Summary
The DNA methylation regulators DNMT3A and TET2 are recurrently mutated in hematological disorders. Despite possessing antagonistic biochemical activities, loss-of-function murine models show overlapping phenotypes in terms of increased hematopoietic stem cell (HSC) fitness. Here, we directly compared the effects of these mutations on hematopoietic progenitor function and disease initiation. In contrast to Dnmt3a-null HSCs, which possess limitless self-renewal in vivo, Tet2-null HSCs unexpectedly exhaust at the same rate as control HSCs in serial transplantation assays despite an initial increase in self-renewal. Moreover, loss of Tet2 more acutely sensitizes hematopoietic cells to the addition of a common co-operating mutation (Flt3ITD) than loss of Dnmt3a, which is associated with a more rapid expansion of committed progenitor cells. The effect of Tet2 mutation manifests more profound myeloid lineage skewing in committed hematopoietic progenitor cells rather than long-term HSCs. Molecular characterization revealed divergent transcriptomes and chromatin accessibility underlying these functional differences.
Keywords: hematopoietic stem cell, clonal hematopoiesis, DNMT3A, TET2
Highlights
-
•
Tet2-null HSCs exhaust at the same rate as wild-type HSCs in serial transplantation
-
•
Loss of Tet2 sensitizes cells to Flt3ITD mutation more dramatically than Dnmt3a
-
•
Loss of Dnmt3a permits epigenetic plasticity between hematopoietic progenitors
-
•
Tet2 deficiency manifests profound myeloid lineage skewing in progenitor cells
Challen and colleagues show that Dnmt3a and Tet2 loss-of-function mutations manifest distinct molecular and functional consequences in different hematopoietic stem and progenitor cell compartments. Despite producing superficially similar phenotypes in terms of stem cell function and disease initiation, we show here divergent influences on progenitor cell lineage skewing, stem cell self-renewal, and predisposition to malignant transformation.
Introduction
Hematopoiesis is a hierarchy with hematopoietic stem cells (HSCs) at the apex (Orkin and Zon, 2008). Tasked with self-renewal to replenish the stem cell pool, and differentiation to maintain blood production, HSCs must possess functional integrity for the lifetime of an individual. Somatic mutations acquired during aging can adversely affect this balance, resulting in hematologic disorders. Two alleles recurrently mutated in blood diseases are the epigenetic regulators DNA methyltransferase 3 alpha (DNMT3A) and tet methylcytosine dioxygenase 2 (TET2) (Cancer Genome Atlas Research Network, 2013). Tumor evolution analysis suggests that these mutations are established in HSCs of these patients (Abelson et al., 2018, Shlush et al., 2014). In addition, variants in DNMT3A and TET2 are the most prevalent events associated with clonal hematopoiesis (CH), where pathogenic mutations are found in the blood of elderly people lacking overt disease (Buscarlet et al., 2017, Genovese et al., 2014, Jaiswal et al., 2014, Xie et al., 2014). These data suggest that DNMT3A and TET2 mutations confer fitness advantages to HSCs.
Despite similarities in disease phenotypes, DNMT3A and TET2 possess antagonistic biochemical activity. DNMT3A catalyzes addition of methyl groups to DNA forming 5-methylcytosine (Okano et al., 1999), while TET2 promotes DNA demethylation by oxidizing the methyl group to 5-hydroxymethylcytosine (Koh et al., 2011). Each mutation alters the DNA methylome in a predictable manner. In patients with acute myeloid leukemia (AML), DNMT3A mutations yield mild hypomethylation of the genome (Spencer et al., 2017), while TET2 mutations result in hypermethylation (Figueroa et al., 2010). However, loss of function of either enzyme in murine hematopoietic progenitors paradoxically results in similar altered function, including a competitive advantage of mutant cells (Celik et al., 2015, Challen et al., 2012, Challen et al., 2014, Li et al., 2011, Moran-Crusio et al., 2011). The mechanisms contributing to increased fitness of DNMT3A- and TET2-mutant HSCs remain largely undefined. The goal of this study was to directly compare loss-of-function effects of Dnmt3a and Tet2 at the HSC level through functional assays and molecular profiling.
Results
Loss of Dnmt3a and Tet2 Enhances Self-Renewal in HSCs to Different Degrees
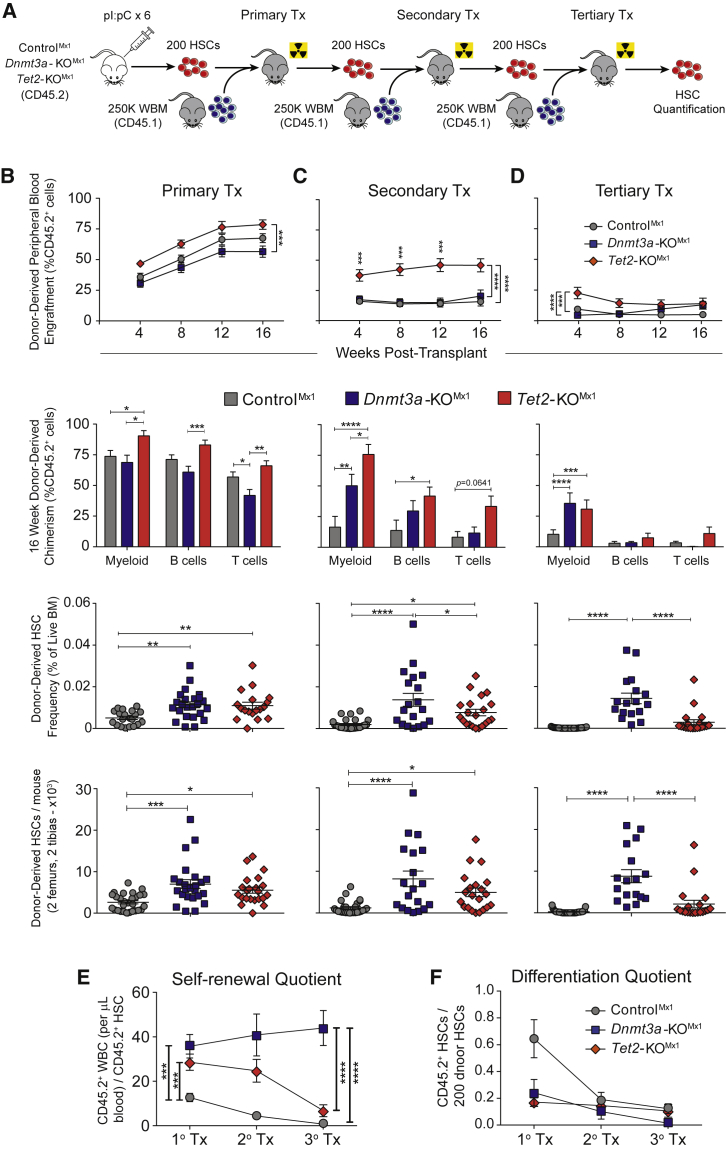
To directly compare the effects of Dnmt3a and Tet2 loss of function on HSC fate, we performed parallel competitive HSC transplants. Floxed Dnmt3a (Kaneda et al., 2004) or Tet2 (Moran-Crusio et al., 2011) mice were crossed with Mx1-Cre (Kuhn et al., 1995). Treatment with polyinosinic:polycytidylic acid (pI:pC) created conditional knockout mice (Dnmt3a-KOMx1 and Tet2-KOMx1). Mx1-Cre;Dnmt3a+/+;Tet2+/+ mice (ControlMx1) were similarly treated with pI:pC. Eight weeks after pI:pC, 200 HSCs (Lineage− c-Kit+ Sca-1+ CD48− CD150+) were transplanted with 2.5 × 105 whole bone marrow (WBM) competitor into wild-type mice (Figure 1A). No differences were noted in HSC abundance in donor mice (Figure S1A). Blood analysis (Figure S1B) revealed significantly higher contribution to all major hematopoietic lineages from Tet2-KOMx1 HSCs compared with control and Dnmt3a-KOMx1 HSCs in primary recipients (Figure 1B). In the bone marrow (BM) 18 weeks post-transplant (Figure S1C), the abundance of both mutant HSC populations was 2-fold higher than ControlMx1 HSCs (Figure 1B).
Figure 1.
Loss of Dnmt3a and Tet2 Enhance Self-Renewal in HSCs to Different Degrees
(A) HSC serial transplantation schematic. In descending column order—contribution of 200 ControlMx1, Dnmt3a-KOMx1 (3aKO), and Tet2-KOMx1 (T2KO) HSCs to peripheral blood, lineage chimerism, HSC frequency, and HSC number in (B) primary (CNT n = 28; 3aKO n = 24; T2KO n = 22), (C) secondary (CNT n = 27; 3aKO n = 19; T2KO n = 21), and (D) tertiary (CNT n = 33; 3aKO n = 23; T2KO n = 19) transplants. (E) Self-renewal and (F) differentiation quotients of indicated HSC genotypes after each transplant. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.001. Mean ± SEM is shown.
Loss of Dnmt3a confers HSCs with unlimited self-renewal (Jeong et al., 2018). To test if Tet2-KOMx1 HSCs possess the same ability, serial competitive HSC transplantation was performed. Two hundred donor-derived (CD45.2+) HSCs were purified from primary recipients and transferred to secondary recipients with 2.5 × 105 fresh (CD45.1+) WBM competitor. Tet2-KOMx1 HSCs maintained significantly higher blood production (Figure 1C), and donor-derived HSCs were increased elevated in recipients of both mutant HSC genotypes (Figure 1C). When examining other progenitor populations (Figure S1D), donor-derived multipotent progenitor 1 ([MPP1] Lineage− c-Kit+ Sca-1+ CD48− CD150−) and MPP3 (Lineage− c-Kit+ Sca-1+ CD48+ CD150−) populations were significantly increased in Tet2-KOMx1 secondary recipients (Figures S1E and S1F). However, after tertiary transplant, Tet2-KOMx1 HSCs surprisingly exhausted to similar levels as control HSCs (Figure 1D).
The ratio of donor-derived HSCs to 200 input HSCs (“self-renewal quotient”) quantifies the average self-renewal of a single test HSC (Challen et al., 2012). Dnmt3a-KOMx1 and Tet2-KOMx1 HSCs both possess greater self-renewal on a per-HSC basis than control HSCs after primary transplant (Figure 1E). But the self-renewal quotient of Tet2-KOMx1 HSCs overlaps with ControlMx1 HSCs at the end of tertiary transplant, in contrast to Dnmt3a-KOMx1 HSCs (Figure 1E). When examining the differentiation capacities of HSCs (16-week WBC count multiplied by percentage of donor-derived peripheral blood cells at 16 weeks divided by the total number of test HSCs, or the “differentiation quotient”) both Dnmt3a-KOMx1 and Tet2-KOMx1 HSCs display a reduced differentiation output on a per-HSC basis (Figure 1F).
Tet2 and Dnmt3a Loss of Function Divergently Influence Rate of Transformation from Same Co-operating Mutation
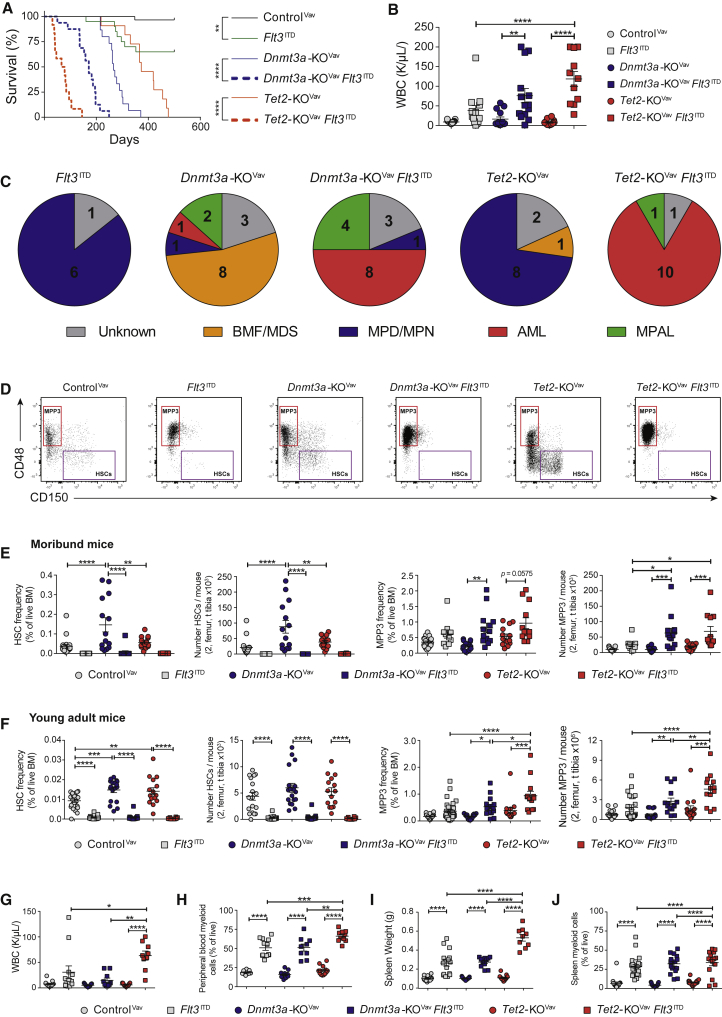
To compare functional contribution of Dnmt3a and Tet2 loss of function with leukemogenesis, we crossed Flt3 internal tandem duplication (Flt3ITD) knockin mice (Lee et al., 2007) to Vav-Cre;Dnmt3afl/fl or Vav-Cre;Tet2fl/fl mice (Dnmt3a-KOVav and Tet2-KOVav). Flt3ITD mutation significantly decreased time to morbidity in both genetic backgrounds, but the magnitude varied. Median survival in a Tet2-deficient background decreased ∼5.2-fold with expression of Flt3ITD, whereas survival of Dnmt3a-KOVav mice only decreased ∼1.5-fold (Figure 2A). Both Dnmt3a-KOVavFlt3ITD/+ and Tet2-KOVavFlt3ITD/+ mice presented with leukocytosis (Figure 2B), anemia, splenomegaly (Figure S2A), and AML (Figure 2C). A proportion of Dnmt3a-KOVavFlt3ITD/+ mice developed mixed phenotype acute leukemia, consistent with the role of Dnmt3a as a T cell leukemia tumor suppressor (Kramer et al., 2017). Dnmt3a-KOVav mice without Flt3ITD predominantly developed BM failure resembling myelodysplastic syndromes, whereas most Tet2-KOVav mice developed myeloproliferative disorders or myeloproliferative neoplasms.
Figure 2.
Tet2 and Dnmt3a Loss of Function Divergently Influence Rate of Transformation from Same Co-operating Mutation
(A) Kaplan-Meier plot comparing time to morbidity between ControlVav (n = 30), Flt3ITD (n = 20), Dnmt3a-KOVav (n = 15), Tet2-KOVav (n = 11), Dnmt3a-KOVavFlt3ITD (n = 16), and Tet2-KOVavFlt3ITD (n = 12) mice.
(B) White blood cell count of day 600 ControlVav and moribund mice of indicated genotypes.
(C) Pathological diagnosis of moribund mice.
(D) Representative flow cytometry plots of moribund mice demonstrating expansion of MPP3 (red box) and depletion of HSCs (purple box) in Flt3ITD genotypes.
(E) Frequency and number of HSCs and MPP3 in moribund mice.
(F–J) (F) Frequency and number of HSCs and MPP3 in 8-week-old ControlVav (n = 18), Flt3ITD (n = 14), Dnmt3a-KOVav (n = 18), Dnmt3a-KOVavFlt3ITD (n = 10), Tet2-KOVav (n = 15), and Tet2-KOVavFlt3ITD (n = 9) mice. Pathological analysis of young adult mice showing (G) WBC counts, (H) peripheral blood myeloid cells, (I) spleen weights, and (J) spleen myeloid cells. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.001. Mean ± SEM is shown.
Analysis of BM progenitors in moribund mice revealed significant expansion of the MPP3 population in Flt3ITD mice (Figure 2D). Flt3ITD depleted HSCs such that even the enhanced self-renewal of Dnmt3a-KOVav could not rescue (Figure 2E). The synergism between Tet2 loss of function and Flt3ITD alleles in promoting MPP3 expansion was already detectable in the BM of young adult mice lacking overt disease (Figure 2F), associated with leukocytosis (Figure 2G), myeloproliferation (Figures 2H and S2B), splenomegaly (Figures 2I and 2J), and lymphoid depletion (Figure S2C). Even though FLT3ITD mutations co-occur in patients with AML with both DNMT3A and TET2 mutations (Cancer Genome Atlas Research Network, 2013), the synergism in Tet2-KOVavFlt3ITD/+ mice show that founding mutations in Dnmt3a and Tet2 have disparate sensitivities to the same co-operating mutation.
Tet2 Mutation Does Not Impart Ectopic Self-Renewal to Hematopoietic Progenitors, but Loss of Dnmt3a Confers Phenotypic Plasticity
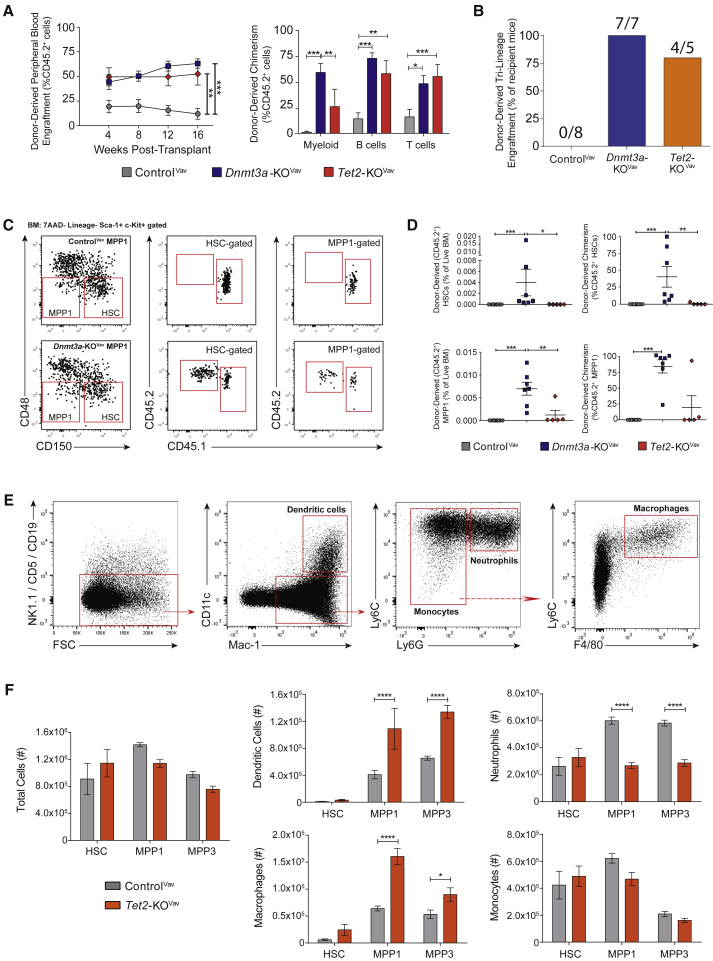
Expansion of the MPP3 population in moribund Flt3ITD mice implicated this BM compartment as the disease-initiating cell population. To test this, 250 MPP3 from young mice were transplanted with 2.5 × 105 wild-type WBM competitor cells. Regardless of genotype, donor-derived cells were barely detectable in the blood (Figure S3A) and failed to engraft the BM and generate disease (data not shown). This demonstrates that neither loss of Dnmt3a nor Tet2, alone or combined with Flt3ITD, imparts self-renewal properties to normally non-self-renewing progenitors.
Transplantation of ControlVav, Tet2-KOVav, and Dnmt3a-KOVav WBM against equal numbers of wild-type BM confirmed the competitive advantage of unfractionated Tet2-KOVav BM (Figures S3B and S3C). But as Tet2-KOMx1 HSC self-renewal was eventually exhausted (Figure 1D), this suggested that a non-HSC progenitor population may contribute to the observed myeloid dominance from Tet2-mutant BM. We performed competitive transplant of 200 MPP1 (Figure 3A). Peripheral blood analysis revealed long-term tri-lineage engraftment among recipients of both Dnmt3a-KOVav and Tet2-KOVav MPP1 (Figure 3B), whereas all recipients of ControlVav MPP1 had <1% donor-derived myeloid cells (Celik et al., 2018). From BM analysis (Figure 3C), Tet2-KOVav MPP1 failed to self-renew but Dnmt3a-KOVav MPP1 reconstituted the BM MPP1 compartment (Figure 3D). Surprisingly, we also observed donor-derived HSCs in all Dnmt3a-KOVav MPP1 recipients (Figures 3C and 3D). Loss of Dnmt3a may bestow epigenetic plasticity between the HSC and MPP1 compartments, reinforcing the importance of Dnmt3a in maintenance of hematopoietic cell type identity.
Figure 3.
Tet2 Mutation Does Not Impart Ectopic Self-Renewal to Hematopoietic Progenitors, but Skews Myeloid Differentiation of Committed Progenitor Cells
(A) Donor-derived peripheral blood cells and 16-week lineage chimerism in recipients of 200 MPP1 from ControlVav (n = 8), Dnmt3a-KOVav (n = 7), and Tet2-KOVav (n = 5) mice.
(B) Frequency of MPP1-transplanted mice with >1% donor-derived engraftment in myeloid, B cell, and T cell lineages.
(C) Representative plots showing donor-derived MPP1 and HSCs in recipients of Dnmt3a-KOVav MPP1.
(D) Frequency and chimerism of donor-derived HSCs and MPP1 in recipients of 200 MPP1.
(E) Representative immunophenotyping of in vitro differentiated progenitor cells.
(F) Immunophenotypic populations produced via in vitro differentiation of progenitor cells from ControlVav and Tet2-KOVav mice (n = 4 per population of each genotype). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.001. Mean ± SEM is shown.
Loss of Tet2 Skews Myeloid Differentiation of Committed Progenitor Cells
As increased myeloid output from Tet2-deficient HSCs could not be attributed to differences in self-renewal, proliferation (Figure S3D), or apoptosis (Figure S3E), we hypothesized that epigenetic dysfunction may skew myeloid differentiation from hematopoietic progenitors. To quantify this, in vitro assays were performed with purified HSCs, MPP1, and MPP3. In methylcellulose, all genotypes initially had the same number of colonies (Figure S3F). Both mutant alleles sustained some colony-forming potential in MPP1 and MPP3 in serial plating. A third passage distinguished Dnmt3a-KOVav HSCs with a significant increase in colony number (Figure S3F). The lack of serial replating of Tet2-KOVav MPP3 in vitro was consistent with engraftment failure in vivo.
Progenitors were then cultured with hematopoietic cytokines in OP9 stroma-coated plates. After 2 weeks, analysis with myeloid markers Gr-1 and CD11b (Mac-1) showed clear differences from Tet2-KOVav progenitors. A Gr-1hi population was lacking while Gr-1mid and Gr-1lo populations were more prevalent (Figure S3G). Further immunophenotyping (Figure 3E) revealed a disproportionate production of myeloid cells. Loss of Tet2 increased dendritic cell and macrophage production from MPP1 and MPP3 at the expense of neutrophils (Figure 3F). This suggests that the primary function of Tet2 mutations in hematopoietic progenitors is not to increase self-renewal, but rather skew myeloid output.
Dnmt3a and Tet2 Loss of Function Alter Hematopoietic Progenitor Function through Distinct Molecular Mechanisms
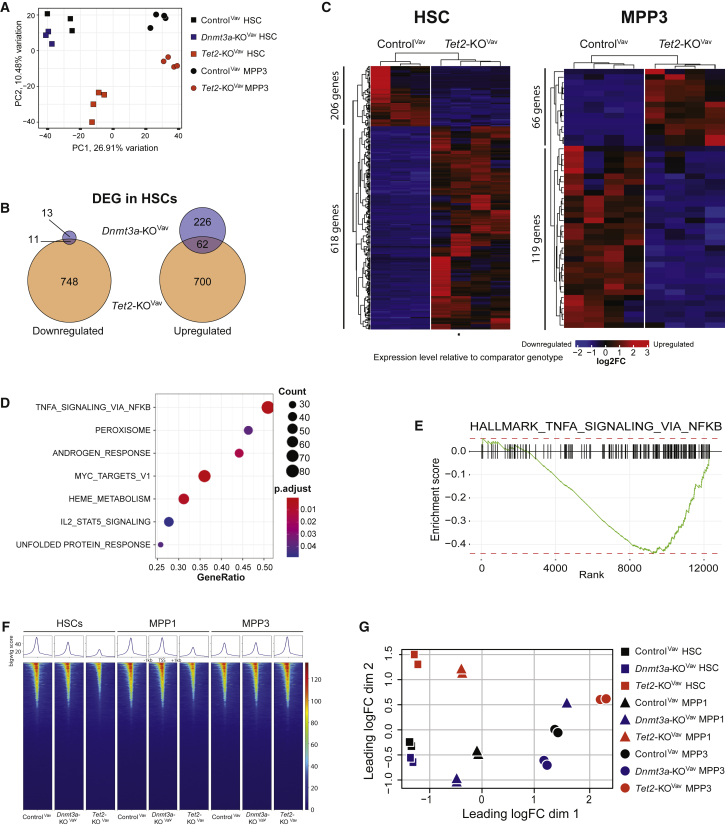
Transcriptional profiling was performed to elucidate mechanisms underlying the functional differences between HSCs over serial transplantation. Unfortunately, the diminution of ControlMx1 and Tet2-KOMx1 HSCs at later transplant stages permitted only two replicates (Table S1). As a supplement, HSCs were purified from ControlVav, Dnmt3a-KOVav, and Tet2-KOVav adult mice (Table S2). The gene expression profiles of ControlVav and Dnmt3a-KOVav HSCs were remarkably similar (Figure S4A), despite their functional differences. Tet2-KOVav HSCs showed a larger dysregulated expression signature (Figure 4A). Of the 24 genes downregulated in Dnmt3a-KOVav HSCs, 11 also showed significant repression in Tet2-KOVav HSCs (Figure 4B). Genes, such as Mki67 and Hmgb2 also showed consistent downregulation in the mutant HSCs across serial transplant (Figure S4B) and may contribute to the differentiation block. A total of 62 out of 288 (21.5%) genes upregulated in Dnmt3a-KOVav HSCs were shared with Tet2-KOVav HSCs, including jun and Fos, which form the AP-1 transcription factor complex important in HSC stress response (Mallaney et al., 2019). Transcriptional analysis was also performed on ControlVav and Tet2-KOVav MPP3 from young adult mice to understand the myeloid lineage skewing arising from loss of Tet2. Although there were fewer differentially expressed genes (DEGs) than the HSC comparison of the same genotypes (Figure 4C), gene set enrichment analysis revealed significantly dysregulated gene sets (Figure 4D). The major difference was diminished nuclear factor κB signaling in Tet2-KOVav MPP3 (Figure 4E).
Figure 4.
Dnmt3a and Tet2 Loss of Function Alter Hematopoietic Progenitor Function through Distinct Molecular Mechanisms
(A) Principal-component analysis plot of gene expression in ControlVav HSCs (n = 3) and MPP3 (n = 4), Dnmt3a-KOVav HSCs (n = 3), and Tet2-KOVav HSCs (n = 4) and MPP3 (n = 4).
(B) Venn diagrams displaying DEG overlap in Dnmt3a-KOVav and Tet2-KOVav HSCs compared with ControlVav HSCs.
(C) Heatmaps displaying DEGs (p < 0.05, fold-change >1 or <−1) between ControlVav and Tet2-KOVav HSCs and MPP3.
(D) Gene set enrichment analysis showing differentially regulated pathways between ControlVav and Tet2-KOVav MPP3.
(E) Gene score enrichment plot of “Hallmark TNFα Signaling via NFkB” gene set in Tet2-KOVav MPP3.
(F) ATAC-seq heatmaps from ControlVav, Dnmt3a-KOVav, and Tet2-KOVav mice. Signals displayed are peaks 1 kb up- and downstream of transcription start sites of protein coding genes.
(G) Multi-dimensional scaling plot with distances approximating the largest log2 fold-changes in the top 500 peaks between ATAC-seq samples.
ATAC sequencing (ATAC-seq) was performed to examine chromatin accessibility in HSCs, MPP1, and MPP3. The most striking difference was global reduction of open chromatin in Tet2-KOVav HSCs and MPP1 (Figure 4F), consistent with the function of Tet2 in promoting DNA demethylation at enhancers (Sardina et al., 2018, Wang et al., 2018). However, Tet2-KOVav MPP3 recovered chromatin accessibility above ControlVav levels, including addition of new peaks at 800 hematopoietic enhancers and 1,232 promoters enriched for myeloid function (Figure S4C). The chromatin landscape of ControlVav and Dnmt3a-KOVav progenitors revealed fewer differences. A multi-dimensional scaling analysis showed the Dnmt3a-KOVav MPP1 cluster closer to HSCs than ControlVav MPP1 (Figure 4G), supporting the notion of a reduced epigenetic barrier between these cell types in the absence of Dnmt3a. Moreover, areas with reduced chromatin accessibility in Dnmt3a-KOVav MPP1 (Table S3) involved genes involved in hematopoietic lineage specification, such as Fli1 and Izkf1, which may contribute to the differentiation block.
Discussion
Somatic mutations in DNMT3A and TET2 comprise approximately 70% of all variants in age-related CH. The persistence of these mutations (Young et al., 2016) coupled with their recurrence in blood malignancies of diverse lineages suggests a stem cell origin. Mouse model studies suggest a similar increase in HSC self-renewal after inactivation of both genes. Previous studies demonstrate a competitive advantage for Tet2-deficient cells in BM transplantation assays (Li et al., 2011, Moran-Crusio et al., 2011). Although this is assumed to be due to enhanced HSC self-renewal, these experiments have been performed with WBM or less pure populations of progenitors. Our results using highly purified HSCs show that Tet2 loss of function modestly increased self-renewal on a per-HSC basis during early passages of transplantation, but this effect was transient and Tet2-deficient HSCs exhausted to comparable levels as control HSCs after tertiary transplant. This is in stark contrast to Dnmt3a-deficient HSCs which show no signs of self-renewal diminution. Moreover, by transplanting defined numbers of HSCs, our quantification shows that, on a per-cell basis, Tet2-mutant HSCs show a similar differentiation deficit as Dnmt3a-mutant HSCs. This was not anticipated given the high peripheral blood chimerism observed from transplantation of Tet2-null WBM, particularly in the myeloid lineage. Rather, our data suggest that increased myeloid output results from skewed differentiation in progenitors lacking Tet2. More open chromatin at enhancers of pro-myeloid differentiation genes in Tet2-KOVav MPP3 may contribute to this phenotype.
Our data are instructive for how these mutations are propagated in humans with chromatin immunoprecipitation. DNMT3A mutations are highly specific for the HSC compartment, with the increase in self-renewal potential allowing these mutations to be efficiently propagated in humans with age. The fact that the mutations do not cause massive transcriptional changes still allows these mutant HSCs to function as effective stem cells, but with greater self-renewal to withstand external stresses that would normally force HSC depletion. Although Tet2 loss of function does not induce ectopic self-renewal in either normal or malignant progenitor cells, this mutation alters the myeloid output of progenitors and potentially sensitizes them to secondary mutations that further drive proliferation. This aligns with evidence in humans showing that these mutations do not have equal potential. Analysis of individuals with CH shows DNMT3A mutations can be found in all blood lineages, whereas TET2 mutations are often absent from T cells (Buscarlet et al., 2018), supportive of differential effects on progenitor lineage differentiation. Our data suggest a model for TET2-mutant CH whereby the mutation is acquired in HSCs, but the functional effects of myeloproliferation are realized by more downstream progenitors.
Experimental Procedures
Detailed methods are provided in Supplemental Information.
Mice and Transplantation
The Institutional Animal Care and Use Committee at Washington University approved all animal procedures. All mice were C57Bl/6 background. Dnmt3afl/fl (Kaneda et al., 2004) and Tet2fl/fl (Moran-Crusio et al., 2011) mice were crossed to Flt3ITD (Lee et al., 2007), Vav-Cre (Georgiades et al., 2002), and Mx1-Cre (Kuhn et al., 1995) strains. To induce Mx1-Cre, six doses (300 μg) of polyinosinic:polycytidylic acid (pI:pC; Sigma, no. p1530) were administered every 48 h via intraperitoneal injection to 8-week-old mice. Mice were allowed to recover for 6 weeks after the last pI:pC injection before sacrifice for HSC purification. Transplant recipients (C57Bl/6 CD45.1, The Jackson Laboratory strain no. 002014), received a split dose of irradiation (10.5 Gy) ∼4 h apart. Cells were transplanted via retro-orbital injection.
RNA-Seq Data, Quality Control, and Analysis
Reads were aligned with STAR v.2.5.4b with Gencode release M20 (GRCm38.p6) genome assembly. Unambiguous read counts were calculated by HTSEQ-count. Data were imported into Noiseq v.2.28.0 for differential gene expression analysis with TMM normalization and batch correction. RNA-seq data are available under GEO GSE139911.
ATAC-Seq
Open chromatin was profiled via the Omni-ATAC method (Corces et al., 2017). Reads were aligned to mm10 with BWA mem v.0.7.17. Duplicates were removed with Picard tools MarkDuplicates v.2.0.1 and bams were processed with snakePipes v.1.3.1. Differential chromatin accessibility was assessed using Rsubread v.1.34.7 and EdgeR v.3.26.8. Peaks were intersected with enhancers using bedtools v.2.25.0. Bigwig tracks and heatmaps were created via deepTools2 v.3.3.1. ATAC-seq data are available under GEO GSE139911.
Statistics
Student’s t test, and one- and two-way ANOVA were used for statistical comparisons where appropriate. Kruskal-Wallis test was used for non-normal data. Survival curves were analyzed using a Mantel-Cox log rank test. Significance is indicated using the following convention: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. All graphs represent mean ± SEM.
Author Contributions
Conceptualization, G.A.C.; Data Curation, E.L.O.; Formal Analysis, E.L.O. and G.A.C.; Funding Acquisition, E.L.O. and G.A.C.; Investigation, E.L.O., A.C.K., C.M., H.C., W.K.K., J.F., E.H., C.R.C.Z., and G.A.C.; Project Administration, G.A.C.; Software, E.L.O.; Supervision, G.A.C.; Visualization, E.L.O. and G.A.C.; Writing – Original Draft, E.L.O.; Writing – Review & Editing, G.A.C.
Acknowledgments
The authors have no conflicting financial interests. This work was supported by the NIH (R01DK102428), the American Society of Hematology, the Longer Life Foundation, the Edward Mallinckrodt, Jr. Foundation, and Gabrielle's Angel Foundation (to G.A.C.). E.L.O. was supported by NIH 5T32CA113275-10 and NIH F31DK114951. C.M. was supported by NIH T32HL007088 and NIH DK111058-01. H.C. was supported by an Edward P. Evans Foundation Young Investigator Award. G.A.C. is a scholar of the Leukemia and Lymphoma Society.
Published: March 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.02.011.
Supplemental Information
Normalized RNA-seq gene expression values (counts per million, cpm) of ControlMx1, Dnmt3a-KOMx1, and Tet2-KOMx1 HSCs across serial competitive transplantation.
Normalized RNA-seq gene expression values (transcripts per million, tpm) of ControlVav, Dnmt3a-KOVav, and Tet2-KOVav HSCs and MPP3s.
Differential regions of open chromatin from ATAC-seq analysis of ControlVav, Dnmt3a-KOVav, and Tet2-KOVav HSCs, MPP1s, and MPP3s.
References
- Abelson S., Collord G., Ng S.W.K., Weissbrod O., Mendelson Cohen N., Niemeyer E., Barda N., Zuzarte P.C., Heisler L., Sundaravadanam Y. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. doi: 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscarlet M., Provost S., Zada Y.F., Barhdadi A., Bourgoin V., Lepine G., Mollica L., Szuber N., Dube M.P., Busque L. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. doi: 10.1182/blood-2017-04-777029. [DOI] [PubMed] [Google Scholar]
- Buscarlet M., Provost S., Zada Y.F., Bourgoin V., Mollica L., Dube M.P., Busque L. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood. 2018;132:277–280. doi: 10.1182/blood-2018-01-829937. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik H., Koh W.K., Kramer A.C., Ostrander E.L., Mallaney C., Fisher D.A.C., Xiang J., Wilson W.C., Martens A., Kothari A. JARID2 functions as a tumor suppressor in myeloid neoplasms by repressing self-renewal in hematopoietic progenitor cells. Cancer Cell. 2018;34:741–756.e8. doi: 10.1016/j.ccell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik H., Mallaney C., Kothari A., Ostrander E.L., Eultgen E., Martens A., Miller C.A., Hundal J., Klco J.M., Challen G.A. Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood. 2015;125:619–628. doi: 10.1182/blood-2014-08-594564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen G.A., Sun D., Jeong M., Luo M., Jelinek J., Berg J.S., Bock C., Vasanthakumar A., Gu H., Xi Y. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen G.A., Sun D., Mayle A., Jeong M., Luo M., Rodriguez B., Mallaney C., Celik H., Yang L., Xia Z. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell. 2014;15:350–364. doi: 10.1016/j.stem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces M.R., Trevino A.E., Hamilton E.G., Greenside P.G., Sinnott-Armstrong N.A., Vesuna S., Satpathy A.T., Rubin A.J., Montine K.S., Wu B. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M.E., Abdel-Wahab O., Lu C., Ward P.S., Patel J., Shih A., Li Y., Bhagwat N., Vasanthakumar A., Fernandez H.F. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G., Kahler A.K., Handsaker R.E., Lindberg J., Rose S.A., Bakhoum S.F., Chambert K., Mick E., Neale B.M., Fromer M. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades P., Ogilvy S., Duval H., Licence D.R., Charnock-Jones D.S., Smith S.K., Print C.G. VavCre transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis. 2002;34:251–256. doi: 10.1002/gene.10161. [DOI] [PubMed] [Google Scholar]
- Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M., Park H.J., Celik H., Ostrander E.L., Reyes J.M., Guzman A., Rodriguez B., Lei Y., Lee Y., Ding L. Loss of Dnmt3a immortalizes hematopoietic stem cells in vivo. Cell Rep. 2018;23:1–10. doi: 10.1016/j.celrep.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M., Okano M., Hata K., Sado T., Tsujimoto N., Li E., Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- Koh K.P., Yabuuchi A., Rao S., Huang Y., Cunniff K., Nardone J., Laiho A., Tahiliani M., Sommer C.A., Mostoslavsky G. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A.C., Kothari A., Wilson W.C., Celik H., Nikitas J., Mallaney C., Ostrander E.L., Eultgen E., Martens A., Valentine M.C. Dnmt3a regulates T-cell development and suppresses T-ALL transformation. Leukemia. 2017;31:2479–2490. doi: 10.1038/leu.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R., Schwenk F., Aguet M., Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Tothova Z., Levine R.L., Anderson K., Buza-Vidas N., Cullen D.E., McDowell E.P., Adelsperger J., Frohling S., Huntly B.J. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Cai X., Cai C.L., Wang J., Zhang W., Petersen B.E., Yang F.C., Xu M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallaney C., Ostrander E.L., Celik H., Kramer A.C., Martens A., Kothari A., Koh W.K., Haussler E., Iwamori N., Gontarz P. Kdm6b regulates context-dependent hematopoietic stem cell self-renewal and leukemogenesis. Leukemia. 2019;33:2506–2521. doi: 10.1038/s41375-019-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K., Reavie L., Shih A., Abdel-Wahab O., Ndiaye-Lobry D., Lobry C., Figueroa M.E., Vasanthakumar A., Patel J., Zhao X. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Orkin S.H., Zon L.I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardina J.L., Collombet S., Tian T.V., Gomez A., Di Stefano B., Berenguer C., Brumbaugh J., Stadhouders R., Segura-Morales C., Gut M. Transcription factors drive tet2-mediated enhancer demethylation to reprogram cell fate. Cell Stem Cell. 2018;23:905–906. doi: 10.1016/j.stem.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush L.I., Zandi S., Mitchell A., Chen W.C., Brandwein J.M., Gupta V., Kennedy J.A., Schimmer A.D., Schuh A.C., Yee K.W. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D.H., Russler-Germain D.A., Ketkar S., Helton N.M., Lamprecht T.L., Fulton R.S., Fronick C.C., O'Laughlin M., Heath S.E., Shinawi M. CpG island hypermethylation mediated by DNMT3A is a consequence of AML progression. Cell. 2017;168:801–816.e13. doi: 10.1016/j.cell.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ozark P.A., Smith E.R., Zhao Z., Marshall S.A., Rendleman E.J., Piunti A., Ryan C., Whelan A.L., Helmin K.A. TET2 coactivates gene expression through demethylation of enhancers. Sci. Adv. 2018;4:eaau6986. doi: 10.1126/sciadv.aau6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Lu C., Wang J., McLellan M.D., Johnson K.J., Wendl M.C., McMichael J.F., Schmidt H.K., Yellapantula V., Miller C.A. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.L., Challen G.A., Birmann B.M., Druley T.E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normalized RNA-seq gene expression values (counts per million, cpm) of ControlMx1, Dnmt3a-KOMx1, and Tet2-KOMx1 HSCs across serial competitive transplantation.
Normalized RNA-seq gene expression values (transcripts per million, tpm) of ControlVav, Dnmt3a-KOVav, and Tet2-KOVav HSCs and MPP3s.
Differential regions of open chromatin from ATAC-seq analysis of ControlVav, Dnmt3a-KOVav, and Tet2-KOVav HSCs, MPP1s, and MPP3s.