Summary
Ovarian cancer (OC) represents the most dismal gynecological cancer. Pathobiology is poorly understood, mainly due to lack of appropriate study models. Organoids, defined as self-developing three-dimensional in vitro reconstructions of tissues, provide powerful tools to model human diseases. Here, we established organoid cultures from patient-derived OC, in particular from the most prevalent high-grade serous OC (HGSOC). Testing multiple culture medium components identified neuregulin-1 (NRG1) as key factor in maximizing OC organoid development and growth, although overall derivation efficiency remained moderate (36% for HGSOC patients, 44% for all patients together). Established organoid lines showed patient tumor-dependent morphology and disease characteristics, and recapitulated the parent tumor's marker expression and mutational landscape. Moreover, the organoids displayed tumor-specific sensitivity to clinical HGSOC chemotherapeutic drugs. Patient-derived OC organoids provide powerful tools for the study of the cancer's pathobiology (such as importance of the NRG1/ERBB pathway) as well as advanced preclinical tools for (personalized) drug screening and discovery.
Keywords: organoids, ovarian cancer, disease modeling, neuregulin-1, high-grade serous ovarian cancer, ERBB
Highlights
-
•
Organoids are established from ovarian cancer (OC)
-
•
Neuregulin-1 (NRG1) is identified as key component for OC organoid growth
-
•
OC organoids capture disease hallmarks and recapitulate patient tumor characteristics
-
•
OC organoids are amenable to drug screening and mechanistic (NRG1/ERBB) research
In this article, Vankelecom and colleagues developed a well-defined protocol to establish organoid cultures from ovarian cancer (OC), particularly from the prevalent high-grade serous OC. The organoids capture disease hallmarks and patient tumor characteristics and provide powerful experimental models to decipher the cancer's pathobiology (such as the role of NRG1/ERBB), as well as preclinical tools for (personalized) drug screening and discovery.
Introduction
Ovarian cancer (OC) is the most lethal gynecological cancer. In more than 80% of patients, the disease is not discovered until advanced stage and metastasis (Narod, 2016). After primary debulking surgery and adjuvant chemotherapy, 70%–80% of the patients show tumor relapse with increasing chemoresistance (Pignata et al., 2017). Most OC cases display an epithelial phenotype (epithelial OC [EOC]), with 75% of the patients diagnosed with high-grade serous OC (HGSOC) of FIGO stage III or IV (i.e., showing extensive metastatic spread) (Jelovac and Armstrong, 2011). HGSOC causes up to 80% of the mortality among OC patients, and thus represents the most outstanding clinical challenge in gynecological oncology. Etiology and site of origin of EOC, whether it is ovarian surface epithelium (OSE) or fallopian tube epithelium (FTE) (or both), are still under intense debate (Kim et al., 2018).
Mechanisms underlying EOC pathobiology are poorly understood, and therapeutic efficiency and patient survival are not significantly improving (Timmermans et al., 2018). Most studies have been done using cancer cell lines that perform poorly in recapitulating histopathological and molecular phenotype of the tumor of origin and of EOC nature in general, thereby lacking clinical translatability (Lengyel et al., 2014). Patient tumor-derived xenografts, growing in immune-deficient mice, better mimic the original tumor but their establishment is inefficient, lengthy, and costly, and is ethically questionable (Sachs and Clevers, 2014). Therefore, more appropriate experimental and preclinical EOC models are needed.
A powerful research tool to model and study human cancer in vitro is provided by the innovative organoid technology. Organoids represent in vitro self-developing three-dimensional (3D) tissue reconstructions, reproducing key features of the tissue of origin (Clevers, 2016). In recent studies it has been demonstrated that organoids can be developed from multiple divergent cancer types such as colon, prostate, breast, and endometrial cancer. These tumor-derived organoids maintain type- and patient-specific characteristics (Boretto et al., 2019, Gao et al., 2014, Sachs et al., 2018, Van De Wetering et al., 2015). To derive organoids, patients’ tumor biopsies are dissociated into fragments and cells, embedded in a 3D extracellular matrix scaffold (such as Matrigel), and cultured in a cocktail of growth and signaling factors, which must be defined and optimized for each individual cancer type.
In the present study, we established organoids from OC that recapitulate disease and patients’ tumor characteristics. Our study independently confirms and expands the recent report by Kopper et al. (2019), although overall derivation efficiency is lower. Importantly, it adds new developed organoid lines to the growing OC organoid biobank, which is an essential impetus to enable the deciphering of the cancer's complex nature, pathogenesis, therapy resistance, and drug sensitivity, and to move the field forward toward more efficient (patient-tailored) treatments.
Results
Establishing Expandable Organoids from EOC
EOC biopsies (predominantly HGSOC; Table 1) were dissociated and cells seeded in OC organoid culture medium-1 (OCOM1; Table S1), the composition of which was based on the medium previously defined to derive organoids from endometrium and endometrial cancer (Boretto et al., 2017, Boretto et al., 2019). However, organoid development efficiency was low (33%) and expandability was limited to 1–2 passages (Figures S1A and S1B). Therefore, we systematically tested culture medium components to improve EOC organoid establishment and growth. Reducing the concentration of the transforming growth factor β (TGFβ) pathway inhibitor A83-01, raising the level of nicotinamide, and changing the source of RSPO1 from cell line-conditioned medium to recombinant protein (culture medium referred to as OCOM2; Figure S1A and Table S1) increased the expandability of developed organoid lines (to 3–7 passages; data not shown) but did not improve formation efficiency (Figures S1A and S1B). Further modification of the medium involving (1) omission of basic fibroblast growth factor (bFGF) and FGF10, (2) addition of insulin-like growth factor 1 (IGF1) and hepatocyte growth factor (HGF), known to stimulate growth of OC cell lines (Aune et al., 2011), and (3) reduction of the p38 mitogen-activated protein kinase inhibitor (p38i) SB203580 (OCOM3; Figure S1A and Table S1), shown to be beneficial for establishing organoids from other cancer types such as endometrial and breast cancer (Boretto et al., 2019, Sachs et al., 2018), improved formation efficiency (Figure S1A and S1B) but did not further increase expandability (data not shown). TGFα, reported to induce cell proliferation in cancerous OSE (Sheng et al., 2010), did not advance organoid growth initiation (data not shown), while RSPO1 was found to be essential (Figure S1B; comparable with Kopper et al., 2019 and Hill et al., 2018). Finally, we found that addition of NRG1 (OCOM4; Figure S1A and Table S1) significantly increased the number of organoids formed (Figure S1C), thereby independently (without prior knowledge) confirming, and in addition quantitatively supporting, the recent finding by Kopper et al. (2019). This beneficial effect of NRG1 is also in line with previous studies showing a potential (paracrine) growth-stimulatory effect of NRG1 in OC tumors and cell lines (Gilmour et al., 2002, Sheng et al., 2010). We further zoomed in on the effect of NRG1 and found a significant increase in the number of proliferating (Ki67+) cells in the organoid cultures as well as of the size of the organoids (Figure S1D). Taken together, by thoroughly probing multiple medium components, we eventually defined a culture medium (OCOM4) that strongly enhanced the EOC organoid formation efficiency (from 33% to 56%; Figure S1A). Interestingly, addition of NRG1 also increased the passageability of the EOC-derived organoids (Figure S1E). Although the number of organoids formed at tumor seeding (passage 0 [P0]) in OCOM4 was not inferior to the culture medium used in Kopper et al. (2019) (Figure S1F; “Kopper” medium, see Table S1), overall organoid derivation efficiency over total number of patients remained lower (for a detailed comparison, see Table S2). Possible reasons are described in the Discussion. Of note, organoid formation efficiency did not significantly differ between freshly obtained and cryopreserved biopsies (Figure S1G and Table 1), thereby underscoring the possibility to store clinical samples pending organoid establishment (as described for some other cancers, in particular mouse xenograft and human breast tumors; Walsh et al., 2016). Furthermore, organoids could be derived from EOC biopsies of both chemo-naive patients (obtained by primary debulking surgery) and chemotherapy-treated patients (obtained by interval debulking surgery after prior neoadjuvant chemotherapy) (Figure S1G).
Table 1.
Overview of EOC Patients and Samples and of Established Organoid Lines
| Patient/EOC Sample No. | Fresh/Cryo | EOC Typea | Patient Treatmentb | EOC Organoid Linec | Organoid Morphology | Passaging Time |
|---|---|---|---|---|---|---|
| 1 | fresh | malignant mixed mesonephric tumor IC2 | PDS | – | ||
| 2 | fresh | HGSOC IVA | IDS | – | ||
| 3 | fresh | HGSOC IVB | PDS | EOC-O_1 | cystic/low-cohesive | short-term |
| 4 | fresh | HGSOC IIIC | IDS | EOC-O_2 | dense/cystic | long-term |
| 5 | fresh | HGSOC IIIC | IDS | – | ||
| 6 | fresh | HGSOC IVB | IDS | – | ||
| 7 | fresh | HGSOC IIIC | IDS | – | ||
| 8 | cryo | HGSOC IVB | IDS | – | ||
| 9 | fresh | LGSOC IVB | IDS | EOC-O_3 | dense/low-cohesive | short-term |
| 10 | fresh | HGSOC IVB | TDS | EOC-O_4 | dense | long-term |
| 11 | cryo | HGSOC IIIC | PDS | – | ||
| 12 | fresh | HGSOC IIIC | IDS | – | ||
| 13A | fresh | HGSOC IVB (omentum) | IDS | – | ||
| 13B | cryo | HGSOC IVB (ovary) | IDS | EOC-O_5 | dense | short-term |
| 14 | fresh | clear cell ovarian cancer IIIA1(i) | PDS | EOC-O_6 | dense/cystic | short-term |
| 15 | cryo | HGSOC IVB | IDS | EOC-O_7 | cystic | long-term |
| 16 | cryo | HGSOC IVB | IDS | – | ||
| 17 | cryo | HGSOC IIIB | SDS | – | ||
| 18 | fresh | HGSOC IIIC | IDS | EOC-O_8 | low-cohesive | long-term |
| 19 | fresh | HGSOC IVB | IDS | – | ||
| 20 | fresh | HGSOC IVB | IDS | – | ||
| 21Ad | fresh | HGSOC IIIC (ovary) | IDS | EOC-O_9 | cystic | long-term |
| 21B | fresh | HGSOC IIIC (omentum) | IDS | – | ||
| 21C | fresh | HGSOC IIIC (rectum) | IDS | – | ||
| 22 | cryo | mucinous cystadenocarcinoma IC1 | PDS | EOC-O_10 | cystic | short-term |
| 23A | cryo | HGSOC IVB (ovary) | PDS | EOC-O_11 | cystic | long-term |
| 23B | cryo | HGSOC IVB (omentum) | PDS | – | ||
| 24 | cryo | HGSOC IVB | IDS | EOC-O_12 | dense | short-term |
| 25 | cryo | HGSOC IIIC | IDS | – | ||
| 26 | cryo | LGSOC IIIC | PDS | EOC-O_13 | dense/low-cohesive | short-term |
| 27 | cryo | HGSOC IIB | PDS | – |
HGSOC, high-grade serous ovarian cancer; LGSOC, low-grade serous ovarian cancer. In case of additional out-of-the-ovary sampling from the same patient, the different surgical sites are specified.
IDS, interval debulking surgery; PDS, primary DS; SDS, secondary DS; TDS, tertiary DS.
–, no organoid derivation.
Eventual healthy tissue organoids.
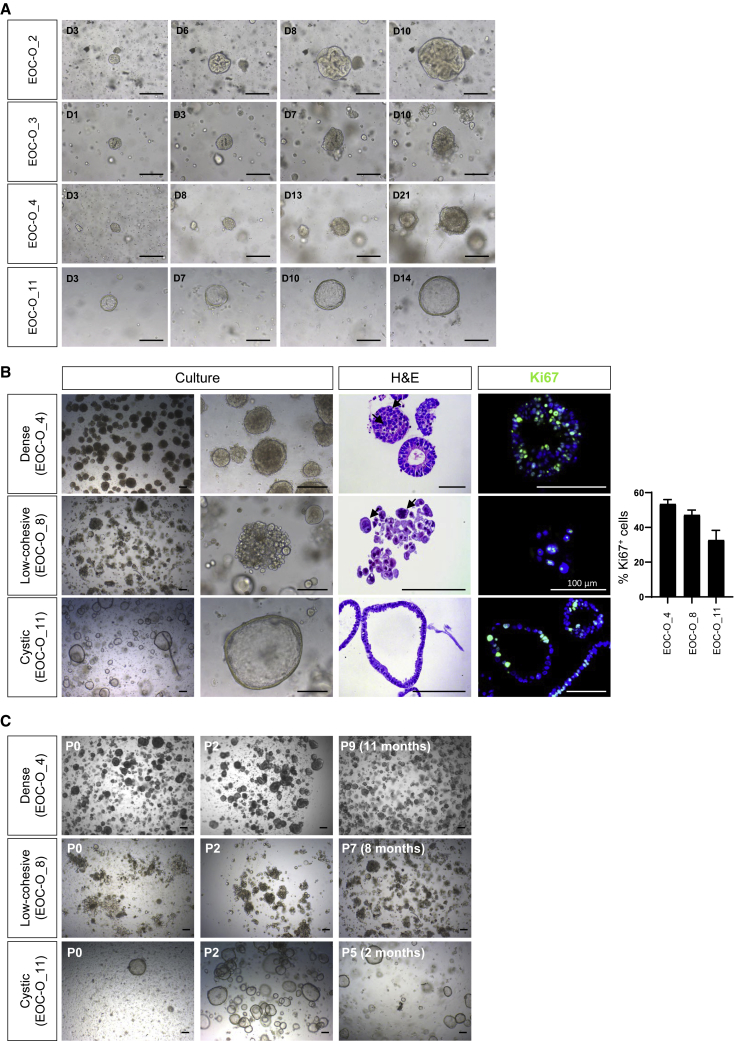
In the culture conditions as optimized above, EOC-derived organoids typically developed within 2–4 weeks, at a rate varying in accordance with individual patients’ tumors (Figure 1A). Organoid morphology also differed between patients’ EOC samples, displaying either a dense phenotype with no or only small lumen, a disorganized configuration of low cellular cohesiveness (“low-cohesive”), or a cystic phenotype with a (single) cell layer bordering a large lumen (Figure 1B; comparable with Kopper et al., 2019). The different organoid types all showed substantial proliferative activity (Ki67+; Figure 1B) and could be expanded, either short-term (up to 4 passages) or long-term (more than 4 passages, up to 1 year and more) (Figure 1C and Table 1). Although the overall efficiency of establishing organoids from HGSOC patients was lower than that in Kopper et al. (2019), the percentage of long-term passageable organoids among the established lines was comparable (see Table S2). The organoids retained their proliferative activity in later passages without any signs of decreased cell viability (as assessed by immunostaining for the apoptosis marker cleaved-caspase 3; Figure S1E). All organoid lines established were cryopreserved and biobanked (Table 1).
Figure 1.
Establishing Organoid Cultures from Ovarian Cancer
(A) Organoid development from EOC (passage 0, P0), showing patients’ tumor-associated differences in growth rate. Representative bright-field images are shown at different days (D) after seeding. Scale bars, 200 μm.
(B) Distinct morphology of patients’ EOC organoid lines. Representative images of organoid culture and individual organoids (bright-field), of H&E staining, and of Ki67 immunofluorescence analysis (DAPI as nuclear stain) are shown. Some high-grade nuclear atypia are indicated by arrows. Bar graph (right) depicts the proportion of Ki67+ cells in the organoid lines as indicated (mean ± SEM, n = 3–5 independent experiments per line). Scale bars, 200 μm unless indicated otherwise.
(C) Long-term expansion of EOC organoid lines. Representative bright-field images of different passages (P) are shown. Scale bars, 200 μm.
EOC-Derived Organoids Reproduce Disease and Original Tumor Phenotype
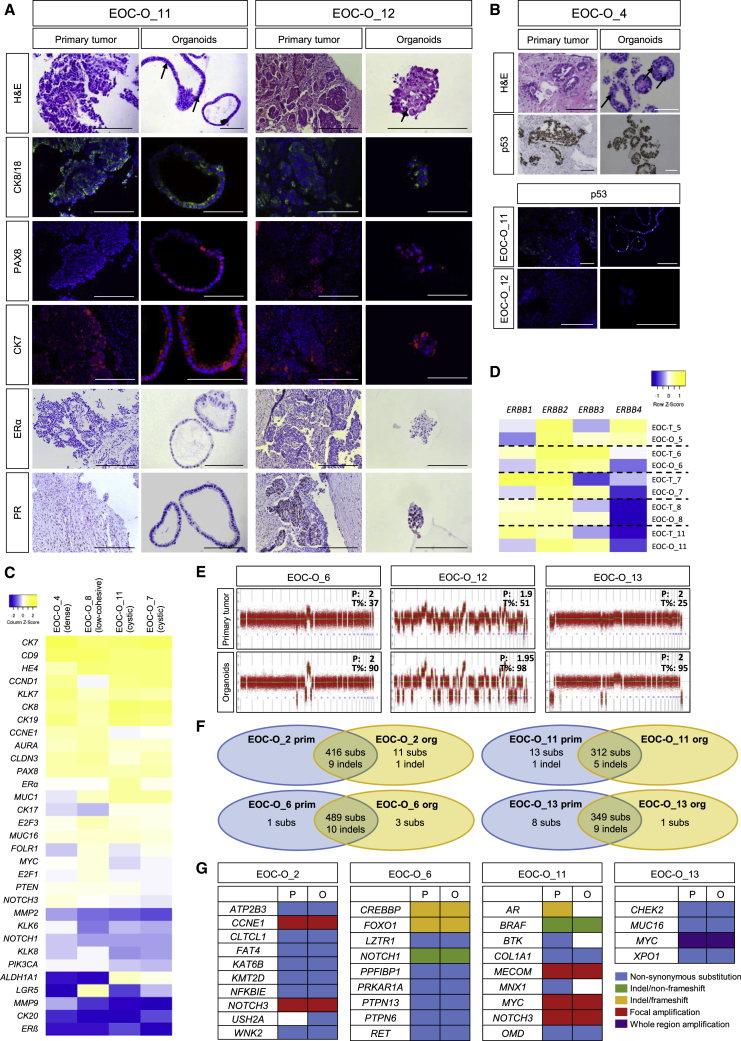
First, histological (hematoxylin-eosin [H&E]) analysis was performed showing high-grade nuclear atypia in the primary tumor samples as characteristic for EOC (particularly HGSOC) (Figures 2A, 2B, and S2A). High-grade nuclear atypia were also observed in the EOC-derived organoids (Figures 1B, 2A, 2B, and S2A). Moreover, multinucleated giant cells found in the primary tissue were also present in the corresponding organoids (Figure S2A; EOC-O_8). Second, epithelial markers (cytokeratin 8 [CK8], CK18, E-cadherin) were positive in the primary tumor and prominently expressed in the organoids, thereby demonstrating their (tumor) epithelial nature as typical for organoid models (Figures 2A and S2B; Table 2) (Sachs and Clevers, 2014). Third, expression of the HGSOC markers PAX8 (Hardy et al., 2018, Wang et al., 2015) and CK7 (Cathro and Stoler, 2002) was observed in primary tumor, which was effectively recapitulated in the organoids (Figure 2A and Table 2). The tumor-suppressor protein p53 is often mutated in HGSOC (The Cancer Genome Atlas Research Network, 2011) and the p53 immunostaining profile is used for pathological diagnosis. The aberrant p53 overexpression pattern (i.e., intense nuclear staining) in primary tumors was mirrored in corresponding organoid lines (Figure 2B and Table 2). Also, complete absence of p53 immunostaining (“null pattern”) in the primary tumor was recapitulated in the derived organoids (Figure 2B, EOC-O_12; Table 2). The expression profile of estrogen receptor α (ERα) and progesterone receptor (PR), clinically variable in HGSOC (Voutsadakis, 2016), was also retained in the corresponding organoid lines (Figure 2A and Table 2). Finally, we performed additional immunohistological and immunofluorescence analyses on organoid lines for which no or insufficient primary tissue was available, and found expression of the HGSOC markers (Figure S2C and Table 2).
Figure 2.
EOC-Derived Organoids Capture Disease and Primary Tumor Phenotype
(A) Organoids reproduce the primary tumor's molecular and cellular phenotype. Representative pictures of H&E staining and immunostaining of disease-associated protein markers in primary tumor and organoids are shown (DAPI and hematoxylin as nuclear stain). The primary tissue shows abundant high-grade nuclear atypia (H&E), which are also found in the organoids (some indicated with arrows). Scale bars, 200 μm.
(B) Organoids reproduce the primary tumor's p53 phenotype. Representative pictures of H&E staining and p53 immunostaining in primary tumor and organoids are shown (DAPI and hematoxylin as nuclear stain). The primary tissue shows abundant high-grade nuclear atypia (H&E), which are also found in the organoids (some indicated with arrows). Scale bars, 200 μm.
(C) Organoids show EOC (HGSOC)-associated gene expression profile. Heatmap of expression of genes, as quantified by qRT-PCR and presented as relative expression to GAPDH (ΔCt) (visualized as color-coded row Z score), in organoids from different patients (with different morphology) is shown. Colors range from blue (low expression) to yellow (high expression).
(D) ERBB expression profile in primary tumors (EOC-T) and corresponding organoids (EOC-O) as quantified by qRT-PCR and presented as relative expression to GAPDH (ΔCt), visualized as color-coded row Z score. Colors range from blue (low expression) to yellow (high expression).
(E) Organoids capture the mutational profile of the primary tissue. Representative copy-number profiles from three different organoid lines (analyzed at P2–P4) and corresponding primary EOC tumor are shown. Numbers indicate ploidy (P) and tumor cell fraction (T%).
(F) Venn diagrams presenting the number of genetic aberrations (subs, substitutions; indel, insertion/deletion) that are common (intersection) or different between primary tumor and corresponding organoids. Numbers were retrieved from Table S3.
(G) Mutation matrix representing hits in cancer consensus, OC-relevant, homology recombination, and amplification-driver genes as detected by WES in primary tumor and derived organoids. P, primary tumor; O, organoids.
Table 2.
Overview of Immunohistochemical and Immunofluorescent Analysis of HGSOC-Derived Organoids
| E-cadherin | CK8/CK18 | PAX8 | CK7 | p53 | ERα | PR | |
|---|---|---|---|---|---|---|---|
| EOC-O_4 | + | + | + | + | + | +/− | + |
| EOC-O_7a | + | + | + | + | − | +/− | + |
| EOC-O_8 | + | + | + | + | + | +/− | +/− |
| EOC-O_11 | + | + | + | + | +/− | − | +/− |
| EOC-O_12 | + | + | + | + | − | − | + |
+, positive; +/−, some positive cells; −, no positive cells.
Data not shown.
Next, we analyzed in organoid lines of different patients/morphology the gene expression of multiple markers known to be highly or lowly expressed in HGSOC (Figure 2C). Organoids showed prominent expression of CD9, CK19, and HE4, the latter being one of the most frequently upregulated genes in EOC (Hwang et al., 2012, Schummer et al., 1999). In contrast, expression levels of CK20 and ERβ (which is highly expressed in normal OSE; Lazennec, 2006) were very low to absent, typical characteristics of EOC (Cathro and Stoler, 2002, Voutsadakis, 2016). PAX2, an FTE transcription factor that is lost in 85% of EOC (Hardy et al., 2018), was undetectable in the HGSOC organoid lines (Figure S2D). We also analyzed the expression of the ERBB receptor family through which NRG1 acts (Harris et al., 2019). Interestingly, ERBB2 and ERBB3 are highly expressed in the organoids, and expression of ERBB3, one of the cognate receptors of NRG1, is predominantly enriched in comparison with the primary tumor (Figure 2D). The role and clinical significance of ERBB2 (HER2) and ERBB3 (HER3) in OC remain unclear and controversial. The new EOC organoid models provide experimental tools to revive this field (see Discussion). Comparison of ERBB receptor expression in organoids developed with and without NRG1 (OCOM4 and OCOM3, respectively) suggests that the final optimized NRG1-containing culture conditions do not select for an NRG1-dependent (ERBB-expressing) subset among EOC types, since organoids grown in medium without NRG1 (i.e., OCOM3) showed ERBB expression levels similar to those of organoids grown in OCOM4 (Figure S2E).
Taken together, patients’ EOC-derived organoids reproduce the disease's cellular and molecular phenotype and show atypia and protein marker expression as present in the original tumor.
Patient EOC-Derived Organoids Recapitulate Genomic and Mutational Landscape of the Primary Tumor
We investigated whether the organoid lines also recapitulated the genetic make-up of their parent tumor. Low-coverage whole-genome sequencing revealed that the vast majority of somatic copy-number alterations (SCNA) in primary tumors were retained in the corresponding organoid lines (Figure 2E). These data also allowed to bioinformatically evaluate tumor content of primary biopsy and derived organoids, showing a clear enrichment and high tumor purity in the organoids (Figure 2E). Prominent SCNA were also observed (using array comparative genomic hybridization [array CGH]) in other organoid lines (for which primary tissue was not available; Figure S2F), thereby demonstrating their chromosomally aberrant nature with multiple gains or losses as frequently observed in HGSOC (Kuo et al., 2009, The Cancer Genome Atlas Research Network, 2011) and as found here (Figure 2E). Immunostaining analysis of mutant p53 also supported the major tumor content of these organoid lines (Figure S2F). SCNA present in the primary tissue of EOC-O_9 were not retrieved in the derived organoid culture (Figure S2F), indicating that these organoids developed from non-cancerous epithelial cells present in the biopsy, which overtook the culture (as previously also reported for other cancer-derived organoids; Boretto et al., 2019, Gao et al., 2014, Van De Wetering et al., 2015, Yan et al., 2018), further supported by normal-cell histology (round, polarized) of the organoids and the absence of nuclear atypia and nuclear p53 expression, the latter being present in the primary tissue (Figure S2F). Expression of PAX8 and acetylated α-tubulin point to an FTE origin of this organoid line (Figure S2F; Kessler et al., 2015, Kopper et al., 2019).
Next, several tumors from patients’ wild-type for germline BRCA1 (as retrieved from the patients’ pathology reports) were sequenced at the exome base-pair level using whole-exome sequencing (WES), together with the derived organoids. The vast majority (98%) of the genetic alterations detected (i.e., 1,638) were similarly present in both primary tumor and resultant organoid line (Figure 2F and Table S3). In particular, mutations in cancer consensus genes (Sondka et al., 2018), in OC-relevant genes (i.e., genes mutated in >4% of OC; Gao et al., 2013) and in the homologous recombination pathway (Pennington et al., 2014) were identified, which highly corresponded between tumor and organoids (Figure 2G and Table S3). For instance, we found frameshift or non-synonymous mutations in the tumor-suppressor genes CREBBP, FOXO1, PRKAR1A, and CHEK2 (Toss et al., 2015, Wang et al., 2016, Xie et al., 2012, Zhang et al., 2017), identically in primary tumor and derived organoids. Non-synonymous substitutions in the nuclear transport protein XPO1, which regulates export of tumor suppressors, cell-cycle inhibitors, and oncogenes (Azmi et al., 2017), were similarly observed in both tumor and organoids. A non-frameshift insertion and deletion were detected in the key cancer/OC-associated genes BRAF and NOTCH1, respectively, identically in tumor and corresponding organoids. The loss of three mutations in EOC-O_11 organoids (Figure 2G) may point to a selection of (a) subclone(s) in this particular organoid line (as also reported for other cancer-derived organoids; Boretto et al., 2019, Broutier et al., 2017). Finally, focal or whole-region amplification, as being propelled by central driver genes (MYC, MECOM, NOTCH3, CCNE1), was similarly present in tumor and corresponding organoid line (Figure 2G). Of note, WES confirmed the BRCA1 wild-type genotype of the patients/samples analyzed (as prospectively retrieved from the patients’ reports) and also showed a TP53 wild-type genotype (which, retrospectively, was in agreement with the patients’ reports). Developing long-term expandable organoids from patients with high-risk OC predisposition due to a germline BRCA1 mutation (Antoniou et al., 2003) (Figure S2G) provides new in vitro research models that should allow us to investigate the role and impact of BRCA1 mutation in OC progression.
Taken together, the developed patients’ EOC-derived organoids highly recapitulate the genomic constitution of the primary tumor.
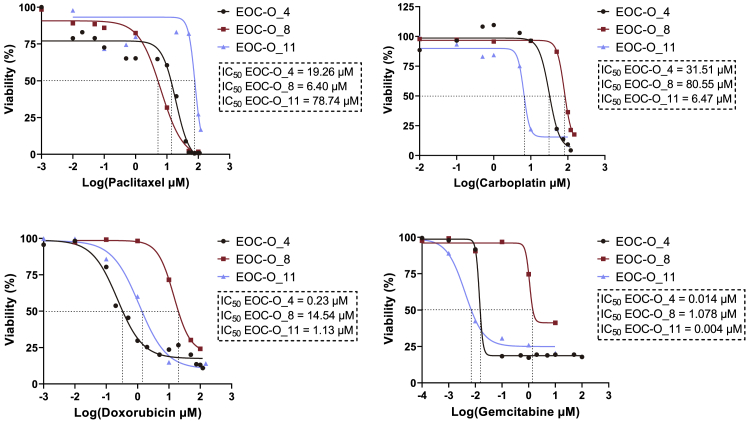
EOC-Derived Organoids Show Tumor-Specific Sensitivity to Clinically Used Chemotherapy
To explore the potential of the EOC-derived organoids for in vitro drug screening applications, we tested the effect of several chemotherapeutic agents standardly used in the clinic to treat HGSOC (i.e., paclitaxel, carboplatin, doxorubicin, and gemcitabine) on established EOC organoids. Drug-response curves revealed distinct sensitivities of the different organoid lines for the drugs (Figure 3), thereby indicating patients’ tumor-dependent responses, and at the same time exposed distinct efficacies of the different drugs on individual tumor organoid lines (Figure S3A). Also, nutlin-3 (currently tested as a targeted therapeutic agent for TP53 wild-type EOC in preclinical settings; Zanjirband et al., 2017) showed different activity depending on the patient’s tumor, and more in particular on the p53 status. EOC-O_7 organoids, derived from a TP53 wild-type tumor (information retrieved from the patients’ pathology report), is sensitive to nutlin-3 (Figure S3B). In contrast, EOC-O_4 and EOC-O_8 organoids, established from TP53 mutant tumors (Figures 2B and S2F; corresponding to the patient pathology reports) showed resistance to nutlin-3 (Figure S3B). Together, our tests show the potential applicability of EOC-derived organoids for drug screening (see also Kopper et al., 2019).
Figure 3.
EOC-Derived Organoids Show Patient-Specific Drug Responses
Dose-response curves of EOC organoid cultures from different patients treated for 72 h with drugs are shown. Cell viability was measured using XTT assay. Mean data points (n = 3 biologically independent experiments, i.e., independent donors, with each dot representing the mean of three technical replicates per donor) are displayed for each drug concentration analyzed. IC50 values are determined (dashed lines) and indicated.
Discussion
In the present study, we established organoids from patients’ EOC (predominantly HGSOC). EOC organoid derivation was not negatively influenced by prior cryopreservation of the clinical biopsy, enabling sample collection and storage pending organoid establishment (as also described for some other cancers, in particular mouse xenograft and human breast tumors; Walsh et al., 2016). We found that NRG1 exerts a beneficial effect on EOC organoid development and growth, including increased proliferative activity. Our data, together with the recent findings of Kopper et al. (2019), support an important impact of NRG1 on OC growth (Gilmour et al., 2002, Sheng et al., 2010) and may provide hints toward NRG1-targeted treatment prospects (Drilon et al., 2018). In particular, we found high expression of ERBB2 (HER2) in tumors and organoids, and high, enriched expression of ERBB3 (HER3) in the organoids. NRG1 acts through ERBB3 (and/or ERBB4), which heterodimerizes with ERBB2 (Harris et al., 2019). The role and clinical significance of HER2 in OC, in contrast to its proven importance in breast cancer, remain unclear and controversial (Serrano-Olvera et al., 2006). A recent meta-analysis revealed a potential prognostic value, although no association was found for serous OC (Luo et al., 2018). Early preclinical studies have defined HER2 as a potential therapeutic target in OC, but have not extensively been followed up nor translated into clinical practice, particularly because the tested HER2-targeted agents failed to show a significant response (Shu et al., 2017). More recent studies suggest that targeting HER2 (e.g., using trastuzumab) sensitizes OC to chemotherapy (Harris et al., 2019, Shu et al., 2017). Given the success of targeting HER2 in breast and gastric cancer, continuing efforts are recommended regarding HER2 in OC, which can now be explored using the EOC organoid models. Also, the role and significance of ERBB3/HER3 (being one of the cognate receptors of NRG1) in OC remain to be determined. HER3 expression might be associated with worse survival in OC, particularly when HER2 is concomitantly overexpressed (Ocana et al., 2013). A recent study revealed an activated NRG1/ERBB3 pathway in OC cell lines (in vitro and as xenograft in vivo which, however, showed distinct death or proliferation-block responses), and in a significant fraction (~30%) of patients’ advanced-stage OC (as studied using tumor cells from ascites) (Sheng et al., 2010). Similar to HER2, targeting HER3 may also potentiate the effect of chemotherapy (Camblin et al., 2019). The EOC organoid models, developed here and in other studies, may revive this domain to decipher the role, impact, and targetability of the NRG1/ERBB2/ERBB3 pathway in OC. Moreover, patient-derived EOC organoids may help to predict individual patient responses and identify the OC patients who may benefit from NRG1/ERBB2/ERBB3-oriented therapy.
Although the initial development of organoids from the tumor biopsy is comparable in our optimized culture medium OCOM4 and the Kopper medium, overall derivation efficiency is lower than in Kopper et al. (2019). Several variables may account for this difference, including patient group variability and heterogeneity (e.g., not covering identical geno-/phenotypes) and biopsy variability (e.g., regarding quality/necrosis, size, tumor abundance). Furthermore, differences in medium components (such as hydrocortisone and forskolin) may, although not affecting the organoid number at initiation, still be important to enhance the efficiency of kick-starting organoid cultures from individual patients. Extensive comparison with the culture conditions of other recent OC organoid studies (Hill et al., 2018, Maru et al., 2019) was not performed.
Despite the now well-demonstrated capacity to establish organoids from EOC, more studies are required to enhance the derivation efficiency (as is also true for other cancer-derived organoids; Boretto et al., 2019, Gao et al., 2014), in particular by scrutinizing and fine-tuning culture conditions to eventually capture more patients and geno-/phenotypes. Although still unsettled and controversial, HER2 overexpression may be present in 10%–30% of all OC types and 20%–40% of HGSOC (which may be higher in advanced stages) (Harris et al., 2019), and HER3 is reported to be expressed in a widely divergent range (from 3% to 90%) of all OC (Davies et al., 2014) and an estimated 20% of serous OC (Rajkumar et al., 1996). It could be possible that our culture conditions (probably also true for Kopper et al., 2019) capture the establishment of organoids from NRG1-dependent (i.e., ERBB-expressing) subtypes of EOC. Although we provide supportive data that the finally optimized culture condition containing NRG1 does not select for NRG1-dependent (ERBB-expressing) OC types, we cannot exclude that initial organoid formation may occur under the influence of endogenous NRG1 (thus indeed thriving ERBB-expressing samples), with exogenous NRG1 enhancing the efficiency by increasing the number of organoids.
The established EOC-derived organoids capture disease cellular characteristics (high-grade nuclear atypia) and molecular phenotype (marker expression). Interestingly, mucin 16 (MUC16) was also found to be expressed in the organoids, and non-synonymous substitutions in MUC16 were similarly observed in both tumor and organoids. MUC16 encodes for cancer antigen 125 (CA-125), which is not detectable in normal OSE and is therefore used as a biomarker during advanced-stage OC follow-up (Thériault et al., 2011), although its significance remains highly controversial (Stewart et al., 2012). Moreover, we found that nuclear atypia, protein marker expression, and SCNA and mutational profile of the tumors was highly conserved in the corresponding organoids although occasional deviations were observed, which may be due to presence of non-tumor cells in the DNA-extracted primary tissue and/or the selection and growth of (a) specific mutant subclone(s) in the culture conditions used (as also reported for other cancer types; Boretto et al., 2019, Broutier et al., 2017, Van De Wetering et al., 2015). Subclone selection might also match what is happening in the patient's cancer at recurrences during consecutive therapies (e.g., selection of the more dominant or chemoresistant subclone[s]). On the other hand, new mutations arising in the organoids might mimic the “natural” mutational evolution of the cancer (being propelled by cancer driver genes such as MYC) (Boretto et al., 2019).
We found that organoids can also be established from EOC tumor cells remaining after chemotherapy. Chemoresistant cells may represent cancer stem cells postulated to drive OC resistance and recurrence (Garson and Vanderhyden, 2015). The (cancer) stem cell markers ALDH1A1 and LGR5 were found to be expressed in some of the organoid lines analyzed. Of note, OSE and FTE contain cells expressing LGR5, which has been suggested to contribute to EOC development (Ng et al., 2014).
Finally, we demonstrated that the EOC-derived organoids are amenable to drug screening and show differential sensitivity of individual patient organoid lines to the chemotherapeutic agents tested. Hence, by predicting patients’ tumor responses to specific drugs using the organoids as “avatars,” the optimal treatment for the individual patient may be selected (as recently reported for other cancer types; Broutier et al., 2017, Huang et al., 2015, Sachs et al., 2018, Van De Wetering et al., 2015). Moreover, the EOC organoid models will be highly instrumental in moving into the field of immunotherapy (e.g., using CAR-T and natural killer cells), as has recently been shown for colorectal cancer organoids (Schnalzger et al., 2019). Since organoids are typically composed of the epithelial compartment of the original tissue, further perfecting the model by adding stromal and immune components of the tumor microenvironment will eventually be needed to reach the organoid model's full potential.
In summary, we established organoid lines from patient-derived EOC that capture disease and patients’ tumor diversity and hallmarks, thereby adding new lines to the existing EOC organoid repertoire, which is essential for gaining deeper insight into the cancer's etiology, pathogenesis, heterogeneity, and chemoresistance, and for identifying new therapeutic targets and screening new drugs, preferably in a patient-tailored manner (also reviewed in Maru and Hippo, 2019). It should be acknowledged that the EOC organoid derivation protocol still deserves further efforts for improvement. The EOC organoid platform has strong potential as an experimental and preclinical research model, and in particular as impetus to revive NRG1/ERBB research in OC, and may eventually identify response-predictive biomarkers, assist in clinical decision making, and provide personalized therapeutic options, particularly for patients in whom standard clinical routes have been exhausted.
Experimental Procedures
Detailed methods are provided in Supplemental Information.
Establishing Organoid Cultures from Patient-Derived EOC Biopsies
EOC biopsies were obtained from patients following standard primary or interval debulking surgery (Table 1). The study was approved by the Ethical Committee Research UZ/KU Leuven (S60589), and written informed consent was obtained from all participating patients. The freshly obtained or cryopreserved tissue was dissociated using collagenase type IV and mechanical shearing. Cells were plated in 70% growth factor-reduced Matrigel/30% Dulbecco’s modified Eagle’s medium/F12 and cultured in defined media (Table S1), and organoids were passaged between 2 and 4 weeks after seeding.
Immunohistochemical Analysis
Tissues and organoids were fixed in paraformaldehyde and paraffin-embedded sections subjected to H&E, immunohistochemical, and/or immunofluorescence staining (for antibodies, see Supplemental Information). Microscopy pictures were taken and proportions of immunoreactive cells counted using Fiji software (http://imagej.net/Citing).
Genomic Analysis
For array comparative genomic hybridization (array CGH), genomic DNA from organoids and primary tissues was labeled with Cy5 and hybridized to Cy3-labeled sex-matched reference DNA. Arrays were scanned using an Agilent microarray scanner, followed by calculation of signal intensities using Agilent Feature Extraction software.
Sequencing was performed on whole-exome and low-coverage whole-genome DNA libraries by Illumina's NextSeq and HiSeq4000, respectively (Table S4). Raw sequencing reads were aligned to the human reference genome and copy-number variations were identified using the low-coverage whole-genome sequencing data while variants were identified in the whole-exome data and further filtered and annotated to retain somatic mutations, using in-house developed bioinformatics pipelines. Tumor content and corresponding ploidy was quantified with ASCAT.
Targeted Sanger Sequencing
The targeted PCR-amplified BRCA1 gene region (for primers, see Table S5) was purified and Sanger sequenced by Eurofins Genomics (Ebersberg, Germany).
Gene Expression Analysis
Organoid RNA was reverse transcribed and subjected to quantitative real-time PCR (qPCR) using gene-specific forward and reverse primers (Table S5). Expression levels were normalized to expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative gene expression levels were calculated as ΔCt values (Ct “target gene” minus Ct “GAPDH”), and the corresponding heatmap was generated by Heatmapper (http://www2.heatmapper.ca/expression/).
Drug Screening
Organoid cultures were treated with a concentration series of paclitaxel, carboplatin, doxorubicin, gemcitabine, or nutlin-3. Cell viability was assayed after 72 h using the XTT assay, and data analysis was performed with GraphPad Prism.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism and are specified in the figure legends. Statistical significance was defined as p < 0.05. All experiments were performed with ≥3 biological replicates unless otherwise indicated.
Author Contributions
N.M. designed the concepts and experiments, performed the experiments and the data analysis, interpreted the results, and co-wrote the manuscript; C.D. designed and performed the experiments, executed the data analysis, and interpreted the results; M.B. contributed to the organoid protocol setup and drug screenings; Z.J. collected patients’ information and samples and helped in concepts, interpretation of the results, and statistical analyses; R.H. collected patients’ information and samples, helped in concepts and interpretation of the results and in maintenance of the organoid cultures; B.B. performed and interpreted the genomic sequencing; F.H. performed a number of gene and protein expression analyses and added conceptual input; I.A. organized and interpreted the genomic sequencing; B.C. added technical and conceptual input; E.V.N. was a collaborating surgeon providing clinical samples; I.V. was a collaborating surgeon providing clinical samples; A-S.V.R. was the pathologist providing expert interpretation of the tumor and organoid histology; D.L. supervised and co-interpreted the genomic sequencing; D.T. was a driving force behind the clinical collaboration to obtain human samples and is a collaborating gynecologist with joint research grants; H.V. designed and supervised the project, co-developed the concepts and ideas, co-designed the experiments, co-analyzed and co-interpreted the data, and wrote the manuscript. All authors critically read and approved the manuscript.
Acknowledgments
We thank the UZ/KU Leuven Genomics Core for their expert assistance in array CGH analysis, and Thomas Van Brussel (D.L.'s group) for technical help in genome sequencing. We are grateful to Lara Vankelecom (University of Ghent, Faculty of Psychology and Educational Sciences, Department of Data Analysis) for expert help with statistical analyses. The computational resources and services used for genome sequencing and analysis were provided by the Flemish Supercomputer Center (VSC), funded by the Hercules Foundation and the Flemish Government, Department of Economy, Science and Innovation (EWI). We are also grateful to InfraMouse (VIB-KU Leuven, Hercules type 3 project ZW09-03) for use of histological instruments and microscopes. This work was supported by grants from the KU Leuven Research Fund and from the Fund for Scientific Research (FWO) - Flanders (Belgium). N.M. is, and B.C. was, a PhD Fellow of the FWO. D.T. is a Senior Clinical Investigator of the FWO.
Published: April 2, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.03.004.
Supplemental Information
References
- Antoniou A., Pharoah P.D.P., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., Loman N., Olsson H., Johannsson O., Borg Å. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune G., Lian A.M., Tingulstad S., Torp S.H., Forsmo S., Reseland J.E., Stunes A.K., Syversen U. Increased circulating hepatocyte growth factor (HGF): a marker of epithelial ovarian cancer and an indicator of poor prognosis. Gynecol. Oncol. 2011;121:402–406. doi: 10.1016/j.ygyno.2010.12.355. [DOI] [PubMed] [Google Scholar]
- Azmi A.S., Li Y., Muqbil I., Aboukameel A., Senapedis W., Baloglu E., Landesman Y., Shacham S., Kauffman M.G., Philip P.A. Exportin 1 (XPO1) inhibition leads to restoration of tumor suppressor miR-145 and consequent suppression of pancreatic cancer cell proliferation and migration. Oncotarget. 2017;8:82144–82155. doi: 10.18632/oncotarget.19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretto M., Cox B., Noben M., Hendriks N., Fassbender A., Roose H., Amant F., Timmerman D., Tomassetti C., Vanhie A. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144:1775–1786. doi: 10.1242/dev.148478. [DOI] [PubMed] [Google Scholar]
- Boretto M., Maenhoudt N., Luo X., Hennes A., Boeckx B.B., Bui B., Heremans R., Perneel L., Kobayashi H., Van Zundert I. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019;21:1041–1051. doi: 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarró L.M., Bradshaw C.R., Allen G.E., Arnes-Benito R., Sidorova O., Gaspersz M.P. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camblin A.J., Tan G., Curley M.D., Yannatos I., Iadevaia S., Rimkunas V., Mino-Kenudson M., Bloom T., Schoeberl B., Drummond D.C. Dual targeting of IGF-1R and ErbB3 as a potential therapeutic regimen for ovarian cancer. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-53322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathro H.P., Stoler M.H. Expression of cytokeratins 7 and 20 in ovarian neoplasia. Am. J. Clin. Pathol. 2002;117:944–951. doi: 10.1309/2T1Y-7BB7-DAPE-PQ6L. [DOI] [PubMed] [Google Scholar]
- Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- Davies S., Holmes A., Lomo L., Steinkamp M.P., Kang H., Muller C.Y., Wilson B.S. High incidence of ErbB3, ErbB4, and MET expression in ovarian cancer. Int. J. Gynecol. Pathol. 2014;33:402–410. doi: 10.1097/PGP.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A., Van Houdt W., Van Gorp J., Taylor-Weiner A., Kester L. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon A., Somwar R., Mangatt B.P., Edgren H., Desmeules P., Ruusulehto A., Smith R.S., Delasos L., Vojnic M., Plodkowski A.J. Response to ERBB3-directed targeted therapy in NRG1-rearranged cancers. Cancer Discov. 2018;8:686–695. doi: 10.1158/2159-8290.CD-17-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:1–20. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Vela I., Sboner A., Iaquinta P.J., Wouter R., Arora V.K., Wongvipat J., Kossai M., Ramazanoglu S., Luendreo P. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson K., Vanderhyden B.C. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction. 2015;149:R59–R70. doi: 10.1530/REP-14-0234. [DOI] [PubMed] [Google Scholar]
- Gilmour L.M.R., Macleod K.G., Mccaig A., Sewell J.M., Gullick W.J., Smyth J.F., Langdon S.P. Neuregulin expression, function and signaling in human ovarian cancer cells. Clin. Cancer Res. 2002;8:3933–3942. [PubMed] [Google Scholar]
- Hardy L.R., Salvi A., Burdette J.E. Unpaxing the divergent roles of PAX2 and PAX8 in high-grade serous ovarian cancer. Cancers (Basel) 2018;10:262. doi: 10.3390/cancers10080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris F.R., Zhang P., Yang L., Hou X., Leventakos K., Weroha S.J., Vasmatzis G., Kovtun I.V. Targeting HER2 in patient-derived xenograft ovarian cancer models sensitizes tumors to chemotherapy. Mol. Oncol. 2019;13:132–152. doi: 10.1002/1878-0261.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.J., Decker B., Roberts E.A., Horowitz N.S., Muto M.G., Worley M.J., Feltmate C.M., Nucci M.R., Swisher E.M., Nguyen H. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8:1404–1421. doi: 10.1158/2159-8290.CD-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Holtzinger A., Jagan I., Begora M., Lohse I., Ngai N., Nostro C., Wang R., Muthuswamy L.B., Crawford H.C. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.R., Jo K., Lee Y., Sung B.J., Park Y.W., Lee J.H. Upregulation of CD9 in ovarian cancer is related to the induction of TNF-α gene expression and constitutive NF-κB activation. Carcinogenesis. 2012;33:77–83. doi: 10.1093/carcin/bgr257. [DOI] [PubMed] [Google Scholar]
- Jelovac D., Armstrong D.K.D. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Hoffmann K., Brinkmann V., Thieck O., Jackisch S., Toelle B., Berger H., Mollenkopf H.-J., Mangler M., Sehouli J. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 2015;6:8989. doi: 10.1038/ncomms9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Park E.Y., Kim O., Schilder J.M., Coffey D.M., Cho C.H., Bast R.C. Cell origins of high-grade serous ovarian cancer. Cancers (Basel) 2018;10:1–28. doi: 10.3390/cancers10110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopper O., de Witte C.J., Lõhmussaar K., Valle-Inclan J.E., Hami N., Kester L., Balgobind A.V., Korving J., Proost N., Begthel H. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 2019;25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- Kuo K.T., Guan B., Feng Y., Mao T.L., Chen X., Jinawath N., Wang Y., Kurman R.J., Shih I.M., Wang T.L. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69:4036–4042. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006;231:151–157. doi: 10.1016/j.canlet.2005.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E., Burdette J., Kenny H., Matei D., Pilrose J., Haluska P., Nephew K., Hales D., Stack M. Epithelial ovarian cancer experimental models. Oncogene. 2014;33:3619–3633. doi: 10.1038/onc.2013.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Xu X., Ye M., Sheng B., Zhu X. The prognostic value of HER2 in ovarian cancer: a meta-analysis of observational studies. PLoS One. 2018;13:1–16. doi: 10.1371/journal.pone.0191972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y., Hippo Y. Current status of patient-derived ovarian cancer models. Cells. 2019;8:505. doi: 10.3390/cells8050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y., Tanaka N., Itami M., Hippo Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol. Oncol. 2019;154:189–198. doi: 10.1016/j.ygyno.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Narod S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016;13:255–261. doi: 10.1038/nrclinonc.2015.224. [DOI] [PubMed] [Google Scholar]
- Ng A., Tan S., Singh G., Rizk P., Swathi Y., Tan T.Z., Huang R.Y.J., Leushacke M., Barker N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014;16:745–757. doi: 10.1038/ncb3000. [DOI] [PubMed] [Google Scholar]
- Ocana A., Vera-Badillo F., Seruga B., Templeton A., Pandiella A., Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J. Natl. Cancer Inst. 2013;105:266–273. doi: 10.1093/jnci/djs501. [DOI] [PubMed] [Google Scholar]
- Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata S., Cecere S.C., Du Bois A., Harter P., Heitz F. Treatment of recurrent ovarian cancer. Ann. Oncol. 2017;28:viii51–viii56. doi: 10.1093/annonc/mdx441. [DOI] [PubMed] [Google Scholar]
- Rajkumar T., Stamp G.W.H., Hughes C.M., Gullick W.J. c-erbB3 protein expression in ovarian cancer. Clin. Mol. Pathol. 1996;49:1–4. doi: 10.1136/mp.49.4.m199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N., Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Sachs N., de Ligt J., Kopper O., Gogola E., Bounova G., Weeber F., Balgobind A.V., Wind K., Gracanin A., Begthel H. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–386.e10. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Schnalzger T.E., de Groot M.H., Zhang C., Mosa M.H., Michels B.E., Röder J., Darvishi T., Wels W.S., Farin H.F. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38:e100928. doi: 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummer M., Ng W.V., Bumgarner R.E., Nelson P.S., Schummer B., Bednarski D.W., Hassell L., Baldwin R.L., Karlan B.Y., Hood L. Comparative hybridization of an array of 21 500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999;238:375–385. doi: 10.1016/s0378-1119(99)00342-x. [DOI] [PubMed] [Google Scholar]
- Serrano-Olvera A., Dueñas-González A., Gallardo-Rincón D., Candelaria M., De la Garza-Salazar J. Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat. Rev. 2006;32:180–190. doi: 10.1016/j.ctrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Sheng Q., Liu X., Fleming E., Yuan K., Piao H., Chen J., Moustafa Z., Thomas R.K., Greulich H., Schinzel A. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T., Li Y., Wu X., Li B., Liu Z. Down-regulation of HECTD3 by HER2 inhibition makes serous ovarian cancer cells sensitive to platinum treatment. Cancer Lett. 2017;411:65–73. doi: 10.1016/j.canlet.2017.09.048. [DOI] [PubMed] [Google Scholar]
- Sondka Z., Bamford S., Cole C.G., Ward S.A., Dunham I., Forbes S.A. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat. Rev. Cancer. 2018;18:696–705. doi: 10.1038/s41568-018-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.L., Rim S.H., Gelb C.A. Physician knowledge and awareness of CA-125 as a screen for ovarian cancer in the asymptomatic, average-risk population. Heal. Educ. Behav. 2012;39:57–66. doi: 10.1177/1090198111407185. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thériault C., Pinard M., Comamala M., Migneault M., Beaudin J., Matte I., Boivin M., Piché A., Rancourt C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011;121:434–443. doi: 10.1016/j.ygyno.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Timmermans M., Sonke G.S., Van de Vijver K.K., van der Aa M.A., Kruitwagen R.F.P.M. No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in The Netherlands. Eur. J. Cancer. 2018;88:31–37. doi: 10.1016/j.ejca.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Toss A., Tomasello C., Razzaboni E., Contu G., Grandi G., Cagnacci A., Schilder R.J., Cortesi L. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed. Res. Int. 2015;2015:341723. doi: 10.1155/2015/341723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsadakis I.A. Hormone receptors in serous ovarian carcinoma: prognosis, pathogenesis, and treatment considerations. Clin. Med. Insights Oncol. 2016;10:17–25. doi: 10.4137/CMO.S32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh A.J., Cook R.S., Sanders M.E., Arteaga C.L., Skala M.C. Drug response in organoids generated from frozen primary tumor tissues. Sci. Rep. 2016;6 doi: 10.1038/srep18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Ma H., Pan Y., Xiao W., Li J., Yu J., He J. PAX2 and PAX8 reliably distinguishes ovarian serous tumors from mucinous tumors. Appl. Immunohistochem. Mol. Morphol. 2015;23:280–287. doi: 10.1097/PAI.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Wang S., Cheng Y., Zheng Y., He Z., Chen W., Zhou W., Duan C., Zhang C. PRKAR1A is a functional tumor suppressor inhibiting ERK/Snail/E-cadherin pathway in lung adenocarcinoma. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep39630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Ushmorov A., Leithäuser F., Guan H., Steidl C., Färbinger J., Pelzer C., Vogel M.J., Maier H.J., Gascoyne R.D. FOXO1 is a tumor suppressor in classical Hodgkin lymphoma. Blood. 2012;119:3503–3511. doi: 10.1182/blood-2011-09-381905. [DOI] [PubMed] [Google Scholar]
- Yan H.H.N., Siu H.C., Law S., Ho S.L., Yue S.S.K., Tsui W.Y., Chan D., Chan A.S., Ma S., Lam K.O. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–897.e11. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Zanjirband M., Curtin N., Edmondson R.J., Lunec J. Combination treatment with rucaparib (Rubraca) and MDM2 inhibitors, Nutlin-3 and RG7388, has synergistic and dose reduction potential in ovarian cancer. Oncotarget. 2017;8:69779–69796. doi: 10.18632/oncotarget.19266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Vlasevska S., Wells V.A., Nataraj S., Holmes A.B., Duval R., Meyer S.N., Mo T., Basso K., Brindle P.K. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B-cell lymphoma. Cancer Discov. 2017;7:323–337. doi: 10.1158/2159-8290.CD-16-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.