Abstract
Depression has been associated with high blood pressure (BP). However, the mechanisms of the relation between depression and high BP are unclear. We therefore examined whether impaired cardiac vagal control, indexed as low levels of resting respiratory sinus arrhythmia (RSA), serves as a route from depression to high BP. The sample included 125 subjects with histories of depression (probands), 123 never-depressed siblings of probands (high-risk siblings), and 156 controls. Resting RSA was assessed at Time 1 (T1) along with BP when subjects were adolescents (Mage = 16.3 years); systolic and diastolic BP (SBP and DBP) were measured again at Time 2 (T2) when subjects were young adults (Mage = 22.3 years). Linear mixed-effects models were used to examine group differences in resting RSA and T2 BP outcomes and to test for RSA mediation of the relation between depression (history or being at high-risk) and BP. Resting RSA was lower among probands than controls but was similar among high-risk siblings and controls, while the subject groups did not differ in T2 SBP or DBP. Controlling for T1 BP, depression history indirectly affected T2 DBP (but not SBP) through resting RSA. The findings suggest that, although the direct detrimental effects of depression on BP are not yet evident in young adulthood, among those with depression histories, impaired cardiac vagal control appears to serve as a mechanism of elevated DBP.
Keywords: depression, blood pressure, cardiac vagal control, respiratory sinus arrhythmia
1. Introduction
Depression has been associated with multiple adverse health outcomes, including hypertension (Meng, Chen, Yang, Zheng, & Hui, 2012). Specifically, hypertension, or high blood pressure (BP), is more prevalent and is detectable at a younger age among adults with lifetime depression than the general population (Goldstein, Fagiolini, Houck, & Kupfer, 2009). In longitudinal studies using community-based samples, depression at baseline and depression history predicted high incidence of hypertension in multi-year follow-ups (Meyer, Armenian, Eaton, & Ford, 2004; Patten et al., 2009). It also has been reported that never-depressed young adults, aged 18–19 years, with family histories of depression had higher BP compared to controls (Mannie et al., 2013), suggesting that hypertension may precede the onset of depression in high-risk populations. However, inverse relations between depression symptoms and BP also have been reported (Bhat, Beilin, Robinson, Burrows, & Mori, 2017; Hildrum, Mykletun, Holmen, & Dahl, 2008). Uncontrolled use of antidepressants may have been one source of the inconsistent findings regarding depression and BP (e.g., Licht et al., 2009).
The relation between depression and hypertension is believed to be mediated by various processes (Brown, Varghese, & McEwen, 2004; Licht et al., 2009; Penninx, 2017; Son, Markovitz, Winders, & Smith, 1997), among which physiological mechanisms may have the most direct courses of action (Goldstein et al., 2015). Impaired cardiac vagal control is one mechanism that has been implicated both in depression and hypertension. Cardiac vagal control refers to the effects of the vagal nerve on the sinus node of the heart, with increasing vagal input being associated with slowing of the heart rate. In turn, cardiac vagal input is modulated by respiratory activity, wherein inhalation blocks vagal input while exhalation releases it (Klabunde, 2011). Thus, at a resting state, heart rate varies as a function of the respiratory cycle, a phenomenon known as respiratory sinus arrhythmia (RSA; Berntson, Cacioppo, Quigley, 1993; Porges, 2007). RSA can be estimated by heart rate variability (HRV) at the frequency of normal average respiration (Berntson et al., 1997). Higher levels of resting RSA indicate greater cardiac vagal control (Berntson et al., 1993; Porges, 2007). High resting RSA is believed to reflect the ability to flexibly adapt to environmental demands, whereas low resting RSA is viewed as an indicator of physiological and behavioral inflexibility (Porges, 2007; Thayer & Lane, 2000).
Indeed, depressed adolescents and adults evidence low resting RSA (for reviews see, Kemp, Quintana, Quinn, Hopkinson, & Harris, 2014; Koenig, Kemp, Beauchaine, Thayer, & Kaess, 2016; Rottenberg, 2007). Moreover, low RSA has been prospectively associated with high levels of depressive symptoms (Huang et al., 2018; Jandackova, Britton, Malik, & Steptoe, 2016). However, the literature is equivocal regarding resting RSA and past depression. For example, Bylsma et al. (2014) reported that adults with remitted depression and controls did not differ in resting RSA. In contrast, Chang et al. (2013) found that resting RSA was lower among remitted adults with severe lifetime depression symptoms than among controls. Compared to controls, another sample of adults with remitted depression had lower levels of a time-domain HRV measure that is highly correlated with RSA (Bassett et al., 2016).
Low resting RSA may be associated with high BP through two physiological processes. First, because resting RSA is linked to various brain regions implicated in BP control (for reviews, see Gianaros & Jennings, 2018; Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012), low RSA levels may indicate deficits in cortical and subcortical BP regulation. Second, acetylcholine, the neurotransmitter released by the vagal nerve fibers, has a vasodilatory function (Klabunde, 2011). Thus, low RSA levels may signal blood vessel constriction and thereby high BP (Clement, Jordaens, & Heyndrickx, 1984).
The association between low RSA and high BP has also been proposed using epidemiological data. For example, resting RSA was found to be lower among individuals with hypertension than normotensives (Singh et al., 1998), and levels of resting RSA were negatively related to prospective increases in BP among individuals with hypertension (Liao et al., 1996) and risk for hypertension among those with normal BP (Schroeder et al., 2003). Therefore, Thayer et al. (2010) had concluded that low levels of resting RSA may be precursors to hypertension. However, the value of resting RSA in predicting high BP has not been explored among individuals with histories of depression.
In research on the health outcomes of depression, one question is whether the findings reflect the consequences of depression or risk factors that exist prior to the onset of the mood disorder. One way to approach that question is to study never-depressed siblings of individuals who have had depression. Such never-depressed siblings are at high risk for eventual depression (Farmer et al., 2000; Ryan et al., 1992) and share genetic and environmental factors with their depressed counterparts. Studying such siblings reflects the assumption that being at high depression risk and having actually experienced depression may elevate BP thought the same RSA mechanisms. This assumption is indirectly supported by findings that young children at high risk for depression have an atypical developmental trajectory of resting RSA (Gentzler, Rottenberg, Kovacs, George, & Morey, 2012).
In light of the literature, our main goal was to examine whether resting RSA is a mechanism of hypertension among young adults with histories of depression and their never-depressed siblings (who are at high risk for depression). RSA was assessed when subjects were adolescents (Time 1); systolic and diastolic BP (SBP and DBP) outcomes were measured when subjects were young adults approximately six years later (Time 2). We hypothesized that (a) at Time 1 (T1), adolescents with histories of depression and their never-depressed high-risk siblings will have lower levels of resting RSA than controls; (b) at Time 2 (T2), subjects with depression history and their siblings will have higher SBP and DBP, compared to controls; and (c) T1 resting RSA will mediate the effects of depression history (and being at high-risk for depression) on T2 SBP and DBP.
2. Method
2.1. Subjects
Our study included 125 young adults with histories of childhood-onset depression (probands), 123 full biological siblings of probands with no lifetime depression (high-risk), and 156 healthy controls. The subjects were a subset of a larger sample, which was recruited between 1997–2006 for a longitudinal study from 23 child mental health facilities in Hungary (Kiss et al., 2007; Tamás et al., 2007). The original study entry criteria included being 7–14 years of age, having a Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) (American Psychiatric Association, 2000) defined depressive disorder, being free of intellectual disability and major medical disorders, and having at least one biological parent and a 7–17.9 year-old full biological sibling willing to participate (Kiss et al., 2007; Tamás et al., 2007). After the original investigation, which focused on genetics, subjects participated in two further studies (T1 and T2), which included the data used in the present report.
The follow-up interval between T1 and T2 ranged from 3.52 to 8.16 years, which was slightly longer, on average, among probands than among siblings and controls (see Table 1). Age of all subjects ranged from 11.20 to 19.08 years at T1, and from 18.00 to 26.66 years at T2. Probands were generally older than siblings and controls (see Table 1).
Table 1.
Characteristics of the Samples
| Variable | Probands (n = 125) |
Siblings (n = 123) |
Controls (n = 156) |
Unadjusted F or χ2 statistics |
|---|---|---|---|---|
| Female (n, %) | 44 (35.2%)a | 69 (56.1%)b | 57 (36.5%)a | 14.31** |
| Age (M years, SD) | ||||
| T1 | 16.84 (1.46)a | 15.96 (1.97)b | 16.12 (2.06)b | 7.97*** |
| T2 | 23.32 (1.67)a | 22.19 (2.08)b | 21.67 (1.59)c | 30.27*** |
| F.U. Interval (M years, SD) | 6.48 (0.72)a | 6.24 (0.65)b | 5.56 (0.74)c | 65.39*** |
| BMI (M kg/m2, SD) | ||||
| T1 | 21.75 (4.17) | 21.29 (4.34) | 20.75 (3.43) | 2.20 |
| T2 | 24.72 (5.80)a | 24.11 (5.53)a,b | 23.22 (3.75)b | 3.18* |
| Regular smoker (n, %) | ||||
| T1 | 39 (31.2%)a | 16 (13.0%)b | 4 (2.6%)c | 45.99*** |
| T2 | 67 (53.6%)a | 54 (43.9%)b | 37 (23.7%)c | 27.73*** |
| Currently depressed (n, %) | ||||
| T1 | 17 (13.6%)a | 0 (0%)b | 0 (0%)b | 39.61*** |
| T2 | 10 (8.0%)a | 0 (0%)b | 0 (0%)b | 22.89*** |
| RF at T1 (M Hz, SD) | 0.24 (0.06) | 0.25 (0.06) | 0.24 (0.06) | 1.83 |
| RSA at T1 (M ln ms2, SD) | 6.52 (1.10) | 6.64 (1.07) | 6.71 (1.00) | 1.16 |
| BP at T1 (M mmHg, SD) via continuous finger cuff monitoring | ||||
| Systolic | 104.1 (14.1) | 104.2 (13.5) | 106.0 (14.6) | 0.80 |
| Diastolic | 65.1 (7.87) | 66.3 (7.25) | 65.1 (7.10) | 1.03 |
| BP at T2 (M mmHg, SD) via brachial cuff monitoring | ||||
| Systolic | 113.1 (12.0) | 111.4 (11.1) | 111.5 (12.2) | 0.79 |
| Diastolic | 72.5 (7.44)a | 72.6 (8.17)a | 70.2 (7.74)b | 4.60* |
T1 = Time 1; T2 = Time 2; F.U. = Follow-up; BMI = Body mass index; RSA = Respiratory sinus arrythmia; RF = Respiratory frequency; BP = Blood pressure. F.U. Interval is the duration in years between T1 and T2; Currently depressed = in an episode of major depressive disorder or dysthymia.
p < .05;
p < .01;
p < .001;
superscripts with different letters denote significant pair-wise contrasts at p < .05.
Probands had the onset of their first major depressive disorder (MDD) episode at 8.97 years of age (SD = 2.08 years), on average. At T1, 61.6% of the probands had had one MDD episode, 34.4% had two episodes, and 4.0% had three or more episodes; 13.6% were in a current depressive episode (MDD and/or dysthymia). By T2, 36.8% had two MDD episodes, and 17.6% had three or more episodes; 8.0% were in a current depressive episode.
2.2. Diagnostic and Physiological Assessments
The recruitment of subjects has been previously reported in detail (Kiss et al., 2007; Tamás et al., 2007). Psychiatric caseness was initially established based on self‐ and parent‐reported information during the semi‐structured Interview Schedule for Children and Adolescents–Diagnostic version (ISCA‐D). The ISCA‐D is an extension of the ISCA (Sherrill & Kovacs, 2000) and has shown acceptable inter‐rater reliability (Kiss et al., 2007). An age appropriate modification, the semi-structured Interview Schedule for Young Adults: Follow-Up Diagnostic version (ISYA-D), was used at T2. Final DSM-IV diagnoses were derived using a consensus best-estimate diagnostic procedure (Maziade et al., 1992).
Subjects were asked not to take allergy, cold, or asthma medications on the day of the assessment and to abstain from caffeine, alcohol, and tobacco for at least one hour prior to participation at both times. At T1, subjects were comfortably seated in the laboratory while physiological recording sensors were attached after consent was obtained. To estimate resting RSA, subjects were instructed to sit still and watch a 180‐s neutral film that depicted aquatic scenes (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992). Electrocardiography (ECG) was recorded with disposable, pregelled stress‐testing Ag/AgCl spot electrodes (ConMed Andover Medical, Haverhill, MA) using a modified Lead II configuration; respiration was assessed by a respiration transducer placed around the subject’s torso at the abdominal level. Physiological signals were sampled online at 1,000 Hz using a MindWare BioNex system and BioLab software (MindWare Technologies Ltd., Gahanna, OH). In addition, BP was monitored continuously from the index and the middle finger of the nondominated hand of the subject by a finger cuff. T1 BP values were only used as covariates in analyses because this continuous measurement is not the standard BP evaluation method (Muntner et al., 2019).
At T2, subjects were seated on a chair with their arms resting at the level of the heart for five minutes after the consent procedure. Three sitting, brachial BP measurements were then taken from the right arm of the subject at five-minute intervals, using an Omron M6 digital BP monitor (Omron Corp., Kyoto, Japan).
Both study protocols were approved by the Institutional Review Board of the University of Pittsburgh and the review boards of the relevant entities in Hungary.
2.3. Data Reduction
ECG data were inspected manually by trained research assistants. When a missing or ectopic heartbeat occurred between two normal beats, the midpoint was interpolated to correct the artifact. Artifacts were considered as uncorrectable when two or more ectopic beats occurred successively. The exclusion criterion for ECG data processing was that 5% or more of a subject’s signals were influenced by correctable or uncorrectable artifacts during the resting baseline (Berntson et al., 1997). However, no subject met the criterion. RSA analyses were conducted using MindWare 3.0.21 software. The ECG data were tapered using the Hamming window, and were filtered by the 60 Hz notch filter. For each subject, a time series of interbeat intervals (IBIs) was derived from ECG data of the entire 180-s resting period. RSA was defined as the natural log‐transformed values of high‐frequency (0.12–0.40 Hz) HRV spectral power, which was calculated from the IBI time series by a fast Fourier transform. The frequency band for RSA analyses was selected based on previous studies (Berntson et al, 1997; Fleming et al., 2011), and had been confirmed by the respiratory data of the present sample (see Table 1). T2 SBP and DBP were calculated as means of the three measurements.
2.4. Analytic Approach
Statistical analyses were conducted using SAS 9.4 software. Relations between variables were assessed by one-way analyses of variance (ANOVA), χ2 tests, or Pearson correlations as appropriate.
To investigate whether depression history and being at high-risk for depression lead to elevated BP, the effects of group on resting RSA and T2 BP outcomes were estimated in regression equations. Groups were coded as two dummy variables with controls as the reference. Below are the regression equations (terms for intercepts, covariates, and random effects are not shown for simplicity):
| (1) |
| (2) |
| (3) |
In the equations, Y represents T2 BP (the dependent variable; SBP and DBP were examined in separate models); Xprob and Xsib represent the dummy variables of group membership (the independent variables); M represents resting RSA (the mediator). Equation 1 represents total effects (c) of each group on T2 BP; Equation 2 represents the direct effects (c’) of group on T2 BP, after accounting for the effect (b) of RSA; and Equation 3 represents the effects (a) of each group on RSA. Linear mixed-effects models with random intercepts for each family were used to account for dependent observations. Given the goals of our study, we controlled in the mixed models for variables known to influence RSA and T2 BP measurements, including sex, age, body mass index (BMI), T1 BP, time interval between the assessments, research site, smoking, and psychotropic and BP medications. We also controlled for current depression status at both times. Additionally, we conducted contrasts to examine whether resting RSA and T2 BP outcomes differed between probands and siblings. Effect sizes of independent variables were estimated by partial R2.
Mediation analysis of indirect effects (Hayes & Preacher, 2014; Preacher & Selig, 2012) was used to examine whether resting RSA is a mediator through which depression influences T2 BP. The indirect effects were calculated from the parameters in Equation 2 and 3 as aprob* b and asib* b. Monte Carlo simulation was then used to determine 95% confidence interval of each indirect effect (Tofighi, MacKinnon, & Yoon, 2009). This approach provides a natural effect size expressed in units of T2 BP values explained by group, through T1 resting RSA.
3. Results
3.1. Descriptive Characteristics
As shown in Table 1, there were more females among siblings than among probands and controls. There were no group differences in T1 BMI, but probands had higher T2 BMI than controls. Moreover, probands and siblings were more likely to be smokers than controls at both times (see Table 1). At T1, no subject was on BP medication; two probands and three siblings were on psychotropic medication, but there were no group differences (χ2 = 3.57, p = .17). At T2, two subjects in each group were on BP medication, and there were no group differences (χ2 = 0.07, p = .97); four probands and two siblings were on psychotropic medication, but there were no group differences (χ2 = 4.88, p = .09). As shown in Table 2, T2 BP were positively correlated with age and BMI.
Table 2.
Pearson Correlation Coefficients Among Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age at T1 | - | ||||||||
| 2. Age at T2 | .91** | - | |||||||
| 3. F.U. Interval | −.21** | .22** | - | ||||||
| 4. BMI at T1 | .20** | .18** | −.03 | - | |||||
| 5. BMI at T2 | .04 | .09 | .11* | .74** | - | ||||
| 6. RSA at T1 | −.04 | −.07 | −.07 | .08 | .09 | - | |||
| 7. SBP at T1 | .04 | .04 | −.01 | .35** | .30** | .03 | - | ||
| 8. DBP at T1 | −.02 | .03 | .11* | .23** | .20** | −.05 | .63** | - | |
| 9. SBP at T2 | .09 | .11* | .04 | .32** | .42** | .02* | .34** | .22** | - |
| 10. DBP at T2 | .07 | .13* | .12* | .22** | .31** | −.10* | .33** | .38** | .65** |
T1 = Time 1; T2 = Time 2; F.U. = Follow-up; BMI = Body mass index; RSA = Respiratory sinus arrythmia; SBP = Systolic blood pressure; DBP = Diastolic blood pressure. F.U. interval is the duration in years between T1 and T2.
p < .05;
p < .01
3.2. Resting RSA
Based on the unadjusted analysis, there were no group differences in resting RSA at T1 (see Table 1). However, the mixed-effects model revealed that probands had lower resting RSA than controls, β = −0.320, SE = 0.140, p = .023, partial R2β = .020, but that siblings and controls did not differ in that regard, β = −0.145, SE = 0.131, p = .27. Additionally, probands and siblings had similar resting RSA, β = −0.175, SE = 0.138, p = .21.
3.3. Blood Pressure Variables
As for T2 SBP, the unadjusted analysis indicated no group differences (see Table 1). Likewise, in the mixed-effects model, T2 SBP was similar across probands and controls, β = −0.923, SE = 1.350, p = .49, siblings and controls, β = 0.980, SE = 1.220, p = .42, and probands and siblings, β = −1.903, SE = 1.145, p = .10.
Although the unadjusted analysis revealed that probands and siblings had higher T2 DBP than controls (see Table 1), the group differences disappeared in the mixed-effects model. Specifically, T2 DBP did not differ across probands and controls, β = 0.815, SE = 1.050, p = .44, siblings and controls, β = 1.608, SE = 0.949, p = .09, or probands and siblings, β = −0.793, SE = 0.906, p = .38.
3.4. Mediation by RSA of the Relation between Depression and Blood Pressure
Based on the mixed-effects models, T1 resting RSA predicted T2 DBP, β = −0.737, SE = 0.331, p = .026, partial R2β = .010, but did not predict T2 SBP, β = −0.202, SE = 0.424, p = .63. In other words, there was an indirect effect of depression history (probands vs. controls), but no indirect effect of being at high-risk for depression (high-risk siblings vs. controls; see Figure 1).
Figure 1.
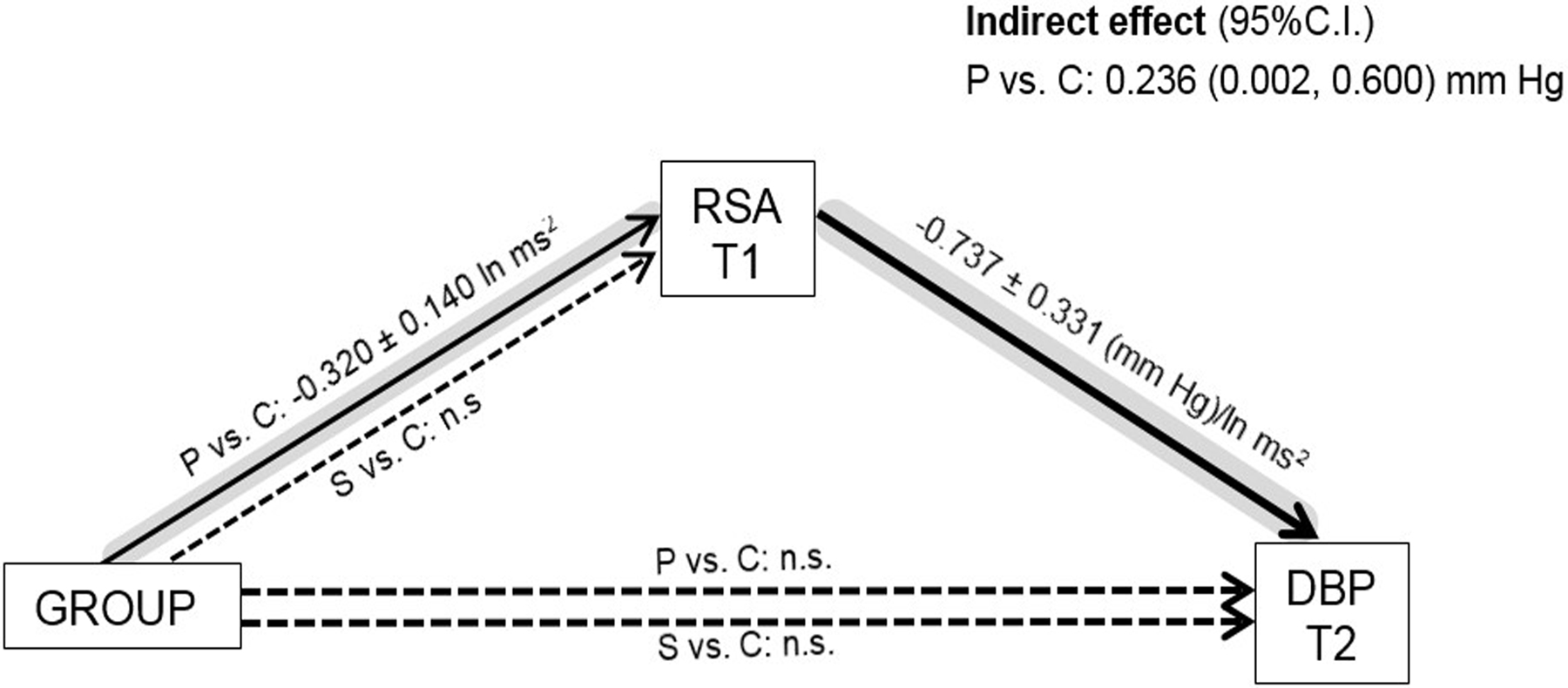
Mediation of group effects on T2 diastolic blood pressure (DBP). Group has two dummy-coded parameters (double lines): Probands (P) vs. Controls (C), and Siblings (S) vs. Controls. RSA = Respiratory sinus arrythmia. T1 = Time 1; T2 = Time 2, about six years later. Coefficients ± standard errors are displayed only for statistically significant effects (solid lines) after adjusting for sex, age, body mass index, T1 blood pressure, time interval between assessments, research site, smoking, psychotropic and blood pressure medications, and current depression status in mixed-effects models. Dashed lines represent insignificant (n.s.) effects. The path with an indirect effect is highlighted in grey.
4. Discussion
The primary goal of our study was to examine whether low resting RSA functions as a mechanism of high BP among young adults with histories of depression. The possibility that RSA is one route through which depression may lead to high BP has implications for the prevention of hypertension. We also investigated this potential mechanism among never-depressed siblings who are at high risk for depression.
Our hypotheses were partially supported. Probands had lower levels of resting RSA than controls. Although there were no group differences in BP outcomes, an indirect effect of group membership (probands vs. controls) on DBP (but not SBP) indicated that resting RSA mediates the relation between past depression and DBP. However, resting RSA did not affect BP outcomes among high-risk siblings.
Our results are in line with reports that impaired cardiac vagal control and depression are associated (Kemp et al., 2014; Koenig et al., 2016). Given that we controlled in the analyses for the presence of current depressive episodes, lower levels of resting RSA among probands relative to controls (but not among high-risk siblings) appear to represent a consequence (“scar”) of past depression. The scar may reflect the physiological effects of dysregulated emotions during past depressive episodes, as low resting RSA has been proposed as an index of emotion dysregulation (Beauchaine & Thayer, 2015; Thayer & Lane, 2000).
After adjusting for covariates, the three groups did not differ in T2 BP outcomes. Therefore, as we already reported (Rottenberg et al., 2014; Vértes et al., under review), although probands had higher BMI and were more likely to be smokers relative to controls, the direct and overt detrimental effects of depression (or being at high risk for depression) on BP may not yet be detectable at the average age of 23 years. Follow-up at older ages will be needed to detect early signs of hypertension.
However, we found an indirect effect of past depression on DBP through resting RSA. Given that resting RSA was measured approximately six years earlier than BP, our results indicate true mediation. This finding coheres with the notion that poor cardiac vagal control may be a precursor to hypertension (Thayer et al., 2010). However, we did not find a similar effect of resting RSA on SBP, which may reflect the different physiological underpinnings of the two BP indices. SBP reflects arterial pressure that results from cardiac contraction, which is driven by sympathetic activation rather than vagal input. In contrast, DBP mirrors cardiac muscle relaxation and blood vessel functioning, which are associated with vagal activity (Clement et al., 1984; Klabunde, 2011). Thus, resting RSA may be more directly related to DBP than SBP. While research on hypertension has focused on SBP (for a review see, Strandberg & Pitkala, 2003), elevated DBP may reflect blood vessel damage and is thus an important practical index of cardiovascular health (Blank, Mann, James, West, & Pickering, 1995; Franklin, 2007). Therefore, more attention should be paid to low levels of resting RSA and elevated DBP in the prevention of and intervention for hypertension.
It is notable that resting RSA did not mediate the effects of depression risk on BP outcomes, indicating that the contributions of impaired cardiac vagal control to elevated BP are not yet detectable prior to the onset of depression. Our results seem inconsistent with the report that young adults at familial risk for depression had higher SBP than did controls (Mannie et al., 2013). The inconsistency may be explained by different samples and analytic approaches. While Mannie et al. (2013) studied young people who had parental histories of depression, the familial risk in our high-risk group derived from siblings’ depression histories. Moreover, Mannie et al. (2013) treated sex as a between-subject factor, and the analyses of BP were not adjusted for covariates. In contrast, we controlled in our analyses for a set of covariates, including sex.
The present study has clinical implications for the prevention of hypertension. Because cardiac vagal control (reflected by RSA) is modifiable, it could be considered as a target in programs to promote healthy BP. Resting RSA can be enhanced by exercise (Routledge, Campbell, McFetridge-Durdle, & Bacon, 2010), meditation (Krygier et al., 2013), and biofeedback (Del Pozo, Gevirtz, Scher, & Guarneri, 2004; Nolan et al., 2005). Moreover, as noted above, low RSA may reflect dysregulated emotions during depression (e.g., Carnevali, Thayer, Brosschot, & Ottaviani, 2018; Ottaviani et al., 2016). Thus, improving emotion regulation may also be considered as a way to improve RSA and maintain normal BP.
Our findings need to be evaluated in light of several design weaknesses. For example, time intervals between the assessments were variable among subjects; T1 BP was assessed using a finger cuff; only three T2 BP readings were obtained during a single visit. Although we controlled in the analyses for subjects’ ages to minimize the confounding effects of development, chronological age is an imperfect surrogate for development. However, our study has several strong features, including a longitudinal design, a large sample size, statistical control of confounds, comprehensive psychiatric evaluation of subjects, and inclusion of a high-risk group. Therefore, the strengths of our study outweigh its weaknesses; and it has documented a route through which depression may lead to hypertension in some populations.
Acknowledgments:
We would like to thank Drs. Krisztina Kapornai, Enikő Kiss, and Ildikó Baji, the research group at the University of Szeged, and clinicians and physicians across various research sites in Hungary for their contributions to this study. We also thank Dr. J. Richard Jennings for his helpful comments on the final manuscript. This study was supported by NIH grants P01 MH056193, R01 MH084938, and R01 HL122648.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC: Author. [Google Scholar]
- Bassett D, Bear N, Nutt D, Hood S, Bassett S, & Hans D (2016). Reduced heart rate variability in remitted bipolar disorder and recurrent depression. Australian & New Zealand Journal of Psychiatry, 50(8), 793–804. 10.1177/0004867416652734 [DOI] [PubMed] [Google Scholar]
- Beauchaine T, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. 10.1111/j.1469-8986.1993.tb01731.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … & van Der Molen MW (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Bhat S, Beilin L, Robinson M, Burrows S, & Mori T (2017). Relationships between depression and anxiety symptoms scores and blood pressure in young adults. Journal of Hypertension, 35, 1983–1991. 10.1097/HJH.0000000000001410 [DOI] [PubMed] [Google Scholar]
- Blank S, Mann S, James G, West J, & Pickering T (1995). Isolated elevation of diastolic blood pressure: Real or artifactual? Hypertension, 26, 383–389. 10.1161/01.hyp.26.3.383 [DOI] [PubMed] [Google Scholar]
- Brown E, Varghese F, & McEwen B (2004). Association of depression with medical illness: Does cortisol play a role? Biological Psychiatry, 55, 1–9. 10.1016/S0006-3223(03)00473-6 [DOI] [PubMed] [Google Scholar]
- Bylsma L, Salomon K, Taylor‐Clift A, Morris B, & Rottenberg J (2014). RSA reactivity in current and remitted major depressive disorder. Psychosomatic Medicine, 76(1), 66–73. 10.1097/PSY.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, Thayer JF, Brosschot JF, & Ottaviani C (2018). Heart rate variability mediates the link between rumination and depressive symptoms: A longitudinal study. International Journal of Psychophysiology, 131, 131–138. 10.1016/j.ijpsycho.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Chang H, Chang C, Chen C, Kuo T, Lu R, & Huang S (2013). Heart rate variability in patients with fully remitted major depressive disorder. Acta Neuropsychiatrica, 25(1), 33–42. 10.1111/j.1601-5215.2012.00658.x [DOI] [PubMed] [Google Scholar]
- Clement D, Jordaens L, & Heyndrickx G (1984). Influence of vagal nervous activity on blood pressure variability. Journal of Hypertension, 2(supplement 3), 391–393. [PubMed] [Google Scholar]
- Del Pozo J, Gevirtz R, Scher B, & Guarneri E (2004). Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. American Heart Journal, 147(3), 545–550. 10.1016/j.ahj.2003.08.013 [DOI] [PubMed] [Google Scholar]
- Farmer A, Harris T, Redman K, Sadler S, Mahmood A, & McGuffin P (2000). Cardiff Depression Study: A sib-pair study of life events and famility in major depression. The British Journal of Psychiatry, 176(2), 150–155. 10.1192/bjp.176.2.150 [DOI] [PubMed] [Google Scholar]
- Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, … & Mant D (2011). Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. The Lancet, 377(9770), 1011–1018. 10.1016/S0140-6736(10)62226-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin S (2007). The importance of diastolic blood pressure in predicting cardiovascular risk. Journal of the American Society of Hypertension, 1(1), 82–93. 10.1016/j.jash.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Gentzler A, Rottenberg J, Kovacs M, George CJ, & Morey J (2012). Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology, 54, 556–567. 10.1002/dev.20614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, & Jennings JR (2018). Host in the machine: A neurobiological perspective on psychological stress and cardiovascular disease. American Psychologist, 73(8), 1031–1044. 10.1037/amp0000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Carnethon M, Matthews K, McIntyre R, Miller G, Raghuveer G, … & McCrindle B (2015). Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: A scientific statement from the American Heart Association. Circulation, 132, 965–986. 10.1161/CIR.0000000000000229 [DOI] [PubMed] [Google Scholar]
- Goldstein B, Fagiolini A, Houck P, & Kupfer D (2009). Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disorders, 11, 657–662. 10.1111/j.1399-5618.2009.00735.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, & Preacher KJ (2014). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67(3), 451–470. 10.1111/bmsp.12028 [DOI] [PubMed] [Google Scholar]
- Huang M, Shah A, Su S, Goldberg J, Lampert RJ, Levantsevych OM, … & Vaccarino V (2018). Association of depressive symptoms and heart rate variability in Vietnam war-era twins: A longitudinal twin difference study. JAMA psychiatry, 75(7), 705–712. 10.1001/jamapsychiatry.2018.0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildrum B, Mykletun A, Holmen J, & Dahl A (2008). Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. The British Journal of Psychiatry, 193, 108–113. 10.1192/bjp.bp.107.045013 [DOI] [PubMed] [Google Scholar]
- Jandackova VK, Britton A, Malik M, & Steptoe A (2016). Heart rate variability and depressive symptoms: a cross-lagged analysis over a 10-year period in the Whitehall II study. Psychological Medicine, 46(10), 2121–2131. 10.1017/S003329171600060X [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Kemp A, Quintana D, Quinn C, Hopkinson P, & Harris A (2014). Major depressive disorder with melancholia displays robust alterations in resting state heart rate and its variability: Implications for future morbidity and mortality. Frontiers in Psychology, 5, 1387 10.3389/fpsyg.2014.01387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E, Gentzler A, George CJ, Kapornai K, Tamás Z, Kovacs M, & Vetró Á (2007). Factors influencing mother–child reports of depressive symptoms and agreement among clinically referred depressed youngsters in Hungary. Journal of Affective Disorders, 100(1), 143–151. 10.1016/j.jad.2006.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde R (2011). Cardiovascular Physiology Concepts. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Koenig J, Kemp A, Beauchaine T, Thayer JF, & Kaess M (2016). Depression and resting state heart rate variability in children and adolescents—A systematic review and meta-analysis. Clinical Psychology Review, 46, 136–150. 10.1016/j.cpr.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Krygier JR, Heathers JA, Shahrestani S, Abbott M, Gross JJ, & Kemp AH (2013). Mindfulness meditation, well-being, and heart rate variability: a preliminary investigation into the impact of intensive Vipassana meditation. International Journal of Psychophysiology, 89(3), 305–313. 10.1016/j.ijpsycho.2013.06.017 [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Barnes R, Tyroler H, Rautaharju P, Holme I, & Heiss G (1996). Association of cardiac automatic function and the development of hypertension: The ARIC Study. American Journal of Hypertension, 9(12), 1147–1156. 10.1016/s0895-7061(96)00249-x [DOI] [PubMed] [Google Scholar]
- Licht C, de Geus E, Seldenrijk A, van Hout H, Zitman F, van Dyck R, & Penninx B (2009). Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension, 53, 631–638. 10.1161/HYPERTENSIONAHA.108.126698 [DOI] [PubMed] [Google Scholar]
- Mannie Z, Williams C, Diesch J, Steptoe A, Leeson P, & Cowen P (2013). Cardiovascular and metabolic risk profile in young people at familial risk of depression. The British Journal of Psychiatry, 203, 18–23. 10.1192/bjp.bp.113.126987 [DOI] [PubMed] [Google Scholar]
- Maziade M, Roy M, Fournier J, Cliche D, Merette C, Caron C, … & Raymond V (1992). Reliability of best‐estimate diagnosis in genetic linkage studies of major psychoses: Results from the Quebec pedigree studies. American Journal of Psychiatry, 149(2), 1674–1686. 10.1176/ajp.149.12.1674 [DOI] [PubMed] [Google Scholar]
- Meng L, Chen D, Yang Y, Zheng Y, & Hui R (2012). Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. Journal of Hypertension, 30, 842–851. 10.1097/HJH.0b013e32835080b7 [DOI] [PubMed] [Google Scholar]
- Meyer C, Armenian H, Eaton W, & Ford D (2004). Incident hypertension associated with depression in the Baltimore Epidemiologic Catchment area follow-up study. Journal of Affective Disorders, 83, 127–133. 10.1016/j.jad.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Muntner P, Shimbo D, Carey R, Charleston J, Gaillard T, Misra S, … & Wright J (2019). Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension, 73, e35–e66. 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R, Kamath M, Floras J, Stanley J, Pang C, Picton P, & Young Q (2005). Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. American Heart Journal, 149(6), 1137e1–1137e7. 10.1016/j.ahj.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, & Brosschot JF (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142(3), 231–259. 10.1037/bul0000036 [DOI] [PubMed] [Google Scholar]
- Patten S, Williams J, Lavorato D, Campbell N, Eliasziw M, & Campbell T (2009). Major depression as a risk factor for high blood pressure: Epidemiologic evidence from a national longitudinal study. Psychosomatic Medicine, 71, 273–279. 10.1097/PSY.0b013e3181988e5f. [DOI] [PubMed] [Google Scholar]
- Penninx B (2017). Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neuroscience & Biobehavioral Reviews, 74, 277–286. 10.1016/j.neubiorev.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K, & Selig J (2012). Advantages of Monte Carlo confidence intervals for indirect effects. Communication Methods and Measures, 6(2), 77–98. 10.1080/19312458.2012.679848 [DOI] [Google Scholar]
- Rottenberg J (2007). Cardiac vagal control in depression: A critical analysis. Biological Psychology, 74, 200–211. 10.1016/j.biopsycho.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Yaroslavsky I, Carney R, Freeland K, George CJ, Baji I, … Kovacs M (2014). The association between major depression disorder in childhood and risk factors for cardiovascular disease in adolescence. Psychosomatic Medicine, 76(2), 122–127. 10.1097/PSY.0000000000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge FS, Campbell TS, McFetridge-Durdle JA, & Bacon SL (2010). Improvements in heart rate variability with exercise therapy. Canadian Journal of Cardiology, 26(6), 303–312. 10.1016/S0828-282X(10)70395-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N, Williamson D, Iyengar S, Orvaschel H, Reich T, Dahl R, & Puig-Antich J (1992). A secular increase in child and adolescent onset affective disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 31(4), 600–605. 10.1097/00004583-199207000-00004 [DOI] [PubMed] [Google Scholar]
- Schroeder E, Liao D, Chambless L, Prineas R, Evans G, & Heiss G (2003). Hypertension, blood pressure, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Hypertension, 42, 1106–1111. 10.1161/01.HYP.0000100444.71069.73 [DOI] [PubMed] [Google Scholar]
- Sherrill J, & Kovacs M (2000). Interview schedule for children and adolescents (ISCA). Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 67–75. 10.1097/00004583-200001000-00018 [DOI] [PubMed] [Google Scholar]
- Singh J, Larson M, Tsuji H, Evans J, O’Donnell C, & Levy D (1998). Reduced heart rate variability and new-onset hypertension: Insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension, 32, 293–297. 10.1161/01.hyp.32.2.293 [DOI] [PubMed] [Google Scholar]
- Son B, Markovitz J, Winders S, & Smith D (1997). Smoking, nicotine dependence, and depressive symptoms in the CARDIA study: Effects of educational status. American Journal of Epidemiology, 145, 110–116. 10.1093/oxfordjournals.aje.a009081 [DOI] [PubMed] [Google Scholar]
- Strandberg T, & Pitkala K (2003). What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Current Opinion in Nephrology and Hypertension, 12, 293–297. 10.1097/01.mnh.0000069868.94246.ef [DOI] [PubMed] [Google Scholar]
- Tamás Z, Kovacs M, Gentzler A, Tepper P, Gádoros J, Kiss E, Kapornai K, & Vetró Á (2007). The relations of temperament and emotion self-regulation with suicidal behaviors in a clinical sample of depressed children in Hungary. Journal of Abnormal Child Psychology, 35(4), 640–652. 10.1007/s10802-007-9119-2 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers III JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane D (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto S, & Brosschot J (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141, 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon D, & Yoon M (2009). Covariances between regression coefficient estimates in a single mediator model. The British Journal of Mathematical and Statistical Psychology, 62, 457–484. 10.1348/000711008X331024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértes M, Dósa E, Yang X, George CJ, Merkely B, Kiss E, … & Kovacs M (2019). Blood pressure and its short-term variability among young adults at high or low risk for depression. Manuscript submitted for publication. [Google Scholar]


