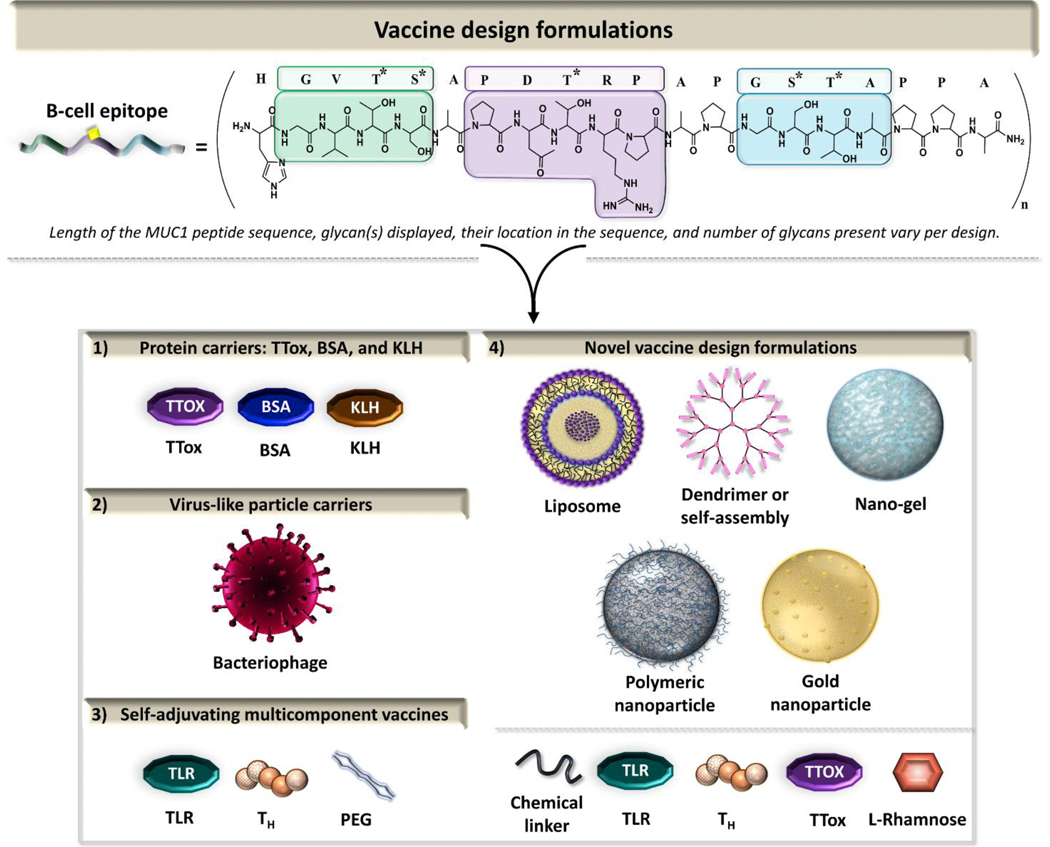
There are several examples of synthetic TACA-MUC1 vaccine designs developed over the last several years that assist in specificity aid eliciting an anti-antigen response [31, 120, 122, 128, 129]. The first prophylactic cancer vaccine clinical trial based on a non-viral antigen, MUC1, in healthy individuals at-risk for colon cancer has been recently been completed [130]. The study identified 13 fully-human IgG type antibodies with specificity for several tumor-associated MUC1 epitopes with a wide range of binding affinities. These antibodies are promising candidates for development of immunotherapeutic drugs. This review focuses on the MUC1 O-glycopeptide vaccines bearing Tn, sTn, TF, and sTF antigens, in combination with carrier platforms (bacterial toxins and virus-like particle), adjuvants such as those targeting pattern recognition receptors [Toll-like receptors (TLRs) and C-type lectin receptors (CLRs)], and/or novel nanoparticle-based delivery systems (Fig. 6).
Fig. 6.
Vaccine formulations represented in this review.
4.1. Protein carriers: TTox/BSA/KLH
Strong antigen-specific immune responses were induced when synthetic mono- and multiglycosylated MUC1 epitopes were paired with BSA or TTox as protein carriers in the presence or absence of a spacer between the two components [122]. These conjugates bearing Tn, sTn, TF, and/or sTF proved to be efficient vaccines, inducing very strong immune responses in mice with predominant IgG isotype antibodies.
BSA-conjugated glycopeptide vaccines, based on MUC1 20-mer tandem repeat sequence bearing either Tn or TF antigen at Thr9 (PDT*RP region), displayed a specific enhanced immune response [131]. On the contrary, the same MUC1 sequence, when bearing Tn antigen at Ser15 (GS*TA) elicited a lower immune response [131]. These differences were attributed to the conformational differences amongst Ser and Thr O-glycosylation points [132–136]. The glycosidic linkage of GalNAc-α-Thr is rather rigid in solution compared to GalNAc-α-Ser. Overall, higher antibody specific titers were generated when both the PDT*RP and GS*TA region were glycosylated with either two Tn antigens, or with one TF and one Tn antigen. Even though divalent glycan presentation elicited high IgG antibody levels, it is important to recognize that the monoglycosylated PDT*RP epitope bearing TF antigen produced almost the same antibody titer levels. Favorability for the TF antigen was seen in both glycan positions. By keeping Tn or TF antigen at Thr within the PDT*RP epitope and introducing sTn or 2,6-sTF glycans at Ser within the GS*TA epitope similar results were obtained [133]. It was concluded that TF favors the PDT*RP region while sialoglycans show immune preference in the GS*TA region. These BSA-conjugated glycopeptide vaccines induced IgG-isotype antibodies, which bound to TA MUC1 expressed on MCF-7 tumor cells. The role of glycosylation within GS*T*A epitope was further evaluated with either BSA or TTox-conjugated to extended 22-mer MUC1 epitopes bearing sTn glycan at either Ser or its neighboring Thr residue [137]. The TTox vaccine conjugate bearing the sTn glycan at the Thr residue was selective for not only tumor cell lines expressing TA MUC1 but also for native tumor tissues, showing for the first-time the diagnostic value of these antibodies. Further extension of MUC1 to include the PDTRP epitope surrounded by two STAPPA motifs (27-mer epitope), and glycosylation with the Tn antigen within the GS*TA epitope, resulted in a strong immune response that demonstrated the IgG class switch [121]. The 27-mer conjugated vaccine induced antibodies that bind with high specificity to TA MUC1 on tumor cells and was successfully used in diagnosis of pancreatic cancer. Recently, a 22-mer MUC1 peptide sequence was functionalized with the Tn antigen at Thr within the PDT*RP region and sTn at Thr within the GST*A region and coupled to TTox [138]. The vaccines induced strong immune responses in wild-type and human MUC1-transgenic mice without autoimmune side effects again the self-antigen. Vaccination with TTox carrier conjugates containing 2,3-sTF within the GST*A region either with or without Tn glycan attached to Thr within PDT*RP region induced robust immune responses [119]. The observed class switch to IgG subtype indicates an acquisitioned immunological memory. Although the incorporation of Tn antigen within PDT*RP region had less impact on the total antibody titers, breast cancer tumor cells (MCF-7) incubated with the antisera from mice immunized with the divalent MUC1 vaccine showed increased antibody binding. This was attributed to induction of a more tumor-selective epitope conformation influenced by glycosylation. These results support the hypothesis that the glycosylation pattern plays a key role in tumor specificity.
TF antigen mimetics (fluorine-substituted a nalogues in the 6 and 6’ positions of the pyranose rings) were incorporated within GVT*S epitope of MUC1 20-mer sequence and conjugated to TTox [139]. These two component synthetic vaccines overrode natural tolerance of the immune system and induced a sufficiently strong immune response in mice. Highly-specific antibodies selectively targeted the tumor-associated MUC1 structures and strongly bound breast cancer cells of the MCF-7 cell line.
Recently, a synthetic MUC1 glycopeptide library consisting of 18 predesigned epitopes of different lengths (20-, 40-, 60-, and 100-mer) carrying Tn, sTn, TF, and/or sTF glycans were used to generate novel anti-MUC1 monoclonal antibodies [140] that utilized BSA and keyhole limpet hemocyanin (KLH) conjugated protein carriers. Two novel antibodies with pre-designed carbohydrate specificities for GalNAc with either an unsubstituted O-6 position or its sialylated version were established. Both antibodies recognized specific O-glycan structures at the PDT*RP motif, however, one antibody recognized Tn, T, and 2,3-sTF glycans, while the other antibody recognized sialic acid at the same position (sTn, 2,6-sTF, and di-sialylated TF).
A synthetic 7-mer triglycosylated-Tn MUC1 vaccine utilizing only the immunodominant PDT*RPAP region in conjugation with PEG, diethyl squarate linker, and BSA afforded similar IgG antibody levels as the non-glycosylated version [141]. However, when MCF-7 breast cancer cells were challenged with the antisera from the triglycosylated MUC1 vaccine there was stronger binding to the cells in comparison to the non-glycosylated version. The overall findings suggest that use of the immunodominant PDT*RP region in a vaccine design, glycosylated or not, assists in overcoming weak immunogenicity [141].
Enhancement of vaccine specificity can be further achieved by including multi-component architecture in vaccine design. Mannose receptors expressed on the surface of DC and APC cells provide an integral bridge between innate and adaptive immunity. Thus, it was hypothesized that attaching mannose ligands to MUC1 glycopeptide vaccines would lead to more efficient internalization and presentation to TH-cells and ultimately to endocytosis. The vaccine design included a 22-mer MUC1 peptide sequence with Tn anchored at Ser in the GS*TA region, conjugated to TTox at the C-terminus, and either a di-mannose or a tetra-mannose receptor ligand at the N-terminal side of a MUC1 epitope [142]. The mannosylated vaccine induced much stronger specific IgG immune responses in mice than the non-mannosylated vaccine control. The tetravalent mannosyl candidate was recognized better by APCs resulting in antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) [142].
4.2. Virus-like particle carriers: RNA Bacteriophage Qβ viral carrier
Due to the inherently weak nature of TACA-MUC1 epitopes alone in generating strong T cell responses and long-lasting memory cells, there is need for T helper cell protein carriers. Bacteriophage Qβ viral carriers offer safe immunostimulatory effects that can display a repetitive array of antigens on its immunogenic surface that produce an effective immune response. Additionally, these virus-like particles (VLPs) self-assemble into viral envelope proteins in which TA MUC1 peptides can be inserted using covalent conjugation. Several MUC1 glycopeptides were synthesized, made up of one full length tandem repeat region of MUC1 (20- and 22-mer peptides), that were monoglycosylated with the Tn antigen in the PDT*RP or GST*A region [143]. These Qβ vaccine constructs elicited strong immune responses in wild type mice, however, IgG antibodies from tolerant human MUC1 transgenic (Tg) mice did not bind tumor cells strongly. Ultimately, the 9-mer sequence SAPDT*RPAP was identified as immunogenic and a critical protective epitope capable of inducing an immune response in the immunotolerant environment. A Qβ-MUC1 vaccine conjugate with SAPDT*RPAP sequence induced a strong IgG antibody response and exhibited high tumor binding and killing activities. The antibodies were selective toward human breast cancer tissues, suggesting its high translational potential. This same research group recently developed vaccines of a similar design utilizing the TF and sTn antigens which also elicited strong immune responses in immune-tolerant human MUC1.Tg mice [144]. The Tn and sTn glycosylated MUC1 vaccines displayed similar IgG binding levels against the cancer cells. The TF glycosylated MUC1 vaccine produced about four times higher titer levels than the other constructs. Additionally, the Qβ-MUC1-TF vaccine showed in vivo efficiency in a lung tumor mice model [144] and has great potential as anticancer vaccine.
4.3. Self-adjuvating multicomponent vaccines
The inherent immunogenicity of carrier proteins is known to suppress the immune response toward the desired MUC1 glycopeptide epitopes. Thus, a new approach incorporates TLRs ligands as part of novel fully synthetic self-adjuvating vaccines. TLRs are expressed on immune cells, macrophages and DC, and act as a bridge between innate and adaptive immunity [145, 146].
Boons and co-workers have designed a tricomponent vaccine that incorporates a TLR2 ligand, Pam3CysSerK4, MUC1 peptide sequence bearing Tn antigen within the TSAPDT*RPAP epitope, and an additional T helper cell epitope derived from poliovirus [147, 148]. This vaccine candidate elicited a robust IgG antibody response able to kill cancer cells by antibody-dependent cell-mediated cytotoxicity (ADCC). The superior properties of the vaccine candidate were also attributed to its ability to produce cytokines at the site where the vaccine interacts with immune cells, thus facilitating maturation of relevant immune cells. Furthermore, it was found that the presence of Pam3CysSerK4 type of TLR ligand and an additional T cell epitope is essential for optimal activity and high titers of IgG [149]. The immune response of the same tricomponent vaccine with a novel Tn antigen mimic (a-methyl serine), was evaluated [150]. The incorporation of the Tn glycan mimetic resulted in more proteolytically resistant construct and an unchanged conformation, however, the immunogenicity didn’t exceed that of the threonine analogue.
It was found that replacement of the TLR2 ligand with a TLR9 ligand, CpG oligodeoxynucleotide (CPG-ODN 1826) was not beneficial [151]. The IgG antibody response of the TLR2 candidate indicated a mixture of Th1/Th2 immune response with high antibody titers, whereas the TLR9 design produced much lower levels. The incorporation of an sTn antigen at the Thr9 position in the TLR2 based vaccine Construct led to potent humoral and cellular immune responses [152]. Interestingly, the levels of IgG stimulation compared to the Tn glycosylated epitope [151], were lower and the IgM levels were higher, highlighting the superiority of Tn glycosylated vaccine version. Recently, the same group reported use of longer MUC1-derived peptide sequence that incorporates a Tn antigen within the PDT*RP region [153]. This longer MUC1 sequence contains multiple epitopes that can activate B-cells, cytotoxic T-lymphocytes, and helper T cells. It was hypothesized that the exogenous T helper cell epitope is not necessary for efficient immune activation. Ultimately, it was shown that by omitting the artificial linkers and exogenous T helper cell epitopes this vaccine candidate was able to activate T cells and induce high IgG titer levels. This study highlights the importance of the MUC1 peptide sequence (proper length) and glycosylation (glycan position and/or combination of glycans) in eliciting humoral and cellular immune responses.
TLR2 receptor ligands were utilized by the Payne group [154–156] in development of self-adjuvating vaccines with or without exogenous helper T cell epitopes. The ability of lipopeptide, Pam2Cys (MALP-2 analog) to induce specific antibodies when conjugated to the 20-mer nonglycosylated (GVTSAPDTRPAPGSTAPPAH) or pentaglycosylated MUC1 epitope (GVT*S*APDT*RPAPGS*T*APPAH) via a PEG spacer was evaluated [154]. The pentaglcosylated MUC1 bears Tn or TF antigens. The glycosylated vaccine constructs were shown to induce high titers of class-switched IgG antibodies despite the lack of helper T cell epitopes. More importantly, only the Tn glycosylated vaccine candidate displayed cross-reactivity, while the TF glycosylated antibodies were specific and only recognized the epitope to which they were raised. The inclusion of the helper T cell epitope, PADRE, in this two-component vaccine construct with use of the extended 27-mer MUC1 epitope glycosylated at Thr in the PDT*RP region and at Ser in the GS*TA region with a Tn antigen boosted the antibody response and resulted in elevated cytokine production [157].
Li and co-workers [158], utilized a 20-mer MUC1 tandem repeat sequence with a Tn antigen in the PDT*RP region conjugated to the fibroblast stimulating lipopeptide 1 (FSL-1, Pam2-CGDPKHPKSF) that supports recognition through TLR2 and TLR6 with or without helper T cell epitopes. Immunological results indicate that the tricomponent glycosylated vaccine design stimulated humoral and cellular immune responses that were almost 1.5 times higher than the candidate without the T helper epitope and the antibodies generated from tricomponent vaccine mice bound MCF-7 cancer cells much more effectively.
4.4. Novel delivery systems (self-assembled, nanoparticles, liposomes, polymeric)
Since pathogens such as viruses and bacteria often display multiple copies of ligands on their surfaces, the immune system prefers their multivalent displays. Consequently, when designing a vaccine, it is advantageous to apply a multivalent approach to stimulate B cell receptor crosslinking that ultimately, results in enhanced antibody production. Recently, novel nanostructured scaffolds decorated with multiple copies of an antigen for eliciting robust immune responses have been designed [159]. This review highlights the architecture and immune response of a diverse set of recently developed nanostructured MUC1 vaccine scaffolds.
Peptide-based self-assembly principle has been used in the field of drug delivery[160, 161], and most recently in vaccine design. Self-assembling peptide, Q11 (Ac-QQKFQFQFEQQ-Am) [162], was conjugated to a tandem repeat sequence of MUC1 glycosylated with a Tn antigen at either the PDT*RP region or the GST*A region or at both positions [163]. These vaccines elicited a high level of antibody response without any adjuvant. They appeared to act through a T cell independent pathway and were associated with the activation of cytotoxic T cells. Most recently, a novel design was proposed by the same group [164]. The synthetic monoglycosylated MUC1 epitopes with Tn linked at Thr in the PDT*RP region were conjugated to a Nap-GFFYK, an unnatural D-amino acid nanovector carrier [164]. These vaccine candidates aggregated into particles of varied diameter (40 to 200 nm) and produced high IgG titer levels that were MUC1-Tn specific. The Nap-nanovector alone was not immunogenic and the IgG class switch was verified by immunizing mice with only MUC1-Tn, confirming they had high levels of IgM antibodies while the nanovector vaccine had minimal IgM antibodies. It was also concluded that complement dependent cytotoxity (CDC) pathway was developed. Additionally, when antisera antibodies from the nanoparticle vaccine immunized mice where presented against MUC1 expressing MCF-7 tumor cells, high binding was observed [164]. Humoral and cellular immune responses were induced as verified by cytokine release analysis.
Hyperbranched polymers form well-defined multivalent platforms that offer unique opportunities for enhancing immunogenicity by optimal multiple antigen presentation. The Kunz research group [165], developed such a fully synthetic multivalent MUC1 polymer vaccine based on hyperbranched polyglycerol (HPG). This globular polymeric carrier, with dendrimer-like structure, was chosen because of its favorable properties, biocompatibility, non-immunogenicity, and solubility in water. In addition to HPG, the vaccine conjugates contained a 22-mer MUC1 glycopeptide with Tn antigen conjugated in the GST*A region or the diglycosylated version with additional Tn conjugated in the PDT*RP region and tetanus toxoid T cell epitope P2 (QYIKANSKFIGITEL). Two types of the HPG-based vaccines were prepared, one carrying 3 monoglycosylated MUC1 epitopes, and other with 6 diglycosylated MUC1 epitopes, offering a more dense antigen presentation. To understand the immunological potential of these vaccines, mice were immunized with the two different vaccines paired with adjuvant. As a control, a diglycosylated MUC1 peptide conjugated to BSA was used. The more dense vaccine candidate produced higher IgG titer levels overall, but it was noted that the end-point titer levels didn’t reach levels elicited by other vaccines that use TTox. However, the MHC-II mediated immune response was observed, and immunological memory was developed. The vaccine carrying the diglycosylated MUC1 sequence consistently had higher IgG subtype titer levels in comparison to the monoglycosylated one, and in addition, exhibited the ability of inducing ADCC and CDC cytotoxicity. Lastly, the antibodies induced by the multivalent vaccine (diglycosylated version) displayed higher binding percentages to epithelial tumor cells.
Liu and co-workers [166], developed a polymer based vaccine that encased the TTox T cell epitope helper with an immunostimulant γ-polyglutamic acid (γ-PGA), and glycosylated MUC1 sequences with Tn antigen in the PDT*RP and GS*TAP regions. In their studies they made five vaccine combinations that included, 1) one diglycosylated MUC1 tandem repeat sequence that was extended with seven N-terminal lysines, γ-PGA, and TTox, 2) same as 1, with an adjuvant, 3) same as 1, without lysine N-terminal extensions and adjuvant, 4) same as 1 without γ-PGA and an adjuvant added, and 5) a positive control of the MUC1 sequence covalently attached to the TTox epitope. The multilayer spherical vaccine candidates were all determined to have a hydrodynamic radius of about 355 nm. The first and fifth vaccine models produced equivalent amounts of IgG antibody titers and the third and fourth vaccines elicited the worst immune responses. Surprisingly, the second vaccine model that includes Freund’s adjuvant produced about half the amount of IgG antibodies as vaccines one and five. It was proposed that Freund’s adjuvant may destruct the multilayer architecture, resulting in a lower immune response. Importantly, the first vaccine model also induced high levels of IgM antibodies, pointing out that the IgM – IgG switch may not have completely happened. When the mouse antisera were incubated with MCF-7 cells, only the first vaccine design showed binding to the tumor cells and successfully killed MCF-7 cells.
The cationic hydrogel type of nano-based scaffold was used in conjunction with MUC1 peptides bearing Tn antigen within the GST*A region and T cell helper P2 (QYIKANSKFIGITEL) epitope derived from TTox [167] or unmethylated cytidine-phosphate-guanosine (CpG) as internal adjuvant for TLR stimulation. The self-assembled hydrodynamic radius of the nanogel vaccines were between 65.4 and 66.7 nm. While both vaccines were able to elicit co-stimulatory cell markers of the immune system, the CpG version was able to induce T cell proliferation. The CpG version vaccine held a higher overall quality of derived antibodies as confirmed by their selective binding for T47D cancer cells.
Functionalized gold nanoparticles carrying CD4+ T cell peptide epitope P30 (FNNFTVSFWLRVPKVSASHLE) from Tetanus toxoid and MUC1 glycosylated with Tn antigen in the GS*T*A and PDT*RP regions were described by Cai and coworkers [168]. It was determined that the glycopeptide-functionalized gold nanoparticles led to an antibody isotope switch from Th2 to Th1 mediated cellular response. Notably, when the gold nanoparticles were removed from the vaccine, the major immune response was Th2 mediated. Antigen mimics based on O → S/Se replacement at the glycosidic linkage were explored in the design of MUC1-functionalized gold nanoparticles by the Corzana group [169]. The MUC1 epitope consisted of a hexapeptide sequence APDT*(O/S or OSe)-RP. The vaccine conjugate tested without any adjuvant, resulted in a significant humoral immune response. Importantly, the mice antisera recognize cancer cells in biopsies of breast cancer patients with high selectivity.
Recently two groups decorated liposome surfaces with an immune complex-targeting ligand, L-rhamnose (Rha) epitope [170, 171]. Rha epitope was used to improve antigen uptake of a glycopeptide sequence containing a CD8+ T cell epitope. Both groups used 20-mer glycosylated MUC1 epitope with Tn antigen attached in the PDT*RP region, but different TLR targeting ligands, Pam3Cys [170] or Pam3CysSK4 [171]. When the Rha epitope was present in the vaccine the IgG titer levels were twice as high as the vaccine without it, demonstrating that CD4+ T cell help was stimulated more effectively in the presence of the Rha epitope. However, the choice of TLR2 ligand was equally important for in vivo enhancement of the response [171]. Liposomes decorated with natural killer T cell (NKT) agonist, α-galactosylceramide (α-GalCer) were prepared in a similar manner [172]. The presence of α-GalCer was expected to activate NKT cells to release large quantities of various cytokines, which may help B cells to proliferate and undergo antibody class switching. The glycosylated 21-mer MUC1 liposomes with Tn antigen in the PDT*RP region was used in preparation of the N-terminally palmitoyl lipid analogs, Pam-MUC1 or Pam2-MUC1. The vaccine designs that didn’t have α-GalCer used Pam3CSK4 immunostimulating adjuvant instead. Mouse models vaccinated with α-GalCer constructs expressed higher levels of inflammatory cytokines towards Th1 and Th2 subtypes and antibodies from the immunized mice elicited antisera that bound better to MCF-7 cells than the other vaccine models. They were also able to determine that Pam2-MUC1 significantly boosted the immunodominant response and it was determined to be the result of the conjugated palmitoyl lipid chains effectively mediating complement lysis. Promising lipid-dependent immunological responses were also observed when the TLR1/2 agonist Pam3CSK4 was used as an adjuvant.
5. Summary and future perspectives
Several therapeutic MUC1 vaccine designs have shown promise in early clinical trials, nevertheless the observed therapeutic effects were insufficient to reach clinical use. The immune tolerance and heterogeneity of TACA-MUC1 on the surface of tumor cells played a pivotal role in the unsuccessful clinical outcomes. Several glycopeptide epitopes carrying one or multiple TACAs were evaluated in recent studies in wild type and MUC1.Tg mouse models. In addition to varied peptide epitope sequences, different type of TACAs, and their position and density, adjuvants and novel delivery systems were employed in the vaccine designs. Although results were not always easy to compare due to the multiple variables in the experimental design, it has been shown that a diverse array of MUC1 antigen preparations were able to trigger humoral and cellular immunity. However, a better understanding of the immunological determinants of an extended adaptive immune response is still needed. The glycan(s) type, and site(s) of attachment, and/or glycosylation induced conformational changes within the immunodominant MUC1 peptide epitopes are foreseen to play a key role in achieving the full potential of MUC1 glycopeptide anti-cancer vaccines.
HIGHLIGHTS.
MUC1 is aberrantly glycosylated and overexpressed in epithelial cancers.
TACAs of MUC1 include truncated O-glycans and their sialylated counterparts.
Endogenous lectins are receptors for TACAs.
TACAs are targets for prophylactic and immunotherapeutic cancer vaccine design.
Factors that govern the design of synthetic cancer vaccines are described.
Acknowledgement
This work was supported by the National Institutes of Health Grant CA242351 M. C.
ABBREVIATIONS
- TACA(s)
tumor associated carbohydrate antigen(s)
- MUC1
Mucin 1
- Ser
Serine
- Thr
Threonine
- MUC1-N
N-terminal MUC1
- MUC1-C
C-Terminal MUC1
- GalNAc
N-acetylgalactosamine
- Gal
Galactose
- GlcNAc
N-acetylglucosamine
- Neu5Ac
N-acetylneuraminic acid
- Fuc
Fucose
- Tn
GalNAcα-O-Ser/Thr, Thomsen Nouveau, CD175
- TF
Galβ1,3-GalNAcα-O-Ser/Thr, Thomsen-Friedenreich, CD176, T antigen
- sTn
Neu5Acα2,6-GalNAcα-O-Ser/Thr, sialyl Tn, CD175s
- sTF
Neu5Acα2,6- and Neu5Acα2,3-Galβ1,3-GalNAcα-O-Ser/Thr, sialyl TF
- Cosmc
Core 1 β3GalT specific molecular chaperone
- α
Alpha
- β
Beta
- T-synthase
Core 1 β3galactosyltransferase, β3GalT
- Glu
Glucose
- ST6GalNAc-I
α2,6-siayltransferase-1
- C2GnTs
core 2 GlcNAc transferases
- EGFR
epidermal growth factor receptor
- ST3Gal-I
α2,3-sialyl transferase
- MHC
Major histocompatibility complex
- sLea
NeuAcα2,3-Galβ1,3-(Fucα1,4)-GlcNAc-R
- sLex
NeuAcα2,3-Galβ1,3-(Fucα1,3)-GlcNAc-R
- NK
Natural killer
- DC
dendritic cell
- DC-SIGN
Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
- MR
Mannose receptor
- CRD
Carbohydrate recognition domain
- S-type lectin
Galectins
- Siglecs
Sialic acid-binding immunoglobulin-type lectin, I-type lectins
- MGL
Macrophage galactose lectin
- T cell
T lymphocyte
- TH
T helper cells
- mAbs
Monoclonal antibodies
- CD4+
Cluster of differentiation 4 T cells
- CD8+
Cluster of differentiation 8 T cells
- VCAM-1
vascular cell adhesion proton 1
- Tregs
regulatory T cells
- IgM
immunoglobulin M
- IgG
immunoglobulin G
- TTox
tetanus toxoid
- ITIM
tyrosine-based inhibition motif
- PD-L1
programmed cell death-1
- TAM
tumor-associated macrophages
Footnotes
Conflict of interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Hattrup CL, Gendler SJ, Structure and function of the cell surface (tethered) mucins, Annu. Rev. Physiol. 70 (2008) 431–457. [DOI] [PubMed] [Google Scholar]
- [2].Gendler SJ, Spicer A, Epithelial mucin genes, Annu. Rev. Physiol. 57 (1995) 607–634. [DOI] [PubMed] [Google Scholar]
- [3].Nath S, Mukherjee P, MUC1: a multifaceted oncoprotein with a key role in cancer progression, Trends Mol. Med. 20(6) (2014) 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gendler S, MUC1, The renaissance molecule, J. Mammary Gland Biol. 6(3) (2001) 339–353. [DOI] [PubMed] [Google Scholar]
- [5].Hanisch F, Muller S, MUC1: the polymorphic appearance of a human mucin, Glycobiology 10(5) (2000) 439–449. [DOI] [PubMed] [Google Scholar]
- [6].Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D, Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin, J. Biol. Chem. 265(25) (1990) 15286–15293. [PubMed] [Google Scholar]
- [7].Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD, Simple sugars to complex disease--mucin-type O-glycans in cancer, Adv. Cancer Res. 126 (2015) 53–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aryal RP, Ju T, Cummings RD, The endoplasmic reticulum chaperone Cosmc directly promotes in vitro folding of T-synthase, J. Biol. Chem. 285(4) (2010) 2456–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].K.B. Lloyd J; Kudryashov V; Yin B; Papadimitriou J, Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines, J. Biol. Chem. 271(52) (1996) 33325–33334. [DOI] [PubMed] [Google Scholar]
- [10].Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, Ho SB, Dell A, Fukuda M, Marth JD, Glycosyltransferase function in core 2-type protein O-glycosylation, Mol. Cell Biol. 29(13) (2009) 3770–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chugh S, Gnanapragassam VS, Jain M, Rachagani S, Ponnusamy MP, Batra SK, Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets, Biochim. Biophys. Acta 1856(2) (2015) 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parry S, Hanisch FG, Leir SH, Sutton-Smith M, Morris HR, Dell A, Harris A, N-Glycosylation of the MUC1 mucin in epithelial cells and secretions, Glycobiology 16(7) (2006) 623–634. [DOI] [PubMed] [Google Scholar]
- [13].Burchell JM, Beatson R, Graham R, Taylor-Papadimitriou J, Tajadura-Ortega V, O-linked mucin-type glycosylation in breast cancer, Biochem. Soc. Trans. 46(4) (2018) 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, Kudelka MR, Cutler C, Zeng J, Wang J, Sun X, Heimburg-Molinaro J, Smith DF, Cummings RD, Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers, Proteomics Clin. Appl. 7(9–10) (2013) 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brockhausen I, Yang J-M, Burchell J, Whitehouse C, Taylor-Papadimitrio J, Mechanisms underlying aberrant glycosylation of MUCl mucin in breast cancer cells, Eur. J. Biochem. 233 (1995) 607–617. [DOI] [PubMed] [Google Scholar]
- [16].Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD, Cosmc is an essential chaperone for correct protein O-glycosylation, PNAS 20 (2010) 9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tongzhong J, Aryal R, Kudelka M, Wang Y, Cummings R, The Cosmc connection to the Tn antigen in cancer, Cancer Biomark. 14 (2014) 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rivinoja A, Kokkonen N, Kellokumpu I, Kellokumpu S, Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen, J. Cell Physiol. 208(1) (2006) 167–174. [DOI] [PubMed] [Google Scholar]
- [19].Hauselmann I, Borsig L, Altered tumor-cell glycosylation promotes metastasis, Front Oncol. 4 (2014) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu J, Gu J, Significance of beta-galactoside alpha2,6 sialyltranferase 1 in cancers, Molecules 20(5) (2015) 7509–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chia J, Goh G, Bard F, Short O-GalNAc glycans: regulation and role in tumor development and clinical perspectives, Biochim. Biophys. Acta 1860(8) (2016) 1623–1639. [DOI] [PubMed] [Google Scholar]
- [22].Karsten U, von Mensdorff-Pouilly S, Goletz S, What makes MUC1 a tumor antigen?, Tumor Biol. 26(4) (2005) 217–220. [DOI] [PubMed] [Google Scholar]
- [23].Pinto R, Carvalho AS, Conze T, Magalhaes A, Picco G, Burchell JM, Taylor-Papadimitriou J, Reis CA, Almeida R, Mandel U, Clausen H, Soderberg O, David L, Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation, J. Cell Mol. Med. 16(7) (2012) 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Julien S, Videira PA, Delannoy P, Sialyl-Tn in cancer: (How) did we miss the target?, Biomolecules 2(4) (2012) 435–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Freitas D, Campos D, Gomes J, Pinto F, Macedo JA, Matos R, Mereiter S, Pinto MT, Polonia A, Gartner F, Magalhaes A, Reis CA, O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype, EBioMedicine 40 (2019) 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Springer G, T and Tn, General carcinoma autoantigens, Science 224 (2017) 1198–1206. [DOI] [PubMed] [Google Scholar]
- [27].Munkley J, The role of sialyl-Tn in cancer, Int. J. Mol. Sci. 17(275) (2016) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cazet D, Julien S, Bobowski M, Burchell J, Delannoy P, Tumour-associated carbohydrate antigens in breast cancer, Breast Cancer Res. 12(204) (2010) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sindrewicz P, Lian LY, Yu LG, Interaction of the oncofetal Thomsen-Friedenreich antigen with galectins in cancer progression and metastasis, Front Oncol. 6(79) (2016) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cao Y, Schlag P, Karsten U, Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation., Virchows Arch 431(3) (1997) 159–166. [DOI] [PubMed] [Google Scholar]
- [31].Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R, Latest developments in MUC1 immunotherapy, Biochem. Soc. Trans. 46(3) (2018) 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tarp MA, Clausen H, Mucin-type O-glycosylation and its potential use in drug and vaccine development, Biochim. Biophys. Acta 1780(3) (2008) 546–563. [DOI] [PubMed] [Google Scholar]
- [33].Kimura T, Finn OJ, MUC1 immunotherapy is here to stay, Expert Opin. Biol. Th. 13(1) (2013) 35–49. [DOI] [PubMed] [Google Scholar]
- [34].Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM, The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research, Clin. Cancer Res. 15(17) (2009) 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sharon N, Lis H, Lectins as cell recognition molecules, Science 246 (1989) 227–234. [DOI] [PubMed] [Google Scholar]
- [36].Ghazarian H, Idoni B, Oppenheimer SB, A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics, Acta Histochem. 113(3) (2011) 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rambaruth ND, Dwek MV, Cell surface glycan-lectin interactions in tumor metastasis, Acta Histochem. 113(6) (2011) 591–600. [DOI] [PubMed] [Google Scholar]
- [38].Nardy AFFR, Freire-de-Lima L, Freire-de-Lima CG, Morrot A, The sweet side of immune evasion: Role of glycans in the mechanisms of cancer progression, Front. Oncol. 6(54) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cornelissen LAM, van Vliet SJ, A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer, Biomolecules 6(2) (2016) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cagnoni AJ, Perez Saez JM, Rabinovich GA, Marino KV, Turning-off signaling by siglecs, selectins, and galectins: chemical inhibition of glycan-dependent interactions in cancer, Front Oncol. 6(109) (2016) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].RodrÍguez E, Schetters STT, van Kooyk Y, The tumour glyco-code as a novel immune checkpoint for immunotherapy, Nat. Rev. Immunol. 18 (2018) 204–211. [DOI] [PubMed] [Google Scholar]
- [42].Taylor M, Drickamer K, Schnaar R, Etzler M, Varki A, Discovery and classification of glycan-binding proteins. https://www.ncbi.nlm.nih.gov/books/NBK453061/, 2017. (accessed 09/09/20192019).
- [43].Brown GD, Willment JA, Whitehead L, C-type lectins in immunity and homeostasis, Nat. Rev. Immunol. 18(6) (2018) 374–389. [DOI] [PubMed] [Google Scholar]
- [44].Borsig L, Selectins in cancer immunity, Glycobiology 28(9) (2018) 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Laubli H, Borsig L, Selectins promote tumor metastasis, Semin. Cancer Biol. 20(3) (2010) 169–177. [DOI] [PubMed] [Google Scholar]
- [46].Kojima N, Handa K, Newman W, Sen-itirohHakomori, Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells, Biochem. Bioph. Res. Co. 182 (1992) 1288–1295. [DOI] [PubMed] [Google Scholar]
- [47].Cascio S, Finn OJ, Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis, Biomolecules 6(4) (2016) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McDermott KM, Crocker PR, Michael AH, Burdick D, Hinoda Y, Hayashi T, Imai K, Hollingsworth MA, Overexpression of MUC1 reconfigures the binding properties of tumor cells, Int. J. Cancer 94 (2001) 783–791. [DOI] [PubMed] [Google Scholar]
- [49].Napoletano C, Zizzari IG, Rughetti A, Rahimi H, Irimura T, Clausen H, Wandall HH, Belleudi F, Bellati F, Pierelli L, Frati L, Nuti M, Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation, Eur. J. Immunol. 42(4) (2012) 936–945. [DOI] [PubMed] [Google Scholar]
- [50].Jégouzo SAF, Quintero-Martínez A, Ouyang X, dos Santos Á, Taylor ME, Drickamer K, Organization of the extracellular portion of the macrophage galactose receptor: A trimeric cluster of simple binding sites for N-acetylgalactosamine, Glycobiology 23(7) (2013) 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ozaki K, Lee RT, Lee YC, Kawasaki T, The differences in structural specificity for recognition and binding between asialoglycoprotein receptors of liver and macrophages, Glycoconj. J. 12(3) (1995) 268–274. [DOI] [PubMed] [Google Scholar]
- [52].Marcelo F, Garcia-Martin F, Matsushita T, Sardinha J, Coelho H, Oude-Vrielink A, Koller C, Andre S, Cabrita EJ, Gabius HJ, Nishimura S, Jimenez-Barbero J, Canada FJ, Delineating binding modes of Gal/GalNAc and structural elements of the molecular recognition of tumor-associated mucin glycopeptides by the human macrophage galactose-type lectin, Chemistry 20(49) (2014) 16147–16155. [DOI] [PubMed] [Google Scholar]
- [53].Beatson R, Maurstad G, Picco G, Arulappu A, Coleman J, Wandell HH, Clausen H, Mandel U, Taylor-Papadimitriou J, Sletmoen M, Burchell JM, The breast cancer-associated glycoforms of MUC1, MUC1-Tn and sialyl-Tn, are expressed in COSMC wild-type cells and bind the C-type lectin MGL, PLoS One 10(5) (2015) e0125994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P, Tumour-associated macrophages as treatment targets in oncology, Nat. Rev. Clin. Oncol 14(7) (2017) 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Saeland E, van Vliet SJ, Backstrom M, van den Berg VC, Geijtenbeek TB, Meijer GA, van Kooyk Y, The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma, Cancer Immunol. Immun. 56(8) (2007) 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Vliet SJ, van Liempt E, Geijtenbeek TB, van Kooyk Y, Differential regulation of C-type lectin expression on tolerogenic dendritic cell subsets, Immunobiology 211(6–8) (2006) 577–585. [DOI] [PubMed] [Google Scholar]
- [57].Zizzari IG, Martufi P, Battisti F, Rahimi H, Caponnetto S, Bellati F, Nuti M, Rughetti A, Napoletano C, The macrophage galactose-type C-type lectin (MGL) modulates regulatory T cell functions, PLoS ONE 10(7) (2015) e0132617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].van Kooyk Y, Ilarregui JM, van Vliet SJ, Novel insights into the immunomodulatory role of the dendritic cell and macrophage-expressed C-type lectin MGL, Immunobiology 220(2) (2015) 185–192. [DOI] [PubMed] [Google Scholar]
- [59].Napoletano C, Zizzari IG, Rughetti A, Rahimi H, Irimura T, Clausen H, Wandall HH, Belleudi F, Bellati F, Pierelli L, Frati L, Nuti M, Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation, Eur. J. Immunol. 42(4) (2012) 936–945. [DOI] [PubMed] [Google Scholar]
- [60].Napoletano C, Rughetti A, Agervig Tarp MP, Coleman J, Bennett EP, Picco G, Sale P, Denda-Nagai K, Irimura T, Mandel U, Clausen H, Frati L, Taylor-Papadimitriou J, Burchell J, Nuti M, Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells, Cancer Res. 67(17) (2007) 8358–8367. [DOI] [PubMed] [Google Scholar]
- [61].Rughetti A, Rahimi H, Belleudi F, Napoletano C, Battisti F, Zizzari IG, Antonilli M, Bellati F, Wandall HH, Benedetti Panici P, Burchell JM, Torrisi MR, Nuti M, Microvesicle cargo of tumor-associated MUC1 to dendritic cells allows cross-presentation and specific carbohydrate processing, Cancer Immunol. Res. 2(2) (2014) 177–186. [DOI] [PubMed] [Google Scholar]
- [62].Zizzari IG, Napoletano C, Battisti F, Rahimi H, Caponnetto S, Pierelli L, Nuti M, Rughetti A, MGL receptor and immunity: when the ligand can make the difference, J. Immunol. Res. 2015 (2015) 450695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].van Vliet SJ, Aarnoudse C, Broks-van den Berg V, Boks M, Geijtenbeek T, van Kooyk Y, MGL-mediated internalization and antigen presentation by dendritic cells: A role for tyrosine-5, Eur. J. Immunol. 37 (2007) 2075–2081. [DOI] [PubMed] [Google Scholar]
- [64].Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y, The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells, J. Immunol. 168(5) (2002) 2118–2126. [DOI] [PubMed] [Google Scholar]
- [65].Ding D, Yao Y, Zhang S, Su C, Zhang Y, C-type lectins facilitate tumor metastasis, Oncol. Lett. 13(1) (2017) 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Unger WW, van Beelen AJ, Bruijns SC, Joshi M, Fehres CM, van Bloois L, Verstege MI, Ambrosini M, Kalay H, Nazmi K, Bolscher JG, Hooijberg E, de Gruijl TD, Storm G, van Kooyk Y, Glycan-modified liposomes boost CD4+ and CD8+ T-cell responses by targeting DC-SIGN on dendritic cells, J. Control Release 160(1) (2012) 88–95. [DOI] [PubMed] [Google Scholar]
- [67].Jiang Y, Zhang C, Chen K, Chen Z, Sun Z, Zhang Z, Ding D, Ren S, Zuo Y, The clinical significance of DC-SIGN and DC-SIGNR, which are novel markets expressed in human colon cancer, PLoS One 9(12) (2014) e114748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, Pontillo M, Mercalli A, Di Carlo V, Allavena P, Piemonti L, Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell, J. Immunol. 172(12) (2004) 7341–7349. [DOI] [PubMed] [Google Scholar]
- [69].Barondes SH, Cooper DN, Gitt MA, Galectins H,. Structure and function of a large family of animal lectins, J. Biol. Chem. 269(33) (1994) 20807–20810. [PubMed] [Google Scholar]
- [70].Johannes L, Jacob R, Leffler H, Galectins at a glance, J. Cell Sci. 131(9) (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [71].Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu F-T, Iacobelli S, Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response?, Trends Immunol. 23(6) (2002) 313–320. [DOI] [PubMed] [Google Scholar]
- [72].Dimitroff CJ, Galectin-binding O-glycosylations as regulators of malignancy, Cancer Res. 75(16) (2015) 3195–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Newlaczyl AU, Yu L-G, Galectin-3 – A jack-of-all-trades in cancer, Cancer Lett. 313(2) (2011) 123–128. [DOI] [PubMed] [Google Scholar]
- [74].Takenaka Y, Fukumori T, Raz A, Galectin-3 and metastasis, Glycoconj. J. 19(7) (2002) 543–549. [DOI] [PubMed] [Google Scholar]
- [75].Liu F-T, Rabinovich GA, Galectins as modulators of tumour progression, Nat. Rev. Cancer 5(1) (2005) 29–41. [DOI] [PubMed] [Google Scholar]
- [76].Ruvolo PP, Galectin 3 as a guardian of the tumor microenvironment, Biochim. Biophys. Acta 1863(3) (2016) 427–437. [DOI] [PubMed] [Google Scholar]
- [77].Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA, Sundblad V, Rabinovich GA, Salatino M, Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease, Cancer Res. 73(3) (2013) 1107–1117. [DOI] [PubMed] [Google Scholar]
- [78].Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM, Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion, J. Biol. Chem. 282(1) (2007) 773–781. [DOI] [PubMed] [Google Scholar]
- [79].Newlaczyl AU, Yu LG, Galectin-3--a jack-of-all-trades in cancer, Cancer Lett. 313(2) (2011) 123–128. [DOI] [PubMed] [Google Scholar]
- [80].Zhao Q, Guo X, Nash GB, Stone PC, Hilkens J, Rhodes JM, Yu LG, Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface, Cancer Res. 69(17) (2009) 6799–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP, The role of thomsen-friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium, Cancer Res. 61 (2001) 4851–4857. [PubMed] [Google Scholar]
- [82].Piyush T, Chacko AR, Sindrewicz P, Hilkens J, Rhodes JM, Yu LG, Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells, Cell Death Differ. 24(11) (2017) 1937–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JonathanM, Yu L-G, Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis, Mol. Cancer 9(154) (2010) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rodriguez MC, Yegorova S, Pitteloud JP, Chavaroche AE, Andre S, Arda A, Minond D, Jimenez-Barbero J, Gabius HJ, Cudic M, Thermodynamic switch in binding of adhesion/growth regulatory human galectin-3 to tumor-associated TF antigen (CD176) and MUC1 glycopeptides, Biochemistry 54(29) (2015) 4462–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yongye AB, Calle L, Arda A, Jimenez-Barbero J, Andre S, Gabius HJ. Martinez-Mayorga K, Cudic M, Molecular recognition of the Thomsen-Friedenreich antigen-threonine conjugate by adhesion/growth regulatory galectin-3: nuclear magnetic resonance studies and molecular dynamics simulations, Biochemistry 51(37) (2012) 7278–7289. [DOI] [PubMed] [Google Scholar]
- [86].Thiemann S, Baum LG, Galectins and immune responses-just how do they do those things they do?, Annu. Rev. Immunol. 34 (2016) 243–264. [DOI] [PubMed] [Google Scholar]
- [87].Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG, Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death, J. Immunol. 176(2) (2006) 778–789. [DOI] [PubMed] [Google Scholar]
- [88].Perillo NL, Pace KE, Seilhamer JJ, Baum LG, Apoptosis of T cells mediated by galectin-1, Nature 378(6558) (1995) 736–739. [DOI] [PubMed] [Google Scholar]
- [89].Fukumori T, Takenaka Y, Yoshii T, Kim H-RC, Hogan V, Inohara H, Kagawa S, Raz A, CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis, Cancer Res. 63 (2003) 8302–8311. [PubMed] [Google Scholar]
- [90].Agrawal B, Gupta N, Konowalchuk JD, MUC1 mucin: A putative regulatory (checkpoint) molecule of T cells, Front Immunol. 9 (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rubinstein N, Alverez M, Zwirner N, Toscano M, IIarregui J, Bravo A, Mordoh J, Fainboim L, Podhajcer O, Rabinovich GA, Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection: A potential mechanism of tumor-immune privilege, Cancer Cell 5 (2004) 241–251. [DOI] [PubMed] [Google Scholar]
- [92].Gordon-Alonso M, Hirsch T, Wildmann C, van der Bruggen P, Galectin-3 captures interferongamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration, Nat. Commun 8(793) (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Varki A, Angata T, Siglecs--the major subfamily of I-type lectins, Glycobiology 16(1) (2006) 1R–27R. [DOI] [PubMed] [Google Scholar]
- [94].Bornhofft KF, Goldammer T, Rebl A, Galuska SP, Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins, Dev. Comp. Immunol. 86 (2018) 219–231. [DOI] [PubMed] [Google Scholar]
- [95].Lubbers J, Rodriguez E, van Kooyk Y, Modulation of immune tolerance via siglec-sialic acid interactions, Front Immunol. 9(2807) (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll T, Crocker PR, Taylor-Papadimitriou J, Burchell JM, The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9, Nat. Immunol. 17(11) (2016) 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Demoulins T, Schneider C, Wehrli M, Hunger RE, Baerlocher GM, Simon HU, Romero P, Munz C, von Gunten S, Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance, J. Clin. Invest. 124(4) (2014) 1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, Paulson JC, Nycholat CM, Schnaar RL, Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties, Glycobiology 27(7) (2017) 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Monzavi-Karbassi B, Pashov A, Kieber-Emmons T, Tumor-associated glycans and immune surveillance, Vaccines (Basel) 1(2) (2013) 174–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Smorodin EP, Kurtenkov OA, Sergeyev BL, Lilleorg AL, Chuzmarov VI, Antibodies to tumor-associatedcarbohydrate epitopes in sera of cancer patients and blood donors, Exp. Oncol 23 (2001) 109–113. [Google Scholar]
- [101].Diaz-Zaragoza M, Hernandez-Avila R, Viedma-Rodriguez Rubi, Arenas-Aranda D, Osta-Saloma P, Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer, Oncol. Rep. 34 (2015) 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y, Circuiting anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer, Int. J. Cancer 03(1) (2003) 97–100. [DOI] [PubMed] [Google Scholar]
- [103].Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA, Taylor-Papadimitriou J, Burchell J, Clausen H, Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes, Cancer Res. 70(4) (2010) 1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fletcher R, Wang YJ, Schoen RE, Finn OJ, Yu J, Zhang L, Colorectal cancer prevention: Immune modulation taking the stage, Biochim. Biophys. Acta Rev. Cancer 1869(2) (2018) 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Finn OJ, A believer’s overview of cancer immunosurveillance and immunotherapy, J. Immunol. 200(2) (2018) 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fremd C, Stefanovic S, Beckhove P, Pritsch M, Lim H, Wallwiener M, Heil J, Golatta M, Rom J, Sohn C, Schneeweiss A, Schuetz F, Domschke C, Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients, Oncoimmunology 5(1) (2016) e1057387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Finn OJ, The dawn of vaccines for cancer prevention, Nat. Rev. Immunol. 18(3) (2018) 183–194. [DOI] [PubMed] [Google Scholar]
- [108].Finn OJ, Cancer vaccines: Between the idea and the reality, Nat. Rev. Immunol. 3 (2003) 630–641. [DOI] [PubMed] [Google Scholar]
- [109].Lollini PL, Cavallo F, Nanni P, Quaglino E, The promise of preventive cancer vaccines, Vaccines (Basel) 3(2) (2015) 467–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Dunn G, Bruce A, Ikeda H, Old L, Schreiber R, Cancer immunoediting: from immunosurveillance to tumor escape, Nat. Immunol. 3 (2002) 991–998. [DOI] [PubMed] [Google Scholar]
- [111].Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD, Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens, Nature 515(7528) (2014) 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mehla K, Tremayne J, Grunkemeyer JA, O’Connell KA, Steele MM, Caffrey TC, Zhu X, Yu F, Singh PK, Schultes BC, Madiyalakan R, Nicodemus CF, Hollingsworth MA, Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors, Cancer Immunol. Immun. 67(3) (2018) 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Barnd D, Lan M, Metzgar R, Finn O, Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells, Proc. Natl. Acad. Sci. U S A 86 (1989) 7159–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Moyer TJ, Zmolek AC, Irvine DJ, Beyond antigens and adjuvants: formulating future vaccines, J. Clin. Invest. 126(3) (2016) 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten A, Snijdewint F, van Kamp G, Schol D, Reis C, Price M, Livingston P, Hilgers J, Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and N-acetylgalactosamine (GalNAc) peptides, Int. J. Cancer 86 (2000) 702–712. [DOI] [PubMed] [Google Scholar]
- [116].Soares MM, Mehta V, Finn OJ, Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection, J. Immunol. 166(11) (2001) 6555–6563. [DOI] [PubMed] [Google Scholar]
- [117].Acres B, Apostolopoulos V, Balloul J, Wreschner D, Xing P, Ali-Hadji D, Bizouarne N, Kieny M, McKenzie I, MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines, Cancer Immunol. Immun. 48 (2000) 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ryan SO, Turner MS, Gariepy J, Finn OJ, Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses, Cancer Res. 70(14) (2010) 5788–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Straßburger D, Glaffig M, Stergiou N, Bialas S, Besenius P, Schmitt E, Kunz H, Synthetic MUC1 antitumor vaccine with incorporated 2,3-sialyl-T carbohydrate antigen inducing strong immune responses with isotype specificity, ChemBioChem 19 (2018) 1142–1146. [DOI] [PubMed] [Google Scholar]
- [120].Hossain MK, Wall KA, Immunological evaluation of recent MC1 glycopeptide cancer vaccines, Vaccines (Basel) 4(3) (2016) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Palitzsch B, Gaidzik N, Stergiou N, Stahn S, Hartmann S, Gerlitzki B, Teusch N, Flemming P, Schmitt E, Kunz H, A synthetic glycopeptde vaccine for the induction of a monoclonal antibody that differentiates between normal and tumor mammary cells and enables the diagnosis of human pancreatic cancer, Angew Chem. Int. Ed. Engl. 55(8) (2016) 2894–2898. [DOI] [PubMed] [Google Scholar]
- [122].Gaidzik N, Westerlind U, Kunz H, The development of synthetic antitumour vaccines from mucin glycopeptide antigens, Chem. Soc. Rev. 42(10) (2013) 4421–4442. [DOI] [PubMed] [Google Scholar]
- [123].Movahedin M, Brooks TM, Supekar NT, Gokanapudi N, Boons GJ, Brooks CL, Glycosylation of MUC1 influences the birEng of a therapeutic antibody by altering the conformational equilibrium of the antigen, Glycobiology 27(7) (2017) 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Rangappa S, Artigas G, Miyoshi R, Yokoi Y, Hayakawa S, Garcia-Martin F, Hinou H, Nishimura S-I, Effects of the multiple O-glycosylation states on antibody recognition of the immunodominant motif in MUC1 extracellular tandem repeats, MedChemComm 7(6) (2016) 1102–1122. [Google Scholar]
- [125].Zhou D, Xu L, Huang W, Tonn T, Epitopes of MUC1 tandem repeats in cancer as revealed by acrystallography: toward glycopeptide signature-guided therapy, Molecules 23(6) (2018) 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Martinez-Saez N, Peregrina JM, Corzana F, Principles of mucin structure: implications for the rational design of cancer vaccines derived from MUC1-glycopeptides, Chem. Soc. Rev. 46(23) (2017) 7154–7175. [DOI] [PubMed] [Google Scholar]
- [127].Coltart DM, Royyuru AK, Williams LJ, Glunz PW, Sames D, Kuduk SD, Schwarz JB, Chen X-T, Danishefsky SJ, D.H. Live, Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains, J. Am. Chem. Soc. 124 (2002) 9833–9844. [DOI] [PubMed] [Google Scholar]
- [128].Nativi C, Renaudet O, Recent progress in antitumoral synthetic vaccines, ACS Med. Chem. Lett. 5(11) (2014) 1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].McDonald DM, Byrne SN, Payne RJ, Synthetic self-adjuvanting glycopeptide cancer vaccines, Front Chem. 3(60) (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lohmueller JJ, Sato S, Popova L, Chu IM, Tucker MA, Barberena R, Innocenti GM, Cudic M, Ham JD, Cheung WC, Polakiewicz RD, Finn OJ, Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and immunotherapeutic potential, Sci. Rep. 6(31740) (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cai H, Huang Z-H, Shi L, Zou P, Zhao Y-F, Kunz H, Li Y-M, Synthesis of Tn/T antigen MUC1 glycopeptide BSA conjugates and their evaluation as vaccines, Eur. J. Organic Chemistry 2011 (20–21) (2011) 3685–3689. [Google Scholar]
- [132].Martinez-Saez N, Castro-Lopez J, Valero-Gonzalez J, Madariaga D, Companon I, Somovilla VJ, Salvado M, Asensio JL, Jimenez-Barbero J, Avenoza A, Busto JH, Bernardes GJ, Peregrina JM, Hurtado-Guerrero R, Corzana F, Deciphering the non-equivalence of serine and threonine O-glycosylation points: implications for molecular recognition of the Tn antigen by an anti-MUC1 antibody, Angew Chem. Int. Ed. Engl. 54(34) (2015) 9830–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Cai H, Huang ZH, Shi L, Sun ZY, Zhao YF, Kunz H, Li YM, Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response, Angew Chem. Int. Ed. Engl. 51(7) (2012) 1719–1723. [DOI] [PubMed] [Google Scholar]
- [134].Westerlind U, Schroder H, Hobel A, Gaidzik N, Kaiser A, Niemeyer CM, Schmitt E, Waldmann H, Kunz H, Tumor-associated MUC1 tandem-repeat glycopeptide microarrays to evaluate serum- and monoclonal-antibody specificity, Angew Chem. Int. Ed. Engl. 48(44) (2009) 8263–8267. [DOI] [PubMed] [Google Scholar]
- [135].Dziadek S, Griesinger C, Kunz H, Reinscheid UM, Synthesis and structural model of an alpha(2,6)-sialyl-t glycosylated MUC1 eicosapeptide under physiological conditions, Chemistry 12(19) (2006) 4981–4993. [DOI] [PubMed] [Google Scholar]
- [136].Corzana F, Jimenez-Barbaro G, Luis M.G.d., Asensio J, Jimenez-Barbero J, Peregrina J, Avenoza A, Serine versus threonine glycosylation: The methyl group causes a drastic alteration on the carbohydrate orientation and on the surrounding water shell, J. Am. Chem. Soc. 129 (2007) 9458–9467. [DOI] [PubMed] [Google Scholar]
- [137].Gaidzik N, Kaiser A, Kowalczyk D, Westerlind U, Gerlitzki B, Sinn HP, Schmitt E, Kunz H, Synthetic antitumor vaccines containing MUC1 glycopeptides with two immunodominant domains-induction of a strong immune response agains breast tumor tissues, Angew Chem. Int. Ed. Engl. 50(42) (2011) 9977–9981. [DOI] [PubMed] [Google Scholar]
- [138].Stergiou N, Glaffig M, Jonuleit H, Schmitt E, Kunz H, Immunization with a synthetic human MUC1 glycopeptide vaccine against tumor-associated MUC1 breaks tolerance in human MUC1 transgenic mice, ChemMedChem 12(17) (2017) 1424–1428. [DOI] [PubMed] [Google Scholar]
- [139].Hoffmann-Roder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, Gerlitzki B, Schmitt E, Kunz H, Synthetic antitumoi vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue, Angew Chem. Int. Ed. Engl. 49(45) (2010) 8498–8503. [DOI] [PubMed] [Google Scholar]
- [140].Naito S, Takahashi T, Onoda J, Uemura S, Ohyabu N, Takemoto H, Yamane S, Fujii I, Nishimura SI, Numata Y, Generation of Novel Anti-MUC1 Monoclonal Antibodies with Designed Carbohydrate Specificities Using MUC1 Glycopeptide Library, ACS Omega 2(11) (2017) 7493–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Li M, Yu F, Yao C, Wang PG, Liu Y, Zhao W, Synthetic and immunological studies on trimeric MUC1 immunodominant motif antigen-based anti-cancer vaccine candidates, Org. Biomol. Chem. 16(6) (2010. 993–999. [DOI] [PubMed] [Google Scholar]
- [142].Glaffig M, Stergiou N, Hartmann S, Schmitt E, Kunz H, A synthetic MUC1 anticancer vaccine containing mannose ligands for targeting macrophages and dendritic cells, ChemMedChem 13(1) (2018) 25–29. [DOI] [PubMed] [Google Scholar]
- [143].Wu X, Yin Z, McKay C, Pett C, Yu J, Schorlemer M, Gohl T, Sungsuwan S, Ramadan S, Baniel C, Allmon A, Das R, Westerlind U, Finn MG, Huang X, Protective epitope discovery and design of MUC1-based vaccine for effective tumor protections in immunotolerant mice, J. Am. Chem. Soc. 140(48) (2018) 16596–16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wu X, McKay C, Pett C, Yu J, Schorlemer M, Ramadan S, Lang S, Behren S, Westerlind U, Finn MG, Huang X, Synthesis and immunological evaluation of disaccharide bearing MUC-1 glycopeptide conjugates with virus-like particles, ACS Chem. Biol. 14 (2019) 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Dowling JK, Mansell A, Toll-like receptors: the swiss army knife of immunity and vaccine development, Clin. Transl. Immunology 5(5) (2016) e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Whiteside TL, The tumor microenvironment and its role in promoting tumor growth, Oncogene 27(45) (2008) 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ, Robust immune responses elicited by a fully synthetic three-component vaccine, Nat. Chem. Biol. 3(10) (2007) 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, Boons GJ, Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine, Proc. Natl. Acad. Sci. U S A 109(1) (2012) 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Buskas T, Ingale S, Boons GJ, Towards a fully synthetic carbohydrate-based anticancer vaccine: synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated tn antigen, Angew Chem. Int. Ed. Engl. 44(37) (2005) 5985–5988. [DOI] [PubMed] [Google Scholar]
- [150].Martinez-Saez N, Supekar NT, Wolfert MA, Bermejo IA, Hurtado-Guerrero R, Asensio JL, Jimenez-Barbero J, Busto JH, Avenoza A, Boons GJ, Peregrina JM, Corzana F, Mucin architecture behind the immune response: design, evaluation and conformational analysis of an antitumor vaccine derived from an unnatural MUC1 fragment, Chem. Sci 7(3) (2016) 2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Abdel-Aal AB, Lakshminarayanan V, Thompson P, Supekar N, Bradley JM, Wolfert MA, Cohen PA, Gendler SJ, Boons GJ, Immune and anticancer responses elicited by fully synthetic aberrantly glycosylated MUC1 tripartite vaccines modified by a TLR2 or TLR9 agonist, ChemBiochem 15(10) (2014) 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Thompson P, Lakshminarayanan V, Supekar NT, Bradley JM, Cohen PA, Wolfert MA, Gendler SJ, Boons GJ, Linear synthesis and immunological properties of a fully synthetic vaccine candidate containing a sialylated MUC1 glycopeptide, Chem. Commun. (Camb) 51(50) (2015) 10214–10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Supekar N, Lakshminarayanan V, Capicciotti C, Sirohiwal A, Madsen C, Wolfert M, Cohen P, Gendler S, Boons G, Synthesis and immunological evaluation of a multicomponent cancer vaccine candidate containing a long MUC1 glycopeptide, ChemBioChem 19(2) (2018) 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].McDonald DM, Wilkinson BL, Corcilius L, Thaysen-Andersen M, Byrne SN, Payne RJ, Synthesis and immunological evaluation of self-adjuvanting MUC1-macrophage activating lipopeptide 2 conjugate vaccine candidates, Chem. Commun. (Camb) 50(71) (2014) 10273–10276. [DOI] [PubMed] [Google Scholar]
- [155].Wilkinson BL, Day S, Chapman R, Perrier S, Apostolopoulos V, Payne RJ, Synthesis and immunological evaluation of self-assembling and self-adjuvanting tricomponent glycopeptide cancer vaccine candidates, Chemistry 18(51) (2012) 16540–16548. [DOI] [PubMed] [Google Scholar]
- [156].Wilkinson BL, Day S, Malins LR, Apostolopoulos V, Payne RJ, Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer, Angew Chem. Int. Ed. Engl. 50(7) (2011) 1635–1639. [DOI] [PubMed] [Google Scholar]
- [157].McDonald DM, Hanna CC, Ashhurst AS, Corcilius L, Byrne SN, Payne RJ, Synthesis of a self-adjuvanting MUC1 vaccine via diselenide-selenoester ligation-deselenization, ACS Chem. Biol. 13(12) (2018) 3279–3285. [DOI] [PubMed] [Google Scholar]
- [158].Li M, Wang Z, Yan B, Yin X, Zhao Y, Yu F, Meng M, Liu Y, Zhao W, Design of a MUC1-based tricomponent vaccine adjuvanted with FSL-1 for cancer immunotherapy, MedChemComm (2019) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Frey S, Castro A, Arsiwala A, Kane RS, Bionanotechnology for vaccine design, Curr. Opin. Biotechnol 52 (2018) 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Habibi N, Kamaly N, Memic A, Shafiee H, Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery, Nano Today 11(1) (2016) 41–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wang Q, Jiang N, Fu B, Huang F, Liu J, Self-assembling peptide-based nanodrug delivery systems, Biomater. Sci (2019) 1–24. [DOI] [PubMed] [Google Scholar]
- [162].Rudra JS, Tian YF, Jung JP, Collier JH, A self-assembling peptide acting as an immune adjuvant, Proc. Natl. Acad. Sci. U S A 107(2) (2010) 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Huang ZH, Shi L, Ma JW, Sun ZY, Cai H, Chen YX, Zhao YF, Li YM, A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy, J. Am. Chem. Soc. 134(21) (2012) 8730–8733. [DOI] [PubMed] [Google Scholar]
- [164].Liu Y, Wang Y, Yu F, Zhang Z, Yang Z, Zhang W, Wang PG, Zhao W, Potentiating the immune response of MUC1 -based antitumor vaccines using a peptide-based nanovector as a promising vaccine adjuvant, Chem. Commun. (Camb) 53(68) (2017) 9486–9489. [DOI] [PubMed] [Google Scholar]
- [165].Glaffig M, Palitzsch B, Stergiou N, Schull C, Strassburger D, Schmitt E, Frey H, Kunz H, Enhanced immunogenicity of multivalent MUC1 glycopeptide antitumour vaccines based on hyperbranched polymers, Org. Biomol. Chem. 13(40) (2015) 10150–10154. [DOI] [PubMed] [Google Scholar]
- [166].Liu YF, Sun ZY, Chen PG, Huang ZH, Gao Y, Shi L, Zhao YF, Chen YX, Li YM, Glycopeptide nanoconjugates based on multilayer self-assembly as an antitumor vaccine, Bioconjug. Chem 26(8) (2015) 1439–1442. [DOI] [PubMed] [Google Scholar]
- [167].Hartmann S, Nuhn L, Palitzsch B, Glaffig M, Stergiou N, Gerlitzki B, Schmitt E, Kunz H, Zentel R, CpG-loaded multifunctional cationic nanohydrogel particles as self-adjuvanting glycopeptide antitumor vaccines, Adv. Healthcare Mater. 4(4) (2015) 522–527. [DOI] [PubMed] [Google Scholar]
- [168].Cai H, Degliangeli F, Palitzsch B, Gerlitzki B, Kunz H, Schmitt E, Fiammengo R, Westerlind U, Glycopeptide-functionalized gold nanoparticles for antibody induction against the tumor associated mucin-1 glycoprotein, Bioorg. Med. Chem. 24(5) (2016) 1132–1135. [DOI] [PubMed] [Google Scholar]
- [169].Companon I, Guerreiro A, Mangini V, Castro-Lopez J, Escudero-Casao M, Avenoza A, Busto JH, Castillon S, Jimenez-Barbero J, Asensio JL, Jimenez-Oses G, Boutureira O, Peregrina JM, Hurtado-Guerrero R, Fiammengo R, Bernardes GJL, Corzana F, Structure-based design of potent tumor-associated antigens: modulation of peptide presentation by single-atom O/S or O/Se substitutions at the glycosidic linkage, J. Am. Chem. Soc. 141(9) (2019) 4063–4072. [DOI] [PubMed] [Google Scholar]
- [170].Karmakar P, Lee K, Sarkar S, Wall KA, Sucheck SJ, Synthesis of a liposomal MUC1 glycopeptide-bbased immunotherapeutic and evaluation of the effect of l-rhamnose targeting on cellular immune responses, Bioconjug. Chem 27(1) (2016) 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hossain MK, Vartak A, Karmakar P, Sucheck SJ, Wall KA, Augmenting vaccine immunogenicity through the use of neural human anti-rhamnose antibodies, ACS Chem. Biol. 13(8) (2018) 2130–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Du JJ, Zou SY, Chen XZ, Xu WB, Wang CW, Zhang L, Tang YK, Zhou SH, Wang J, Yin XG, Gao XF, Liu Z, Guo J, Liposomal antitumor vaccines targeting mucin 1 elicit a lipid-dependent immunodominant response, Chem. Asian J 14(12) (2019) 2116–2121. [DOI] [PubMed] [Google Scholar]