Abstract
Neonatal necrotizing enterocolitis (NEC) is a serious gastrointestinal disease with high death rate in premature infants. Fish oil (FO) and its constituents have been shown to ameliorate intestinal inflammation and mucosal damage. However, the underlying mechanism of action is not known. In the present study, we divided Sprague-Dawley rats into three groups: control group, NEC model group, and FO pre-feeding+NEC model group. Briefly, one week before NEC modeling, in addition to being fed with milk, the FO pre-feeding+NEC modeling group was fed with FO, the NEC group was fed with saline, and the control group was only inserted a gastric-tube for 7 days. Subsequently, histological assay, Western blot, and ELISA were performed. Pretreatment with FO attenuated the NEC symptoms, alleviated intestinal pathological injury, and decreased the expressions of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Furthermore, pretreatment with FO reduced the expressions of endoplasmic reticulum stress (ERS) related proteins, caspase-12, and glucose-regulated protein 78 (GRP78). In addition, intestinal histopathological scores showed a significant positive correlation with intestinal expressions of IL-6, TNF-α, and caspase-12. Collectively, these results indicate that ERS pathway might be involved in the effect of FO in alleviating intestinal mucosal inflammation and injury in rats with NEC
Subject terms: Immunization, Inflammatory bowel disease
Introduction
Neonatal necrotizing enterocolitis (NEC) is a serious gastrointestinal disease that affects premature infants and is associated with a high mortality rate. Various factors such as premature birth, formula feeding, intestinal ischemia, and bacterial colonization are known to activate the intestinal inflammatory response that ultimately induces NEC1. A variety of inflammatory factors such as platelet activating factor (PAF), tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-8 have been implicated in the pathogenesis of NEC2. Elevated levels of IL-6 and IL-8 were found in infants with NEC3. During the process of NEC, TNF-α was shown to induce significant mitochondrial dysfunction and mitochondrial apoptosis, which eventually led to apoptosis of intestinal epithelial cells4. Recently, the anti-inflammatory effects of docosahexaenoic acid (DHA) and arachidonic acid were shown to prevent NEC in premature infants5. Insulin-like growth factor I6 and berberine7 were shown to attenuate inflammation and ameliorate the clinical symptoms and progression of NEC. Taken together, the mechanism for alleviating NEC effectively is worthy of study.
The endoplasmic reticulum (ER) is an organelle with characteristic flattened, membrane-enclosed sacs or tube-like structures. ER includes rough ER and smooth ER. Rough ER is studded with ribosomes and plays a role in protein synthesis, while the smooth ER is involved in lipid synthesis and metabolism. Harmful stimuli such as hypoxia, infection, oxidative stress, and harmful metabolites disrupt homeostasis in ER and cause ER dysfunction which further induces endoplasmic reticulum stress (ERS). ERS triggers the expressions of unfolded protein response (UPR) and glucose-regulated protein 78 (GRP78) in the cytoplasm. Hence, GRP78 is a classical marker of ERS8. In addition, caspase-12, which is located on the cytoplasmic side of the ER, was shown to eliminate damaged cells that could not be repaired in time; this process was mediated via an ER-specific apoptosis pathway9. Failure of UPR to restore ER homeostasis leads to cellular apoptosis and local inflammation. Lu et al.10. demonstrated that ERS and UPR in NEC were associated with increased levels of IL-6 and IL-8 along with severe epithelial injury. Therefore, the relationship between ERS and NEC needs to be further clarified.
Fish Oil (FO) is rich in DHA, eicosapentaenoic acid (EPA), and ω-3 polyunsaturated fatty acids (ω-3 PUFAs). ω-3 PUFAs in FO were shown to ameliorate inflammation and mucosal damage in ulcerative colitis11. In addition, it was shown to attenuate intestinal inflammation and reduce the incidence of NEC in neonatal rats12. It was also shown to prevent ERS-mediated apoptosis of primary rat hepatocytes13. The anti-inflammatory effects of DHA are mediated via modulation of ER homeostasis14. DHA was shown to protect astrocytes against ischemic injury15 and muscle cells against palmitate-induced atrophy via inhibition of ERS16. EPA enhanced the integrity of the intestinal mucosal epithelial barrier via up-regulation of the expression of tight junction proteins in a rat model of heatstroke17. In addition, it was shown to attenuate statin-induced ERS in cultured myoblast cells18. These findings indicate the protective effect of FO in intestinal cells. However, further studies are required to unravel the underlying mechanism of the protective effect.
In the present study, we investigated the ERS-coupled inflammatory response by determining the expressions of ERS-related marker proteins and pro-inflammatory cytokines in intestinal tissue of neonatal rats with NEC. We further evaluated the repressive effect of FO on ERS-coupled intestinal inflammation with an aim to uncover the therapeutic potential of FO against NEC.
Materials and methods
Animals and experimental design
A total of 96 one-day-old neonatal Sprague-Dawley (SD) rats (clean grade, body weight: 5–10 g) were randomly divided into three equal groups (control, NEC, and FO pre-feeding +NEC groups, n = 32). Rats in the control group were fed with milk throughout the experiment, and were inserted with gastric tube once a day for 7 days; subsequently, they were administered an equal amount of saline by gavage once a day at 12:00 Hrs for three days. In addition to being fed with milk, rats in the NEC group were administered saline by gavage for seven days till the day of performing NEC modeling with “formula feeding + hypoxia + cold stimulation + LPS gavage” combined multi-factor method for three days. In addition to milk, rats in the FO pre-feeding +NEC group were pre-fed with FO (contains 35% of DHA and EPA totally, 0.6 mL/100 g/d) (Sigma, St. Louis, MO, USA) by gavage for seven days (based on our preliminary experiment) till the day of performing NEC modeling. All rats in each group were housed in a single cage at a temperature of 25 °C–30 °C and 50–60% humidity with a 12 h light: 12 h dark cycle. During the experiments, the behavior of animals and the state of their feces were recorded. NEC modeling was performed according to the protocols published by Hunter et al.19 and Welak et al.20 Briefly, all rats were administered 0.4 mL formula milk four times (08:00 Hrs, 12:00 Hrs, 16:00 Hrs, 20:00 Hrs) a day by gavage. At 08:00 Hrs and 20:00 Hrs, rats in the NEC group and FO pre-feeding +NEC group were placed in hypoxic chambers (nitrogen flow: 15 L/min) twice a day for three days. The rats were placed in the hypoxic chambers for 90 s when the oxygen concentration in the chamber was down to 0, and subsequently removed to 4 °C for 10 mins. At 12:00 Hrs, rats in the NEC group and FO pre-feeding +NEC group were administered 10 mg/kg BW lipopolysaccharide (LPS, Sigma, St. Louis, MO, USA) by gavage at 12:00 Hrs every day for three days.
At 0 h, 24 h, 48 h, and 72 h of NEC modeling, eight rats in each group were randomly sacrificed and their intestinal samples were collected. This study was carried out in accordance with the Guiding Principles for the Care and Use of Laboratory Animals of the Soochow University. Animals were treated in accordance with Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press). The experimental protocols were approved by the Ethics Committee for Animal Experiments at the Soochow University (NO.2013LW003).
Histological assay
Intestinal tissues (2 cm proximal to the ileocecal region) were collected and fixed in 4% paraformaldehyde. Paraffin sections (thickness: 5 μm) were prepared for hematoxylin & eosin (HE) staining prior to histopathological examination. The intestinal histopathological damage was scored by a single-blinded evaluator according to a standard described by Nadler et al.21 (Table 1). Three random fields (40×) from each sample were evaluated and the rats were diagnosed as NEC when the score was greater than two.
Table 1.
Standard criteria for intestinal histopathological scoring.
| Score | Histological morphology |
|---|---|
| 0 | Intestinal villus and mucosa epithelium are intact |
| 1 | Slight separation between the sub-mucosa and the lamina propria |
| 2 |
Moderate separation between the sub-mucosa and the lamina propria OR sub-mucosal edema OR muscular layer edema |
| 3 |
Marked separation between the sub-mucosa and lamina propria OR severe sub-mucosal edema OR severe muscular layer edema OR villous fall off |
| 4 |
Disappearance of intestinal villi OR intestinal villus necrosis |
For immunostaining, the intestinal cross sections were incubated overnight with caspase-12 (SC-21747, Santa Cruz Biotechnology Inc, Dallas, TX, USA) or GRP78 (MB0050, Bioworld, USA) primary antibody at 4 °C. Subsequently, biotin anti-rabbit IgG and 3,3’-diaminobenzidine (DAB) were used for visualization. The results were expressed as gray value analysis in five random fields (40×) from each section by using Image-Pro-Plus 5.0 software.
Western Blot
The total protein from intestinal samples (0.1 g) was extracted by lysis using 0.3 mL RIPA buffer (Beyotime, Jiangsu, China) with 1% PMSF (ST506, Beyotime, Jiangsu, China). The total protein was separated by SDS-PAGE, followed by electro-transfer to a polyvinylidene fluoride membrane (PVDF, Millipore, Billerica, MA, USA). The PVDF membrane was incubated overnight with caspase-12 primary antibody (1:1000) at 4 °C. After incubation with secondary antibody (1:3000) for 1 h at 37 °C, the membranes were visualized using an ECL system (Bio Rad Laboratones, Inc., Hercules, CA, USA). The amount of protein was determined using the Image J software. The data were expressed as the gray value of the bands normalized to the gray value of the corresponding β-actin bands. Each experiment was performed in triplicate.
ELISA
The intestinal tissue was homogenized in ice-cold physiological saline (PBS). The supernatant was harvested following centrifugation at 5000 rpm for 15 min at 4 °C and was stored at −80 °C. The levels of intestinal cytokines IL-6 and TNF-α were determined using a commercial ELISA kit (R&D system, US) according to the manufacturer’s instructions. The OD values were determined at a wavelength of 450 nm. Standard curve was used to calculate the concentrations of IL-6 and TNF-α. Each sample was analyzed in triplicate
Data analysis
Data are presented as mean ± standard error of the mean (SEM). Differences between various groups were assessed using one-way ANOVA. SPSS 20.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analyses. Least significant difference (LSD) post hoc multiple comparisons test was used to determine the statistical differences. P values <0.05 were considered indicative of statistical significance.
Ethical approval and informed consent
1. Approval: The experimental protocols were approved by the Ethics Committee for Animal Experiments at the Soochow University (NO.2013LW003).
2. Accordance: The methods were carried out in accordance with the relevant guidelines and regulations.
Results
FO attenuates intestinal mucosal injury in rats with NEC
In contrast to the control group, rats in the NEC group developed symptoms such as abdominal distension, diarrhea, respiratory distress, and watery stools. At necropsy performed at 72 h of NEC modeling (Fig. 1), the intestines of rats in the NEC group showed black appearance with signs of luminal flatulence. Histopathological analysis (Fig. 2, Table 2) revealed gradual aggravation of intestinal mucosal injury with progression of NEC modeling. At 72 h of NEC modeling, most of the intestinal villi in the NEC group exhibited disordered arrangement, fall off, or necrosis. Further, separation between sub-mucosa and lamina propria, extensive neutrophil infiltration in the mucosa, and sub-mucosal edema were observed. Moreover, the pathological scores of NEC group were significantly elevated during NEC progression. Symptoms in the FO + NEC group (such as diarrhea, respiratory distress, watery stool, and luminal flatulence) were much milder than those in the NEC group. At 24 h, 48 h and 72 h, the intestinal pathological scores in the FO + NEC group were lower than those in the NEC group by 38.79%, 27.49%, and 36.20%, respectively (P < 0.05 at all 3 time-points).
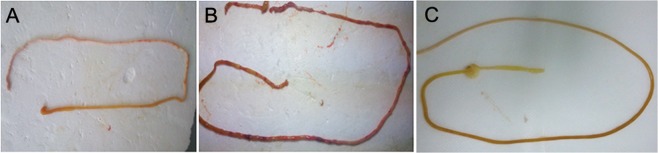
Figure 1.
Necropsy findings of rats with NEC. (A) Intestinal morphology in the control group shows no signs of obvious intestinal luminal flatulence. (B) At 72 h of NEC modeling, the small intestine in NEC group showed black appearance with signs of luminal flatulence. (C) At 72 h of NEC modeling, the small intestine in the FO + NEC group exhibited deep yellow appearance with no obvious signs of intestinal luminal flatulence.
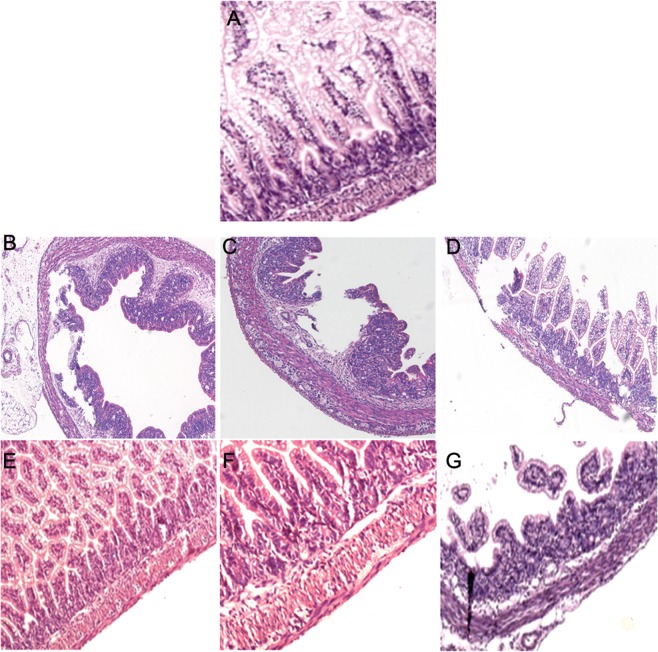
Figure 2.
Histopathological analysis of intestine of rats with NEC. (A) Intestinal tissue of the control group showing intact intestinal villi and mucosal epithelium. (B–D) At 24 h, 48 h, and 72 h of NEC modeling, the intestinal mucosa in NEC group exhibited separation between the sub-mucosa and the lamina propria, intestinal villus fall off, and mucosal neutrophil infiltration. (E–G) At 24 h, 48 h, and 72 h of NEC modeling, FO pre-feeding attenuated NEC-induced intestinal mucosal pathological damage.
Table 2.
Intestinal histopathological scores of rats in various study groups (n = 8).
| Group | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Control | 0.04 ± 0.12 | 0.08 ± 0.15 | 0.08 ± 0.15 | 0.12 ± 0.17 |
| NEC | 0.04 ± 0.12 | 1.16 ± 0.31*Δ | 1.71 ± 0.45*Δ | 2.79 ± 0.66*Δ |
| FO + NEC | 0.04 ± 0.12 | 0.71 ± 0.28*#Δ | 1.24 ± 0.34*#Δ | 1.78 ± 0.56*#Δ |
*P < 0.05 versus control group; #P < 0.05 versus NEC group; △P < 0.05 versus the immediately preceding time –point.
FO attenuated intestinal mucosal inflammation in rats with NEC
Consistently lower expressions of intestinal IL-6 and TNF-α were observed in the control group (Tables 3 and 4). During NEC modeling, the expression levels of IL-6 and TNF-α showed a gradual increase over time. The IL-6 levels in the NEC group at 24, 48, and 72 h were higher than the respective levels in the control group by 7.98%, 51.89%, and 71.90%, respectively (P < 0.05 at all 3 time-points). Similarly, the TNF-α level in the NEC group were higher than those in the control group by 4.59%, 33.98%, and 69.16%, respectively. High expression levels of IL-6 and TNF-α were observed in the FO + NEC group at 24 h, 48 h and 72 h; however, the IL-6 levels in the FO + NEC group at 24, 48, and 72 h were lower than those in the NEC group by 4.30%, 9.40%, and 13.70%, respectively (P < 0.05), while the TNF-α levels in FO + NEC group were lower than those in the NEC group by 3.07%, 8.26%, and 11.37%, respectively.
Table 3.
Rat intestinal IL-6 expression in various study groups (pg/mL, n = 8).
| Group | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Control | 146.34 ± 8.49 | 149.55 ± 12.59 | 151.21 ± 11.69 | 155.87 ± 12.01 |
| NEC | 147.26 ± 10.81 | 161.49 ± 12.57Δ | 229.68 ± 13.64*Δ | 267.94 ± 20.94*Δ |
| FO + NEC | 150.05 ± 9.39 | 154.54 ± 10.50 | 208.09 ± 15.42*#Δ | 231.24 ± 18.16*#Δ |
*P < 0.05 versus control group; #P < 0.05 versus NEC group; △P < 0.05 versus the immediately preceding time –point.
Table 4.
Rat intestinal TNF-α expression in various study groups (pg/mL, n = 8).
| Group | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Control | 146.32 ± 7.57 | 149.11 ± 8.73 | 149.40 ± 9.78 | 151.04 ± 10.12 |
| NEC | 146.10 ± 8.75 | 155.95 ± 9.24Δ | 200.16 ± 12.97*Δ | 255.50 ± 16.05*Δ |
| FO + NEC | 145.74 ± 9.12 | 151.17 ± 10.43 | 183.63 ± 13.78*#Δ | 226.46 ± 15.01*#Δ |
*P < 0.05 versus control group; #P < 0.05 versus NEC group; △P < 0.05 versus the immediately preceding time –point.
Effect of FO on intestinal caspase-12 protein expression in rats with NEC
In the present study, caspase-12-positive cells exhibited yellow-brown staining in the cytoplasm, and were mainly distributed around the intestinal mucosal epithelial cells and in the sub-mucosa (Fig. 3). The results of Western blotting showed that the protein expressions of caspase-12 in the NEC group at 24 h, 48 h and 72 h of NEC modeling were greater than those in the control group by 22.02%, 45.25%, and 77.12%, respectively (P < 0.05 at all 3 time-points) (Fig. 4, Table 5). After pretreatment with FO, the expressions of caspase-12 at 24 h, 48 h, and 72 h of NEC modeling were greater than those in the control group by 6.61%–40.28%, but were lower than those in the NEC group by 12.63%, 17.57%, and 20.80%, respectively (P < 0.05 at all 3 time-points).
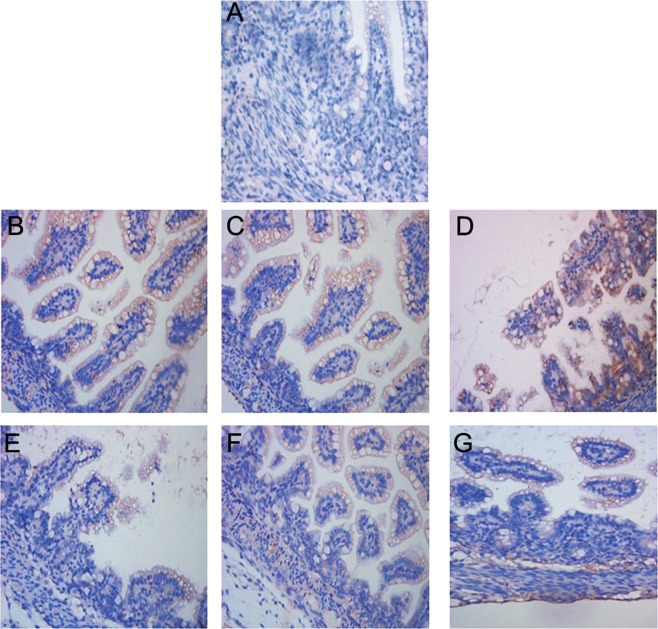
Figure 3.
Expression analysis of caspase-12 in intestine of rats with NEC. (A) Immunostaining for intestinal caspase-12 protein in the control group showing caspase-12-positive cells mainly located in the intestinal epithelial cells and the sub-mucosa. (B–D) At 24 h, 48 h, and 72 h of NEC modeling, the Caspase-12-positive cells in the NEC group are mainly located around the intestinal epithelial cells, mucosa and the sub-mucosa. (E–G) At 24 h, 48 h, and 72 h of NEC modeling, the expression of intestinal caspase-12 protein in the intestinal mucosa in the FO + NEC group is markedly reduced.
Figure 4.
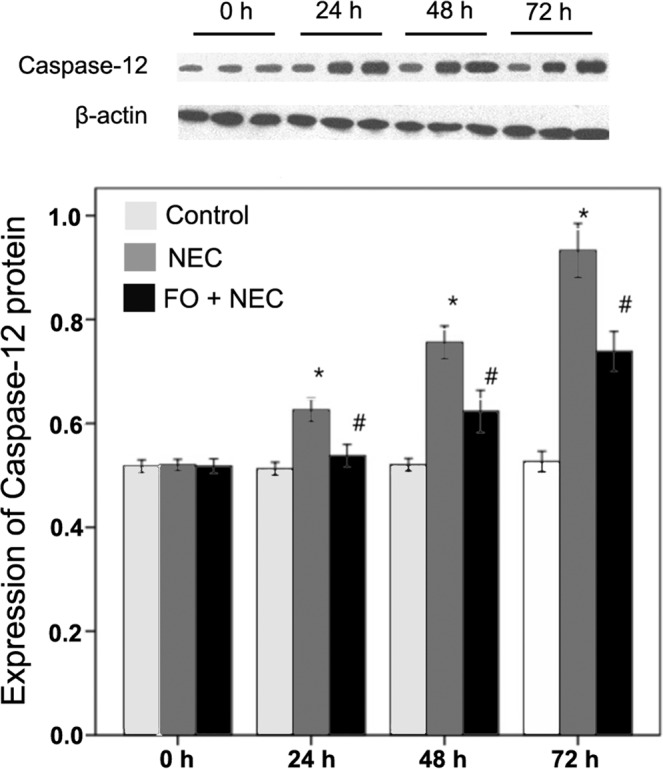
Quantitative analysis of intestinal caspase-12 protein by Western blot. The histogram represents the ratio of optical density of caspase-12 to that of β-actin. *P < 0.05 for NEC versus control group; #P < 0.05 for FO + NEC versus NEC group.
Table 5.
Gray value analysis of intestinal caspase-12 protein expression in rats (n = 8).
| Group | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Control | 0.5176 ± 0.0146 | 0.5126 ± 0.0120 | 0.5204 ± 0.0146 | 0.5266 ± 0.0237 |
| NEC | 0.5198 ± 0.0131 | 0.6255 ± 0.0266*Δ | 0.7559 ± 0.0379*Δ | 0.9327 ± 0.0622*Δ |
| FO + NEC | 0.5177 ± 0.0171 | 0.5465 ± 0.0315*#Δ | 0.6231 ± 0.0487*#Δ | 0.7387 ± 0.0458*#Δ |
*P < 0.05 versus control group; #P < 0.05 versus NEC group; △P < 0.05versus the immediately preceding time -point.
Effect of FO on intestinal GRP78 protein expression in rats with NEC
On immunostaining, GRP78-positive cells exhibited yellow-brown staining in the cytoplasm and were mainly located around the intestinal mucosal epithelial cells (Fig. 5). The gray value analysis of GRP78 showed that the GRP78 protein expressions in the NEC group at 24 h, 48 h, and 72 h of NEC modeling were greater than those in the control group by 22.95%, 45.26%, and 38.62%, respectively (P < 0.05 at all 3 time-points) (Table 6). The GRP78 protein expressions in the FO + NEC group at 24 h, 48 h and 72 h of NEC modeling were greater than those in the control group by 14.59%, 29.85, and 27.15, respectively (P < 0.05 for all) and lesser than those in the NEC group by 6.80%, 10.61%, and 8.27%, respectively (P < 0.05 at all 3 time-points).
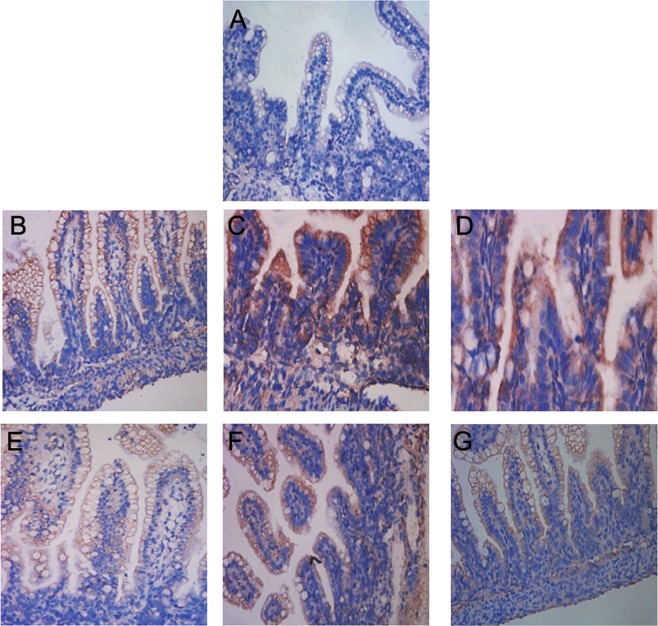
Figure 5.
Expression of GRP78 in intestine of rats with NEC. (A) The expression of GRP78 protein in the control group; GRP78-positive cells are mainly distributed in the intestinal epithelial cells and the sub-mucosa. (B–D) Immunostaining for intestinal GRP78 protein in the NEC group at 24 h, 48 h, and 72 h of NEC modeling shows GRP78-positive cells mainly located around the intestinal epithelial cells, mucosa and the sub-mucosa. (E–G) At 24 h, 48 h and 72 h of NEC modeling, the immunostaining of intestinal GRP78 protein in the FO + NEC group. FO pre-feeding reduced the expression of GRP78 protein in the intestinal mucosa.
Table 6.
Gray value analysis of intestinal GRP78 protein expression in rats (n = 8).
| Group | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Control | 119.58 ± 5.82 | 120.14 ± 6.96 | 119.61 ± 6.02 | 120.51 ± 8.22 |
| NEC | 117.72 ± 7.50 | 147.71 ± 9.01*Δ | 173.75 ± 11.88*Δ | 167.05 ± 10.75* |
| FO + NEC | 118.13 ± 5.90 | 137.67 ± 6.18*#Δ | 155.31 ± 8.18*#Δ | 153.23 ± 9.58*# |
*P < 0.05 versus control group; #P < 0.05 versus NEC group; △P < 0.05 versus the immediately preceding time –point.
Correlation between histopathological score and IL-6, TNF-α or caspase-12 in intestine of rats with NEC
In the NEC model, the expressions of GRP78 and caspase-12 protein in the intestine of rats showed a progressive increase over time during the process of the NEC (Table 7). Under NEC modeling, there was a rapid increase in caspase-12 protein at 24 h and it reached a peak at 72 h, while the GRP78 protein reached a peak at 48 h. The NEC group showed the greatest increase in the expressions of caspase-12 protein at 72 h of NEC modeling (77.12% greater than that in the control group) and GRP78 protein at 48 h of NEC modeling (45.26% greater than that in the control group). In addition, in the NEC group, we observed a strong positive correlation between intestinal histopathological score and the expression levels of IL-6, TNF-α, and caspase-12 [correlation coefficients at 24, 48, 72 hours: 0.914, 0.894, and 0.926, respectively (P < 0.05 for all), Table 7].
Table 7.
Correlation of histopathological score with intestinal expressions of IL-6, TNF-α, and caspase-12 in rats.
| Score | Sample amount | IL-6 (pg/mL) | TNF-α (pg/mL) | caspase-12 (gray value from Western blot) |
|---|---|---|---|---|
| ≤1 | 64 | 152.14 ± 11.66 | 149.95 ± 10.50 | 0.5245 ± 0.03 |
| 1∼2 | 20 | 212.11 ± 22.86 | 196.67 ± 23.38 | 0.6976 ± 0.06 |
| 2∼3 | 9 | 254.44 ± 10.19 | 236.59 ± 12.53 | 0.8428 ± 0.07 |
| 3∼4 | 3 | 288.29 ± 4.50 | 265.51 ± 16.94 | 0.9929 ± 0.03 |
| r | 0.914 | 0.894 | 0.926 | |
| p | <0.001 | <0.001 | <0.001 |
Discussion
It is well known that ERS and UPR activation are closely related to intestinal inflammation in mouse and human inflammatory bowel diseases28. In our study, the levels of ERS-related molecules (caspase-12 and GRP78) in the intestinal tissue increased during progression of NEC modeling. Furthermore, we observed a positive correlation of caspase-12 with IL-6 or TNF-α, which suggests that ERS is associated with intestinal inflammation and plays a crucial role in the pathogenesis of NEC. Our results are consistent with those of Lu et al.10 who reported increased expressions of GRP78 and inflammatory factors (IL-6 and IL-8) in intestinal tissue of children with NEC. In contrast, in our study, the intestinal pathological scores in the FO + NEC group were lesser than those in the NEC group by 38.79%. Further, during NEC modeling, the FO + NEC group showed decrease in caspase-12, GRP78, IL-6, and TNF-α by 20.80%, 10.61%, 13.70%, and 11.37%, respectively. Similarly, in a study by Zheng et al.29, DHA treatment significantly decreased the expression of GRP78 in mouse hepatocytes. These results indicate that the protective effect of FO on the intestinal tract of rats with NEC may be mediated via inhibition of inflammatory response which is associated with ERS.
In the present study, the intestinal pathological score in the NEC group showed a positive correlation with the levels of IL-6 and TNF-α. This result suggests that the level of intestinal pro-inflammatory cytokines may reflect the severity of intestinal mucosal damage in rats with NEC. In a study by Halpern et al23., treatment with anti-TNF-α reduced the incidence of NEC from 80% to 17%, which suggests that TNF-α plays an important role as a mediator in NEC progression. Pre-treatment of rats with FO in our study (FO + NEC group) remarkably reduced the level of IL-6 and TNF-α in rats with NEC at 48 h of modeling. In addition, the intestinal tissue pathological scores in the FO + NEC group were much lower than those in the NEC group. Consistent with these findings, ω-3 PUFAs were earlier shown to ameliorate postoperative intestinal inflammation and dysmotility24. DHA administration was shown to attenuate activation of inflammasomes induced by macrophage-derived TNF-α25. In patients with ulcerative colitis, EPA supplementation reduced mucosal inflammation, promoted differentiation of goblet cells, and modulated the intestinal microbiota composition26. In addition, IL-1 receptor associated kinase inhibitors were shown to reduce the levels of TNF-α, IL-1β, and IL-6, and further inhibit the inflammatory response in rats with NEC27. All these findings indicate a potential anti-NEC effect of FO.
In our study, we observed aggravation of NEC symptoms over time during NEC processing; at 72 h of NEC modeling, the phenomenon of intestinal luminal flatulence was observed at necropsy. However, mild intestinal luminal flatulence was observed in the group pretreated with FO. These results indicate that pre-treatment of rats with FO alleviated the symptoms of NEC. Similarly, administration of DHA and EPA was shown to reduce the incidence of colitis in an earlier study on rats22. In addition, insulin-like growth factor I intervention reduced the symptoms and the incidence rate of NEC in rats6. As DHA and EPA are two main ingredients of FO, we speculate that FO can be considered as a potential therapeutic agent for NEC.
In conclusion, FO pre-feeding of newborn rats attenuated NEC-induced intestinal mucosal damage and alleviated the accompanying clinical symptoms. Thus, FO may be considered as a single potential agent for alleviating the clinical symptoms of NEC. The protective effect of FO on the intestinal tract of rats with NEC may be mediated via inhibition of intestinal inflammatory response which is associated with ERS. However, the detailed signaling mechanisms that mediate the effect of FO on ERS require further study.
Acknowledgements
This study was supported by the General Program of National Natural Science Foundation of China (No. 81771626; 81971423); Jiangsu Provincial Key Talent Program for Women and Children Health (FRC201731); the Diagnosis and Treatment Technology Project of Clinical Key Disease in Suzhou province (LCZX201612); Minsheng Technology - Key Technology Application Research Project (SS201644).
Author contributions
X.L.Z. substantially contributions to conception and design in the experiments; N.X.C., L.L.Y., P.C., M.L.C. and J.W. conducted the experiments, acquisition of data, analysis and interpretation of data; X.P.Z. drafted the article and revised it. All authors participated in the analysis and interpretation of data, and final approved the version to be published.
Data availability
The data supporting the results of the present investigation are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaoli Zhu and Ningxun Cui.
Contributor Information
Xueping Zhu, Email: zhuxueping4637@hotmail.com.
Jian Wang, Email: wj196312@vip.163.com.
References
- 1.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Seminars in perinatology. 2008;32:100–106. doi: 10.1053/j.semperi.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clinics in perinatology. 2013;40:109–124. doi: 10.1016/j.clp.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodha A, Howlett A, Ahmed T, Moore AM. The Role of Interleukin-6 and Interleukin-8 Circulating Cytokines in Differentiating between Feeding Intolerance and Necrotizing Enterocolitis in Preterm Infants. American journal of perinatology. 2017;34:1286–1292. doi: 10.1055/s-0037-1603329. [DOI] [PubMed] [Google Scholar]
- 4.Baregamian N, et al. Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxidative medicine and cellular longevity. 2009;2:297–306. doi: 10.4161/oxim.2.5.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijendran V, et al. Long-chain polyunsaturated fatty acids attenuate the IL-1beta-induced proinflammatory response in human fetal intestinal epithelial cells. Pediatric research. 2015;78:626–633. doi: 10.1038/pr.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian F, Liu GR, Li N, Yuan G. Insulin-like growth factor I reduces the occurrence of necrotizing enterocolitis by reducing inflammatory response and protecting intestinal mucosal barrier in neonatal rats model. European review for medical and pharmacological sciences. 2017;21:4711–4719. [PubMed] [Google Scholar]
- 7.Fang C, et al. Berberine ameliorates neonatal necrotizing enterocolitis by activating the phosphoinositide 3-kinase/protein kinase B signaling pathway. Experimental and therapeutic medicine. 2018;15:3530–3536. doi: 10.3892/etm.2018.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuo K, et al. The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecologic oncology. 2013;128:552–559. doi: 10.1016/j.ygyno.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 10.Lu P, et al. Endoplasmic reticulum stress, unfolded protein response and altered T cell differentiation in necrotizing enterocolitis. PloS one. 2013;8:e78491. doi: 10.1371/journal.pone.0078491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto N, Torres MI, Rios A, Gil A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. The Journal of nutrition. 2002;132:11–19. doi: 10.1093/jn/132.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Caplan MS, et al. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatric research. 2001;49:647–652. doi: 10.1203/00006450-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Dong L, Yang X, Shi H, Zhang L. alpha-Linolenic acid prevents endoplasmic reticulum stress-mediated apoptosis of stearic acid lipotoxicity on primary rat hepatocytes. Lipids in health and disease. 2011;10:81. doi: 10.1186/1476-511X-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snodgrass RG, et al. Docosahexaenoic acid and palmitic acid reciprocally modulate monocyte activation in part through endoplasmic reticulum stress. The Journal of nutritional biochemistry. 2016;32:39–45. doi: 10.1016/j.jnutbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Begum G, Kintner D, Liu Y, Cramer SW, Sun D. DHA inhibits ER Ca2+ release and ER stress in astrocytes following in vitro ischemia. Journal of neurochemistry. 2012;120:622–630. doi: 10.1111/j.1471-4159.2011.07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodworth-Hobbs, M. E. & Perry, B. D. Docosahexaenoic acid counteracts palmitate-induced endoplasmic reticulum stress in C2C12 myotubes: Impact on muscle atrophy. 5 (2017). [DOI] [PMC free article] [PubMed]
- 17.Xiao G, et al. Eicosapentaenoic Acid Enhances Heatstroke-Impaired Intestinal Epithelial Barrier Function In Rats. Shock (Augusta, Ga.). 2015;44:348–356. doi: 10.1097/SHK.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 18.Ohta M, Kawano H, Notsu T, Naba H, Imada K. Eicosapentaenoic acid attenuates statin-induced ER stress and toxicity in myoblast. Biochemical and biophysical research communications. 2012;424:301–307. doi: 10.1016/j.bbrc.2012.06.111. [DOI] [PubMed] [Google Scholar]
- 19.Hunter CJ, et al. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infection and immunity. 2009;77:1031–1043. doi: 10.1128/IAI.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Welak SR, et al. Intestinal NADPH oxidase 2 activity increases in a neonatal rat model of necrotizing enterocolitis. PloS one. 2014;9:e115317. doi: 10.1371/journal.pone.0115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadler EP, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. The Journal of surgical research. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsuka Y, et al. omega-3 fatty acids attenuate mucosal inflammation in premature rat pups. Journal of pediatric surgery. 2011;46:489–495. doi: 10.1016/j.jpedsurg.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Halpern MD, et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. American journal of physiology. Gastrointestinal and liver physiology. 2006;290:G757–764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 24.Wehner S, et al. Preoperative short-term parenteral administration of polyunsaturated fatty acids ameliorates intestinal inflammation and postoperative ileus in rodents. Langenbeck’s archives of surgery. 2012;397:307–315. doi: 10.1007/s00423-011-0862-z. [DOI] [PubMed] [Google Scholar]
- 25.Chen, C. Y. & Chen, C. Y. Omega-3 polyunsaturated fatty acids reduce preterm labor by inhibiting trophoblast cathepsin S and inflammasome activation. 132, 2221–2239 (2018). [DOI] [PubMed]
- 26.Prossomariti, A. et al. Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. 7, 7458 (2017). [DOI] [PMC free article] [PubMed]
- 27.Hou Y, Lu X, Zhang Y. IRAK Inhibitor Protects the Intestinal Tract of Necrotizing Enterocolitis by Inhibiting the Toll-Like Receptor (TLR) Inflammatory Signaling Pathway in Rats. Medical science monitor: international medical journal of experimental and clinical research. 2018;24:3366–3373. doi: 10.12659/MSM.910327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuckin MA, Eri RD, Das I, Lourie R, Florin TH. ER stress and the unfolded protein response in intestinal inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2010;298:G820–832. doi: 10.1152/ajpgi.00063.2010. [DOI] [PubMed] [Google Scholar]
- 29.Zheng, J. et al. Docosahexaenoic Acid Ameliorates Fructose-Induced Hepatic Steatosis Involving ER Stress Response in Primary Mouse Hepatocytes. Nutrients. 8 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the results of the present investigation are available from the corresponding author upon reasonable request.