Summary
HIV-associated neurocognitive disorders (HAND) affect over half of HIV-infected individuals, despite antiretroviral therapy (ART). Therapeutically targetable mechanisms underlying HAND remain elusive, partly due to a lack of a representative model. We developed a human-induced pluripotent stem cell (hiPSC)-based model, independently differentiating hiPSCs into neurons, astrocytes, and microglia, and systematically combining to generate a tri-culture with or without HIV infection and ART. Single-cell RNA sequencing analysis on tri-cultures with HIV-infected microglia revealed inflammatory signatures in the microglia and EIF2 signaling in all three cell types. Treatment with the antiretroviral compound efavirenz (EFZ) mostly resolved these signatures. However, EFZ increased RhoGDI and CD40 signaling in the HIV-infected microglia. This activation was associated with a persistent increase in transforming growth factor α production by microglia. This work establishes a tri-culture that recapitulates key features of HIV infection in the CNS and provides a new model to examine the effects of infection, its treatment, and other co-morbid conditions.
Keywords: hiPSC, HIV, microglia, astrocyte, neuron, model, tri-culture, EIF2, neuroinflammation, efavirenz
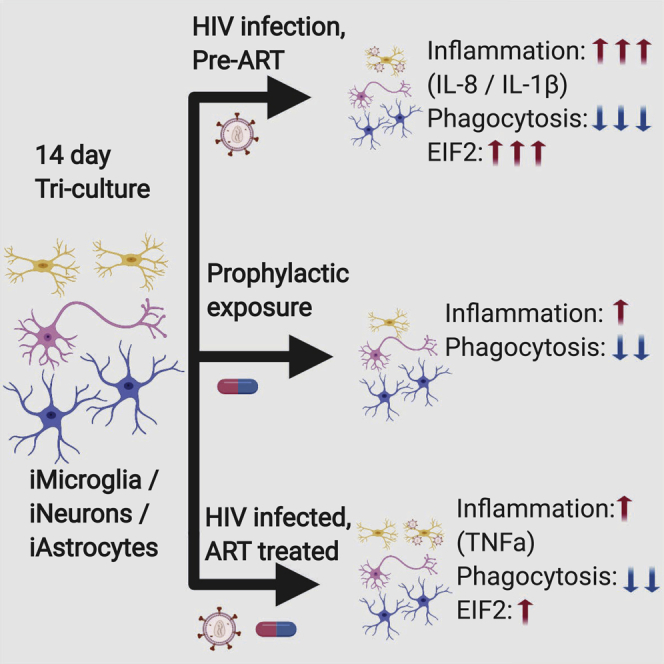
Graphical Abstract
Highlights
-
•
Describes a tri-culture of hiPSC-derived microglia, astrocytes, and neurons
-
•
HIV infection leads to increased EIF2 signaling in all three cell types
-
•
Efavirenz treatment alone creates an inflammatory signature by RNA expression
-
•
Infected iMicroglia increased inflammation response and reduced synaptophagocytosis
Anderson, Jordan-Sciutto, and colleagues developed an hiPSC-derived tri-culture of microglia, astrocytes, and neurons to investigate the cell-cell interactions during HIV infection and antiretroviral treatment. Infection led to EIF2 signaling activation across all cell types and an especially high inflammatory response and reduced synaptophagocytosis by microglia. In addition, with efavirenz treatment, RhoGDI and CD40 signaling were distinctly activated in the microglia.
Introduction
HIV-associated neurocognitive disorders (HAND) are a chronic, progressing spectrum of diseases that lead to a range of neurologic disorders, including HIV-associated dementia. The major pathologic manifestation that persists in HAND patients with antiretroviral treatment (ART) is synaptodendritic damage and the accumulation of microglia, the resident immune cell of the central nervous system (CNS); however, the mechanisms underlying synaptic damage remain elusive. Synapse loss is associated with infiltration of macrophages from outside of the CNS and activation of microglia. Both HIV-infected macrophage populations can release cytokines, viral proteins, and excitotoxins, which can lead to synaptic damage (Saylor et al., 2016) and are potential reservoirs for the virus (Castellano et al., 2017). Although patients on ART experience milder forms of HAND, they still experience chronic inflammation (Kolson, 2017). Infected microglia and uninfected, but active microglia may be working in tandem to slowly release proinflammatory cytokines and reactive oxygen species that, over time, can lead to synaptodendritic damage (Sui et al., 2007, Turchan–Cholewo et al., 2009). Additional aspects of inflammatory responses have been implicated in HAND, including the unfolded protein response (UPR) and its resultant activation of EIF2 (Akay et al., 2012, Jiang et al., 2017, Lindl et al., 2007).
Although the major pathological manifestation of HAND is synaptodendritic damage, the response during initial exposure to HIV and ART is unknown largely because current models for HIV-mediated neuroinflammation are limited by species differences and human tropism of the virus. For instance, the HIV-1 Tat transgenic mouse model exhibits neuroinflammation and behavioral deficits, but it only expresses a single viral protein from astrocytes (Kim et al., 2003). In addition, while isolated microglia from human patients can provide important insights, this is only a snapshot of the end stage of the disease. Therefore, the development of an in vitro human system allowing the interaction of the main cell types involved in HAND is needed to further understand the neuropathogenesis and develop novel therapeutics.
We have developed a human-induced pluripotent stem cell (hiPSC)-based model. Whereby, we separately differentiate hiPSCs into forebrain-like excitatory neurons, astrocytes, and microglia, and then combine them to create a tri-culture, with or without HIV infection of the microglia and with or without ART. Our protocol rapidly produces microglia-like cells (iMg) that express multiple classical markers in mono-culture, productively infect with HIV, and respond to ART. In addition, we have developed a differentiation protocol for functional astrocyte-like cells (iAst) that express hallmark proteins. Utilizing this system, we investigated the effects of HIV infection (Inf), infection with the antiretroviral efavirenz (EFZ) (Inf + EFZ), and EFZ treatment alone (Uninf + EFZ), compared to uninfected tri-cultures (Uninf) and to each other. Interestingly, acute HIV infection reduced synaptophagy by both infected iMg and uninfected iMg in the cultures. Inf also caused gene expression changes consistent with activated inflammatory cytokine signaling in iMg and activation of EIF2 signaling in iMg, iAst, and iPSC-derived neurons (iNrn). Although Inf + EFZ reduced many inflammatory markers, EIF2 signaling activation persisted in the iNrn, and RhoGDI and CD40 signaling persisted in the iMg. In addition, EFZ treatment alone invoked its own discrete inflammatory response, reaffirming the toxic effects of EFZ (Ciccarelli et al., 2011) and revealing pathways that may contribute to the toxicity. Our system, which recapitulates key findings in patient studies, provides a platform to mechanistically understand responses to HIV seen in human studies and further reveal the complex roles of the individual cell types during infection ± ART, how ART alone can elicit an inflammatory response, and the prominent role microglia play in the early inflammation response to HIV infection in the brain.
Results
iMicroglia Exhibit Similar Gene and Protein Expression as Other iPSC-Derived Microglia
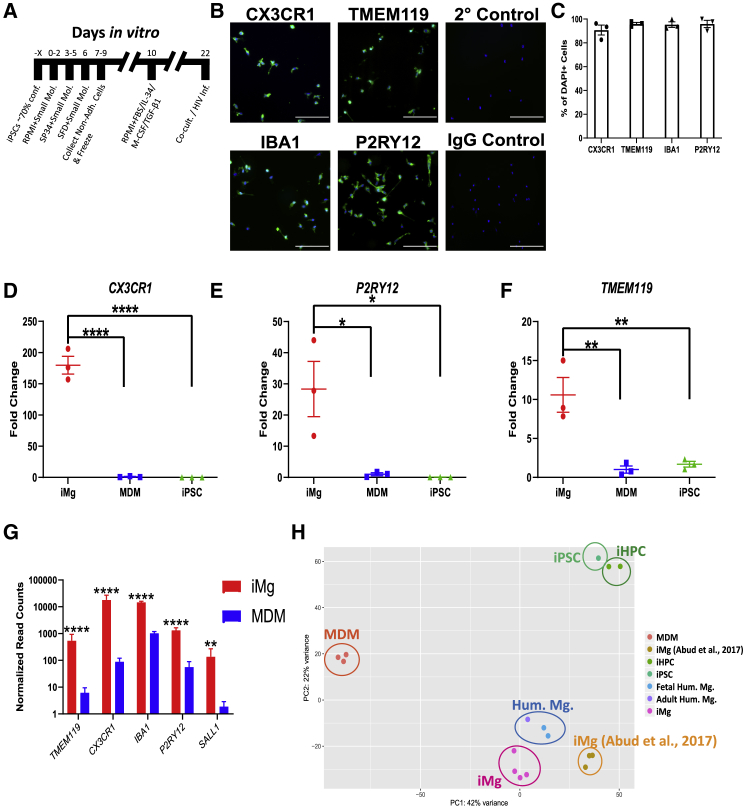
To develop a tractable system for studying HAND in vitro, we adapted two previously published protocols to generate iMg from four separate iPSC lines (Figure 1A). First, iPSCs are differentiated into CD41+ CD235+ common myeloid progenitors (CMPs) through a 9-day process (Paluru et al., 2014). Next, using a modified 11-day protocol (Abud et al., 2017) CMPs are differentiated into a highly pure population of ramified iMg that express CX3CR1, IBA1, TMEM119, and P2RY12 (Figures 1B, 1C, and S1A). Based on the Abud protocol, we used the small molecules interleukin-34 (IL-34) and colony-stimulating factor 1 (CSF-1), as these are the ligands for CSF1R and are necessary for microglia development in vivo (Easley-Neal et al., 2019), and transforming growth factor β1 (TGF-β1), as it helps induce an in vivo pattern of gene expression (Gosselin et al., 2017). Fetal bovine serum was implemented to increase viability. iMg exhibited a 179-, 28-, and 11-fold increase in CX3CR1 (p < 0.0001), P2RY12 (p < 0.05), and TMEM119 (p < 0.01), respectively, over monocyte-derived macrophages (MDMs) by qRT-PCR (Figures 1D–1F). Microglial identity was further confirmed by RNA sequencing (RNA-seq) (Figures 1G and S1B). Importantly, iMg also express CCR5 (Figure S1C), one of the co-receptors necessary for HIV infection (Deng et al., 1996). iMg also lacked expression of myeloid progenitor markers (Figure S1F). Finally, we compared our iMg with human MDMs as well as datasets from primary human adult and fetal microglia, iPSCs, induced hematopoietic progenitor cells, and iMicroglia from Abud and colleagues (Abud et al., 2017). Principal-component analysis revealed closer clustering of our iMg (pink) to primary human microglia (blue) than previously published iPSC-derived microglia (light orange) (Figure 1H).
Figure 1.
Generation and Characterization of iMg
(A) Timeline for iMg differentiation.
(B) Immunostaining for microglia specific markers: CX3CR1, TMEM119, IBA1, and P2RY12, secondary and IgG controls. All cultures were stained for DAPI. Scale bar represents 50 μm.
(C) Percentage of DAPI(+) cells that are double-positive for microglia marker. n = 3 cell lines, error bars represent SEM.
(D–F) qRT-PCR validation for expression of CX3CR1 (D), P2RY12 (E), and TMEM119 (F) in iMg. Probes normalized to GAPDH expression. n = 3 cell lines, one-way ANOVA, Dunnett's post hoc analysis, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; error bars represent SEM.
(G) Bulk RNA-seq normalized read counts of specific microglia markers from iMg and MDMs. n = 4 iMg lines, n = 3 MDMs. Benjamini-Hochberg, false discovery rate (FDR) = 0.01, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; error bars represent SEM.
(H) Principal-component analysis (PCA) for monocyte-derived macrophages, iMicroglia, and (Abud et al., 2017) iMg, iPSC, iHPC, and primary human microglia. Each dot represents a bulk RNA-seq sample. MDM, monocyte-derived macrophage; iHPC, induced hematopoietic progenitor cells. iPSC, iHPC, Hum. Mg., and iMg samples were all retrieved from Abud et al. (2017).
iMg Are Productively Infected with HIV and Respond to Multiple Antiretrovirals
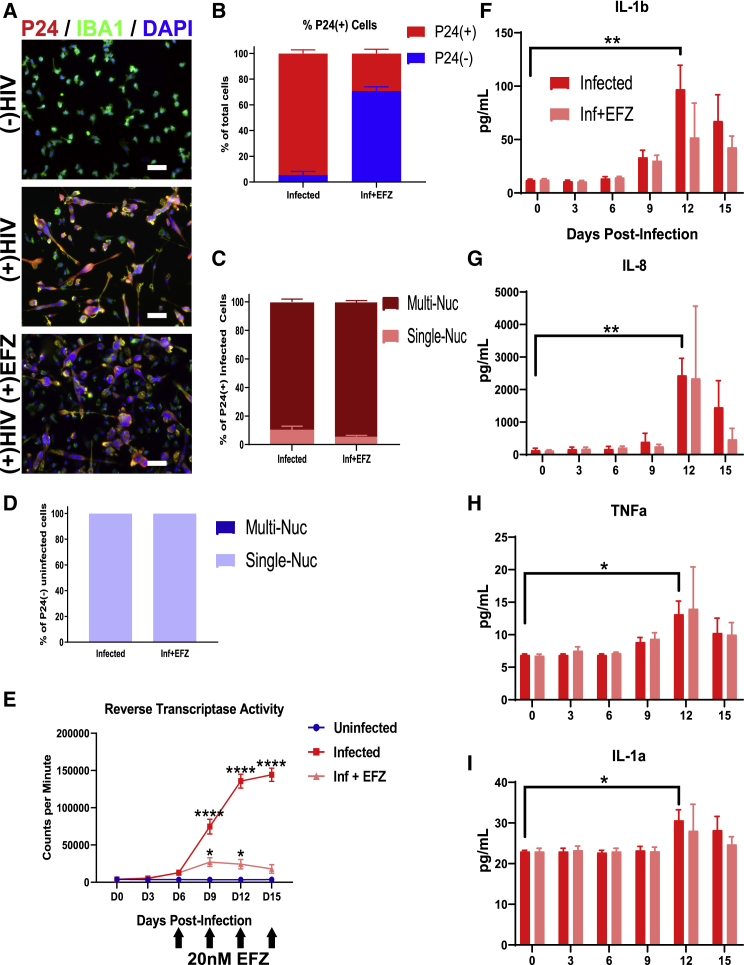
To establish the effect of HIV infection and ART on microglia, we infected iMg mono-culture with 50 ng/mL of the CSF-derived, R5-tropic JAGO strain of HIV (Chen et al., 2002). After a 15-day infection, a time point based on previous studies of MDMs (O'Donnell et al., 2006), 94.5% ± 5.5% of the iMg were positive for the HIV capsid protein P24 and exhibited vast multinucleation (Figures 2A and 2B). Most (89.1% ± 3.8%) P24(+) iMg were multinucleated (Figure 2C), while no P24(−) iMg were multinucleated (Figure 2D), showing that multinucleation is exclusively associated with infection. Reverse transcriptase activity showed peak viral production occurred near day 15 (Figure 2E).
Figure 2.
HIV-Infected iMicroglia Produce an Inflammatory Response, and Respond to EFZ
(A) Immunostaining showing reduced percentage of P24+ (red) IBA1+ (green) iMg in HIV-infected mono-cultures + EFZ treatment compared with infected cultures with no EFZ treatment. Scale bar represents 50 μm.
(B) Percentage of P24+ cells in mono-culture iMg at D15 for infected and Inf + EFZ conditions. n = 4 infections from 3 cell lines, error bars represent SEM.
(C) Percent of P24+ single nucleated and multinucleated iMg in mono-culture for infected and Inf + EFZ conditions. n = 4 infections from 3 cell lines.
(D) Percent of P24(−) single nucleated and multinucleated iMg in mono-culture for Inf and Inf + EFZ conditions. n = 4 infections from 3 cell lines.
(E) Reverse transcriptase activity of Uninf, Inf, and Inf + EFZ (20 nM) iMg show productive infection and response to EFZ. n = 3 independent infections of WT6, one-way ANOVA, Dunnett's post hoc analysis; ∗p < 0.05, ∗∗∗∗p < 0.0001; error bars represent SEM.
(F–I) Cytokine analysis of infected iMg mono-culture displays increase in IL-1b (F), IL-8 (G), TNF-α (H), and IL-1a (I) production in the infected iMg. n = 3 independent infections of WT6, one-way ANOVA, Dunnett's post hoc analysis; ∗p < 0.05, ∗∗p < 0.01; error bars represent SEM.
We initially infected iMg from iPSCs of four individuals. Remarkably, the iMg of one individual showed limited infection (Figure S1E). Subsequent genotyping revealed heterozygosity for the CCR5Δ32 mutation, known to reduce infectivity by HIV (Liu et al., 1996) (Figure S1D). This line was thus excluded from subsequent studies, but this example demonstrates the validity of our system to model known regulators of human infection.
To determine if ART can suppress infection in the iMg, we examined the effects of the antiretroviral drug EFZ, a non-nucleoside reverse transcriptase inhibitor (De Clercq, 2004). EFZ remains a first-line drug in many parts of the world (Taramasso et al., 2018). Because EFZ blocks HIV reverse transcription, it had an advantage in allowing the study of non-productively infected, HIV-exposed cells alongside productively infected cells, a scenario thought to occur in brains of ART-treated HIV+ people. At day 15, EFZ reduced the infection rate by two-thirds to 29.2% ± 6.3% (Figures 2A and 2B). Of those iMg infected in the EFZ condition, rates of multinucleation were over 90% and similar to the Inf condition (Figure 2C), and 100% of P24(−) iMg in the Inf+ EFZ culture were single nucleated (Figure 2D). Reverse transcriptase activity was severely reduced with EFZ treatment (Figure 2E). The combined impaired reverse transcriptase and reduced percentage of P24(+) cells shows that EFZ effectively suppressed new infection, allowing us to study both infected and uninfected iMg in the same culture.
Infected iMg Produced Proinflammatory Cytokines at Peak Infection, Which Is Tempered by EFZ Treatment
HIV infection in the CNS leads to changes in cytokine profiles (Ginsberg et al., 2018). Therefore, we quantified the changes in production of relevant cytokines in iMg over the course of infection ± EFZ. Infection led to increased production of several proinflammatory cytokines, specifically IL-1b (p < 0.01), IL-1a (p < 0.05), transforming growth factor α (TNF-α) (p < 0.05), and most prominently, IL-8 (p < 0.01) (Figures 2F–2I). However, the two other cytokines tested: IL-6 and IL-10, did not change across any condition (Figures S2A and S2B). It was expected that the anti-inflammatory IL-10 would not increase, but surprising that IL-6 did not increase (Shah et al., 2011).
Infected iMg cultures exposed to EFZ had less production of IL-1b, IL-8, TNF-α, and IL-1a (Figures 2F–2I), suggesting a reduced inflammatory reaction, which recapitulates what is seen in ART-treated patients. Uninf, Uninf + EFZ, and the DMSO vehicle control did not elucidate a cytokine reaction across the six cytokines tested (Figures S2C–S2G).
Infected iMg Have Impaired Cell-Cycle Regulation and DNA Repair and Increased Expression of Inflammatory Genes
To investigative overall gene expression changes during infection, we performed bulk RNA-seq on three iMg lines ± HIV infection, which revealed significant changes in cell-cycle regulation and DNA repair (Figure S3A), consistent with our observation that HIV infection of iMg results in multinucleation. Although no inflammatory pathways were identified by Ingenuity Pathway Analysis, many inflammatory genes involved in the complement system, nuclear factor κB (NF-κB) signaling, and TNF-α signaling were significantly upregulated, as well as IL1b, CCL8, and FOS (Figure S3C).
Collectively, these data show that the iMg exhibit an inflammatory response similar to that seen in vivo with increased production of IL-8, IL-1b, IL-1a, and TNF-α. This inflammatory response is strongly attenuated with EFZ. RNA-seq analysis revealed changes in cell cycle and inflammation between uninfected and infected iMg as expected.
Generation of iPSC-Derived Tri-cultures of iNeurons, iAstrocytes, and iMicroglia
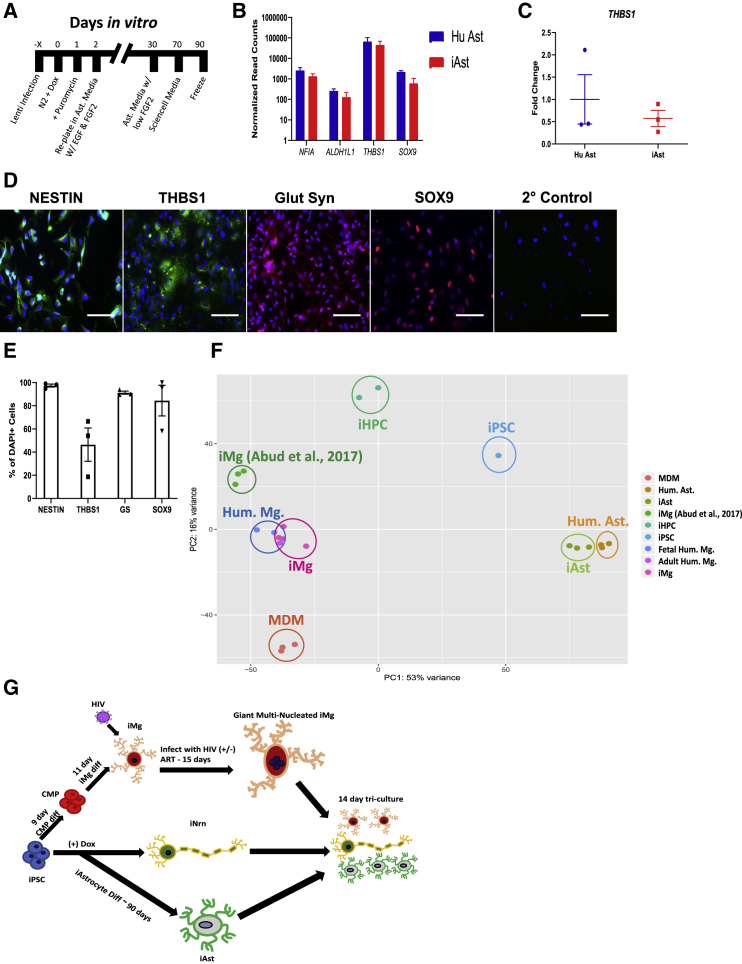
iNrn were generated by an established protocol that generates a homogeneous population of glutamatergic, forebrain-like excitatory neurons (Zhang et al., 2013). iPSC-derived astrocytes (iAst) were generated by brief exposure of iPSCs to the NGN2 transcription factor used to drive iNrn differentiation, and that in vivo is expressed by cortical progenitors before their conversion from neurogenesis to gliogenesis. We noticed that, after 2 days of NGN2 production, cells express the neural progenitor markers Nestin and NCAM and the astrocyte marker SOX9 (Kang et al., 2012) (Figure S4A). Because the NGN2 protocol produces a homogeneous population of neurons, we posited that shifting the differentiation at the neural progenitor stage would yield a relatively homogeneous population of iAst. We thus shifted the differentiation to astrocytes by removing the NGN2-inducing agent doxycycline at day 2, while promoting astrocytic differentiation and proliferation with fibroblast growth factor 2 (FGF-2), epidermal growth factor (EGF), and fetal bovine serum (FBS) (Michler-Stuke et al., 1984). After 70 days the iAst were switched to Sciencell Astrocyte Media (Figure 3A). After 90 days, RNA-seq revealed similar overall gene expression between iAst and fetal human astrocytes (Hu Ast), including several key astrocyte genes (Figures 3B and 3F). qRT-PCR validation of THBS1 confirmed similar expression Hu Ast and iAst (Figure 3C). We confirmed several of these genes at the protein level, including Nestin, glutamine synthetase, THBS1, an important protein in early synaptogenesis (Christopherson et al., 2005), and SOX9, revealing a relatively homogeneous population (Figures 3D and 3E). The iAst exhibited modest glutamate uptake and propagated Ca2+ waves in a gap junction-dependent manner, demonstrated by halted Ca2+ propagation with 100 μM carbenoxolone, a gap junction blocker (Figures S4F–S4I and Video S1). While in mono-culture, the iAst did not detectably express GFAP, GLT-1, or GLAST (Figures S4B–S4D), in tri-culture, single-cell RNA-seq (scRNA-seq) showed expression of GLT-1 in iAst (Figure S4E). As neuronal activity regulates GLT-1 in astrocytes (Swanson et al., 1997), iAst have a more in vivo-like phenotype when in the more physiologically relevant tri-culture.
Figure 3.
Generation and Characterization of iAst and Formation of Tri-cultures
(A) Timeline for iAstrocyte differentiation.
(B) Bulk RNA-seq normalized read counts of select astrocyte specific genes from primary human astrocytes and iAst. n = 3 cell lines, Benjamini-Hochberg, FDR = 0.01, error bars represent SEM.
(C) qRT-PCR validation for expression of THBS1 in Hu Ast and iAst. Probe normalized to GAPDH expression. n = 3 cell lines, two-tailed t test, error bars represent SEM.
(D) Immunostaining for the astrocyte-specific markers: Nestin, thrombospondin-1, glutamine synthetase, SOX9, and secondary control. Scale bar represents 50 μm.
(E) Percentage of DAPI(+) cells that are double-positive for astrocyte marker. n = 3 cell lines, error bars represent SEM.
(F) PCA for monocyte-derived macrophages, iMicroglia, primary human astrocytes, and iAstrocytes, and (Abud et al., 2017) iMg, iPSC, iHPC, and primary human microglia. Each dot represents a bulk RNA-seq sample. MDM, monocyte-derived macrophage; Hum. Ast., primary fetal human astrocytes; iHPC, induced hematopoietic progenitor cells. iPSC, iHPC, Hum. Mg., iMg samples were all retrieved from Abud et al. (2017).
(G) Flowchart for differentiations into iMg, iAst, and iNrn and combination into tri-culture ± HIV infection and ART.
We also exposed the iAst in mono-culture to the most highly expressed cytokines in the HIV-infected iMg, to determine whether the cytokines produced by infected iMg can elicit an inflammatory response in the iAst. We exposed the iAst to IL-1b and IL-8 at 10 ng/mL for 8 h and then analyzed the supernatants on the same six-cytokine panel. We found that, of the six cytokines tested, the iAst produced increased amounts of IL-1a (Figure S3B), suggesting that the iMicroglia can elicit an inflammatory response in the iAst.
We next sought to study iMg in an in vitro setting amenable to the study of synaptic phagocytosis and the influences of HIV and ART on gene expression in forebrain cells. To accomplish this we independently generated iNrn, iAst, and iMg cells, and then placed them in mixed cultures. For this study, analysis was performed 14 days after all 3 cell types are combined (Figure 3G), as our intention was to focus on the acute phase of exposure of forebrain-like neurons, astrocytes, and microglia to productively HIV-infected microglia. Having validated mono-cultures and assembled tri-cultures, we next used scRNA-seq to investigate cell-type-specific gene expression changes during infection ± EFZ.
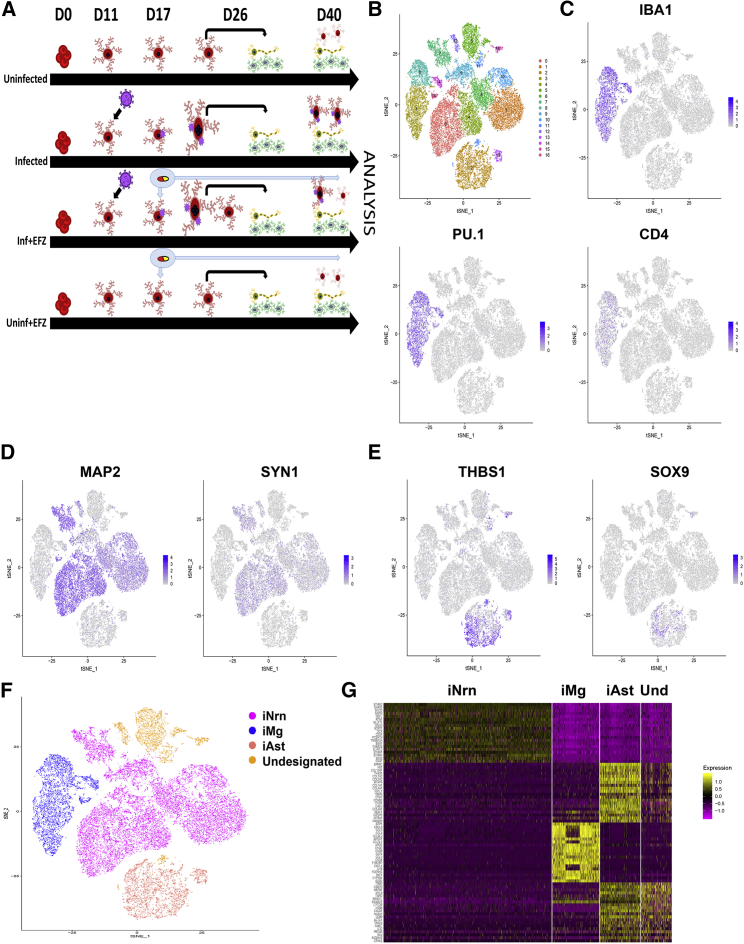
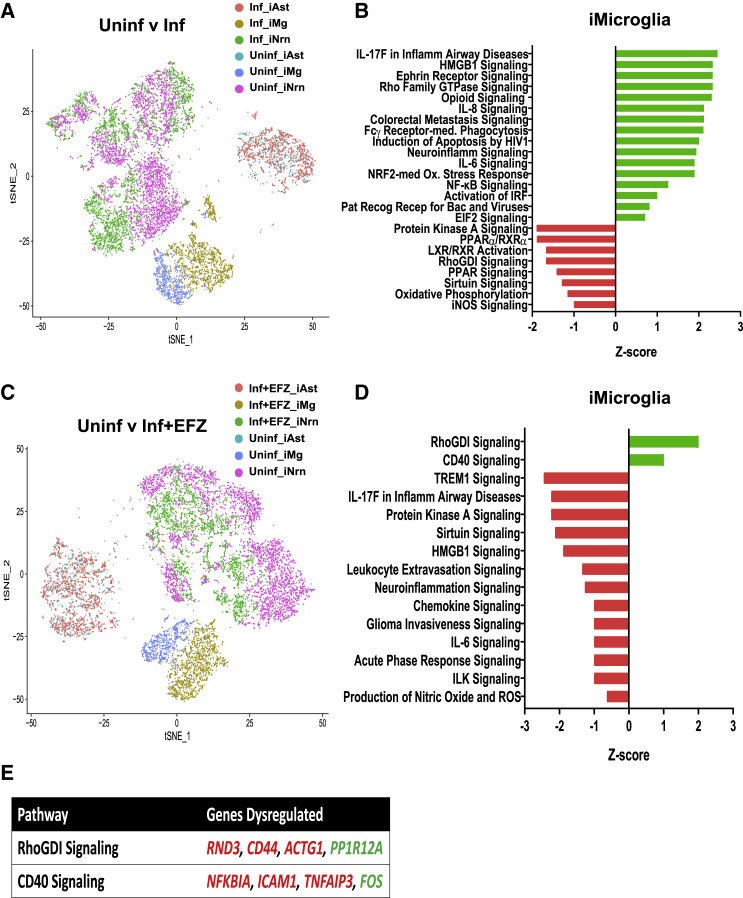
scRNA-Seq Identified Each of the Three Cell Types in all Four Conditions
To create the tri-culture, we began the iNrn differentiation, added the iAst post-puromycin selection at D5 of the iNrn differentiation, and added the iMg at D7 of the iNrn differentiation, so that the iAst had time to acclimate before addition of HIV-infected iMg. The tri-culture was maintained for an additional 14 days, to D21 of the iNrn differentiation in NBM/B27 with neurotrophin 3 (NT-3) and brain-derived neurotrophic factor (BDNF) as described before (Zhang et al., 2013). To assay gene expression changes in each of the three cell types during HIV infection ± EFZ, we conducted scRNA-seq on four conditions: Uninf, Inf, Inf + EFZ, and Uninf + EFZ (Figure 4A). Cells were sequenced from the Uninf (n = 6,564), Inf (n = 7,431), Inf + EFZ (n = 7,111), and Uninf + EFZ (n = 10,071) conditions, with comparable numbers of each cell type per condition (Figure S5D). All conditions were then aggregated and initially analyzed through the Cellranger pipeline (10× Genomics, v.3.0.1). Secondary analysis was performed using the Seurat package in R. We generated 16 unbiased clusters (Figure 4B). First, we separated the clusters by cell type, then broke down each cell cluster by condition. We assigned clusters to one of the three cell types by expression of several key genes: iMg by expression of IBA1, PU.1, and CD4; iNrn by expression of MAP2 and SYN1; and iAst by expression of THBS1 and SOX9 (Figures 4C–4E). There were four clusters (clusters 5, 10, 12, and 13) that did not fit into any of the three cell types by expression of the chosen markers (Figures 4B and 4F). To determine what the fourth cell type might represent, we examined the expression of the top 20 genes in each of the 4 cell types (Figure 4G). The gene expression pattern of the undesignated cells best matched the iAst, but scRNA-seq did not capture expression of THBS1 or SOX9, suggesting these cells represent less mature versions of the iAst. Hence, they were excluded from further analysis (Figure S5A). We then separated each cell type by condition (Figure S5B). We examined expression of genes related to inflammation and found the largest change in the iMg among the three cell types (Figure S5C).
Figure 4.
scRNA-Seq Identified Each of the Three Cell Types in all Four Conditions
(A) Timeline from start of CMP differentiation through tri-culture for the four conditions.
(B) t-SNE of unbiased clustering of combined scRNA-seq from all four conditions.
(C–E) Expression patterns of cell-type-specific markers for microglia (C), neurons (D), and astrocytes (E).
(F) t-SNE clustering by cell type based on cell-type-specific marker expression. One cluster did not align with any of the three cell types by the expression patterns chosen in (C–E).
(G) Heatmap of top 20 genes expressed in each cell type
Inflammatory Pathways and EIF2 Signaling Were Dysregulated among all Three Cell Types during Infection, but iMg Were Most Affected
To understand the gene expression changes among the three cell types during HIV infection ± EFZ, we first compared the Inf condition with Uninf. Several inflammatory pathways were significantly activated in Inf iMg compared with Uninf iMg, including IL-8 and NF-κB signaling. One of the top pathways dysregulated in Inf iMg compared with Uninf was the EIF2 pathway (Figure 5B). EIF2 signaling is involved in the UPR and, more broadly, the integrated stress response (ISR) (Janssens et al., 2014). The EIF2 pathway was not only dysregulated in iMg, but also was consistently increased in the iAst and iNrn (Figures S6A and S6B). However, only iMg had increased expression of ATF4 mRNA, the transcription factor involved downstream of ISR activation (Masuda et al., 2013) in Uninf v Inf (Figure S5C). Previously, we have shown that the ISR, particularly the PERK arm, is activated in neurons and astrocytes from human brain samples with HAND (Akay et al., 2012, Lindl et al., 2007).
Figure 5.
iMg Activate RhoGDI and CD40 in Response to HIV Infection with EFZ Treatment
(A) t-SNE plot of Uninf and Inf conditions.
(B) Ingenuity Pathway Analysis of iMg between Uninf and Inf conditions. Uninf is baseline. Benjamini-Hochberg FDR = 0.05, Fisher's exact <0.05, Z score cutoff ±0.5.
(C) t-SNE plot of Uninf and Inf + EFZ conditions.
(D) Ingenuity Pathway Analysis of iMg between Uninf and Inf + EFZ conditions. Uninf is baseline. Benjamini-Hochberg FDR = 0.05, Fisher's exact <0.05, Z score cutoff ±0.5.
(E) Specific genes dysregulated that are involved in the RhoGDI and CD40 pathways in Inf + EFZ iMg compared with Uninf iMg. Red genes are downregulated. Green genes are upregulated.
Inf + EFZ Caused Distinct Increased Activation of RhoGDI and CD40 Pathways
We next compared Uninf with Inf + EFZ, expecting to see a dampened immune response compared with Inf. Many of the top affected pathways in Inf iMg were related to inflammation, and there is a stark difference between Inf iMg and Inf + EFZ iMg (Figures 5B and 5D). However, the Inf + EFZ had a much milder inflammatory reaction, where RhoGDI and CD40 signaling were the only upregulated pathways compared with Uninf iMg (Figures 5D and 5E). RhoGDI negatively regulates Rac, which functions in multiple inflammation pathways (Wilkinson and Landreth, 2006), and CD40 activates NF-κB signaling (D'Aversa et al., 2008). A milder inflammatory response corroborates well with lesser disease severity seen in HAND patients who are taking ART (Saylor et al., 2016). Interestingly, EIF2 signaling was only activated in the iNrn in Inf + EFZ (Figures 5D, S6C, and S6D).
The scRNA-seq data suggest that the microglia were most affected by the infection ± EFZ, with major changes to EIF2 signaling, inflammatory, oxidative damage, and phagocytic gene pathways. However, the distinct activation of RhoGDI and CD40 in the Inf + EFZ condition suggests that the combination of Inf + EFZ creates a unique effect not seen with infection or EFZ alone.
Inf + EFZ Had an Attenuated, but Distinct Inflammatory Response Compared with Inf
Inf iMg had increased activity in several inflammatory pathways, including IL-6 signaling, neuroinflammation signaling, and Fcγ receptor-mediated phagocytosis compared with Inf + EFZ, suggesting that the Inf + EFZ had an overall lower immune response. However, the EIF2 signaling pathway, part of the ISR, was lower in Inf iMg compared with Inf + EFZ (Figure S6E).
Remarkably, EFZ treatment alone created distinct changes in immune-related signaling pathways in the iMg (Figure S6F), creating a unique cassette of dysregulated pathways not fully recapitulated in any other comparison with Uninf. These results show a stark difference in responses to EFZ treatment alone and EFZ treatment with infection, suggesting combinatorial and probably interacting effects of infection and EFZ treatment.
iMg Synaptophagocytosis Is Impaired in Inf, Inf + EFZ iMg, and Uninf + EFZ iMg
To further explore the differences in iMg responses to infection ± EFZ and EFZ treatment alone, we interrogated the ability of iMg to phagocytose synapses. Fcγ receptor-mediated phagocytosis pathway was activated in the Inf iMg as well as the Uninf + EFZ iMg compared with Uninf iMg (Figures 5B and S6F). In addition, HIV-infected macrophages and uninfected macrophages exposed to HIV display decreased phagocytic capabilities, specifically through the Fcγ receptor-mediated pathway, due to Nef and Tat inhibiting endocytosis (Debaisieux et al., 2015, Mazzolini et al., 2010). However, human microglia synaptophagocytosis in the context of HIV infection has not been tested.
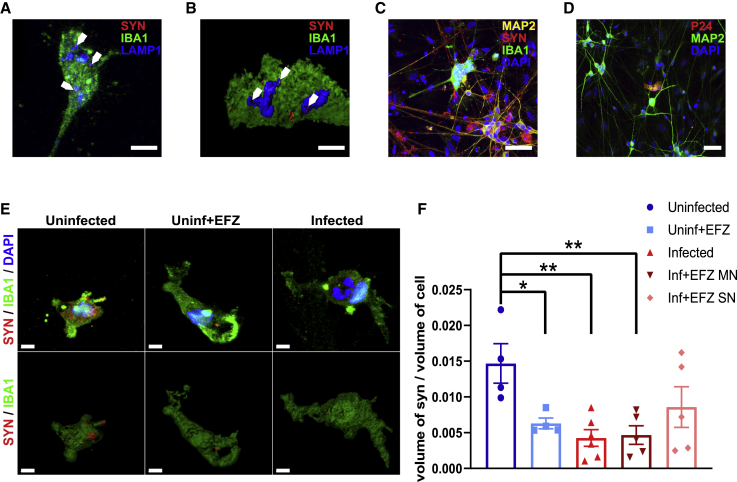
In tri-cultures evaluated 14 days after addition of the iMg, we found colocalization of SYN1 in the LAMP1+ lysosomes of the iMg (Figures 6A and 6B), suggesting that the iMg phagocytize synaptic material. We confirmed that iMg are in contact with iNrn (Figure 6C), and importantly, only the iMg are infected as only they, and not the iNrn (Figure 6D) or iAst, show P24 immunofluorescence. In addition, 1 out of 1,208 iAst in the Inf condition had reads for p24 from the scRNA-seq. These results suggest that it is unlikely that astrocytes are productively infected but may internalize HIV particles from the infected microglia at very low levels. We were also able to delineate infected from uninfected iMg in the Inf + EFZ condition by multinucleation, which is present in nearly all P24 + iMg (Figures 2A and S7B).
Figure 6.
HIV-Infected iMicroglia Have Reduced Synaptophagocytosis, which Is Ameliorated by EFZ Treatment
(A and B) Immunostaining (A) and surface reconstruction of a side view (B) of iMg in an iNrn co-culture displaying synaptic phagocytosis by colocalization of synaptophysin+ (red) puncta within LAMP1+ (blue) lysosomes in IBA1+ (green) iMg. Sale bar represents 10 μm.
(C) Giant multinucleated IBA1+ (green) iMg potentially interacting with synaptophysin+ (red) synapses on MAP2+ (yellow) dendrites. Sale bar represents 50 μm.
(D) Multinucleated iMg, but not MAP2+ (green) iNrn or iAst, are P24+ (red) in the tri-culture after 14 days. Sale bar represents 50 μm.
(E) Representative surface reconstructions of synaptophysin signal (red) within IBA1+ (green) iMg in the Uninf, Uninf + EFZ, and Inf tri-cultures. SN, single nucleated; MN, multinucleated. Scale bar represents 5 μm.
(F) Synaptophagy is significantly decreased in infected iMg ± EFZ and uninfected iMg + EFZ compared with control. n = 4 (Uninf = 4, Uninf + EFZ = 4, Inf = 6, Inf + EFZ = 6) independent differentiations of WT6, one-way ANOVA, Dunnett's post hoc analysis; ∗p < 0.05; error bars represent SEM.
The Inf iMg phagocytosed 70.9% less synapses than Uninf iMg (p < 0.01). In addition, in the Inf + EFZ condition, the infected (multinucleated) had 68.2% reduced phagocytosis (p < 0.01). However, the uninfected (single nucleated) iMg in the Inf + EFZ condition showed trends toward reduction in synaptophagy but was not statistically significant (p = 0.1). Finally, Uninf + EFZ condition iMg phagocytosed 57.1% less than Uninf iMg (p < 0.05) (Figures 6E and 6F). Multiple factors from the virus itself, the immune response, and the effects of antiretrovirals are acting on the iMg during HIV infection. All these factors may play a role in reducing synaptic phagocytosis and warrant future study.
To confirm that differences in apparent iMg synaptophagy are not secondary to reduced numbers of synapses overall in a given culture condition, we also measured local and random (50-μm radius areas with no iMg) synapse density to ensure the uninfected microglia were not in a more synapse dense area (Figures S7A and S7C). In fact, there was no significant difference in iMg-proximal or random synapse density across all conditions (Figures S7D and S7E). This finding aligns with previous studies that demonstrate inhibition of phagocytosis by viral proteins Tat and Nef in both infected and uninfected macrophages (Debaisieux et al., 2015, Mazzolini et al., 2010) and is also consistent with ART-related impairment of phagocytosis (Giunta et al., 2011).
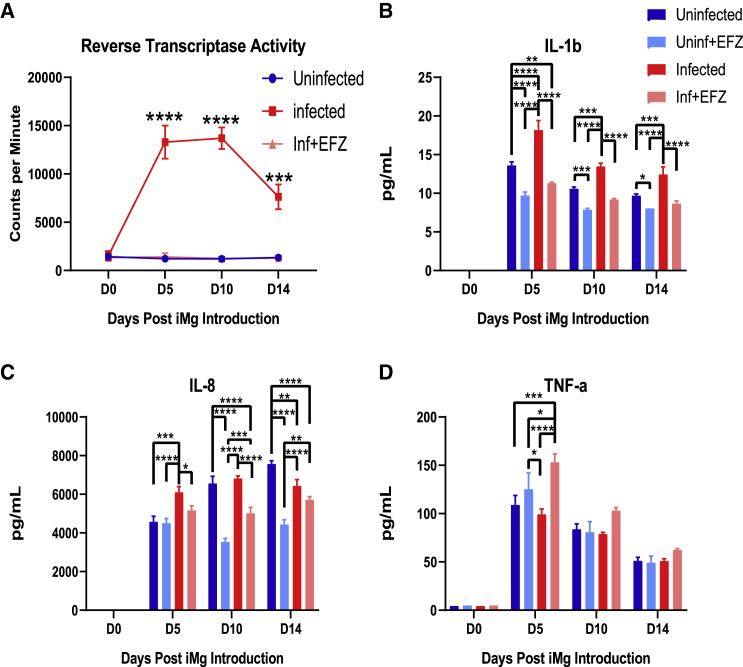
EFZ Reduced the Production of IL-8 and IL-1b by Infected iMg, but Enhanced TNF-α
To validate the activation of cytokine and inflammatory pathways in infected microglia ± EFZ, we compared cytokine production between each condition in tri-culture. Starting at the time of iMg addition, we collected supernatant every 5 days until day 14 of tri-culture. Reverse transcriptase activity confirmed that the iMg in the infected condition remain productively infected (Figure 7A). IL-8 and IL-1b were significantly increased in the infected tri-culture compared with uninfected control 5 days after iMg introduction (p < 0.0001, p < 0.001). IL-1b remained increased over uninfected at each time point; however, IL-8 decreases by day 10 (Figures 7B and 7C). The Inf + EFZ condition had consistent, significant reduced cytokine production of IL-8 from D10 through D14 (p < 0.0001) and IL-1b at D5 (p < 0.01) compared with Uninf. Uninf + EFZ had the lowest levels of cytokine production across all the groups (Figures 7B–7D). In addition, there was no increase in TNF-α in the Inf tri-culture. However, the Inf + EFZ condition did have increased TNF-α compared with all other conditions at D5 (Figure 7D). The Inf and Inf + EFZ tri-cultures also did not exhibit increases in IL-6 or IL-10, similar to our culture findings (Figures S2H and S2I). These data combined with the scRNA data suggest there are distinct influences on the inflammatory response during infection alone and infection with EFZ. Inf iMg exclusively had increased expression of CXCL8 and IL1B genes (Figure S5C). In addition, IL-8 and NF-κB signaling were activated only in Inf iMg compared with Uninf by scRNA-seq (Figure 5B) Interestingly, TNF-α regulates AP-1 (FOS) (Clark et al., 2005), a CD40 signaling pathway gene that is upregulated in Inf + EFZ iMg when compared with the Uninf (Figures S5C and 5D). These results further suggest distinct inflammatory reactions from Inf and Inf + EFZ that are observed in the iMg.
Figure 7.
Inf + EFZ Mitigates IL-1b and IL-8 Production, but Increases TNF-α Production
(A) Reverse transcriptase activity of Uninf, Inf, and Inf + EFZ tri-culture. n = 3 infections of WT6, one-way ANOVA, Dunnett's post hoc analysis; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; error bars represent SEM.
(B–D) Cytokine analysis of Uninf, Uninf + EFZ, Inf, and Inf + EFZ iMg displays increase in IL-1b (B) and IL-8 (C) production in the Inf tri-culture, and Inf + EFZ had increased TNF-α (D). n = 3 infections of WT6, two-way ANOVA, Tukey's post hoc analysis; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; error bars represent SEM.
Discussion
We describe an hiPSC tri-culture model to investigate the interdependent and individual roles of microglia, astrocytes, and neurons in the context of HIV infection. In recent years, there have been several new differentiation protocols for these cell types (Abud et al., 2017, Santos et al., 2017, Zhang et al., 2013) and several iPSC/primary cell co-cultures that have studied various neurological disorders (Lin et al., 2018, Park et al., 2018). This model advances previous works in exploring gene expression changes with scRNA-seq in the context of viral infection and relevant drug treatments to begin to unfurl a particularly difficult disease to study.
This model recapitulates several previous in vivo findings, such as increases in IL-8 and IL-1b production in microglia and EIF2 signaling in astrocytes and neurons (Akay et al., 2012, Ginsberg et al., 2018). The consistent activation of EIF2 signaling in all three cell types not only recapitulates previous findings, but also suggests microglia have dysregulated EIF2 signaling. ISR and EIF2 activation has been implicated in establishing initial viral replication (Jiang et al., 2017). A recent study also found stress response genes, including the ISR-associated gene ATF4, upregulated in the brains of older HIV-positive patients (Solomon et al., 2019). The UPR and consequently EIF2 activation has been implicated in multiple neurodegenerative disorders (Scheper and Hoozemans, 2015), these findings bolster the possibility that UPR and ISR activation play important roles in HAND development or at least the initial response to HIV in the brain.
We also found that the gene pathways associated with IL-8, IL-1b, and TNF-α production were upregulated in iMg, suggesting that the microglia are the main culprit in the initial response to HIV infection. However, iMg infection seems to lead to reduced phagocytosis of synapses. This falls in line with previously reported impaired phagocytosis by HIV-infected or exposed macrophages (Debaisieux et al., 2015, Mazzolini et al., 2010). While random synapse density was variable, there was consistent reduced synaptic particles in Inf iMg, suggesting that there was reliably a surplus of synapses. Although not significant, Uninf + EFZ cultures had consistently less overall synapse density, suggesting that the reduced phagocytosis may be due to a global reduction in synapses. In addition, we cannot rule out the possibility that the Inf or Uninf + EFZ were more efficient in degrading the synaptic particles. Ideally, future studies using live-imaging are warranted to resolve this issue. How iMg phagocytosis would be affected or if inflammation patterns would change in longer-term cultures remain to be determined.
In addition, we discovered a starkly different immune response at the gene and functional level in Inf versus Inf + EFZ, characterized by distinct CD40/RhoGDI pathway activation and TNF-α production. CD40 and RhoGDI activation could be controlled by the increased production of TNF-α that was only found in the Inf + EFZ condition. This persistent immune activation highlights the need for further studies into the role that antiretrovirals play in propagating the chronic inflammation seen in HIV patients today (Kolson, 2017). The mitigated reaction we observed with EFZ treatment is consistent with human studies showing a reduced severity of HAND with the widespread use of antiretroviral therapies (Saylor et al., 2016), but also with its persistence despite control of viral load. Furthermore, we revealed a substantial immune response to EFZ treatment alone. This warrants further study, particularly due to the recommended prophylactic use of ART in HIV-negative patients (Spinner et al., 2016).
hiPSC cultures are particularly useful for studying HIV neuropathology, since human primary neuronal cells and postmortem tissue are limited in availability and not amenable to molecular manipulation. In addition, HIV only infects human cells, rendering the interpretation of results from animal models more convoluted. Hence, mechanistic studies of the influences of HIV and ART on human neural cells are limited. This tri-culture system allows us to better study the mechanisms of early HIV infection in the brain. However, there are caveats to this system that must be considered. Each of the iPSC-derived cell types are similar to their in vivo counterparts by gene and protein expression, as well as function, but are not exact. In addition, the iCells' gene expression profile at the end stage of differentiation is relatively immature and more closely represents early stages of development in vivo. Still, our culture system allows reductionist study of three key cell types over weeks of infection, but it may be refined by inclusion of additional cell types to further optimize modeling HAND in the adult and/or chronic setting.
This tri-culture has validated several findings in the field, as well as, produced multiple findings for HIV neuropathology. However, this model is not restricted to HIV neuropathology and can also be utilized to study other neurological disorders. This highly tractable, reductive system can be genetically and pharmacologically modified at any stage. These differentiations could also be used on patient-derived cells, creating a disease-relevant, patient-specific tri-culture system. Similar cultures have been developed (Haenseler et al., 2017, Park et al., 2018), but not to this complexity or with a focus on viral infections. The innovation of the tri-culture is not the individual differentiations of iMg or iAst, as there are many published differentiations. Rather, it is an all hiPSC tri-culture that reliably recapitulated the intricate interactions among multiple cell types during HIV infection and revealed new, potential therapeutic targets. Alternatively differentiated iNrn, iMg, or iAst or additional cell types could be implemented. The tri-culture can be another instrumental tool in understanding the workings of complex neurological disorders and in developing novel therapeutic strategies.
Experimental Procedures
iNeuron Differentiation of iPSCs
iPSCs were transfected with two plasmids VSVG.HIV.SIN.cPPT.CMV.mNgn2.WPRE and VSVG.HIV.SIN.cPPT.CMV.rTTA.WPRE, produced by Marius Wernig (Stanford University) and packaged by the University of Pennsylvania Viral Vector Core. Cells were exposed to 1 μg/mL polybrene (Sigma-Aldrich TR-1003). The medium is fully exchanged 6 h after exposure. iPSCs are differentiated according to a previously published method (Zhang et al., 2013). In brief, after transfection, iPSCs were exposed to N2 medium containing 5 mL N2 Supplement-B (STEMCELL Technologies 07156), 0.5 mL 55 mM β-mercaptoethanol (Life Technologies 21985-023), 0.5 mL primocin (Invivogen ant-pm-2), BDNF (10 ng/mL, PeproTech 450-02), NT-3 (10 ng/mL, PeproTech 450-03), laminin (200 ng/mL, Sigma L2020), and doxycycline (2 μg/mL, Sigma D3072) in DMEM/F12 (Gibco 11,320-033) for 24 h (DIV0). Cells were then exposed to puromycin (5 μg/mL, Sigma P9620) for 24 h in the same N2 medium (DIV1). iNeurons were re-plated 24 h later (DIV2) to experiment-appropriate plates coated with Matrigel GFR (Corning 354230) (1:20 DMEM). Cells were washed 2× in PBS and lifted with StemPro accutase (Thermo Fisher Scientific, A11105-01) for 5 min at 37°C. Cells were spun down at 1,000 rpm for 5 min at room temperature. Cells were resuspended and plated in iN medium (Neurobasal-A medium; Invitrogen, A24775-01) with 5 mM glucose (Sigma, G5146), 10 mM sodium pyruvate (Sigma, P5280), glutamax (Life Technologies, 35050-061), penicillin/streptomycin (Thermo Fisher Scientific, 15140-148), BDNF and NT-3 (10 ng/mL), and doxycycline (2 μg/mL) through 9 days. Ara-C (2 μM, Sigma, C6645) was added on DIV3, and there was a full medium exchange 24 h later (DIV4). Doxycycline was discontinued at DIV10 for the rest of the 21-day differentiation.
iAstrocyte Differentiation of iPSCs
iPSCs transfected with the NGN2 virus were put through the first 2 days of the iNeuron differentiation. On day 3, cells were exposed to astrocyte differentiation medium: N2 medium (without BDNF, NT-3, laminin, and doxycycline), 10% FBS (HyClone SH30071.03HI), B-27 with vitamin A (Thermo Fisher Scientific, 17504044), FGF-2 (20 ng/mL, R&D Systems, 233-FB-025) and EGF (20 ng/mL, R&D Systems, 236-EG-200). After 30 days, EGF was removed and FGF2 reduced to 5 ng/mL. After 70 total days, cells were switched into Astrocyte Medium (Sciencell, 1801). Experiments were performed after 90 total days of differentiation.
iMicroglia Differentiation of iPSCs
iPSCs were differentiated into CMPs according to the published protocol (Paluru et al., 2014) by the Human Pluripotent Stem Cell Core (CHOP). CMPs were plated at 333k cells/well in a 24-well CellBIND plate (Corning 3337). CMPs were differentiated in iMg medium (RPMI 1640 medium, GE Healthcare Life Sciences, SH30027.01) with 10% FBS (HyClone, SH30071.03HI), recombinant human IL-34 (100 ng/mL, R&D Systems, 5265-IL-010), CSF-1 recombinant human protein (25 ng/mL, Thermo Fisher Scientific, PHC9504), and recombinant human TGF-β1 (50 ng/mL, PeproTech, 100-21). Half medium changes were performed every 2 days for 11 days.
Tri-culture Combination
iNeurons were differentiated as described above and re-plated on DIV2 to Matrigel (Corning, 354230) coated (1:20 DMEM) Nunc Lab-Tek II 8-well chamber slides (Thermo Fisher Scientific, 62407-296) at 70k cells/well in iN medium. On DIV5 of iNeuron differentiation, the iAstrocytes were added at 50k cells/well. On DIV7 of iNeuron differentiation, iMicroglia were added at 100k cells/well. Cultures were taken out to DIV21 of iNeuron differentiation.
Statistics
GraphPad Prism 8 was used for statistical analysis. One-way ANOVA and Dunnett's post hoc, two-way ANOVA and Tukey's post hoc, two-way ANOVA and Sidak's post hoc, two-tailed t test, or two-tailed paired t test were used as indicated. p values are indicated in figures as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n.s., not significant.
Author Contributions
Conceptualization, S.A.A., K.L.J.-S., and S.K.R.; Methodology, S.A.A., K.L.J.-S., and S.K.R.; Investigation, S.K.R.; Formal Analysis, S.K.R., M.V.G., N.P.S., and J.P.G.; Writing – Original Draft, S.K.R., S.A.A., K.L.J.-S., F.C.B., and K.S.W.; Funding Acquisition, S.A.A. and K.L.J.-S.; Resources, S.A.A., K.L.J.-S., F.C.B., and H.H.; Supervision, K.J.S. and S.A.A.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (R21 NS107594 02) and the Penn Center for Aids Research (CFAR) and Penn Mental Health AIDS Research Center (PMHARC) (5-P30-MH-097488-05). We thank the Human Pluripotent Stem Cell Core (CHOP) for providing CMPs, Center for Applied Genomics for performing and analyzing RNA-seq, CFAR for providing HIV strains, and PMHARC for analyzing cytokines. We thank Elizabeth Krizman for performing western blots and glutamate uptake assays, who was partially supported by the Intellectual and Developmental Disabilities Research Center at CHOP/Penn U54 HD086984, and Herbert M. Lachman, MD, for providing iPSC lines.
Published: March 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.02.010.
Contributor Information
Stewart A. Anderson, Email: sande@pennmedicine.upenn.edu.
Kelly L. Jordan-Sciutto, Email: jordank@upenn.edu.
Accession Numbers
The datasets generated during and/or analyzed during this study are available in the GEO repository https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc&equals;GSE143687. The accession number for the data reported in this paper is GEO:GSE143687.
Supplemental Information
References
- Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay C., Lindl K.A., Shyam N., Nabet B., Goenaga-Vazquez Y., Ruzbarsky J., Wang Y., Kolson D.L., Jordan-Sciutto K.L. Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex. Neuropathol. Appl. Neurobiol. 2012;38:175–200. doi: 10.1111/j.1365-2990.2011.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano P., Prevedel L., Eugenin E.A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 2017;7:12866. doi: 10.1038/s41598-017-12758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Sulcove J., Frank I., Jaffer S., Ozdener H., Kolson D.L. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J. Virol. 2002;76:9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson K.S., Ullian E.M., Stokes C.C., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., Barres B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N., Fabbiani M., Di Giambenedetto S., Fanti I., Baldonero E., Bracciale L., Tamburrini E., Cauda R., De Luca A., Silveri M.C. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403. doi: 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- Clark J., Vagenas P., Panesar M., Cope A.P. What does tumour necrosis factor excess do to the immune system long term? Ann. Rheum. Dis. 2005;64(suppl 4):iv70. doi: 10.1136/ard.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aversa T.G., Eugenin E.A., Berman J.W. CD40-CD40 ligand interactions in human microglia induce CXCL8 (interleukin-8) secretion by a mechanism dependent on activation of ERK1/2 and nuclear translocation of nuclear factor-kappaB (NFkappaB) and activator protein-1 (AP-1) J. Neurosci. Res. 2008;86:630–639. doi: 10.1002/jnr.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem. Biodivers. 2004;1:44–64. doi: 10.1002/cbdv.200490012. [DOI] [PubMed] [Google Scholar]
- Debaisieux S., Lachambre S., Gross A., Mettling C., Besteiro S., Yezid H., Henaff D., Chopard C., Mesnard J.M., Beaumelle B. HIV-1 Tat inhibits phagocytosis by preventing the recruitment of Cdc42 to the phagocytic cup. Nat. Commun. 2015;6:6211. doi: 10.1038/ncomms7211. [DOI] [PubMed] [Google Scholar]
- Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R.E., Hill C.M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Easley-Neal C., Foreman O., Sharma N., Zarrin A.A., Weimer R.M. CSF1R ligands IL-34 and CSF1 are differentially required for microglia development and maintenance in white and gray matter brain regions. Front. Immunol. 2019;10:2199. doi: 10.3389/fimmu.2019.02199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg S.D., Alldred M.J., Gunnam S.M., Schiroli C., Lee S.H., Morgello S., Fischer T. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann. Neurol. 2018;83:406–417. doi: 10.1002/ana.25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B., Ehrhart J., Obregon D.F., Lam L., Le L., Jin J., Fernandez F., Tan J., Shytle R.D. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol. Brain. 2011;4:23. doi: 10.1186/1756-6606-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Skola D., Coufal N.G., Holtman I.R., Schlachetzki J.C.M., Sajti E., Jaeger B.N., O'Connor C., Fitzpatrick C., Pasillas M.P. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356 doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenseler W., Sansom S.N., Buchrieser J., Newey S.E., Moore C.S., Nicholls F.J., Chintawar S., Schnell C., Antel J.P., Allen N.D. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Reports. 2017;8:1727–1742. doi: 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S., Pulendran B., Lambrecht B.N. Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 2014;15:910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Santos Rocha C., Hirao L.A., Mendes E.A., Tang Y., Thompson G.R., 3rd, Wong J.K., Dandekar S. HIV exploits antiviral host innate GCN2-ATF4 signaling for establishing viral replication early in infection. MBio. 2017;8 doi: 10.1128/mBio.01518-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P., Lee H.K., Glasgow S.M., Finley M., Donti T., Gaber Z.B., Graham B.H., Foster A.E., Novitch B.G., Gronostajski R.M. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 2012;74:79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.O., Liu Y., Ruan Y., Xu Z.C., Schantz L., He J.J. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolson D. Neurologic complications in persons with HIV infection in the era of antiretroviral therapy. Top. Antivir. Med. 2017;25:97–101. [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-T., Seo J., Gao F., Feldman H.M., Wen H.-L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl K.A., Akay C., Wang Y., White M.G., Jordan-Sciutto K.L. Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol. Appl. Neurobiol. 2007;33:658–669. doi: 10.1111/j.1365-2990.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Masuda M., Miyazaki-Anzai S., Levi M., Ting T.C., Miyazaki M. PERK-eIF2alpha-ATF4-CHOP signaling contributes to TNFalpha-induced vascular calcification. J. Am. Heart Assoc. 2013;2:e000238. doi: 10.1161/JAHA.113.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini J., Herit F., Bouchet J., Benmerah A., Benichou S., Niedergang F. Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood. 2010;115:4226–4236. doi: 10.1182/blood-2009-12-259473. [DOI] [PubMed] [Google Scholar]
- Michler-Stuke A., Wolff J.R., Bottenstein J.E. Factors influencing astrocyte growth and development in defined media. Int. J. Dev. Neurosci. 1984;2:575–584. doi: 10.1016/0736-5748(84)90035-2. [DOI] [PubMed] [Google Scholar]
- O'Donnell L.A., Agrawal A., Jordan-Sciutto K.L., Dichter M.A., Lynch D.R., Kolson D.L. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J. Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluru P., Hudock K.M., Cheng X., Mills J.A., Ying L., Galvão A.M., Lu L., Tiyaboonchai A., Sim X., Sullivan S.K. The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells. Stem Cell Res. 2014;12:441–451. doi: 10.1016/j.scr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Wetzel I., Marriott I., Dréau D., D’Avanzo C., Kim D.Y., Tanzi R.E., Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018 doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R., Vadodaria K.C., Jaeger B.N., Mei A., Lefcochilos-Fogelquist S., Mendes A.P.D., Erikson G., Shokhirev M., Randolph-Moore L., Fredlender C. Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Reports. 2017;8:1757–1769. doi: 10.1016/j.stemcr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D., Dickens A.M., Sacktor N., Haughey N., Slusher B., Pletnikov M., Mankowski J.L., Brown A., Volsky D.J., McArthur J.C. HIV-associated neurocognitive disorder––pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016;12:234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper W., Hoozemans J.J. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 2015;130:315–331. doi: 10.1007/s00401-015-1462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Verma A.S., Patel K.H., Noel R., Rivera-Amill V., Silverstein P.S., Chaudhary S., Bhat H.K., Stamatatos L., Singh D.P. HIV-1 gp120 induces expression of IL-6 through a nuclear factor-kappa B-dependent mechanism: suppression by gp120 specific small interfering RNA. PLoS One. 2011;6:e21261. doi: 10.1371/journal.pone.0021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Chettimada S., Misra V., Lorenz D.R., Gorelick R.J., Gelman B.B., Morgello S., Gabuzda D. White matter abnormalities linked to interferon, stress response, and energy metabolism gene expression changes in older HIV-positive patients on antiretroviral therapy. Mol. Neurobiol. 2019 doi: 10.1007/s12035-019-01795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner C.D., Boesecke C., Zink A., Jessen H., Stellbrink H.-J., Rockstroh J.K., Esser S. HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection. 2016;44:151–158. doi: 10.1007/s15010-015-0850-2. [DOI] [PubMed] [Google Scholar]
- Sui Z., Sniderhan L.F., Schifitto G., Phipps R.P., Gelbard H.A., Dewhurst S., Maggirwar S.B. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J. Immunol. 2007;178:3226–3236. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- Swanson R.A., Liu J., Miller J.W., Rothstein J.D., Farrell K., Stein B.A., Longuemare M.C. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramasso L., Biagio A.D., Maggiolo F., Tavelli A., Caputo S.L., Bonora S., Zaccarelli M., Caramello P., Costantini A., Viscoli C. First-line antiretroviral therapy with efavirenz plus tenofovir disiproxil fumarate/emtricitabine or rilpivirine plus tenofovir disiproxil fumarate/emtricitabine: a durability comparison. HIV Med. 2018;19:475–484. doi: 10.1111/hiv.12628. [DOI] [PubMed] [Google Scholar]
- Turchan–Cholewo J., Dimayuga V.M., Gupta S., Gorospe R.M.C., Keller J.N., Bruce–Keller A.J. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid. Redox Signal. 2009;11:193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B.L., Landreth G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J. Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78 doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.