Abstract
In recent decades, neuropeptides have been found to play a major role in communication along the gut-brain axis. Various neuropeptides are expressed in the central and peripheral nervous systems, where they facilitate the crosstalk between the nervous systems and other major body systems. In addition to being critical to communication from the brain in the nervous systems, neuropeptides actively regulate immune functions in the gut in both direct and indirect ways, allowing for communication between the immune and nervous systems. In this mini review, we discuss the role of several neuropeptides, including calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase-activating polypeptide (PACAP), corticotropin-releasing hormone (CRH) and phoenixin (PNX), in the gut-brain axis and summarize their functions in immunity and stress. We choose these neuropeptides to highlight the diversity of peptide communication in the gut-brain axis.
Abbreviations: NPY, neuropeptide Y; SP, substance P; α-MSH, α-melanocyte-stimulating hormone; VIP, vasoactive intestinal peptide; LPS, lipopolysaccharides; TRPV1, transient receptor potential vanilloid receptor-1; CGRP, calcitonin gene-related peptide; CNS, central nervous system; CRLR, calcitonin receptor like receptor; RAMP1, receptor activity-modifying protein1; PACAP, pituitary adenylate cyclase-activating polypeptide; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotrophic hormone; HPA axis, hypothalamic–pituitary–adrenal axis; PNX, phoenixin
Keywords: Neuropeptide, Gut-brain axis, Immunity, Antimicrobial peptides, Stress, Hypothalamic–pituitary–adrenal axis
1. Introduction
Trillions of bacteria colonize the human gut and the gut microbiota is essential for human health [1], [2], [3], [4]. While commensal bacteria reside in the host, providing key benefits, including protection against invasion, opportunistic or virulent bacteria are eliminated from the gut by the local innate immune system [5]. Increasing evidence points to appropriate gut microbiota not only making critical contribution to the immune system, but also having a profound impact on brain function [6], [7], [8]. When virulent pathogens invade, metabolic products such as lipopolysaccharides (LPS) can directly affect the function of enteric neurons, spinal sensory neurons and the vagus nerve through activation of Toll-like receptors or translocation and release of neuropeptides and hormones [9]. Gut permeability is perhaps the most important factor in initiating microbial interaction with the rest of the body [10].
The gut is the largest immune competent organ in our body, and contains close to 100 million neurons [11], [12]. Most of the nerve structures belong to the enteric nervous system (ENS), which regulates gut functions autonomously. The remaining extrinsic nerves connect the central nervous system with the gut and belong either to the afferent gut-brain or the efferent brain-gut axis [13], [14]. Bidirectional communication between the brain and the gut has long been recognized [7], [8]. To summarize the concept of the bidirectional deep interaction between central and peripheral nervous systems and the immune system, one can note that most of the neuropeptides, produced by neurons during immune response versus infectious agents or malignant cells, have neuroendocrine-like activity that can influence both brain and gut functions. The neuropeptides calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP) have been studied within this framework.
Neuropeptides are important due to their ability to regulate a range of diverse biological activities. As neurotransmitters, neuropeptides are components of the autonomic nervous system and act locally at peripheral sites; as neuromodulators, neuropeptides could act on central regulatory centers; and as neurohormones and hormones, neuropeptides could reach the immune system, peripheral vessels, organs and glands through the circulatory system [17]. It is reported that neuropeptides show close relationships and deep interactions between the neuropeptidergic and immunological systems within the host immune homeostasis. Neuropeptides, such as substance P (SP) [16], vasoactive intestinal peptide (VIP) [15], and α-melanocyte-stimulating hormone (α-MSH), released by nervous fibers in the intestine, exhibit a variety of proinflammatory or anti-inflammatory effects that are required for the modulation of innate and adaptive immune response [18], [19].
The influence of the gut microbiota on several aspects of central nervous system (CNS) function is increasingly supported by a growing body of experimental data [20], [21], [22]. The mechanism of this influence is complex and involves multiple direct and indirect pathways. The direct link between the microbiota and hypothalamic–pituitary–adrenal (HPA) axis association with stress shows the significant role of microbiota in CNS function [23], [24], [25], [26]. While inflammatory diseases, such as inflammatory bowel disease (IBD), could lead to mental disorder, stress could also induce inflammation through increasing intestinal permeability [27]. In this article, we will review the current evidence in the literature that points towards roles of neuropeptides in gut-brain axis and their influence on host immune system and psychiatric disorders. We will also review the possible mechanisms through which gut microbiota might be involved in the pathogenesis of these disorders with example neuropeptides. While there are numerous neuropeptides involved in communication along the gut-brain axis, many of which are mentioned in this mini review, a couple select neuropeptides are chosen for in depth analysis to demonstrate the diversity of gut-brain axis communications, as a full description of all gut-brain axis neuropeptides is outside the scope of this mini-review.
2. Role of gut-brain axis in immune response
Starting from the study on capsaicin activity, the role of neuropeptides in the connection between the neuroendocrine and immune system has been receiving increasing attention [28], [29]. Neuropeptides from the gut-brain axis have two key roles on the immune system: enhance innate host defense and direct antimicrobial function [30], [31]. In initiating microbial interactions with the intestine, metabolic products such as lipopolysaccharide (LPS) created by pathogenic microorganisms can increase the gut permeability and alter the activity of the ENS and CNS [32]. The intestinal barrier, which could act against this invasion, consists of multiple layers that includes gut flora and external mucus layer, epithelial layer, and lamina propria [33]. Immune cells such as lymphocytes, macrophages, plasma cells, antigen presenting cells, and mast cells are mainly concentrated on the epithelial layer and lamina propria [32].
Many neurotransmitters and neuropeptides bind directly to their receptors expressed in human T cells (also termed T lymphocytes), and also in various other immune cells, including B cells, dendritic cells, macrophages, and microglia, and subsequently induce various very potent immune effects [34], [35]. Recent studies show that neuropeptides such as neuropeptide Y, Somatostatin, GnRH-I, GnRH-II and CGRP could bind to their receptors in normal peripheral human T cells and trigger or elevate significantly a kaleidoscope of T cell functions and features crucial for health-keeping and disease-fighting tasks [36], [37].
Conventionally neuropeptides are considered as signaling molecules but have recently been shown to be pleiotropic molecules that are integral components of the nervous and immune system [38]. Common characteristics between some neuropeptides and antimicrobial peptides (AMPs), such as shared signal sequence, similarities in size, cationic charge or amphipathic design, suggest that neuropeptides might also serve an additional function in antimicrobial immunity [39]. Table1 shows some significant neuropeptides that have been proven to have antimicrobial activity, such as substance P (SP), neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP).
Table 1.
Common antimicrobial neuropeptides.
| Neuropeptide | Origin | Sequence | Antimicrobial Activity |
|---|---|---|---|
| Neuropeptide Y (NPY) | Human | Pro-neuropeptide Y (30–64) | Against Gram-positive bacteria, Gram-negative bacteria, fungi and parasites [114] |
| Calcitonin gene-related peptide (CGRP) | Human | Calcitonin gene-related peptide 1 (83–119) | Against Gram-positive bacteria, Gram-negative bacteria and fungi [31] |
| Substance P (SP) | Human | SP (1–11) | Against Gram-positive bacteria, Gram-negative bacteria and fungi [31], [114] |
| Vasoactive intestinal peptide (VIP) | Human | VIP peptides (125–152) | Against Gram-positive bacteria, Gram-negative bacteria, fungi and parasites [115] |
| α-Melanocyte-stimulating hormone (αMSH) | Human | α-MSH (1–13) | Against Gram-positive bacteria, Gram-negative bacteria, fungi and parasites [116] |
| Pituitary adenylate cyclase-activating peptide (PACAP) | Human/Catfish | PACAP (131–169, PACAP-38)/PACAP (131–158, PACAP-27) | Against Gram-positive bacteria, Gram-negative bacteria and fungi [53] |
| Adrenomedullin (AM) | Human | Adrenomedullin (1–52) | Against Gram-positive bacteria, Gram-negative bacteria and parasites [38], [117] |
| NDA-1 | hydra | NDA-1 (1–71) | Against Gram-positive bacteria, Gram-negative bacteria [53] |
| Enkelytin | Human | Proenkephalin-A (209–237) | Against Gram-positive bacteria [118] |
The negatively charged bacterial membranes make it a target of antimicrobial neuropeptides compared to the membranes of plants and animals, which have no net charge. After binding to the bacterial membrane, antimicrobial neuropeptides could displace membrane lipids to depolarize the normally energized bacterial membrane, causing fatal problems for the bacteria by altering the membrane structure to create physical holes that cause cellular contents to leak out, or even by entering into the interior of the target bacterial cell to activate deadly processes, such as induction of hydrolases that degrade the cell wall. While conventional antibiotics are easier for the bacteria to become drug-resistant, it is less likely for bacteria to become resistant against a neuropeptide antibiotic as its target is the bacterial membrane, the principal cell structure of bacteria [40]. Since the increased emergence of multi-resistant human pathogenic bacteria has become a worldwide problem, the antimicrobial neuropeptides open up new avenues for future therapeutic application of antibiotic-resistant infection.
2.1. Neuroimmune connector, calcitonin gene-related peptide (CGRP)
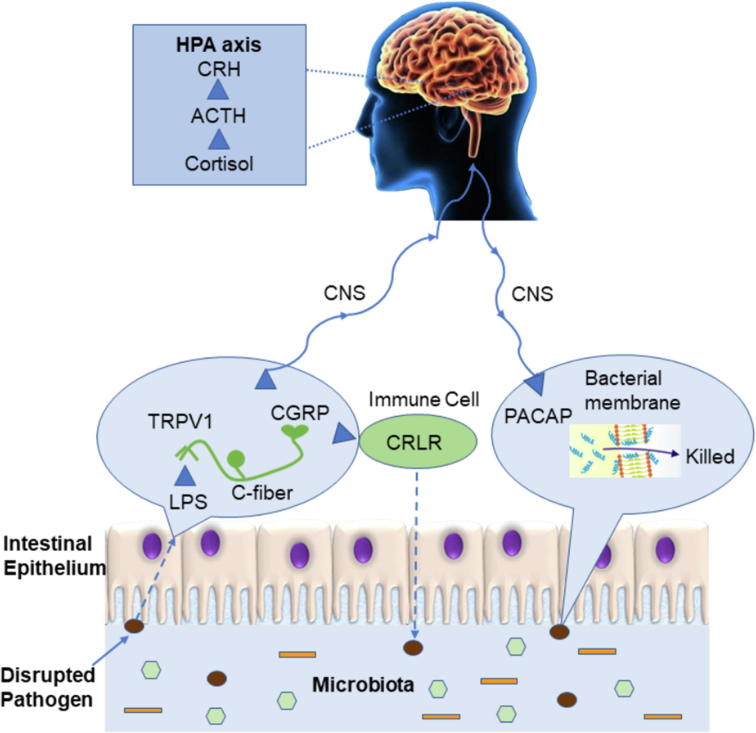
Neuropeptide calcitonin gene-related peptide (CGRP) is a member of the calcitonin peptide family that is expressed in both peripheral and central neurons. It is released from afferent fibers at the site of stimulation when gut environment is changing [41]. As shown in Fig. 1, once infection stimulates transient receptor potential vanilloid receptor-1 (TRPV1) on the surface of C fibers, CGRP is secreted from sensory nerves, which are distributed in C fibers and upstream. After release, CGRP actives host defense and immune response at different sites by binding to its receptor, calcitonin receptor like receptor (CRLR), and its receptor activity-modifying protein1 (RAMP1) found on T and B lymphocytes, macrophages, mast cells, and dendritic cells among others [42], [43]. Since CGRP is released at the site of stimulation, regulating innate immune activation and mediating information flow to the rest of the nervous system, it is exemplified as a neuroimmune connector [41].
Fig. 1.
The bi-directional communication between the gut and the brain during pathogen infection and the potential function of neuropeptides on the host immunity and stress. LPS, lipopolysaccharides; TRPV1, transient receptor potential vanilloid receptor-1; CGRP, calcitonin gene-related peptide; CNS, central nervous system; CRLR, calcitonin receptor like receptor; PACAP, pituitary adenylate cyclase-activating polypeptide; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotrophic hormone; HPA axis, hypothalamic–pituitary–adrenal axis.
CGRP has a regulatory effect on both dendritic cell and T-cell functions, down-regulating pro-inflammatory cytokine tumor necrosis factor α (TNF-α) and promoting anti-inflammatory cytokine, interleukin 10 (IL-10) [44]. Dendritic cells are known to express the CGRP receptor CalcR and respond to CGRP in an anti-inflammatory manner [34]. Recently, a study shows that CGRP treatment significantly reduced TNF-α release while upregulating IL-10 release in LPS-activated dendritic cells. This data suggest that TRPV1 activation of dendritic cells plays a role in their homeostasis and regulation through the release of CGRP [45]. The role of CGRP might also be different in the acute and later stages of inflammation. In acute stages of inflammation, CGRP that is present in peripheral tissues is known to enhance inflammatory responses by dilation of blood vessels, extravasation of inflammatory cells, and activation of secretion of inflammatory cytokines, while in the later stage of inflammation, the proinflammatory activities of macrophages and lymphocytes can be inhibited by the action of CGRP, leading to the suppression of inflammatory responses [46].
The CGRP signaling has been implicated in the cAMP, PKC, ERK, and p38 signal transduction pathways [42]. A recent study has shown that CGRP may play an important role in biological defenses including infection and inflammation by integrating the nervous system, hematopoiesis, and immunity. On the one hand, after initiation of inflammation, CGRP stimulates hematopoiesis in the bone marrow cell, which may compensate proliferating hematopoietic cells including monocytes, which are recruited to the local inflammatory tissues. On the other hand, in chronic inflammation, expression of the CGRP receptor CRLR may be reduced and the loss of CGRP function may inhibit hematopoiesis with reduction of myeloid cells, which could terminate the inflammatory responses by reduction of tissue-invaded macrophages [47].
CGRP is one of the main neurotransmitters involved in immune function and is a key responder to tissue damage that is perceived as “pain” [48]. A recent study with Drosophila shows that diuretic hormone 31 (DH31) and CGRP display a similar activity that triggers muscle contractions during bacterial infection which may cause intestinal pain. The traditional treatment may prescribe drugs that inhibit the visceral spasms which probably slows down the elimination of the pathogens that are responsible for the discomfort. Therefore, a deeper understanding of the CGRP physiological mechanism would be helpful to design novel drugs and adapt medical practices to treat the visceral pain and diarrhea associated with bacterial infection [49].
2.2. Antibacterial neuropeptide, pituitary adenylate cyclase-activating peptide (PACAP)
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a member of the secretin/glucagon superfamily, with potent anti-inflammatory and potent cytoprotective properties [50], [51]. It is most abundant in the brain, but there are significant levels in other organs, including the thymus, spleen, lymph nodes, and duodenal mucosa [52]. In the current work, analysis of C. gariepinus PACAP-38 primary structure reveals the high cationic nature of the peptide, which displays a net charge of +9 at physiological pH and provides evidence of antimicrobial activity of PACAP against a wide spectrum of Gram-negative and Gram-positive bacteria and fungi (Fig. 1) [53]. The basic structure of PACAP consists of 38 amino acids. The C-terminal domain of PACAP-38 is involved in the stabilization of the α-helix, and the N-terminal domain of PACAP plays an essential role for binding affinity and biological activity [54]. It has a significant component of hydrophobic residues with a structurally amphipathic arrangement, both hallmarks of canonical AMP. Its sequence has been remarkably conserved throughout evolution, from fish to mammals, suggesting that this peptide fulfills important biological functions in a broad spectrum of organisms [54]. PACAP-27 is a native isoform of PACAP-38. They both had antimicrobial activity against the Gram-negative bacteria E. coli in the radial diffusion assay, with PACAP-38 having the highest potency, i.e. the lowest minimum inhibitory concentration (MIC). While PACAP-38 has potent sterilizing activity against P. aeruginosa in the more stringent broth dilution assay and antimicrobial activity against the Gram-positive bacteria S. aureus in the radial diffusion assay, PACAP-27 did not have any detectable effect against S. aureus. The difference of antimicrobial activity between PACAP-38 and PACAP-27, shows that while the N-terminal of PACAP plays an essential role in the bacterial binding process, the α-helix on the C-terminal end might also play an important role [55].
Although PACAP has highly potent antimicrobial activity against a wide spectrum of bacteria and fungi, as a drug, PACAP38 is limited by its short half-life [56]. After studying the microbial activity of PACAP and its analogs (PACAP3-38), the result shows that they all have potent antimicrobial activity against a panel of Gram-positive and Gram-negative pathogens. While PACAP-38 and PACAP-27 were almost completely cleaved by DPP IV in less than 30 min, there was no indication that any analog tested was cleaved at all by DPP IV even after 72 h. Additionally, they have low toxicity against human RBCs. The potency and species selectivity of the antimicrobial activity of PACAP analogs also improved. PACAP (9–38), which adopts a π-helix conformation rather than an α-helical conformation like PACAP38, exhibits an increased specificity toward Burkholderia cenocepacia J2315 compared to other tested bacteria [57]. Besides the mammals, the antimicrobial activities of PACAP have also been explored in the non-mammalian vertebrate immune system. Current work provides evidence of antimicrobial activity of Clarias gariepinus PACAP against a wide spectrum of Gram-negative and Gram-positive bacteria and fungi of interest for human medicine and aquaculture, in which computational prediction studies support the putative PACAP therapeutic activity [53]. Overall, the current study contributes to a better understanding of PACAP and its function on the immune system and stimulates renewed interest in PACAP as a new therapeutic agent for the treatment of microbial species.
3. Role of gut-brain axis in stress response and psychological disorders
While many neuropeptides are involved in communication along the gut-brain axis in the immune response, neuropeptides also are key players in the response to stress. From neuropeptides and neurotransmitters secreted from the brain to gut peptides secreted from enteroendocrine (EEC) cells in the gastrointestinal (GI) tract, neuropeptides and peptide hormones have critical functions in bidirectional communication in the stress response. EEC cells, which are a small portion of epithelial cells in the gut, are regulated through the gut microbiome [58], [59], allowing for bidirectional communication between the brain and the microbiome. Many neuropeptides, such as corticotropin-releasing hormone (CRH) and neuropeptide Y, among others, are involved in communication along the gut-brain axis, including in the response to harmful circumstances, namely stress [60]. Furthermore, the microbiome and signaling along the gut-brain axis can also affect behaviors related to psychological disorders, such as anxiety and depression [61], [62], [63]. Many endogenous peptides, such as neurotensin, oxytocin, amylin, neuropeptide Y, and cholecystokinin-8, are well studied in psychological disorders [60], [64].
3.1. Connection between the gut and psychological disorders
Diseases like obesity and inflammatory bowel disease (IBD), are commonly studied due to the involvement of the microbiome and bidirectional communication along the gut-brain axis in these diseases. As the connection between the gut-brain axis and obesity has recently been reviewed [65], this mini review will focus on IBD. IBD affects a diverse population of patients world-wide, especially in western countries. Although IBD is not considered fatal, its effects and associated [66], [67]. Studies have shown that these psychological disorders are more prevalent in IBD individuals compared to healthy controls [68], [69]. Furthermore, stress was correlated with symptoms of IBD, indicating a sensitivity to stress in IBD [70]. Thus, the bi-directional communication between the gut and the brain in IBD influences symptoms of the disease, including the effect of psychological diseases and stress on the gastrointestinal tract. Communication in the gut-brain axis in stress involves a wide range of molecules participating in either brain to gut or gut to brain communication. Here, the focus will be on brain to gut communication during stress via neuropeptides.
Communication during stress in the gut-brain axis commonly occurs through the hypothalamic–pituitary–adrenal (HPA) axis. HPA is the main pathway for stress response and is involved in psychological disorders [71]. This pathway starts with release of corticotropin-releasing hormone (CRH) from the hypothalamus, which then initiates the release of adrenocorticotrophic hormone (ACTH) from the pituitary. The circulation of ACTH releases glucocorticoid hormones, namely cortisol and corticosterone from the adrenal glands [71]. A discussion about the role of CRH and ACTH peptide hormones in the stress response will be provided in the following section, and in the second section a relatively new peptide with potential anxiolytic effects will be discussed in detail. Here, we will provide a short discussion about other neuropeptide changes due to stress.
3.2. Overview of neuropeptide changes in stress
Neuropeptide Y (NPY) is a key neuropeptide in the enteric nervous system, and has anti-stress properties [72]. The role of NPY in inflammatory responses, pain, emotion, mood, cognition, stress, ingestion, and energy homeostasis has previously been reviewed [73], so only a brief discussion will be presented here. NPY levels have been found to decrease in patients with post-traumatic stress disorder and increase in people exposed to trauma who do not develop or recover from the trauma [73]. In a study in humans with IBD, NPY levels in venous blood samples were found to increase in IBD patients [74]. In rodent models of stress and IBD, NPY levels were also found to be altered. In a dextran sulfate sodium (DSS)-induced colitis model in mice, increased levels of NPY were found in plasma as well as increased NPY expression in the hypothalamus [75], [76]. Another study investigated the effect of electro-acupuncture on two rat models of stress, namely the chronic and acute stress model and the senna gavage and chronic model. This study revealed decreased levels of NPY in the distal colon, hypothalamus, and spinal cord with the two models of stress, but electro-acupuncture restored NPY and other neurotransmitter levels to that of the healthy control group [77]. Finally, a study of minimal traumatic brain injury in rats found that the NPY levels were initially decreased 48 h after trauma, but then were elevated 30 days after trauma [78]. Additional in-depth reviews of the role of the NPY neuropeptide family in stress can be found elsewhere [79], [80].
A variety of neuropeptides are potentially involved in the gut-brain axis’s response to stress. Thyrotropin-releasing hormone was increased in water avoidance stress in both serum and mucosa, and a receptor for the hormone was expressed in the colon mucosal epithelium and myenteric plexus neurons [81]. The role of endogenous opioid signaling has also been investigated, where it was found that there was a switch in the opioid signaling to an excitation effect in dorsal root ganglia [82]. Additionally, apelin and cholecystokinin (CCK) levels were studied during acute restraint stress in rats [83]. Endogenous apelin mediated release of CCK from enteric neurons in stressed rats [83]. Further study of these neuropeptides may provide a deeper understanding of the role of the gut-brain axis in stress response.
3.3. Hypothalamic–pituitary–adrenal axis communication in the stress response
The response to stress along the hypothalamic–pituitary–adrenal axis involves peptide hormones CRH and ACTH. CRH in mammals is a 41 amino acid long peptide. CRH is secreted from the hypothalamus [84], stimulating the release of ACTH, a 39 amino acid peptide, from the pituitary. There are two CRH receptors (CRH-R1 and CRH-R2), which are G protein-coupled receptors from the B1 family. CRH-R1 is highly expressed in the brain, with more limited expression in peripheral tissues, while CRH-R2 has more limited expression in the brain and is more highly expressed in peripheral tissues [85].
In humans, the role of the hypothalamic–pituitary–adrenal axis in stress and IBD is extensively studied, and recent studies in humans examined the effect of administered CRH under different conditions. CRH administration in humans reproduced the effect of public speaking psychological stress, namely increased small intestine permeability [86]. The administration of CRH increased ACTH levels in plasma, which is expected as the function of CRH is to promote ACTH release. Additionally, exogenous administered CRH affects the brain regions associated with emotional-arousal circuitry and pain differently during colorectal distention in control individuals than IBD patients [87]. A follow up study found that hypothalamic–pituitary–adrenal–sympathoadrenal responses to CRH injection were different in control and IBD patients during colorectal distention, indicating that changes in adrenal gland activity in response to ACTH stimulation may be involved with IBD [88].
In rodent studies, different models of IBD and stress are combined to investigate the role of the hypothalamic–pituitary–adrenal axis, including CRH and ACTH. The chronic restraint stress model is one model commonly used in rodents. An investigation of germ-free mice and specific pathogen infected mice under restraint stress revealed higher levels of hypothalamic–pituitary–adrenal axis related compounds, including CRH and ACTH, in germ-free mice [89]. Overall, specific pathogen infected mice exhibited more anxiety behaviors in restraint stress, indicating changes in intestinal microbiota can influence the hypothalamic–pituitary–adrenal axis response to stress [89]. Another study investigated probiotic treatment during restraint stress, revealing that treatment with Lactobacillus helveticus NS8 restored ACTH levels and lessened the anxiety and depression symptoms caused by restraint stress, again revealing the importance of the gut microbiome composition in stress [90]. Maternal separation stress early in development and CRH injection both resulted in changes in colorectal mobility and gut microbiome content by the stressors [91]. Furthermore, buserelin treatment, which was expected to cause enteric neuronal loss, resulted in up to 50% loss of neurons and an increase in CRH immunoreactive neurons in the colon. Despite these physical changes, however, the physiology and function of the gut is still well preserved with regards to the stress response and hypothalamic–pituitary–adrenal axis [92].
In a mouse model of IBD, DDS-induced colitis, the effect of stress, via a water avoidance stress model of mild psychological stress, was studied. This revealed changes in CRH, CRH-R1, NPY, and NPY receptor Y1, among others, revealing altered brain-gut signaling during DDS induced colitis [76]. The role of the hypothalamic–pituitary–adrenal axis in visceral nociception was investigated in rats using a colorectal distention protocol, which demonstrated that CRH in the central nucleus of the amygdala was involved in signaling for visceral nociception through release of noradrenaline after CRH-R1 binding [93]. Thus, changes in the HPA axis occur due to a variety of stressors, and when combined with the important role of the gut microbiome composition in stress, a dynamic gut-brain axis signaling process is revealed.
3.4. Phoenixin – A neuropeptide with multiple roles
In contrast to the well-studied peptides along the HPA axis, novel neuropeptides are still being discovered. Relatively recently, the neuropeptide phoenixin (PNX) was discovered with bioinformatics approach, revealing a 14 amino acid peptide and a 20 amino acid peptide, both of which are amidated [94]. The peptides are highly conserved across species. Phoenixin is primarily localized to the hypothalamus, with peripheral localization to other organs, including the heart, thymus, and stomach [94]. Originally, in studies in rats, phoenixin was found to be involved in reproduction as administration of small interfering RNA (siRNA) against phoenixin in vivo delayed estrus and reduced gonadotrophin-releasing hormone (GnRH) receptor expression, which is involved in regulation of the reproductive system [94]. The G protein-coupled receptor Gpr173 is the putative receptor for PNX binding [95], [96]. While the original function of the peptide was in reproduction, with multiple studies focusing on this, there has been a range of other proposed functions for PNX, including roles in psychological disorders, which will be discussed following description of the pathway and localization of PNX.
In vitro studies have provided insight into the pathway for PNX involvement in reproduction. GnRH is involved in regulation of release of reproductive hormones, such as luteinizing hormone (LH). Evidence suggests that PNX mediates GnRH secretion by regulating the expression of the GnRH receptor [94] through activation of the cyclic AMP/ protein kinase A pathway after PNX binds to the Gpr 173 receptor [96]. Additional research has examined the ability of PNX to affect levels of other molecules, including vasopressin [97], as well as identifying potential regulators of PNX and the PNX receptor [98], [99], [100]. Interestingly, in women with polycystic ovary syndrome, PNX-14 levels, along with LH and androgen, were increased in the serum of patients compared to controls, indicating a potential role of PNX-14 in polycystic ovary syndrome [101].
The specific immunohistological localization and expression analysis revealed numerous localization patterns in the brain, suggesting that phoenixin has a wide range of functions [94], [102], [103]. A study of the localization of pheonixin-14 (as opposed to the initial studies that used an antibody that bound to both the 14 and 20 amino-acid residue peptides with C-terminal amidation) revealed pheonixin-14 was localized to the medial division of the central amygdaloid nucleus in the brain, spinal trigeminal tract, spinocerebellar tract, and cells between crypts of duodenum, jejunum, and ileum [104]. PNX has been implicated in feeding behavior as studies in rats have shown high co-expression with the neuropeptide nesfatin-1, which is involved in food intake, energy expenditure, and glucose homeostasis [103], along with an increase in food intake upon injection of PNX [105], [106]. The peptide has also been implicated in the suppression of visceral pain [102] as well as signaling of itch sensation [107]. The effect of PNX in the heart has also been studied [108].
Furthermore, PNX levels have been investigated in psychological disorders, namely anxiety, and neurocognitive disorders. In mice, PNX-14 was shown to reduce anxiety in the open field and elevated maze test in a dose dependent manner [109]. In humans, a study in obese men revealed a negative association of PNX and anxiety. Interestingly, when the group was divided into minimal and moderate anxiety subgroups, the minimal anxiety group had higher PNX levels, although the p-value was just above the level needed for statistical significance [110]. As depression and perceived stress were not correlated with PNX, the PNX anxiety correlation appears to be specific, but the study in obese men revealed only a moderate correlation between PNX and anxiety, potentially due to other factors [110], [111]. For neurocognitive disorders, PNX improved memory formation and retention through GnRH receptor activation in mice, indicating a potential ability of the neuropeptide to enhance memory [112]. Also, PNX was shown to potentially reverses the effects of Alzheimer’s Disease as it lessened the effects of Aβ1-42 and scopolamine, which cause memory impairment [112]. PNX levels were tested in humans with either mild cognitive impairment, subjective memory complaints, or Alzheimer’s disease, and no significant differences were observed between the groups, although there was no healthy control for comparison [113]. The reported positive effects of PNX on anxiety and cognitive function have the potential to be impactful for treating these disorders. Thus, it will be interesting to follow up with further studies on the role of PNX in anxiety and neurocognitive disorders.
4. Summary
Significant progress has been made over the past decades in recognizing the importance of neuropeptides in bidirectional communication between the brain and the gut and their influence on host immunity and stress. As stated earlier, neuropeptides such as CGRP are involved in host monitoring of the gut environment and have an important function that connects nervous and immune systems during infection. Neuropeptides such as PACAP could directly act on the bacteria membrane to kill the bacteria. The effective defense and low bacterial resistance probability make the antimicrobial neuropeptides an attractive new class of antibiotics. Key findings show that stress influences the composition of the gut microbiota and that bidirectional communication between microbiota and the CNS influences stress reactivity and responses. Neuropeptide CRH acts as a common mediator during this dynamic gut-brain axis signaling process. The positive effects of PNX on anxiety and cognitive function have the potential to be impactful for development of therapeutic strategies to treat these disorders.
We are just beginning to understand the meaning of gut-to-brain microbiome interactions and what role neuropeptides ultimately play for host homeostasis including immunity and stress. Although their implications are described in selected models, some of the precise mechanisms and overall effects are not fully understood. Further progress in understanding the various processes involved in neuropeptide modulation of the interactions between the gut microbiome and the central and peripheral nervous systems is essential to develop effective treatments for immune and stress disorders with neurogenic components. Because our understanding of the interactions of the gut-brain axis continues to expand, novel therapies will be developed to treat gut microbiome-mediated immune and stress diseases. Neuropeptides, their receptors, and the proteases that degrade the same neuropeptides may become the special target of new specific pharmacologic approaches.
Conflict of interest statement
The authors do not have any conflict of interest to declare.
CRediT authorship contribution statement
Pingli Wei: Writing - original draft. Caitlin Keller: Writing - original draft. Lingjun Li: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Acknowledgements
Preparation of this manuscript was supported in part by National Science Foundation (CHE-1710140) and National Institutes of Health grant R01DK071801. LL acknowledges a Vilas Distinguished Achievement Professorship and a Charles Melbourne Johnson Distinguished Chair Professorship, and a UW2020 grant with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin-Madison School of Pharmacy.
References
- 1.John G.K., Mullin G.E. The gut microbiome and obesity. Curr Oncol Rep. 2016;18(7):45. doi: 10.1007/s11912-016-0528-7. [DOI] [PubMed] [Google Scholar]
- 2.Ghaisas S., Maher J., Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 2016;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guinane C.M., Cotter P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol. 2013;6(4):295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M.P., Zaghouani H., Niklas V. Gut microbiota, the immune system, and diet influence the neonatal gut–brain axis. Pediatr Res. 2015;77(1–2):127–135. doi: 10.1038/pr.2014.161. [DOI] [PubMed] [Google Scholar]
- 7.Foster J.A., McVey Neufeld K.-A. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Petra A.I., Panagiotidou S., Hatziagelaki E., Stewart J.M., Conti P., Theoharides T.C. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R.-B. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395(6699):284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 10.Frazier T.H., DiBaise J.K., McClain C.J. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35(5_suppl):14S–20S. doi: 10.1177/0148607111413772. [DOI] [PubMed] [Google Scholar]
- 11.Buhner S. Calcium imaging of nerve-mast cell signaling in the human intestine. Front Physiol. 2017;8:1–12. doi: 10.3389/fphys.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajvanshi A.K. The three minds of the body. Brain, heart and gut. 2011 [Google Scholar]
- 13.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2015;28(2):203. [PMC free article] [PubMed] [Google Scholar]
- 15.Ichihara K., Eng J., Yalow R.S. Ontogeny of immunoreactive CCK, VIP and secretin in rat brain and gut. Biochem Biophys Res Commun. 1983;112(3):891–898. doi: 10.1016/0006-291x(83)91701-1. [DOI] [PubMed] [Google Scholar]
- 16.Barber W.D., Burks T.F. Brain-gut interactions: brain stem neuronal response to local gastric effects of substance P. Am J Physiol. 1987;253(3 Pt 1):G369–G377. doi: 10.1152/ajpgi.1987.253.3.G369. [DOI] [PubMed] [Google Scholar]
- 17.Lotti T., D’Erme A.M., Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32(5):633–645. doi: 10.1016/j.clindermatol.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Sabatino F., Di Zazzo A., De Simone L., Bonini S. The intriguing role of neuropeptides at the ocular surface. Ocul Surf. 2017;15(1):2–14. doi: 10.1016/j.jtos.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Lai N.Y., Mills K., Chiu I.M., Chiu I. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defense HHS Public Access. J Intern Med. 2017;282(1):5–23. doi: 10.1111/joim.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goehler L.E., Gaykema R.P.A., Opitz N., Reddaway R., Badr N., Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19(4):334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Bercik P. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609.e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 22.Sudo N. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Palma G. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015;6(1):7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 25.Golubeva A.V. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015;60:58–74. doi: 10.1016/j.psyneuen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Barouei J., Moussavi M., Hodgson D.M. Effect of maternal probiotic intervention on HPA Axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussaoui N. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J Neurogastroenterol Motil. 2017;23(1):135–143. doi: 10.5056/jnm16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin T., Wu H., Wang Y., Peng H. Capsaicin induces immunogenic cell death in human osteosarcoma cells. Exp Ther Med. 2016;12(2):765–770. doi: 10.3892/etm.2016.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granato M., Gilardini Montani M.S., Filardi M., Faggioni A., Cirone M. Capsaicin triggers immunogenic PEL cell death, stimulates DCs and reverts PEL-induced immune suppression. Oncotarget, Oct. 2015;6(30):29543–29554. doi: 10.18632/oncotarget.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley J.E., Kay N.E., Solomon G.F., Plotnikoff N.P. Neuropeptides: conductors of the immune orchestra. Life Sci. 1987;41(5):527–544. doi: 10.1016/0024-3205(87)90405-x. [DOI] [PubMed] [Google Scholar]
- 31.El Karim I.A., Linden G.J., Orr D.F., Lundy F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200(1–2):11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X., Han Y., Du J., Liu R., Jin K., Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8(32):53829–53838. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarandi S.S., Peterson D.A., Treisman G.J., Moran T.H., Pasricha P.J. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil. 2016;22(2):201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambrecht B.N. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir Res. 2001;2(3):133. doi: 10.1186/rr49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart M.P., Cabanas C., Hogg N., Steinman L., Lider O. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156(5):1810–1817. [PubMed] [Google Scholar]
- 36.Chen A., Ganor Y., Rahimipour S., Ben-Aroya N., Koch Y., Levite M. The neuropeptides GnRH-II and GnRH-I are produced by human T cells and trigger laminin receptor gene expression, adhesion, chemotaxis and homing to specific organs. Nat Med. 2002;8(12):1421–1426. doi: 10.1038/nm1202-801. [DOI] [PubMed] [Google Scholar]
- 37.Timmermans J.-P., Scheuermann D.W., Stach W., Adriaensen D., De Groodt-Lasseel M.H.A. Distinct distribution of CGRP-, enkephalin-, galanin-, neuromedin U-, neuropeptide Y-, somatostatin-, substance P-, VIP- and serotonin-containing neurons in the two submucosal ganglionic neural networks of the porcine small intestine. Cell Tissue Res. May 1990;260(2):367–379. doi: 10.1007/BF00318639. [DOI] [PubMed] [Google Scholar]
- 38.Augustyniak D., Nowak J., Lundy F.T. Direct and indirect antimicrobial activities of neuropeptides and their therapeutic potential. Curr Protein Pept Sci. 2012;13(8):723–738. doi: 10.2174/138920312804871139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 40.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 41.Uddman R., Edvinsson L., Ekblad E., Håkanson R., Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- 42.Russell F.A., King R., Smillie S.-J., Kodji X., Brain S.D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assas B.M., Pennock J.I., Miyan J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci. 2014;8(8):1–9. doi: 10.3389/fnins.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaraee R., Ebtekar M., Ahmadiani A., Sabahi F. Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. Int Immunopharmacol. 2003;3(13–14):1883–1887. doi: 10.1016/S1567-5769(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 45.Assas B.M., Wakid M.H., Zakai H.A., Miyan J.A., Pennock J.L. Transient receptor potential vanilloid 1 expression and function in splenic dendritic cells: a potential role in immune homeostasis. Immunology. 2016;147(3):292–304. doi: 10.1111/imm.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanesch U., Pfrommer U., Grubb B.D., Schaible H.-G. Acute and chronic phases of unilateral inflammation in rat’s ankle are associated with an increase in the proportion of calcitonin gene-related peptide-immunoreactive dorsal root ganglion cells. Eur J Neurosci. 1993;5(2):154–161. doi: 10.1111/j.1460-9568.1993.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 47.Suekane A. CGRP-CRLR/RAMP1 signal is important for stress-induced hematopoiesis. Sci Rep. 2019;9(1):429. doi: 10.1038/s41598-018-36796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benemei S., Nicoletti P., Capone J.G., Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009;9(1):9–14. doi: 10.1016/j.coph.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Benguettat O. The DH31/CGRP enteroendocrine peptide triggers intestinal contractions favoring the elimination of opportunistic bacteria. PLoS Pathog. 2018;14(9):1–26. doi: 10.1371/journal.ppat.1007279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji H. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation by the cAMP-PKA pathway. Hepatology. 2013;57:1225–1237. doi: 10.1002/hep.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reglodi D. PACAP is an endogenous protective factor—insights from PACAP-deficient mice. J Mol Neurosci. 2012;48(3):482–492. doi: 10.1007/s12031-012-9762-0. [DOI] [PubMed] [Google Scholar]
- 52.Delgado M. PACAP in immunity and inflammation. Ann N Y Acad Sci. 2003;992(1):141–157. doi: 10.1111/j.1749-6632.2003.tb03145.x. [DOI] [PubMed] [Google Scholar]
- 53.Lugo J.M. Evidence for antimicrobial and anticancer activity of pituitary adenylate cyclase-activating polypeptide (PACAP) from North African catfish (Clarias gariepinus): Its potential use as novel therapeutic agent in fish and humans. Fish Shellfish Immunol. 2019;86:559–570. doi: 10.1016/j.fsi.2018.11.056. [DOI] [PubMed] [Google Scholar]
- 54.Debbabi S. Antibacterial properties of the pituitary adenylate cyclase-activating polypeptide: A new human antimicrobial peptide. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doan N.D. Design and characterization of novel cell-penetrating peptides from pituitary adenylate cyclase-activating polypeptide. J Controlled Release. 2012;163(2):256–265. doi: 10.1016/j.jconrel.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 56.Green B.D., Irwin N., Flatt P.R. Pituitary adenylate cyclase-activating peptide (PACAP): Assessment of dipeptidyl peptidase IV degradation, insulin-releasing activity and antidiabetic potential. Peptides. 2006;27(6):1349–1358. doi: 10.1016/j.peptides.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Debbabi S. Antibacterial properties of the pituitary adenylate cyclase-activating polypeptide: a new human antimicrobial peptide. PLoS One. 2018;13(11):1–15. doi: 10.1371/journal.pone.0207366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dockray G.J. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol. 2013;13(6):954–958. doi: 10.1016/j.coph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Cohen L.J. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549(7670):48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lach G., Schellekens H., Dinan T.G., Cryan J.F. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15(1):36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burokas A. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 62.Diaz Heijtz R. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong M.-L. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGonigle P. Peptide therapeutics for CNS indications. Biochem Pharmacol. 2012;83(5):559–566. doi: 10.1016/j.bcp.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Niccolai E., Boem F., Russo E., Amedei A. The gut–brain axis in the neuropsychological disease model of obesity: a classical movie revised by the emerging director microbiome. Nutrients. 2019;11(1) doi: 10.3390/nu11010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guthrie E., Jackson J., Shaffer J., Thompson D., Tomenson B., Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol. 2002;97(8):1994–1999. doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 67.Nordin K., Påhlman L., Larsson K., Sundberg-Hjelm M., Lööf L. Health-related quality of life and psychological distress in a population-based sample of Swedish patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37(4):450–457. doi: 10.1080/003655202317316097. [DOI] [PubMed] [Google Scholar]
- 68.Walker J.R. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103(8):1989–1997. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 69.Graff L.A. Stress coping, distress and health perceptions in inflammatory bowel disease and community controls. Am J Gastroenterol. 2009;104(12):2959–2969. doi: 10.1038/ajg.2009.529. [DOI] [PubMed] [Google Scholar]
- 70.Whitehead W.E., Crowell M.D., Robinson J.C., Heller B.R., Schuster M.M. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33(6):825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer R.L., Deak T. A users guide to HPA axis research. Physiol Behav. 2017;178:43–65. doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorsell A. Brain neuropeptide Y and corticotropin-releasing hormone in mediating stress and anxiety. Exp Biol Med. 2010;235(10):1163–1167. doi: 10.1258/ebm.2010.009331. [DOI] [PubMed] [Google Scholar]
- 73.Holzer P., Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014,;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stasi C. Neuroendocrine dysregulation in irritable bowel syndrome patients: a pilot study. J Neurogastroenterol Motil. 2017;23(3):428–434. doi: 10.5056/jnm16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hassan A.M. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front Behav Neurosci. 2014;8:386. doi: 10.3389/fnbeh.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reichmann F., Hassan A.M., Farzi A., Jain P., Schuligoi R., Holzer P. Dextran sulfate sodium-induced colitis alters stress-associated behaviour and neuropeptide gene expression in the amygdala-hippocampus network of mice. Sci Rep. 2015;5(1):9970. doi: 10.1038/srep09970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun J. Electro-acupuncture decreases 5-HT, CGRP and increases NPY in the brain-gut axis in two rat models of Diarrhea-predominant irritable bowel syndrome(D-IBS) BMC Complement Altern Med. 2015;15(1):340. doi: 10.1186/s12906-015-0863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sagarkar S., Mahajan S., Choudhary A.G., Borkar C.D., Kokare D.M., Sakharkar A.J. Traumatic stress-induced persistent changes in DNA methylation regulate neuropeptide Y expression in rat jejunum. Neurogastroenterol Motil. 2017;29(9) doi: 10.1111/nmo.13074. [DOI] [PubMed] [Google Scholar]
- 79.Farzi A., Reichmann F., Holzer P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. 2015;213(3):603–627. doi: 10.1111/apha.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reichmann F., Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99–109. doi: 10.1016/j.npep.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Wang C., Zhang L. The potential role of thyrotropin-releasing hormone in colonic dysmotility induced by water avoidance stress in rats. Neuropeptides. 2018;70:47–54. doi: 10.1016/j.npep.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Guerrero-Alba R. Stress activates pronociceptive endogenous opioid signalling in DRG neurons during chronic colitis. Gut. 2017;66(12):2121–2131. doi: 10.1136/gutjnl-2016-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bülbül M., Sinen O., Bayramoğlu O., Akkoyunlu G. Acute restraint stress induces cholecystokinin release via enteric apelin. Neuropeptides. 2019;73:71–77. doi: 10.1016/j.npep.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 84.Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 85.Ketchesin K.D., Stinnett G.S., Seasholtz A.F. Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans. Stress. 2017;20(5):449–464. doi: 10.1080/10253890.2017.1322575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanuytsel T. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka Y. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka Y., Kanazawa M., Kano M., Tashiro M., Fukudo S. Relationship between sympathoadrenal and pituitary-adrenal response during colorectal distention in the presence of corticotropin-releasing hormone in patients with irritable bowel syndrome and healthy controls. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0199698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huo R. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang S. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 91.Murakami T. Changes in intestinal motility and gut microbiota composition in a rat stress model. Digestion. 2017;95(1):55–60. doi: 10.1159/000452364. [DOI] [PubMed] [Google Scholar]
- 92.Sand E. Buserelin treatment to rats causes enteric neurodegeneration with moderate effects on CRF-immunoreactive neurons and Enterobacteriaceae in colon, and in acetylcholine-mediated permeability in ileum. BMC Res Notes. 2015;8(1):824. doi: 10.1186/s13104-015-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su J., Tanaka Y., Muratsubaki T., Kano M., Kanazawa M., Fukudo S. Injection of corticotropin-releasing hormone into the amygdala aggravates visceral nociception and induces noradrenaline release in rats. Neurogastroenterol Motil. 2015;27(1):30–39. doi: 10.1111/nmo.12462. [DOI] [PubMed] [Google Scholar]
- 94.Yosten G.L.C. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;25(2):206–215. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stein L.M. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Integr Comp Physiol. 2016;311(3):R489–R496. doi: 10.1152/ajpregu.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Treen A.K., Luo V., Belsham D.D. Phoenixin activates immortalized gnrh and kisspeptin neurons through the novel receptor GPR173. Mol Endocrinol. 2016;30(8):872–888. doi: 10.1210/me.2016-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gasparini S. Novel regulator of vasopressin secretion: phoenixin. Am J Physiol Integr Comp Physiol. 2018;314(4):R623–R628. doi: 10.1152/ajpregu.00426.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McIlwraith E.K., Loganathan N., Belsham D.D. Phoenixin expression is regulated by the fatty acids palmitate, docosahexaenoic acid and oleate, and the endocrine disrupting chemical bisphenol a in immortalized hypothalamic neurons. Front Neurosci. 2018;12:838. doi: 10.3389/fnins.2018.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suszka-Świtek A. The Gn RH analogues affect novel neuropeptide SMIM 20/phoenixin and GPR 173 receptor expressions in the female rat hypothalamic–pituitary–gonadal (HPG) axis. Clin Exp Pharmacol Physiol. 2019;46(4):350–359. doi: 10.1111/1440-1681.13061. [DOI] [PubMed] [Google Scholar]
- 100.McIlwraith E.K., Loganathan N., Belsham D.D. Regulation of Gpr173 expression, a putative phoenixin receptor, by saturated fatty acid palmitate and endocrine-disrupting chemical bisphenol A through a p38-mediated mechanism in immortalized hypothalamic neurons. Mol Cell Endocrinol. 2019;485:54–60. doi: 10.1016/j.mce.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 101.Ullah K. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin Chim Acta. 2017;471:243–247. doi: 10.1016/j.cca.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 102.Lyu R.-M. Phoenixin: a novel peptide in rodent sensory ganglia. Neuroscience. 2013;250:622–631. doi: 10.1016/j.neuroscience.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pałasz A., Rojczyk E., Bogus K., Worthington J.J., Wiaderkiewicz R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci Lett. 2015;592:17–21. doi: 10.1016/j.neulet.2015.02.060. [DOI] [PubMed] [Google Scholar]
- 104.Prinz P. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem Biophys Res Commun. 2017;493(1):195–201. doi: 10.1016/j.bbrc.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 105.Schalla M. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides. 2017;96:53–60. doi: 10.1016/j.peptides.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 106.Friedrich T. Intracerebroventricular injection of phoenixin alters feeding behavior and activates nesfatin-1 immunoreactive neurons in rats. Brain Res. 2019;1715:188–195. doi: 10.1016/j.brainres.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 107.Cowan A., Lyu R.-M., Chen Y.-H., Dun S.L., Chang J.-K., Dun N.J. Phoenixin: a candidate pruritogen in the mouse. Neuroscience. 2015;310:541–548. doi: 10.1016/j.neuroscience.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rocca C. Phoenixin-14: detection and novel physiological implications in cardiac modulation and cardioprotection. Cell Mol Life Sci. 2018;75(4):743–756. doi: 10.1007/s00018-017-2661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang J.H. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res. 2015;286:39–48. doi: 10.1016/j.bbr.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 110.Hofmann T. Phoenixin is negatively associated with anxiety in obese men. Peptides. 2017;88:32–36. doi: 10.1016/j.peptides.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Schalla M.A., Stengel A. The role of phoenixin in behavior and food intake. Peptides. 2019;114:38–43. doi: 10.1016/j.peptides.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 112.Jiang J.H. Phoenixin-14 enhances memory and mitigates memory impairment induced by Aβ1-42 and scopolamine in mice. Brain Res. 2015;1629:298–308. doi: 10.1016/j.brainres.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 113.Yuruyen M. Does plasma phoenixin level associate with cognition? Comparison between subjective memory complaint, mild cognitive impairment, and mild Alzheimer’s disease. Int Psychogeriatrics. 2017;29(9):1543–1550. doi: 10.1017/S1041610217000825. [DOI] [PubMed] [Google Scholar]
- 114.Hansen C.J., Burnell K.K., Brogden K.A. Antimicrobial activity of substance P and neuropeptide Y against laboratory strains of bacteria and oral microorganisms. J Neuroimmunol. 2006;177(1–2):215–218. doi: 10.1016/j.jneuroim.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Campos-Salinas J. Therapeutic efficacy of stable analogues of vasoactive intestinal peptide against pathogens. J Biol Chem. May 2014;289(21):14583–14599. doi: 10.1074/jbc.M114.560573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cutuli M., Cristiani S., Lipton J.M., Catania A. Antimicrobial effects of α-MSH peptides. J Leukoc Biol. 2000;67(2):233–239. doi: 10.1002/jlb.67.2.233. [DOI] [PubMed] [Google Scholar]
- 117.Allaker R.P., Kapas S. Adrenomedullin and mucosal defence: interaction between host and microorganism. Regul Pept. 2003;112(1–3):147–152. doi: 10.1016/s0167-0115(03)00033-8. [DOI] [PubMed] [Google Scholar]
- 118.Goumon Y. Characterization of antibacterial COOH-terminal proenkephalin-A-derived peptides (PEAP) in infectious fluids. Importance of enkelytin, the antibacterial PEAP209-237 secreted by stimulated chromaffin cells. J Biol Chem. 1998;273(45):29847–29856. doi: 10.1074/jbc.273.45.29847. [DOI] [PubMed] [Google Scholar]