Abstract
Prospective evidence indicates that functional biomechanics and brain connectivity may predispose an athlete to an anterior cruciate ligament injury, revealing novel neural linkages for targeted neuromuscular training interventions. The purpose of this study was to determine the efficacy of a real-time biofeedback system for altering knee biomechanics and brain functional connectivity. Seventeen healthy, young, physically-active female athletes completed six weeks of augmented neuromuscular training (aNMT) utilizing real-time, interactive visual biofeedback and 13 served as untrained controls. A drop vertical jump and resting state functional magnetic resonance imaging were separately completed at pre and post time points to assess sensorimotor adaptation. The aNMT group had a significant reduction in peak knee abduction moment (pKAM) compared to controls (p = .03, d = .71). The aNMT group also exhibited a significant increase in functional connectivity between the right supplementary motor area and the left thalamus (p = .0473 after false discovery rate correction). Greater percent change in pKAM was also related to increased connectivity between the right cerebellum and right thalamus for the aNMT group (p = .0292 after false discovery rate correction, r2 = .62). No significant changes were observed for the controls (p’s > .05). Our data provide preliminary evidence of potential neural mechanisms for aNMT-induced motor adaptations that reduce injury risk. Future research is warranted to understand the role of neuromuscular training alone and how each component of aNMT influences biomechanics and functional connectivity.
Keywords: Central Nervous System, Anterior Cruciate Ligament, Coordination, Resting-state fMRI, Motion Analysis
1. Introduction
Risk of musculoskeletal injury, specifically anterior cruciate ligament (ACL) injury, is associated with decreased neuromuscular control and coordination during dynamic activities (Griffin et al., 2000; Hewett et al., 2005; Hewett, Torg, & Boden, 2009; Ireland, 2002; Krosshaug, Slauterbeck, Engebretsen, & Bahr, 2007; Zazulak, Hewett, Reeves, Goldberg, & Cholewicki, 2007). Advances in 3D motion analyses and force platforms have permitted the assessment of neuromuscular coordination using the drop vertical jump (DVJ) (Chaudhari et al., 2007; Cruz et al., 2013; DiCesare, Kiefer, Bonnette, & Myer, 2019; Doherty et al., 2016; Earl, Monteiro, & Snyder, 2007; Etnoyer, Cortes, Ringleb, Van Lunen, & Onate, 2013; Ford, Myer, & Hewett, 2003, 2007a; Hewett et al., 2005; Limroongreungrat & Boonkerd, 2019; McLean et al., 2007; Paterno et al., 2010; Popovic et al., 2018; Schmitz et al., 2014; Taylor et al., 2017). During the landing phase of the DVJ, tri-planar hip, knee, and ankle motion that results in the knee collapsing toward the midline, or ‘dynamic valgus,’ is quantified via an external peak knee abduction moment (pKAM) (Carson & Ford, 2011; Ford, Myer, & Hewett, 2007b; Ford et al., 2005; Ford, Shapiro, Myer, Van Den Bogert, & Hewett, 2010; Galloway et al., 2018; Hewett et al., 2005; Malfait et al., 2014; Myer et al., 2010; Myer et al., 2013; Myer et al., 2015). Prospective biomechanical measures of pKAM during landing predict non-contact ACL injury with 73% specificity and 78% sensitivity (Hewett et al., 2005), a finding that has motivated ACL injury prevention programs to improve neuromuscular coordination through neuromuscular training (NMT) (Ireland, 2002; Myer, Chu, Brent, & Hewett, 2008; Sugimoto, Myer, Foss, & Hewett, 2014, 2015; Sugimoto, Myer, McKeon, & Hewett, 2012). However, traditional ACL injury prevention programs are susceptible to non-compliance (Sugimoto, Myer, Bush, et al., 2012), require considerable resources and trained instructors (Hewett, Ford, & Myer, 2006), and have poor dose-response relationships (Sugimoto, Myer, McKeon, et al., 2012) thus requiring high compliance and extended training durations to elicit desirable movement outcomes (Padua et al., 2012). These barriers may explain why ACL injury incidence rates remain high (Beynnon et al., 2014; Sanders et al., 2016) and highlight a need for complementary approaches that enhance athlete compliance and widespread implementation. As motivation appears to be a critical factor for achieving high compliance (Steffen, Myklebust, Olsen, Holme, & Bahr, 2008), a stronger mechanistic understanding of NMT may improve ‘buy-in’ (i.e., increase motivation) to facilitate implicit processes underlying enhanced motor learning (Wulf & Lewthwaite, 2016).
One mechanism that has been historically overlooked regarding NMT is how the central nervous system (CNS), specifically the brain, contributes to ACL injury (Grooms & Onate, 2016). ACL injuries often occur during scenarios that require intricate sensorimotor coordination (Krosshaug, Nakamae, et al., 2007), which exemplify the critical role of the CNS in maintaining functional joint stability. In an exploratory neuroimaging study (Diekfuss, Grooms, Yuan, et al., 2019), females who sustained complete ACL ruptures during their competitive soccer season were matched to healthy teammates. Prospective functional magnetic resonance imaging (fMRI) revealed reduced correlation in blood oxygen level dependent (BOLD) signal activity at rest (i.e., functional connectivity) between the left primary somatosensory cortex (a region that processes sensory nervous system information) and the right posterior lobe of the cerebellum (a region associated with motor control) prior to the ACL injury (Diekfuss, Grooms, Yuan, et al., 2019). Hemispheric lateralization of significant findings (i.e., right versus left cerebellum) could be due to the small sample size in the prior study, precluding the power to detect additional effects, or possibly due to lower limb dominance, which was not reported (Diekfuss, Grooms, Yuan, et al., 2019). Nevertheless, somatosensory-cerebellar connectivity throughout both hemispheres is vital for successful motor coordination and navigation (Manto et al., 2012) and these data identified a potential neural predisposition to ACL injury that could be investigated, and possibly modified, through further research (Diekfuss, Grooms, Yuan, et al., 2019). While ACL injury prevention training has been shown to reduce corticomotor excitability in the gluteus maximus relative to strength training (using transcranial magnetic stimulation) (Powers & Fisher, 2010), the effects of traditional ACL injury prevention training on brain functional connectivity has not been clearly established. Previous research has demonstrated that other forms of motor skill training, ranging from discrete fine motor tasks such as finger tapping (Ma, Narayana, Robin, Fox, & Xiong, 2011) and tool use (i.e., chopstick handling) (Yoo, Sohn, & Jeong, 2013) to more complex balance board training (Taubert, Lohmann, Margulies, Villringer, & Ragert, 2011), drum training (Amad et al., 2016), and aerobic fitness training (Voss et al., 2010), have pronounced effects on functional connectivity. Considering its behavioral relevance and the sensitivity of measures of functional connectivity to changes within the CNS (Guerra-Carrillo, Mackey, & Bunge, 2014), functional connectivity could be a valuable tool to supplement our understanding of ACL injury prevention.
In one preliminary study investigating the role of ACL injury prevention training in relation to high-risk knee biomechanics and neuroplasticity (Grooms et al., 2018), healthy athletes completed six weeks of standard NMT supplemented with real-time biofeedback, or augmented NMT (aNMT). Participants completed pre- and post-training fMRI sessions employing a knee motor control testing paradigm and were separately assessed in 3D motion capture using a virtual-reality simulated soccer scenario for high-risk knee biomechanics (Grooms et al., 2018). aNMT increased sensory and motor planning network activity and decreased motor cortex activity (suggesting improved efficiency), and both neural changes were correlated with a reduction in high-risk knee biomechanics (Grooms et al., 2018). These preliminary data demonstrated neuroplasticity potentially resulting from aNMT and highlighted the need to further examine the adaptability of brain connectivity that may underlie ACL injury risk (Diekfuss, Grooms, Nissen, et al., 2019; Diekfuss, Grooms, Yuan, et al., 2019).
The purpose of the present study was to assess the efficacy of aNMT for improving high-risk landing biomechanics and for increasing sensorimotor, knee-related brain functional connectivity. We hypothesized that (1) aNMT would improve knee biomechanics that are high risk during the DVJ, (2) participation in aNMT would increase sensorimotor functional connectivity between regions related to knee motor control, and (3) improvements in knee biomechanics that are high risk would be associated with increased knee-related, sensorimotor functional connectivity for those who participated in aNMT.
2. Method
As changes in biomechanics and functional connectivity as a function of aNMT have yet to be explored, we used prior, related research to guide our sample size. Specifically, one study examined changes in functional connectivity following 13 sessions (once session per week for 13 weeks) of combined coordinative, cognitive, and visual training (Demirakca, Cardinale, Dehn, Ruf, & Ende, 2016). The authors found significant pre- to post-training functional connectivity changes using an n of 32 (n = 21 training & n = 11 controls) (Demirakca et al., 2016). Another study (Amad et al., 2016) found significant functional connectivity changes following 24 sessions of drum training (three sessions per week for eight weeks) with an n of 31 (n = 15 training, n = 16 controls). As drumming is a cardiovascular-demanding bilateral coordinative exercise (De La Rue, Draper, Potter, & Smith, 2013) (comparable to NMT more generally) and the study by Demirakca et al. (2016) was multimodal (i.e., cognitive, visual, and coordinative demands akin to aNMT), we aimed to enroll a similar number of participants in this preliminary investigation (n ~ 30).
Before engaging in a larger scale, resource-intensive and costly randomized controlled trial, we completed this proof-of-concept preliminary investigation (non-randomized controlled trial) to determine potential efficacy for aNMT to improve biomechanical and functional connectivity. One local high-school volunteered to participate in our proposed study and enrolled 25 female soccer players. Eight of these athletes were not included in the present analyses due to MRI contraindications/poor imaging data (e.g., metal orthodontics/artifact/technical issue). The participants who did not receive (or have usable) neuroimaging data will be presented in a companion manuscript focused on biomechanics only. Raw demographic data for the final 17 participants used in all analyses herein (aNMT group) is presented in Table 1.
Table 1:
Characteristics of Study Participants at First Study Visit (‘pre’ time point)
| Subject ID | Group | Age* | Height (cm)* | Weight (kg)* | pKAM (Nm)* |
|---|---|---|---|---|---|
| 1 | Control | 16 | 171 | 60.2 | −21.60 |
| 2 | Control | 16 | 164 | 60.3 | −7.24 |
| 3 | Control | 17 | 168 | 49.7 | −3.38 |
| 4 | Control | 16 | 170 | 57.0 | −8.44 |
| 5 | Control | 17 | 163 | 62.4 | −3.60 |
| 6 | Control | 16 | 160 | 51.3 | −6.66 |
| 7 | Control | 15 | 169 | 81.6 | −25.31 |
| 8 | Control | 16 | 166 | 68.2 | −6.15 |
| 9 | Control | 16 | 159 | 59.4 | −25.62 |
| 10 | Control | 17 | 162 | 50.6 | −13.25 |
| 11 | Control | 17 | 157 | 52.2 | −5.09 |
| 12 | Control | 15 | 163 | 64.6 | −4.79 |
| 13 | Control | 17 | 158 | 56.8 | −13.60 |
| 14 | aNMT | 16 | 173 | 63.9 | 0.97 |
| 15 | aNMT | 16 | 169 | 53.6 | −15.37 |
| 16 | aNMT | 16 | 171 | 58.6 | −16.33 |
| 17 | aNMT | 15 | 168 | 68.7 | −33.11 |
| 18 | aNMT | 17 | 170 | 58.5 | −21.43 |
| 19 | aNMT | 15 | 174 | 54.5 | −13.87 |
| 20 | aNMT | 17 | 170 | 52.9 | −32.26 |
| 21 | aNMT | 16 | 166 | 57.6 | −22.20 |
| 22 | aNMT | 15 | 165 | 66.8 | −17.81 |
| 23 | aNMT | 14 | 155 | 45.4 | −12.43 |
| 24 | aNMT | 16 | 168 | 59.9 | −14.24 |
| 25 | aNMT | 14 | 151 | 40.9 | −17.74 |
| 26 | aNMT | 15 | 158 | 43.6 | −19.02 |
| 27 | aNMT | 15 | 162 | 49.8 | −6.49 |
| 28 | aNMT | 17 | 161 | 57.3 | −1.48 |
| 29 | aNMT | 16 | 167 | 73.8 | −40.52 |
| 30 | aNMT | 17 | 169 | 87.9 | −34.43 |
aNMT: augmented neuromuscular training
pKAM: peak knee abduction movement (bilateral average of three trials)
No significant between-group differences (all p > .05; two-tailed)
To enhance the study’s research design, we contacted a second high-school (located in the same city as the first high-school) to participate in two neuroimaging sessions separated by approximately seven weeks (i.e., to serve as controls). Thirteen female soccer athletes from the second high-school volunteered to participate and enrolled as controls. These control participants did not differ in age, height, or weight to that of the aNMT group (all p > .05), and their raw demographic data is also presented in Table 1.
The study took place during both high-schools’ soccer offseason, data from both groups were collected simultaneously, and all participants received equal compensation. No participants from either group were involved in any structured conditioning or other ACL prevention programs over the duration of the study period. All participants (n = 30) completed DVJ testing using 3D motion capture and a resting-state fMRI (rs-fMRI) scan at a pre- and post-test time point separated by approximately seven weeks (the aNMT group underwent a six week intervention described below). The institutional ethics committee approved the project and informed consent or assent (if under 18) was obtained from all participants prior to commencing the study.
2.1. Augmented Neuromuscular Training (aNMT)
The aNMT biofeedback group completed six weeks of standard NMT (3 times per week; 18 total sessions) as part of a pre-season conditioning program (Myer, Ford, Palumbo, & Hewett, 2005). NMT consisted of plyometric, core strengthening and balance, resistance, and speed training that are detailed in Myer et al. 2005. NMT sessions were team-based (group training), were provided at no cost to the athlete, and lasted approximately 1.5 hours (45 minutes per NMT station × two stations per session [e.g., plyometric and speed training]). Mean compliance for NMT was 85.0% (+/− 11.8%). The majority of these NMT sessions were further supplemented with an additional exercise that involved participants interacting with a real-time visual biofeedback stimulus (i.e, aNMT) performed on average two times per week for six weeks over the course of the program (i.e., 12 of the total 18 sessions). The aNMT biofeedback protocol consisted of six movements (squat, pistol squat, single-leg romanian dead lift, overhead squat, squat jump, and tuck jump). Participants’ completed each exercise twice (2 separate aNMT sessions) while interacting with the aNMT stimulus for three sets of 10 repetitions for bilateral exercises (squat, overhead squat, squat jump, tuck jump) and three sets of five repetitions per leg for the unilateral exercises (pistol squat, single-leg romanian dead lift). aNMT was run individually for each subject (also at no cost to the athlete) and required an additional 15 minutes to complete. If a participant was absent from the aNMT session (i.e., missed a NMT session that included aNMT), the participant would complete the aNMT session during a subsequent NMT session, such that compliance for aNMT was 100%.
The following text provides further information regarding the aNMT stimulus, but we direct the reader to recent literature for in-depth methodological details (Bonnette et al., 2020; Bonnette, DiCesare, Kiefer, et al., 2019). Briefly, the aNMT stimulus consisted of a dynamic, rectangular shape with vertices that were mapped to key biomechanical variables related to ACL injury risk (Ford, Myer, Schmitt, van den Bogert, & Hewett, 2008; Hewett et al., 2005; Hewett et al., 2009; Myer et al., 2009; Paterno, 2017; Schoenfeld, 2010; Zazulak et al., 2007). These variables included lateral trunk flexion, knee to hip joint extensor moment force ratio, knee abduction moment of force, vertical ground reaction force ratio, and foot center of pressure location. The stimulus shape responded in real time as a function of participants’ movements as their actions led to changes in those biomechanical variables. Variables that were possible to calculate during all six aNMT exercises, such as lateral trunk flexion, were included in each exercise’s aNMT stimulus. When it was not possible to calculate a variable during some aNMT exercises, such as the vertical ground reaction force ratio during the pistol squat and single leg romanian dead lift (unilateral exercises), the variable was not included as part of the aNMT stimulus. Participants were instructed to perform all exercises to achieve a goal rectangle shape, which equated to producing biomechanical parameters associated with ACL injury risk reduction. Deviations toward injury risk factors resulted in specific shape distortions (Figure 1). The variables used for each aNMT session were identical for all participants and did not vary based on an individual participant’s performance. Note that the aNMT stimulus presented in Bonnette, DiCesare, Kiefer, et al. (2019) and Bonnette et al. (2020) was wirelessly transmitted in real-time to video eyeglasses worn by participants, whereas the aNMT stimulus used in the present study was displayed on a projector screen (Grooms et al., 2018).
Figure 1:
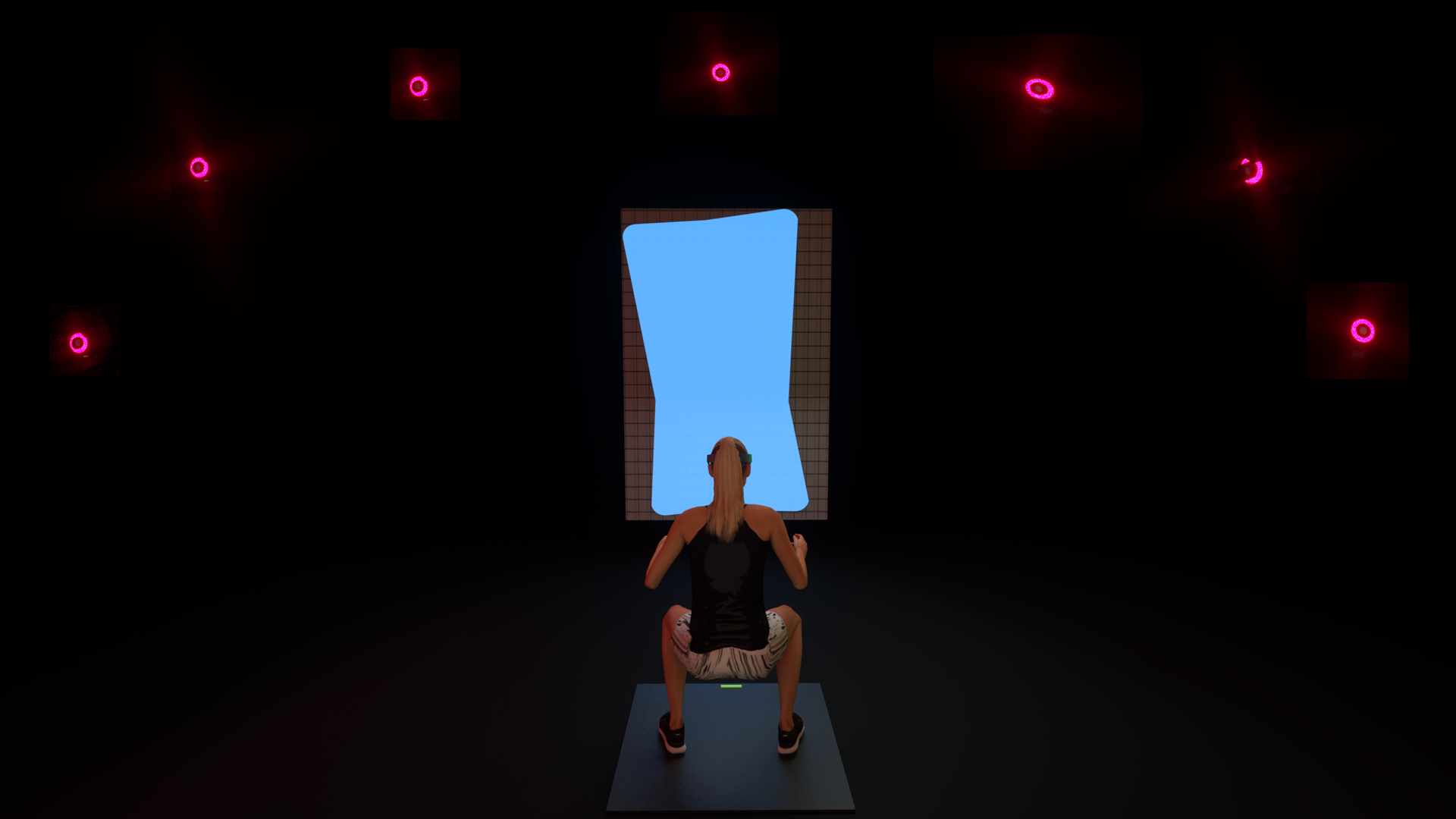
An illustration of a participant performing a double leg squat while interacting with the augmented neuromuscular training stimulus. The stimulus was mapped on to key biomechanical variables that would distort in real-time as a as a function of participants’ movements.
Though the equipment required to produce the aNMT stimulus are not readily accessible outside of a research laboratory at this time (e.g., force plates, 3D motion capture system), ongoing research aims to implement aNMT with new, cost-effective technologies while maintaining similar efficacy and validity of biofeedback. For instance, portable, wireless, and markerless motion analysis systems are commercially available for less than a few thousand dollars (e.g., Microsoft Azure Kinect; Microsoft Corp., Redmond, WA), and efforts are being made to bring 3D motion capture capabilities to consumer devices such as the iPad and iPhone (Apple Inc, Cupertino, CA). Likewise, kinetic data can be obtained from portable force plates (e.g., BODITRAK, BodiTrak Sports, Webster Groves, MO) at lower costs than traditional lab setups. Integration of data across systems could support a real-time biofeedback aNMT stimulus to be presented using standard visual displays (e.g., computer monitors). With respect to NMT more generally, previous literature has reported estimated costs of ACL prevention programs to range from a few dollars to ~ $500.00 (after adjusting for inflation; consumer price index) per athlete per season (Hewett et al., 2006), depending on program specifics (e.g., number of sessions, personnel for training). Incorporating emergent cost-effective technologies with NMT to display real-time biofeedback will also allow for personalized training at a further reduced cost (e.g., by removing the need for expensive clinicians to provide feedback) for easier implementation in non-laboratory settings (e.g., at home, high school gymnasium). However the authors acknowledge that continued research to test these technologies against gold standard 3D motion analysis systems and longitudinal efficacy clinical trials are needed.
2.2. Drop Vertical Jump
To assess whether aNMT improved high-risk knee biomechanics based on the DVJ (Hypothesis 1), participants were outfitted with 31 retroreflective markers and completed a standing trial with all joints in neutral position (Grooms et al., 2018). Participants were then asked to stand on top of a box (31 cm high) with their feet positioned 35 cm apart and instructed to drop directly down off the box and immediately perform a maximum vertical jump, raising both arms while jumping for an overhead target, for three trials (Ford et al., 2003). Marker trajectory and ground reaction force data were recorded using a 44-camera, high-speed (240 Hz) digital motion analysis system (Motion Analysis Corp.) and two embedded force platforms (AMTI, Watertown, MA), post-processed with Visual3D (C-Motion, Inc., Germantown, MD), and analyzed using custom MATLAB scripts (MathWorks, Inc, Natick, MA). Greater pKAM during landing is associated with increased ACL injury risk (Hewett et al., 2005) and therefore pKAM was used as the biomechanical dependent variable of interest. We summed the three pKAM values for each limb (six total) and computed a single, bilateral average (by dividing the summed value by six). Our rationale for using a bilateral average (rather than separate unilateral analyses) was that our complementary dependent variable of interest (functional connectivity) was collected during the resting state (no unilateral active movement to drive a lateralized brain response) and both limbs were equivocally trained during aNMT (primarily bilateral exercises; unilateral exercises were repeated for both limbs).
2.3. Neuroimaging
MRI was conducted on a Phillips 3T Ingenia scanner (Philips Medical Systems, Best, the Netherlands) with a 32-channel, phased array head coil. A magnetization-prepared rapid gradient-echo sequence was used to acquire a high resolution 3D T1-weighted images (sagittal): TR = 8.1 ms, TE = 3.7 ms; field of view = 256 × 256 mm; matrix= 256 × 256; in-plane resolution = 1 × 1 mm; slice thickness = 1 mm; number of slices = 180. To test whether aNMT influenced brain sensorimotor functional connectivity related to knee motor control based on resting-state fMRI (Hypothesis 2), participants were asked to look at a crosshair displayed on a projector screen and remain still for approximately 5.5 minutes. Resting-state fMRI data were acquired with the following parameters: TR = 650 ms; TE = 30 ms; flip angle = 53°; field of view = 200 × 200 mm; acquisition matrix = 68 × 66; SENSE factor = 1.5; reconstructed in-plane resolution = 2.5 × 2.5; slice thickness = 3.5 mm; number of slices = 40; multi band factor = 4.
Processing of the rs-fMRI data were performed using the CONN toolbox (version 17.F, http://www.nitrc.org/projects/conn) (Whitfield-Gabrieli & Nieto-Castanon, 2012). The CONN toolbox utilizes the Statistical Parametric Mapping (SPM) 12 package (Wellcome Institute of Cognitive Neurology, London) for spatial preprocessing which includes realignment and unwarping, normalization to Montreal Neurological Institute (MNI) template space (resampled to 2 mm isotropic), and smoothing (8 mm full-width half-max kernel). Temporal preprocessing steps were completed in CONN and included scrubbing of motion-outlier frames (> 0.9 mm or > ±5 standard deviation in global signal) and regression of the top five principle components of the BOLD signal from cerebrospinal fluid and white matter compartments (aCompCor) (Behzadi, Restom, Liau, & Liu, 2007), as well as zero- and first-order derivatives of realignment parameters and band-pass filtering to a window of 0.008 Hz - 0.09 Hz.
To examine whether aNMT influenced sensorimotor functional connectivity specifically related to knee motor control (Hypothesis 2) and whether such functional connectivity changes were related to improvements in high-risk knee biomechanics (Hypothesis 3), 25 regions of interest (ROI) (10 mm spheres) were derived from previous studies that utilized a knee flexion/extension paradigm (Grooms, Page, & Onate, 2015; Kapreli et al., 2007). Development of these ROIs (left and right sensorimotor-related regions) has been previously described in detail (Diekfuss, Grooms, Yuan, et al., 2019) and were selected due to their prospective relation to ACL injury (Diekfuss, Grooms, Nissen, et al., 2019; Diekfuss, Grooms, Yuan, et al., 2019). Fisher-transformed Pearson correlation coefficients between the average residual BOLD signal time series (extracted from the individual ROIs) were used to define functional connectivity (Biswal, Zerrin Yetkin, Haughton, & Hyde, 1995).
2.4. Data analyses
First, an independent t-test was conducted to assess any significant differences in pKAM at the pre time point. To address Hypothesis 1, percent change scores for the pKAM (pre to post) were then calculated for each group. One outlier was observed for the aNMT group (box plot inspection revealed this subject’s data point was outside 1.5 times the interquartile range [i.e., below the lower whisker]). As this was the only outlier (and this participant had usable neuroimaging data), we replaced this value with the group mean (Tabachnick & Fidell, 2007). Percent change scores for each group were then compared using an independent t-test.
To test hypothesis 2, we identified appropriate ROIs to use as ‘seed’ ROIs from the total 25 used previously (Diekfuss, Grooms, Nissen, et al., 2019; Diekfuss, Grooms, Yuan, et al., 2019). Twenty of the ROIs were initially derived from knee flexion/extension movements within a population that excluded those with a history of musculoskeletal injury (Kapreli et al., 2007), whereas five ROIs were derived from the same knee/flexion paradigm but in a subject who previously had an ACL injury and went on to a second ACL injury (Grooms et al., 2015). As the present study did not track actual ACL injury incidence or study those with a history of ACL injury, we elected to use the 20 ROIs from Kapreli et al. (2007) as seeds, but still included the five ROIs from Grooms et al. (2015) as target ROIs in the analysis. The anatomical ROIs were identified and an omnibus F-test was conducted to determine which of the 20 seed ROIs exhibited significant connectivity differences with one or more of the other 24 ROIs (inclusive of all seed and target ROIs) for the aNMT and control groups, independently. Specifically, one-way ANOVAs were used to identify ‘seed’ ROIs that exhibited a significant group effect in their connectivity patterns. Post-hoc paired-samples t-tests were applied to each pairwise connection between any significant seed ROIs and each other ROI to determine specific pre to post connectivity changes for each group, independently. Alpha level was set at p < .05 and multiple comparison error corrections using the false discovery rate (FDR) approach were used for all F- and t- tests (Chumbley, Worsley, Flandin, & Friston, 2010).
For hypothesis 3, linear regression analyses were conducted to determine whether percent change in pKAM was related to changes in sensorimotor connectivity related to knee motor control. Percent change in pKAM was used as the predictor variable for the pre to post change in functional connectivity for all 25 possible ROI-to-ROI comparisons (300 regressions total) for each group, independently. We included all possible ROI-to-ROI comparisons (including the five ROIs from Grooms et al. 2015) as pKAM is more directly related to ACL injury risk. The FDR approach was used to correct for multiple comparisons set at p < .05 (Chumbley et al., 2010). In other words, this analysis assessed whether the percent change in pKAM (the predictor variable) was associated with pre to post changes in functional connectivity (the predicted variable) for any ROI-to-ROI comparison, with only the relationships surviving the FDR correction being deemed as significant/meaningful. Based on prior literature, we expected our aNMT group to improve biomechanics (Bonnette et al., 2020; Bonnette, DiCesare, Kiefer, et al., 2019; Ericksen et al., 2016; Ericksen, Thomas, Gribble, Doebel, & Pietrosimone, 2015; Ford, DiCesare, Myer, & Hewett, 2015) and, as the strength of resting-state functional connectivity reflects a history of co-activation during active states (Corbetta, 2012; Di, Gohel, Kim, & Biswal, 2013), we expected sensorimotor, knee-related connectivity to strengthen following aNMT, since the aNMT exercises engaged the knee akin to the task-based knee fMRI studies used to derive the ROIs (Grooms et al., 2015; Kapreli et al., 2007). Thus, all statistical analyses noted above were conducted using one-tailed tests. For any significant finding we also included the uncorrected p value when relevant (i.e., the raw p value [puncorrected] before the application of the conservative FDR p value correction used for the connectivity analyses [pcorrected]), means and SDs, and effect sizes to facilitate reader interpretation regarding significance.
3. Results
As noted in Table 1, no significant differences in pKAM at the pre time point were observed between the two groups (p > .05). As shown in Figure 2, the percent change in pKAM was significantly greater (i.e., peak knee moment decreased) for the aNMT group (M = 31.80, SD = 36.53) compared to the control group (M = 2.57, SD = 46.78), t(28) = 1.92, p = .03, d = .71. The omnibus test revealed significant connectivity changes from pre- to post for the left thalamus and all other target ROIs for the aNMT group, F(4, 13) = 7.52, puncorrected = .0023, pcorrected = .0460. Post-hoc analyses revealed significantly increased connectivity between the left thalamus and the right supplementary motor area (SMA) for the aNMT group from pre (M = −.10, SD = .17) to post (M = .07, SD = .18), β = .17, t(16) = 3.37, puncorrected = .0020, pcorrected = .0473. Our regression analyses revealed that greater improvement in the percent change in pKAM was related to increased connectivity at post between the right cerebellum (anterior division) and right thalamus for the aNMT group, t(15) = 4.89, puncorrected = .0001, pcorrected =.0292, r2 = .61 (see Figure 3). No significant pre to post changes in functional connectivity or associations between percent change in pKAM and pre to post change in functional connectivity were observed for the controls (all pcorrected > .05).
Figure 2:
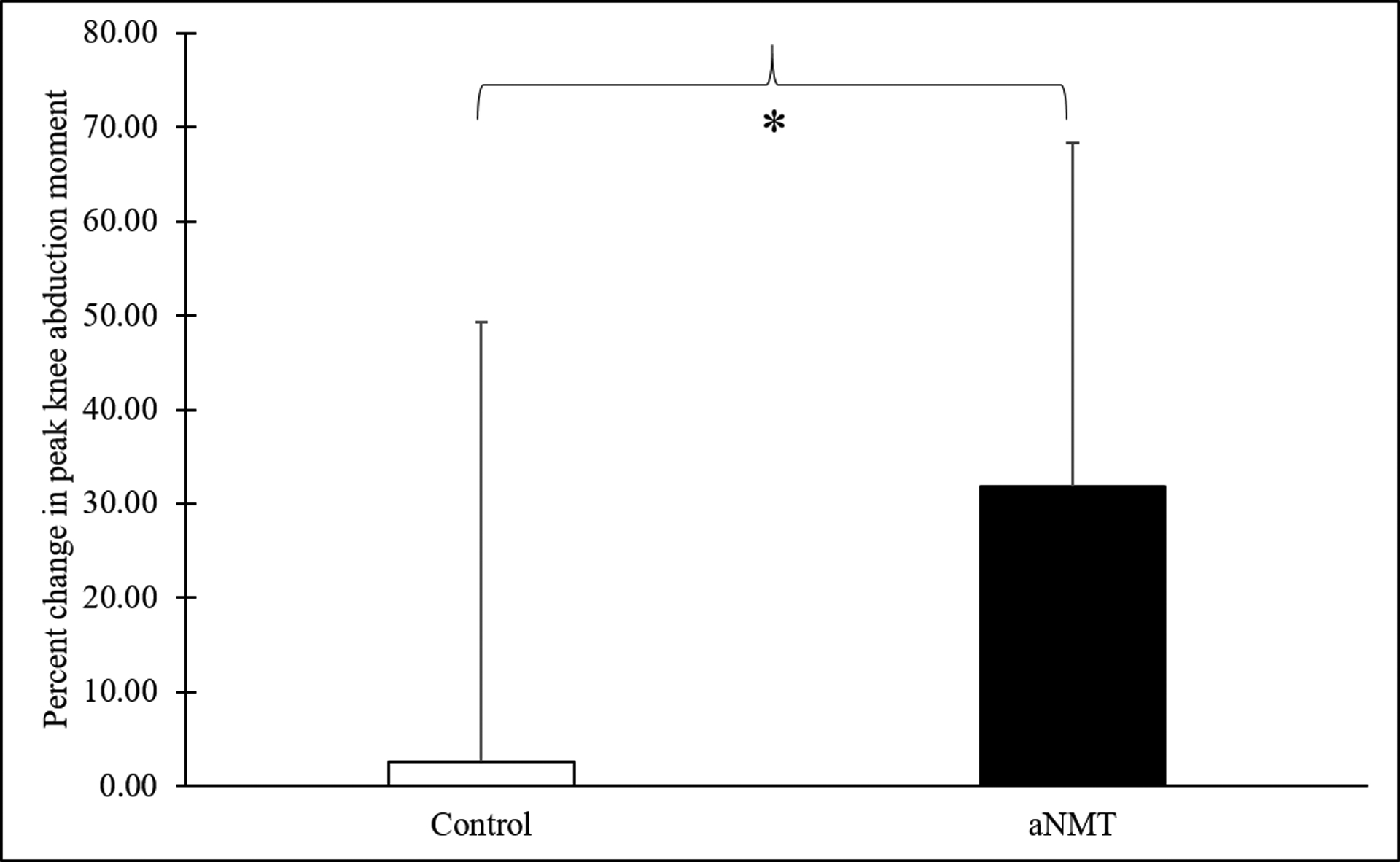
*The percent change in peak knee abduction moment from pre to post was significantly greater (i.e., knee moment decreased) for the augmented neuromuscular training group compared to the control group (p = .03).
Figure 3:
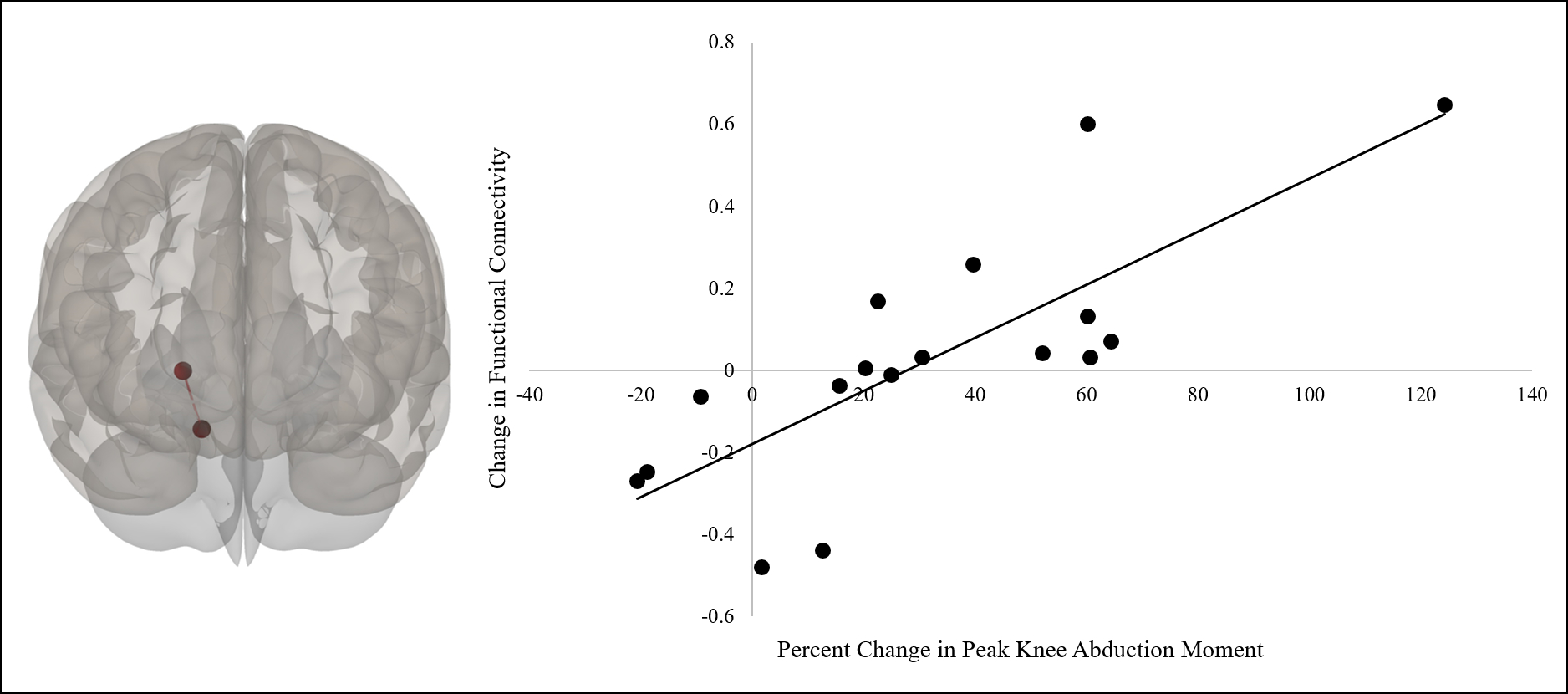
Regression analyses revealed that, for the participants who completed augmented neuromuscular training, greater improvement in the percent change in peak knee abduction moment was related to increased connectivity at post between the right cerebellum (anterior division; more inferior circle) and right thalamus (p =.0292; false discovery rate corrected; more superior circle). Left image is an anterior view of the brain.
4. Discussion
Our biomechanical results are consistent with previous reports demonstrating the capability for altering knee biomechanics while interacting with an aNMT stimulus (Bonnette et al., 2020; Bonnette, DiCesare, Kiefer, et al., 2019) and following six weeks of aNMT (Grooms et al., 2018). Considering depressed functional connectivity within the knee motor network has been noted as a potential neural target to supplement ACL injury prevention (Diekfuss, Grooms, Nissen, et al., 2019; Diekfuss, Grooms, Yuan, et al., 2019), it is noteworthy that our aNMT group, who interacted with a visual stimulus during 6 weeks of training, did significantly increase cortical (SMA)-thalamic connectivity and experienced greater improvements in pKAM that were associated with increased cerebellar-thalamic connectivity. The SMA has been extensively linked to motor control as it is important for initiating and generating movement (Tanji & Hoshi, 2001). The thalamus—traditionally viewed as a ‘brain relay hub’—maintains a critical role in motor control due to its anatomical location between cortical and subcortical motor-related areas, (Bosch-Bouju, Hyland, & Parr-Brownlie, 2013) and the cerebellum is also vitally important to motor control as it contributes to timing of motor activity (Manto et al., 2012).
Resting-state functional connectivity reflects a history of co-activation of spatially distinct brain regions during active states (Corbetta, 2012; Di et al., 2013), which provides a framework for our aNMT-related connectivity findings. The thalamus receives and transmits sensory information from many cortical regions, including the SMA (Rouiller, Tanne, Moret, & Boussaoud, 1999), which plausibly increased activity in response to the sensorimotor demands of aNMT. Neurophysiological studies have demonstrated that integrating two sensory modalities (in our case proprioception and vision) within the same spatially relevant context (i.e., crossmodal integration) involves distinct neural processes (Calvert, 2001). Training with the aNMT biofeedback stimulus required a high degree of cross-modal integration, as the visual feedback changed in real-time with proprioceptive information, which may have strengthened cortical-thalamic connectivity. Neural networks involved in crossmodal operations often include the superior temporal sulcus, the intraparietal sulcus, frontal cortex, parietal regions, and insular/claustrum regions (Calvert, 2001; Calvert, Hansen, Iversen, & Brammer, 2001; Di et al., 2013; Lewis, Beauchamp, & DeYoe, 2000; Macaluso & Driver, 2001). Although our a priori knee network ROI approach precluded investigation into all of the aforementioned regions, our findings of strengthened cortical-thalamic connectivity post-aNMT provide preliminary support that crossmodal integration may be the driving mechanism for aNMT. More specifically, we hypothesize that aNMT may facilitate improved motor planning within the SMA through crossmodal, sensory relay processing enhancements mediated by the thalamus. An interesting area of future investigation may be to evaluate how altered movement mechanics measured during the presentation of the aNMT stimulus (e.g., using a heat map analysis (Bonnette et al., 2020; Bonnette, DiCesare, Kiefer, et al., 2019) contribute to crossmodal-related brain activity in real-time (e.g., using portable electroencephalography).
Our pre to post regression analyses revealed that changes in knee biomechanics were related to strengthened cerebellar-thalamic connectivity for the aNMT group, rather than cortical-thalamic connectivity. On the surface these results may appear divergent, but are likely explained by task constraints. The DVJ is a dynamic, ballistic task that requires a high degree of precision, motor timing, and motor coordination on the part of the athlete that was tested separately from aNMT. The cerebellum contributes to feedforward mechanisms (Pisotta & Molinari, 2014) and sensorimotor timing (Spencer & Ivry, 2013), and may be more involved in the demands of the DVJ, than in than the slower, aNMT-specific NMT exercises. In other words, changes in pKAM as measured by the DVJ may be more sensitive to changes in cerebellar-thalamic connectivity, and may be less related to the above-mentioned crossmodal neural operations occurring during aNMT.
Biofeedback systems are driven by their capability to induce implicit motor learning strategies (i.e., absent of specific explicit instruction) (Bonnette, DiCesare, Diekfuss, et al., 2019; Bonnette et al., 2020; Bonnette, DiCesare, Kiefer, et al., 2019). Implicit learning contributes to reorganization of the motor cortex, (Hirano, Kubota, Koizume, Tanaka, & Funase, 2017) highlighting potential strategies to leverage neuroplasticity. For instance, supplementing ACL injury prevention methods with an external focus (i.e., verbal instruction that directs attention towards the effects of one’s movement (Diekfuss, Rhea, et al., 2019; Wulf, 2013; Wulf, Höβ, & Prinz, 1998)) and/or integrating analogies (i.e., verbal instruction utilizing metaphors (Liao & Masters, 2001)) may enhance functional connectivity through synaptogenic processes (Wulf & Lewthwaite, 2016). Indeed, recent evidence indicates that adopting such motor learning techniques does in fact influence brain activity (Raisbeck, Diekfuss, Grooms, & Schmitz, 2019; Sakurada, Hirai, & Watanabe, 2019). Capitalizing on such implicit processes to enhance motor learning may be especially beneficial for ACL injury prevention methods to engage cortical-thalamic and/or cerebellar-thalamic neural processes. Many musculoskeletal prevention and rehabilitation efforts focus on motor progression using strength training, proprioceptive exercises, range of motion activities, etc. (Ardern et al., 2018; Cavanaugh & Powers, 2017). Motor progression success is often assessed through biomechanical assessment (Hopper, Haff, Joyce, Lloyd, & Haff, 2017; Myer et al., 2005), and while current NMT practices are often beneficial in reducing risk of ACL injury (Petushek, Sugimoto, Stoolmiller, Smith, & Myer, 2019), our data support that neural progression should also be considered as part of current standards of care (Gokeler, Neuhaus, Benjaminse, Grooms, & Baumeister, 2019). Clinicians could potentially leverage motor learning principles (Gokeler et al., 2019; Lewthwaite & Wulf, 2017; Wulf & Lewthwaite, 2016) to engage the appropriate neural networks distinct to various musculoskeletal ailments (Diekfuss, Grooms, Nissen, et al., 2019; Diekfuss, Grooms, Yuan, et al., 2019; Grooms et al., 2017; Silfies, Vendemia, Beattie, Stewart, & Jordon, 2017).
The implicit motor learning strategies that we hypothesize underlie aNMT may also shed light on laterality within the functional connectivity findings. The increase in cortical-thalamic connectivity was localized to the right SMA and left thalamus, whereas the increase in cerebellar-thalamic connectivity was localized to the right hemisphere, only. As the thalamus is important for transmitting information throughout the entire cortex (Sherman & Guillery, 2002), it is interesting that strengthened left and right thalamic connectivity was localized to right sensorimotor regions. Though a clear behavioral role for each hemisphere is inconclusive (i.e., right versus left brain dominance) (Corballis, 2014), there is accumulating evidence that the right hemisphere is particularly involved in intuition and ‘insight-based’ functions (McCrea, 2010), which are highly related to implicit learning in general (Lieberman, 2000). Though cognitive-based measures of intuition were not obtained, many nonverbal coding skills (e.g., processing the tone of a voice) have been shown to rely heavily on networks and associated neural substrates primarily within the right hemisphere (Borod, 1993; Lieberman, 2000; McCrea, 2010). aNMT abstractly displays information that an athlete must use to learn how to coordinate multiple biomechanics variables without explicit instruction (Bonnette et al., 2020; Bonnette, DiCesare, Kiefer, et al., 2019). The aNMT group may have steadily improved their ability to intuitively ‘code’ the perceptual information displayed by the aNMT stimulus, resulting in hyperactive right hemispheric activity that co-activated with the thalamus throughout the intervention. Though lesion and fMRI studies provide neurological data in favor of the right hemisphere for such intuition-based functionality (Goel & Vartanian, 2004; Miller & Tippett, 1996), we stress that such an interpretation is largely speculative. A reasonable alternative explanation could be that participants in the aNMT group varied in terms of right and left side motor dominance (right versus left handedness and footedness) that drove some of the observed effects. Unfortunately, handedness and footedness were not collected in this study, and we emphasize that future large-scale trials should collect these data to further deconstruct the neural mechanisms underlying aNMT-driven biomechanical changes.
4.1. Limitations
While our study provides the first report combining biomechanical and functional connectivity changes that occur following aNMT, the present study is not without its limitations (including our noted failure to include measures of athlete handedness and footedness). First, our control group did not complete a standard NMT (without real-time biofeedback) to deconstruct whether our observed biomechanical and connectivity changes were due to the biofeedback stimulus or NMT, more generally. Second, we elected to examine pre to post changes in connectivity and the associated relationships with biomechanical adaptions independently for each group (rather than longitudinal group-wise differences using ANOVAs) and acknowledge this may not be the strongest statistical model as no interaction effects were examined. However, this approach was chosen to identify proof-of-concept, preliminary neural mechanisms (due to a low sample size) that could guide future, large-scale randomized controlled clinical trials that optimize aNMT for enhanced brain adaptation. Lastly, participants were not randomized to the aNMT or control group at the participant level (one school enrolled in aNMT and a second school enrolled control participants to enhance study design), which raises the potential for bias. Though there were no significant differences in age, height, weight, or pKAM at the pre time point, the pre- to post-aNMT functional connectivity changes and their relation to biomechanical adaptations warrants cautious interpretation due to the present study’s experimental design. Future studies should consider comparing standard NMT to aNMT and/or integrating complementary ‘sham’ conditions (Bonnette, DiCesare, Kiefer, et al., 2019) with larger sample sizes and group randomization to further understand the effect of aNMT on functional connectivity. Further, as superior dose-response relationships are critical for ACL prevention effectiveness (Sugimoto, Myer, Bush, et al., 2012), future large-scale studies should use compliance data as covariates (and/or predictors) and consider additional neuroimaging sessions (e.g., after 2 weeks, 4 weeks, etc.) to further refine the relative effects of aNMT on biomechanics and functional connectivity.
4.2. Conclusion
These data provide preliminary evidence of potential neuroplastic mechanisms underlying changed movement mechanics resulting from NMT, supplemented with real-time, interactive, visual biofeedback. Not only do these data reveal how the brain may be adapting in response to aNMT, but they may help guide researchers who have called for a paradigm shift toward ‘brain-training’ in musculoskeletal rehabilitation (Armijo-Olivo, 2017). In addition to ACL injury prevention, aNMT may be useful for other painful musculoskeletal disorders (e.g., patellofemoral pain) that exhibit alterations in brain structure and function (Silfies et al., 2017). Simple biofeedback modifications (e.g. adjusting the relative gain of visual and sensory information) could potentially leverage neuroplastic mechanisms for enhanced injury prevention and rehabilitation efforts.
Acknowledgements:
The authors would like to thank the athletes, parents, and coaching staff from Madeira and Seton High Schools for their participation in this study. We would also like to thank the SPORT Center staff and interns as well as the IRC technologists, Lacey Haas, Brynne Williams, John Lanier, and Kaley Bridgewater for their support in the success of this project. We also thank Christopher D. Riehm for his assistance in creating Figure 1.
Funding: This project was funded in part with ongoing funding Support from National Institutes of Health/NIAMS Grants U01AR067997 and Cincinnati Children’s Basic Science Research and the Clinical Translational, Outcomes and Health Services Funding.
Footnotes
Conflict of Interest Statement: Gregory D. Myer consults with Commercial entities to support application to the US Food and Drug Administration but has no financial interest in the commercialization of the products. Dr. Myer’s institution receives current and ongoing grant funding from National Institutes of Health/NIAMS Grants U01AR067997, R01 AR070474, and industry sponsored research funding related to brain injury prevention and assessment with Q30 Innovations, LLC, and ElMinda, Ltd. Dr. Myer receives author royalties from Human Kinetics and Wolters Kluwer. Dr. Riley’s institution receives current and ongoing grant funding from National Institutes of Health/NIAMS grant U01AR067997 and NIH/NIDCD grant 1R01DC017301–01. Dr. Riley receives royalties from Springer-Verlag. Drs. Myer, Kiefer and Riley are also an inventors of biofeedback technologies (2017 Non Provisional Patent Pending- Augmented and Virtual reality for Sport Performance and Injury Prevention Application filed 11/10/2016 (62/420,119), Software Copyrighted.) designed to enhance rehabilitation and prevent injuries and has potential for future licensing royalties. Scott Bonnette, Christopher A. DiCesare, Ryan P. MacPherson, Staci Thomas, Brooke Gaad, Kim D. Barber Foss, Katie Kitchen, Dustin R. Grooms, and Jed A. Diekfuss are contributors to the implementation of the biofeedback technology to which the inventors have allocated a portion of the inventor’s share of potential royalties from licensing of technology.
References
- Amad A, Seidman J, Draper SB, Bruchhage MMK, Lowry RG, Wheeler J, … Smith MS (2016). Motor Learning Induces Plasticity in the Resting Brain—Drumming Up a Connection. Cerebral Cortex, 27(3), 2010–2021. 10.1093/cercor/bhw048 [DOI] [PubMed] [Google Scholar]
- Ardern CL, Ekas G, Grindem H, Moksnes H, Anderson A, Chotel F, … Engebretsen L (2018). 2018 International Olympic Committee consensus statement on prevention, diagnosis and management of paediatric anterior cruciate ligament (ACL) injuries. Knee Surg Sports Traumatol Arthrosc, 26(4), 989–1010. 10.1007/s00167-018-4865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armijo-Olivo S (2017). A new paradigm shift in musculoskeletal rehabilitation: why we should exercise the brain? Brazilian journal of physical therapy. 10.1016/j.bjpt.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynnon BD, Vacek PM, Newell MK, Tourville TW, Smith HC, Shultz SJ, … Johnson RJ (2014). The effects of level of competition, sport, and sex on the incidence of first-time noncontact anterior cruciate ligament injury. The American journal of sports medicine, 42(8), 1806–1812. 10.1177/0363546514540862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Magnetic resonance in medicine, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bonnette S, DiCesare CA, Diekfuss JA, Grooms DR, MacPherson RP, Riley MA, & Myer GD (2019). Advancing anterior cruciate ligament injury prevention using real-time biofeedback for amplified sensorimotor integration. Journal of athletic training, 54(9), 985–986. 10.4085/1062-6050-54.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnette S, DiCesare CA, Kiefer AW, Riley MA, Barber-Foss KD, Thomas S, … Myer GD (2020). A technical report on the development of a real-time visual biofeedback system to optimize motor learning and movement deficit correction. Journal of Sports Science & Medicine, 19, 84–94. [PMC free article] [PubMed] [Google Scholar]
- Bonnette S, DiCesare CA, Kiefer AW, Riley MA, Barber Foss KD, Thomas S, … Myer GD (2019). Injury Risk Factors Integrated Into Self-Guided Real-Time Biofeedback Improves High-Risk Biomechanics. Journal of sport rehabilitation, 1–9. 10.1123/jsr.2017-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borod JC (1993). Emotion and the brain—anatomy and theory: An introduction to the Special Section. Neuropsychology, 7(4), 427. [Google Scholar]
- Bosch-Bouju C, Hyland BI, & Parr-Brownlie LC (2013). Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Frontiers in computational neuroscience, 7, 163 10.3389/fncom.2013.00163.eCollection2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA (2001). Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cerebral Cortex, 11(12), 1110–1123. 10.1093/cercor/11.12.1110 [DOI] [PubMed] [Google Scholar]
- Calvert GA, Hansen PC, Iversen SD, & Brammer MJ (2001). Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. Neuroimage, 14(2), 427–438. 10.1006/nimg.2001.0812 [DOI] [PubMed] [Google Scholar]
- Carson DW, & Ford KR (2011). Sex differences in knee abduction during landing: a systematic review. Sports Health, 3(4), 373–382. 10.1177/1941738111410180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, & Powers M (2017). ACL rehabilitation Progression: Where are we now? Current reviews in musculoskeletal medicine, 10(3), 289–296. 10.1007/s12178-017-9426-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari AM, Lindenfeld TN, Andriacchi TP, Hewett TE, Riccobene J, Myer GD, & Noyes FR (2007). Knee and hip loading patterns at different phases in the menstrual cycle: implications for the gender difference in anterior cruciate ligament injury rates. Am J Sports Med, 35(5), 793–800. 10.1177/0363546506297537 [DOI] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, & Friston K (2010). Topological FDR for neuroimaging. Neuroimage, 49(4), 3057–3064. 10.1016/j.neuroimage.2009.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC (2014). Left brain, right brain: facts and fantasies. PLoS biology, 12(1), e1001767 10.1371/journal.pbio.1001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M (2012). Functional connectivity and neurological recovery. Developmental psychobiology, 54(3), 239–253. 10.1002/dev.20507 [DOI] [PubMed] [Google Scholar]
- Cruz A, Bell D, McGrath M, Blackburn T, Padua D, & Herman D (2013). The effects of three jump landing tasks on kinetic and kinematic measures: implications for ACL injury research. Research in sports medicine, 21(4), 330–342. 10.1080/15438627.2013.825798 [DOI] [PubMed] [Google Scholar]
- De La Rue SE, Draper SB, Potter CR, & Smith MS (2013). Energy expenditure in rock/pop drumming. Int J Sports Med, 34(10), 868–872. 10.1055/s-0033-1337905 [DOI] [PubMed] [Google Scholar]
- Demirakca T, Cardinale V, Dehn S, Ruf M, & Ende G (2016). The exercising brain: changes in functional connectivity induced by an integrated multimodal cognitive and whole-body coordination training. Neural plasticity, 2016. 10.1155/2016/8240894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Gohel S, Kim EH, & Biswal BB (2013). Task vs. rest—different network configurations between the coactivation and the resting-state brain networks. Frontiers in human neuroscience, 7, 493 10.3389/fnhum.2013.00493.eCollection2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCesare CA, Kiefer AW, Bonnette SH, & Myer GD (2019). High-Risk Lower-Extremity Biomechanics Evaluated in Simulated Soccer-Specific Virtual Environments. J Sport Rehabil, 1–23. 10.1123/jsr.2018-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekfuss JA, Grooms DR, Nissen KS, Schneider DK, Foss KDB, Thomas S, … Myer GD (2019). Alterations in knee sensorimotor brain functional connectivity contributes to ACL injury in male high-school football players: a prospective neuroimaging analysis. Braz J Phys Ther. 10.1016/j.bjpt.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekfuss JA, Grooms DR, Yuan W, Dudley J, Foss KDB, Thomas S, … Myer GD (2019). Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. Journal of Science and Medicine in Sport, 22(2), 169–174. 10.1016/j.jsams.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekfuss JA, Rhea CK, Schmitz RJ, Grooms DR, Wilkins RW, Slutsky AB, & Raisbeck LD (2019). The Influence of Attentional Focus on Balance Control over Seven Days of Training. Journal of motor behavior, 51(3), 281–292. 10.1080/00222895.2018.1468312 [DOI] [PubMed] [Google Scholar]
- Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Sweeney K, … Delahunt E (2016). Coordination and symmetry patterns during the drop vertical jump in people with chronic ankle instability and lateral ankle sprain copers. Physical therapy, 96(8), 1152–1161. 10.2522/ptj.20150160 [DOI] [PubMed] [Google Scholar]
- Earl JE, Monteiro SK, & Snyder KR (2007). Differences in lower extremity kinematics between a bilateral drop-vertical jump and a single-leg step-down. journal of orthopaedic & sports physical therapy, 37(5), 245–252. 10.2519/jospt.2007.2202 [DOI] [PubMed] [Google Scholar]
- Ericksen HM, Thomas AC, Gribble PA, Armstrong C, Rice M, & Pietrosimone B (2016). Jump–landing biomechanics following a 4-week real-time feedback intervention and retention. Clinical Biomechanics, 32, 85–91. 10.1016/j.clinbiomech.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Ericksen HM, Thomas AC, Gribble PA, Doebel SC, & Pietrosimone BG (2015). Immediate effects of real-time feedback on jump-landing kinematics. journal of orthopaedic & sports physical therapy, 45(2), 112–118. 10.2519/jospt.2015.4997 [DOI] [PubMed] [Google Scholar]
- Etnoyer J, Cortes N, Ringleb SI, Van Lunen BL, & Onate JA (2013). Instruction and jump-landing kinematics in college-aged female athletes over time. Journal of athletic training, 48(2), 161–171. 10.4085/1062-6050-48.2.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KR, DiCesare CA, Myer GD, & Hewett TE (2015). Real-time biofeedback to target risk of anterior cruciate ligament injury: a technical report for injury prevention and rehabilitation. J Sport Rehabil, 2013–0138. 10.2519/jospt.2015.4997 [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, & Hewett TE (2003). Valgus knee motion during landing in high school female and male basketball players. Medicine & Science in Sports & Exercise, 35(10), 1745–1750. 10.1249/01.MSS.0000089346.85744.D9 [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, & Hewett TE (2007a). Reliability of landing 3D motion analysis: implications for longitudinal analyses. Medicine and Science in Sports and Exercise, 39(11), 2021–2028. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, & Hewett TE (2007b). Reliability of landing 3D motion analysis: implications for longitudinal analyses. Medicine & Science in Sports & Exercise, 39(11), 2021–2028. 10.1249/mss.0b013e318149332d [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Schmitt LC, van den Bogert AJ, & Hewett TE (2008). Effect of Drop Height on Lower Extremity Biomechanical Measures in Female Athletes: 859. Medicine & Science in Sports & Exercise, 40(5), S80 10.1249/01.mss.0000321794.25134.e4 [DOI] [Google Scholar]
- Ford KR, Myer GD, Smith RL, Byrnes RN, Dopirak SE, & Hewett TE (2005). Use of an overhead goal alters vertical jump performance and biomechanics. J Strength Cond Res, 19(2), 394–399. 10.1519/15834.1 [DOI] [PubMed] [Google Scholar]
- Ford KR, Shapiro R, Myer GD, Van Den Bogert AJ, & Hewett TE (2010). Longitudinal sex differences during landing in knee abduction in young athletes. Medicine and science in sports and exercise, 42(10), 1923 10.1249/MSS.0b013e3181dc99b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway RT, Xu Y, Hewett TE, Barber Foss KD, Kiefer AW, DiCesare CA, … Myer GD (2018). Age-dependent patellofemoral pain hip and knee risk landing profiles in prepubescent and postpubescent female athletes. The American journal of sports medicine, 46(11), 2761–2771. 10.1177/0363546518788343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, & Vartanian O (2004). Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cerebral Cortex, 15(8), 1170–1177. 10.1093/cercor/bhh217 [DOI] [PubMed] [Google Scholar]
- Gokeler A, Neuhaus D, Benjaminse A, Grooms DR, & Baumeister J (2019). Principles of Motor Learning to Support Neuroplasticity After ACL Injury: Implications for Optimizing Performance and Reducing Risk of Second ACL Injury. Sports Medicine, 1–13. 10.1007/s40279-019-01058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, … Ireland ML (2000). Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 8(3), 141–150. [DOI] [PubMed] [Google Scholar]
- Grooms DR, Kiefer AW, Riley MA, Ellis JD, Thomas S, Kitchen K, … Myer GD (2018). Brain-behavior mechanisms for the transfer of neuromuscular training adaptions to simulated sport: Initial findings from the train the brain project. Journal of Sport Rehabilitation, 27(5), 1–5. 10.1123/jsr.2017-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms DR, & Onate JA (2016). Neuroscience application to noncontact anterior cruciate ligament injury prevention. Sports Health, 8(2), 149–152. 10.1177/1941738115619164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, & Onate JA (2017). Neuroplasticity associated with anterior cruciate ligament reconstruction. journal of orthopaedic & sports physical therapy, 47(3), 180–189. 10.2519/jospt.2017.7003 [DOI] [PubMed] [Google Scholar]
- Grooms DR, Page SJ, & Onate JA (2015). Brain Activation for Knee Movement Measured Days Before Second Anterior Cruciate Ligament Injury: Neuroimaging in Musculoskeletal Medicine. Journal of athletic training, 50(10), 1005–1010. 10.4085/1062-6050-50.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Carrillo B, Mackey AP, & Bunge SA (2014). Resting-state fMRI: a window into human brain plasticity. The Neuroscientist, 20(5), 522–533. 10.1177/1073858414524442 [DOI] [PubMed] [Google Scholar]
- Hewett TE, Ford KR, & Myer GD (2006). Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. The American journal of sports medicine, 34(3), 490–498. 10.1177/0363546505282619 [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Heidt RS, Colosimo AJ, McLean SG, … Succop P (2005). Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes. The American journal of sports medicine, 33(4), 492–501. 10.1177/0363546504269591 [DOI] [PubMed] [Google Scholar]
- Hewett TE, Torg JS, & Boden BP (2009). Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med, 43(6), 417–422. 10.1136/bjsm.2009.059162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Kubota S, Koizume Y, Tanaka S, & Funase K (2017). Different Effects of Implicit and Explicit Motor Sequence Learning on Latency of Motor Evoked Potential Evoked by Transcranial Magnetic Stimulation on the Primary Motor Cortex. Frontiers in human neuroscience, 10, 671 10.3389/fnhum.2016.00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper AJ, Haff EE, Joyce C, Lloyd RS, & Haff GG (2017). Neuromuscular training improves lower extremity biomechanics associated with knee injury during landing in 11–13 year old female netball athletes: A randomized control study. Frontiers in physiology, 8, 883 10.3389/fphys.2017.00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland ML (2002). The female ACL: why is it more prone to injury? Orthopedic Clinics, 33(4), 637–651. 10.1016/s0030-5898(02)00028-7 [DOI] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Kelekis D, Peeters R, … Sunaert S (2007). Lower limb sensorimotor network: issues of somatotopy and overlap. Cortex, 43(2), 219–232. 10.1016/s0010-9452(08)70477-5 [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, … Bahr R (2007). Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. The American journal of sports medicine, 35(3), 359–367. 10.1177/0363546506293899 [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Slauterbeck JR, Engebretsen L, & Bahr R (2007). Biomechanical analysis of anterior cruciate ligament injury mechanisms: three‐dimensional motion reconstruction from video sequences. Scandinavian journal of medicine & science in sports, 17(5), 508–519. 10.1111/j.1600-0838.2006.00558.x [DOI] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, & DeYoe EA (2000). A comparison of visual and auditory motion processing in human cerebral cortex. Cerebral Cortex, 10(9), 873–888. 10.1093/cercor/10.9.873 [DOI] [PubMed] [Google Scholar]
- Lewthwaite R, & Wulf G (2017). Optimizing motivation and attention for motor performance and learning. Current Opinion in Psychology. 10.1016/j.copsyc.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Liao C-M, & Masters RS (2001). Analogy learning: A means to implicit motor learning. Journal of sports sciences, 19(5), 307–319. [DOI] [PubMed] [Google Scholar]
- Lieberman MD (2000). Intuition: a social cognitive neuroscience approach. Psychological bulletin, 126(1), 109 10.1037/0033-2909.126.1.109 [DOI] [PubMed] [Google Scholar]
- Limroongreungrat W, & Boonkerd C (2019). Immediate effect of ACL kinesio taping technique on knee joint biomechanics during a drop vertical jump: a randomized crossover controlled trial. BMC Sports Science, Medicine and Rehabilitation, 11(1), 32 10.1186/s13102-019-0144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, & Xiong J (2011). Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage, 58(1), 226–233. 10.1016/j.neuroimage.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, & Driver J (2001). Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia, 39(12), 1304–1316. 10.1016/s0028-3932(01)00119-1 [DOI] [PubMed] [Google Scholar]
- Malfait B, Sankey S, Azidin R, Deschamps K, Vanrenterghem J, Robinson MA, … Verschueren S (2014). How reliable are lower-limb kinematics and kinetics during a drop vertical jump. Med Sci Sports Exerc, 46(4), 678–685. 10.1249/MSS.0000000000000170 [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, … Mariën P (2012). Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. The Cerebellum, 11(2), 457–487. 10.1007/s12311-011-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea SM (2010). Intuition, insight, and the right hemisphere: Emergence of higher sociocognitive functions. Psychology research and behavior management, 3, 1 10.2147/prbm.s7935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SG, Fellin R, Suedekum N, Calabrese G, Passerallo A, & Joy S (2007). Impact of fatigue on gender-based high-risk landing strategies. Medicine and science in sports and exercise, 39(3), 502–514. 10.1249/mss.0b013e3180d47f0 [DOI] [PubMed] [Google Scholar]
- Miller LA, & Tippett LJ (1996). Effects of focal brain lesions on visual problem-solving. Neuropsychologia, 34(5), 387–398. 10.1016/0028-3932(95)00116-6 [DOI] [PubMed] [Google Scholar]
- Myer GD, Chu DA, Brent JL, & Hewett TE (2008). Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clin Sports Med, 27(3), 425–448, ix. 10.1016/j.csm.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Foss KDB, Goodman A, Ceasar A, Rauh MJ, … Hewett TE (2010). The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clinical biomechanics, 25(7), 700–707. 10.1016/j.clinbiomech.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Foss KDB, Liu C, Nick TG, & Hewett TE (2009). The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clinical journal of sport medicine, 19(1), 3–8. 10.1097/JSM.0b013e318190bddb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Palumbo JP, & Hewett TE (2005). Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res, 19(1), 51–60. 10.1519/13643.1 [DOI] [PubMed] [Google Scholar]
- Myer GD, Stroube BW, DiCesare CA, Brent JL, Ford KR, Heidt RS Jr, & Hewett TE (2013). Augmented feedback supports skill transfer and reduces high-risk injury landing mechanics: a double-blind, randomized controlled laboratory study. The American journal of sports medicine, 41(3), 669–677. 10.1177/0363546512472977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GDD, Ford KR, Di Stasi SL, Foss KD, Micheli LJ, & Hewett TE (2015). High knee abduction moments are common risk factors for patellofemoral pain (PFP) and anterior cruciate ligament (ACL) injury in girls: is PFP itself a predictor for subsequent ACL injury? Br J Sports Med, 49(2), 118–122. 10.1136/bjsports-2013-092536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua DA, DiStefano LJ, Marshall SW, Beutler AI, de la Motte SJ, & DiStefano MJ (2012). Retention of movement pattern changes after a lower extremity injury prevention program is affected by program duration. The American journal of sports medicine, 40(2), 300–306. 10.1177/0363546511425474 [DOI] [PubMed] [Google Scholar]
- Paterno MV (2017). Non-operative Care of the Patient with an ACL-Deficient Knee. Current reviews in musculoskeletal medicine, 10(3), 322–327. 10.1007/s12178-017-9431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, & Hewett TE (2010). Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine, 38(10), 1968–1978. 10.1177/0363546510376053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petushek EJ, Sugimoto D, Stoolmiller M, Smith G, & Myer GD (2019). Evidence-based best-practice guidelines for preventing anterior cruciate ligament injuries in young female athletes: a systematic review and meta-analysis. The American journal of sports medicine, 47(7), 1744–1753. 10.1177/0363546518782460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisotta I, & Molinari M (2014). Cerebellar contribution to feedforward control of locomotion. Frontiers in human neuroscience, 8, 475. doi: 10.3389/fnhum.2014.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic T, Caswell SV, Benjaminse A, Siragy T, Ambegaonkar J, & Cortes N (2018). Implicit video feedback produces positive changes in landing mechanics. Journal of experimental orthopaedics, 5(1), 12 10.1186/s40634-018-0129-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers CM, & Fisher B (2010). Mechanisms underlying ACL injury-prevention training: the brain-behavior relationship. Journal of athletic training, 45(5), 513–515. 10.4085/1062-6050-45.5.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisbeck LD, Diekfuss JA, Grooms DR, & Schmitz R (2019). The Effects of Attentional Focus on Brain Function During a Gross Motor Task. J Sport Rehabil, 1–7. 10.1123/jsr.2018-0026 [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Tanne J, Moret V, & Boussaoud D (1999). Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: a multiple retrograde tracing study. Journal of Comparative Neurology, 409(1), 131–152. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Hirai M, & Watanabe E (2019). Individual optimal attentional strategy during implicit motor learning boosts frontoparietal neural processing efficiency: A functional near‐infrared spectroscopy study. Brain and behavior, 9(1), e01183 10.1002/brb3.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TL, Maradit Kremers H, Bryan AJ, Larson DR, Dahm DL, Levy BA, … Krych AJ (2016). Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. The American journal of sports medicine, 44(6), 1502–1507. 10.1177/0363546516629944 [DOI] [PubMed] [Google Scholar]
- Schmitz RJ, Cone JC, Tritsch AJ, Pye ML, Montgomery MM, Henson RA, & Shultz SJ (2014). Changes in drop-jump landing biomechanics during prolonged intermittent exercise. Sports Health, 6(2), 128–135. 10.1177/1941738113503286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld BJ (2010). Squatting kinematics and kinetics and their application to exercise performance. The Journal of Strength & Conditioning Research, 24(12), 3497–3506. 10.1519/JSC.0b013e3181bac2d7 [DOI] [PubMed] [Google Scholar]
- Sherman SM, & Guillery R (2002). The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 357(1428), 1695–1708. 10.1098/rstb.2002.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silfies SP, Vendemia JMC, Beattie PF, Stewart JC, & Jordon M (2017). Changes in Brain Structure and Activation May Augment Abnormal Movement Patterns: An Emerging Challenge in Musculoskeletal Rehabilitation. Pain Med, 18(11), 2051–2054. 10.1093/pm/pnx190 [DOI] [PubMed] [Google Scholar]
- Spencer RM, & Ivry RB (2013). Cerebellum and timing. Handbook of the cerebellum and cerebellar disorders, 1201–1219. [Google Scholar]
- Steffen K, Myklebust G, Olsen OE, Holme I, & Bahr R (2008). Preventing injuries in female youth football–a cluster‐randomized controlled trial. Scandinavian journal of medicine & science in sports, 18(5), 605–614. 10.1111/j.1600-0838.2007.00703.x [DOI] [PubMed] [Google Scholar]
- Sugimoto D, Myer GD, Bush HM, Klugman MF, McKeon JMM, & Hewett TE (2012). Compliance with neuromuscular training and anterior cruciate ligament injury risk reduction in female athletes: a meta-analysis. Journal of athletic training, 47(6), 714–723. 10.4085/1062-6050-47.6.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto D, Myer GD, Foss KDB, & Hewett TE (2014). Dosage effects of neuromuscular training intervention to reduce anterior cruciate ligament injuries in female athletes: meta-and sub-group analyses. Sports Medicine, 44(4), 551–562. 10.1007/s40279-013-0135-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto D, Myer GD, Foss KDB, & Hewett TE (2015). Specific exercise effects of preventive neuromuscular training intervention on anterior cruciate ligament injury risk reduction in young females: meta-analysis and subgroup analysis. Br J Sports Med, 49(5), 282–289. [DOI] [PubMed] [Google Scholar]
- Sugimoto D, Myer GD, McKeon JM, & Hewett TE (2012). Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br J Sports Med, 46(14), 979–988. 10.1136/bjsports-2014-093461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (Producer). (2007). Using multivariate statistics, 5th ed. Using multivariate statistics, 5th ed. [Google Scholar]
- Tanji J, & Hoshi E (2001). Behavioral planning in the prefrontal cortex. Current opinion in neurobiology, 11(2), 164–170. 10.1016/S0959-4388(00)00192-6 [DOI] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, & Ragert P (2011). Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage, 57(4), 1492–1498. 10.1016/j.neuroimage.2011.05.078 [DOI] [PubMed] [Google Scholar]
- Taylor JB, Ford KR, Schmitz RJ, Ross SE, Ackerman TA, & Shultz SJ (2017). Biomechanical differences of multidirectional jump landings among female basketball and soccer players. The Journal of Strength & Conditioning Research, 31(11), 3034–3045. 10.1519/JSC.0000000000001785 [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, … White SM (2010). Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in aging neuroscience, 2, 32 10.3389/fnagi.2010.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wulf G (2013). Attentional focus and motor learning: a review of 15 years. International Review of Sport and Exercise Psychology, 6(1), 77–104. 10.1080/1750984X.2012.723728 [DOI] [Google Scholar]
- Wulf G, Höβ M, & Prinz W (1998). Instructions for motor learning: Differential effects of internal versus external focus of attention. Journal of motor behavior, 30(2), 169–179. 10.1080/00222899809601334 [DOI] [PubMed] [Google Scholar]
- Wulf G, & Lewthwaite R (2016). Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon Bull Rev Psychonomic Bulletin & Review(2). 10.3758/s13423-015-0999-9 [DOI] [PubMed] [Google Scholar]
- Yoo K, Sohn WS, & Jeong Y (2013). Tool-use practice induces changes in intrinsic functional connectivity of parietal areas. Frontiers in human neuroscience, 7, 49 10.3389/fnhum.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazulak BT, Hewett TE, Reeves NP, Goldberg B, & Cholewicki J (2007). Deficits in neuromuscular control of the trunk predict knee injury risk: prospective biomechanical-epidemiologic study. The American journal of sports medicine, 35(7), 1123–1130. 10.1177/0363546507301585 [DOI] [PubMed] [Google Scholar]


