Abstract
Background
Chemotherapy‐induced peripheral neuropathy (CIPN) is one of the most debilitating long‐term side effects in breast cancer survivors. We conducted a randomized controlled pilot trial to assess the feasibility, safety, and effects of an acupuncture intervention on CIPN in this population.
Patients and Methods
Women with stage I–III breast cancer with grade 1 or higher CIPN after taxane‐containing adjuvant chemotherapy were randomized 1:1 to an immediate acupuncture (IA) arm or to a waitlist control group (CG). Participants in the IA arm received 18 sessions of acupuncture over 8 weeks, then received no additional acupuncture. Patients in the CG arm received usual care over 8 weeks, followed by nine sessions of acupuncture over 8 weeks. Measures including Patient Neurotoxicity Questionnaire (PNQ), Functional Assessment of Cancer Therapy—Neurotoxicity subscale (FACT‐NTX), and Brief Pain Inventory—short form (BPI‐SF) were collected at baseline and at 4, 8, and 16 weeks after enrollment.
Results
Forty women (median age, 54) were enrolled (20 to IA and 20 to CG), with median time between completion of chemotherapy and enrollment of 14 months (range 1–92). At 8 weeks, participants in the IA arm experienced significant improvements in PNQ sensory score (−1.0 ± 0.9 vs. −0.3 ± 0.6; p = .01), FACT‐NTX summary score (8.7 ± 8.9 vs. 1.2 ± 5.4; p = .002), and BPI‐SF pain severity score (−1.1 ± 1.7 vs. 0.3 ± 1.5; p = .03), compared with those in the CG arm. No serious side effects were observed.
Conclusion
Women with CIPN after adjuvant taxane therapy for breast cancer experienced significant improvements in neuropathic symptoms from an 8‐week acupuncture treatment regimen. Additional larger studies are needed to confirm these findings.
Implications for Practice
Chemotherapy‐induced peripheral neuropathy (CIPN) is a toxicity that often persists for months to years after the completion of adjuvant chemotherapy for early breast cancer. In a randomized pilot trial of 40 breast cancer survivors with CIPN, an 8‐week acupuncture intervention (vs. usual care) led to a statistically and clinically significant improvement in subjective sensory symptoms including neuropathic pain and paresthesia. Given the lack of effective therapies and established safety profile of acupuncture, clinicians may consider acupuncture as a treatment option for mild to moderate CIPN in practice.
Keywords: Acupuncture, Chemotherapy‐induced peripheral neuropathy, Breast cancer survivors, Quality of life, Taxane
Short abstract
Chemotherapy‐induced peripheral neuropathy (CIPN) is a common side effect of chemotherapy. This article describes results of a study that evaluated the feasibility and benefits of acupuncture in breast cancer survivors with CIPN symptoms.
Introduction
Chemotherapy‐induced peripheral neuropathy (CIPN) is one of the most common side effects during and after chemotherapy in patients with cancer. Taxanes, platinum agents, and vinca alkaloids often cause CIPN. Symptoms of CIPN vary according to the type and severity of sensory, motor, and autonomic nerve damage and typically include paresthesia and pain.
In breast cancer, paclitaxel and docetaxel are commonly used in the early‐stage and metastatic settings. Rates of CIPN in patients with breast cancer range between 30% and 97% 1, 2, 3, 4, 5, and studies have shown that even 6 years after treatment, 47% of patients still report symptoms of CIPN 6. Persistent CIPN is associated with poor physical function, falls, and greater disability in cancer survivors 6, 7. Several studies report that the risk of falling in women with CIPN symptoms was 1.8 to 2.7 times greater than in women without CIPN symptoms 6, 7, with fall rates of 31.9% to 41.5% 2 in women with symptomatic CIPN.
A number of pharmacologic agents are currently used to treat CIPN in patients with cancer, including antidepressants, anticonvulsants such as gabapentin, and non‐narcotic and narcotic analgesics. However, only duloxetine is currently recommended for treatment of painful CIPN 8. Dietary supplements, such as glutamine, glutathione, vitamin E, and vitamin B12, have also been studied. Most of the treatments that have been evaluated for management of CIPN to date treat only pain and not paresthesia. Additionally, many of the agents currently used to treat CIPN cause side effects such as fatigue, dizziness, insomnia, or nausea, underscoring the need for better treatments to manage CIPN in patients with cancer.
Acupuncture is an ancient medical intervention in which fine metallic needles are inserted into anatomical locations of the body to stimulate the peripheral and the central nervous system. Acupuncture has been studied in clinical trials in patients with cancer and has been shown to be effective in mitigating toxicities of cancer treatment, ranging from chemotherapy‐induced nausea and vomiting to aromatase inhibitor–related musculoskeletal complaints 9, 10. Acupuncture trials in patients with CIPN have suggested that acupuncture may alleviate CIPN symptoms and improve nerve conduction 11, 12, 13, 14, but data are still limited. We conducted a randomized pilot study to evaluate the feasibility and benefits of acupuncture in breast cancer survivors with CIPN symptoms.
Materials and Methods
Trial Design
This was a randomized, waitlist‐controlled pilot study designed to assess the effect of acupuncture versus usual care on CIPN symptoms in patients with stage I–III breast cancer with persistent CIPN after adjuvant chemotherapy.
Participants were randomized 1:1 to an immediate acupuncture arm or to a waitlist control arm. The acupuncture arm received 18 acupuncture treatments over 8 weeks, followed by an 8‐week follow‐up period in which no acupuncture was administered. The waitlist control group received no acupuncture over the first 8 weeks of protocol participation, then received nine acupuncture treatments over the subsequent 8 weeks. Study measures were collected at the time of study enrollment, 4, 8, and 16 weeks after randomization. The primary endpoint of the study was change in the composite Patient Neurotoxicity Questionnaire (PNQ) score between acupuncture and usual care arms at the end of week 8. All participants signed an informed consent prior to randomization. The study was approved by the Institutional Review Board of the Dana‐Farber/Harvard Cancer Center and registered at clinical http://trials.gov (NCT02129686).
Eligibility
Participants were eligible for inclusion if they had histologically confirmed stage I–III breast cancer, had completed adjuvant taxane‐based chemotherapy (alone or in combination), were at least 18 years of age, had an Eastern Cooperative Oncology Group performance status of 0 or 1, and reported grade 1 or greater CIPN symptoms for more than 2 weeks as defined by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. Patients underwent screening for eligibility, including verification of baseline CIPN symptoms, by study staff prior to enrollment. There were no limitations on time from the last paclitaxel administration, but concurrent chemotherapy was not allowed. Exclusion criteria included having metastatic or recurrent disease, history of preexisting peripheral neuropathy prior to chemotherapy, uncontrolled seizure disorder, unstable cardiac disease or myocardial infarction within 6 months prior to study entry, being pregnant or nursing, or having used acupuncture for CIPN within 6 months prior to study entry.
Acupuncture Procedure
Acupuncture was administered by five experienced acupuncturists employing a traditional Chinese medicine style with a standardized protocol administered to all study participants 15. Acupuncture needles (Tai‐Chi, 0.20 × 25 mm, and 0.25 × 40 mm; Lhasa OMS, Inc., Weymouth, MA) were inserted into prespecified acupuncture points. Figure 1 presents the acupuncture protocol with main points. The depth of needling varied based on patient body sizes. After insertion, the needles were manually manipulated to obtain the De Qi sensation, which was defined as the acupuncturist feeling a tugging or grasping sensation from the needle manipulation and the patient feeling soreness, fullness, heaviness, or local distension at local needling sites. Manual acupuncture was used during the first week of study as an introductory phase. Starting in the second week, an electroacupuncture device (AWQ‐104 L, Lhasa OMS, Inc.) was connected with the needles at TW5 and second Baxie and/or SP6 and LR3 bilaterally, according to locations of CIPN symptoms, with alternating 2–10 Hz for 30 minutes each session. The targeted stimulation intensity reported by the participants was at 3 or 4 on a scale from 0 to 10 (0 being feeling nothing at all and 10 being the strongest one can imagine). An inferred heat lamp (TDP CQ‐27, Lhasa OMS, Inc.) was placed above the feet. The use of Qiduang was optional pending the tolerance of participants. A complete list of acupuncture points that were used is provided in the supplemental online material.
Figure 1.
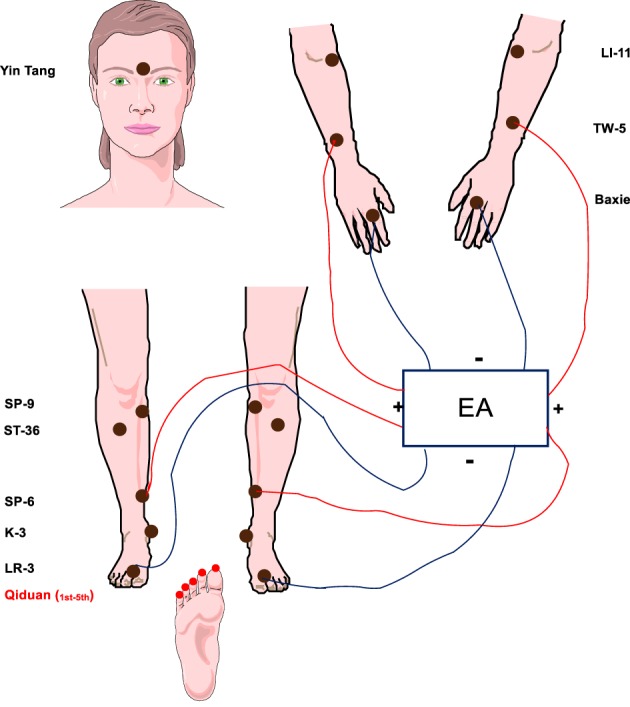
Acupuncture protocol for chemotherapy‐induced peripheral neuropathy. Abbreviation: EA, electroacupuncture.
Adverse effects of the acupuncture intervention were evaluated using NCI‐CTCAE version 4.03 at each visit.
Patient‐Reported Outcome Measures
Outcome measures were collected at baseline (before randomization) and 4, 8, and 16 weeks after enrollment. The primary endpoint was change in CIPN symptoms, which was assessed through PNQ, a questionnaire validated for diagnosis and quantification of CIPN 16, 17. The PNQ was chosen as the primary assessment of neuropathy symptoms based on its capacity to detect both sensory nerve deficit and interference of neuropathy symptoms on activities of daily living, as well as its use to assess CIPN symptoms in other controlled clinical trials in populations with breast cancer 16, 18. A recent review and Delphi survey also concluded that this measure provided a robust patient‐level assessment of neuropathy symptoms 19.
The PNQ consists of two items (sensory and motor) and assesses symptoms of paresthesia such as tingling, numbness, pain, and cold perception in the hands or feet. PNQ grades symptoms from grade A (no neuropathy) to grade E (very severe neuropathy). The PNQ sensory neuropathy grades have been correlated with NCI‐CTCAE sensory neuropathy grade (r = 0.58) and Functional Assessment of Cancer Therapy—Neurotoxicity subscale (FACT‐NTX; r = 0.51), and the time‐dependent distribution of PNQ motor neuropathy grade has been correlated with FACT‐NTX (r = 0.57) 20. Changes in PNQ subscale scores of one unit or greater are regarded as clinically significant 18.
CIPN‐specific quality of life (QOL) was assessed by the FACT‐NTX 11‐item subscale 21, 22. The FACT‐ NTX is a widely used, validated instrument that assesses the impact of neuropathy on health‐related QOL. An increase of five points or more on the FACT‐NTX is considered to be a clinically meaningful improvement in QOL 21, 22. Patients also completed the Brief Pain Inventory—short form (BPI‐SF), an instrument used to evaluate the severity of pain, including neuropathic pain, and the impact on the patient's daily functioning 23. Global QOL was assessed with the European Organisation for Research and Treatment of Cancer Quality‐of‐Life Questionnaire Core 30 (QLQ‐C30) instrument, which has been used widely to assess QOL in patients undergoing a variety of cancer treatments 24, 25. A mean difference of ten points or more is considered clinically significant 26.
Statistical Analysis and Sample Size Calculation
Randomization assignments were generated by the study statistician using a permuted block design. Randomization was stratified by patient age (≥60 years vs. <60 years). The primary endpoint of the study was response rate, defined as the proportion of patients with a one‐unit change in least one item of the PNQ between baseline and week 8. The target sample size was 40 patients. We anticipated a 20% loss from measurement between the time of consent and week 16, resulting in an analytic sample of 32 patients. Based upon prior work, we estimated that 75% of patients in the acupuncture arm would achieve a one‐unit improvement in PNQ scores 27. Fisher's exact test with a sample size of 16 patients per group and two‐sided type I error of .05 would have 83% power if the spontaneous recovery rate in the usual care arm were 20%.
The Wilcoxon rank‐sum test was used to compare changes in PNQ scores between the two intervention arms. PNQ scores were converted from A–E to a 0–4 scale, with 0 being no neuropathy and 4 being very severe neuropathy, for the purposes of these analyses. Power estimates assumed a common SD of 0.9 in the acupuncture and usual care arms 18. A sample of 32 patients (16 per group) would provide 80% power to detect a one‐unit difference in the change of PNQ scores between groups with a two‐sided, type I error rate of .05.
Data were analyzed according to the intention‐to‐treat principle. Baseline demographic, disease characteristics, prior treatment, and comorbidities were compared using the relevant descriptive methods for continuous and categorical variables. The response rate analysis used Fisher's exact test; continuous measures were compared between intervention arms using Wilcoxon rank‐sum tests.
Secondary endpoints include feasibility, defined as (A) the ability to enroll the target sample size and (B) the completion of at least 80% of the planned acupuncture sessions during the 16‐week period. Other secondary endpoints included changes in FACT‐NTX scores, BPI‐SF pain severity, pain interference, average pain, worst pain, and QLQ‐C30 from baseline to week 8.
Standard scoring algorithms were used for each of the assessments. A two‐sided p value less than or equal to .05 was considered statistically significant. All data were analyzed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Accrual and Baseline Patient Characteristics
Patients were enrolled between June 2014 and July 2016. Figure 2 shows the CONSORT diagram. Of 96 potentially eligible patients, 40 (41.2%) were enrolled and randomized (20 to acupuncture and 20 to waitlist control). The reasons for exclusion were refusal to participate (n = 46) and ineligibility (n = 10). Of 40 participants enrolled, two withdrew prior to treatment (5%), two withdrew during treatment (5%), and one withdrew because of physician decision (2.5%). Thirty‐five participants (87.5%) completed the required protocol intervention, and 33 participants had evaluable data at baseline and 8 weeks (16 acupuncture and 17 waitlist control).
Figure 2.
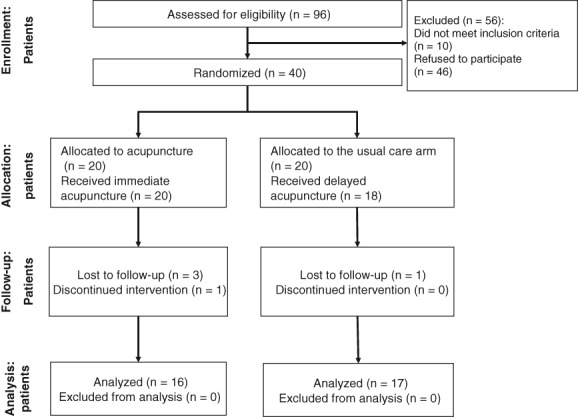
CONSORT diagram.
Patient characteristics are presented in Table 1. All participants were female. The median age was 54 years. The majority of participants were non‐Hispanic white and had grade 1–2 CIPN at baseline. Median time between study enrollment and completion of chemotherapy was 14 months (range 1–92 months). There were no differences between groups in demographic or clinical characteristics. At baseline, CIPN‐targeted medications were used in 82.5% of participants (Table 2). Use of anticonvulsants was slightly higher in the acupuncture arm versus the usual care arm (50% vs. 40%). Use of other agents was similar in both groups.
Table 1.
Baseline characteristics for patients in the acupuncture and usual care groups
| Characteristics | Acupuncture (n = 20), n (%) | Usual care (n = 20), n (%) |
|---|---|---|
| Age, median (range), years | 54.0 (32.0–68.0) | 53.5 (26.0–71.0) |
| Months between Dx and enrollment, median (range) | 17.3 (1.4–92.0) | 13.3 (5.3–92.4) |
| Sex: female | 20 (100) | 20 (100) |
| Race | ||
| White | 16 (80) | 15 (75) |
| Black | 2 (10) | 3 (15) |
| Asian | 2 (10) | 2 (10) |
| Stages | ||
| I | 4 (20) | 7 (35) |
| II | 11 (55) | 5 (25) |
| III | 5 (25) | 8 (40) |
| Hormone receptor status | ||
| ER− | 7 (35) | 6 (30) |
| ER+ | 13 (65) | 14 (70) |
| PR− | 7 (35) | 12 (60) |
| PR+ | 13 (65) | 8 (40) |
| Prior therapies | ||
| Mastectomy | 9 (45) | 9 (45) |
| Breast conservation | 11 (55) | 11 (55) |
| Anthracycline | 18 (90) | 13 (65) |
| Taxane | 20 (100) | 20 (100) |
| Trastuzumab | 6 (30) | 5 (25) |
| Radiation | 14 (70) | 17 (85) |
| Hormonal therapy | 11 (55) | 10 (50) |
| Pretreatment NCI‐CTCAE neuropathy grade | ||
| Grade 1 | 9 (45) | 9 (45) |
| Grade 2 | 9 (45) | 8 (40) |
| Grade 3 | 1 (5) | 0 (0) |
| Missing | 1 (5) | 3 (15) |
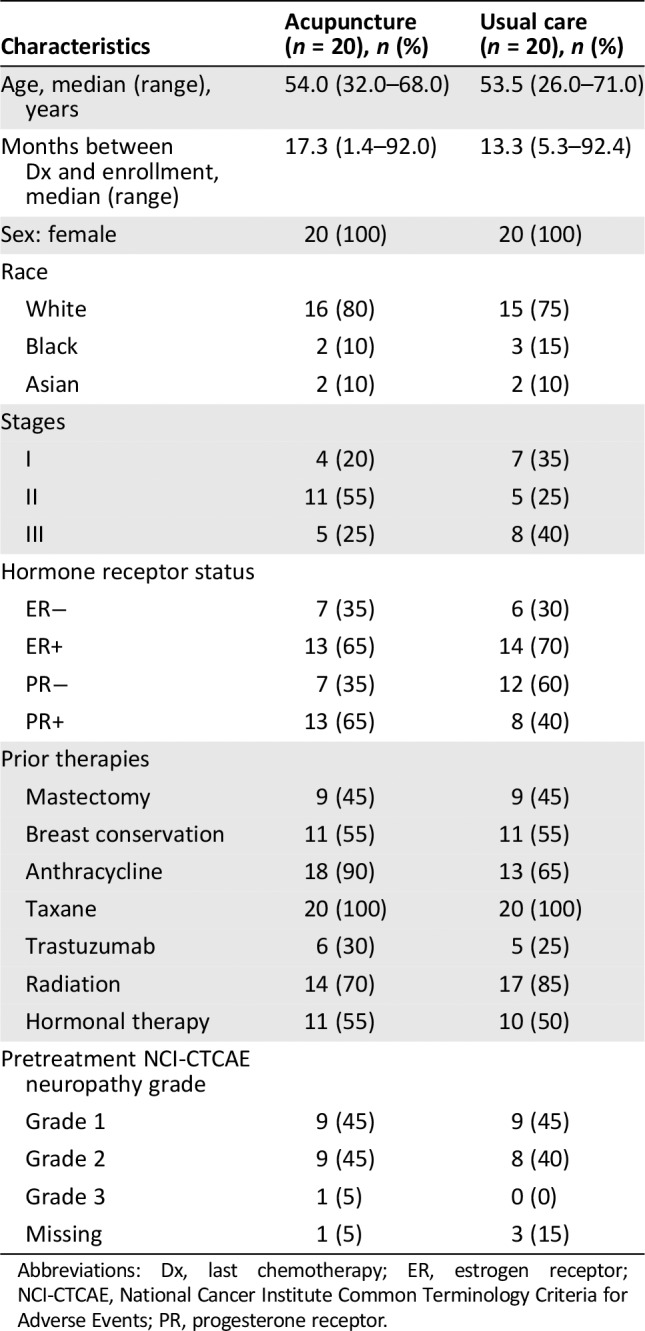
Abbreviations: Dx, last chemotherapy; ER, estrogen receptor; NCI‐CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; PR, progesterone receptor.
Table 2.
Use of CIPN‐targeted medications
| Time point, CIPN‐targeted medication | Acupuncture (n = 20), n (%) | Usual care (n = 20), n (%) |
|---|---|---|
| Baseline | ||
| Anticonvulsants | 10 (50) | 8 (40) |
| NSAIDs | 6 (30) | 7 (35) |
| Opioids | 2 (10) | 4 (20) |
| Antidepressants | 3 (15) | 3 (15) |
| Week 8 | ||
| Anticonvulsants | 10 (50) | 8 (40) |
| NSAIDs | 6 (30) | 7 (35) |
| Opioids | 2 (10) | 4 (20) |
| Antidepressants | 3 (15) | 3 (15) |
| Week 16 | ||
| Anticonvulsants | 10 (50) | 8 (40) |
| NSAIDs | 6 (30) | 7 (35) |
| Opioids | 2 (10) | 4 (20) |
| Antidepressants | 3 (15) | 3 (15) |
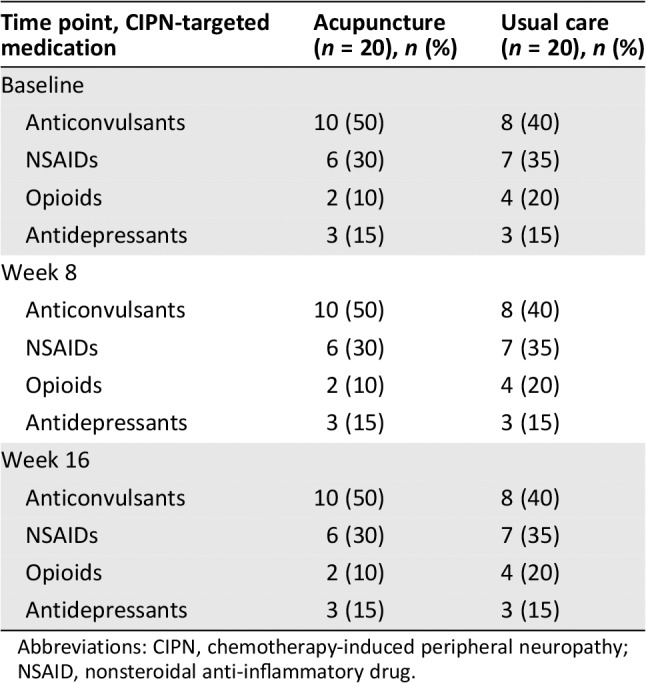
Abbreviations: CIPN, chemotherapy‐induced peripheral neuropathy; NSAID, nonsteroidal anti‐inflammatory drug.
Primary Outcome
CIPN Sensory and Motor Symptoms
Mean ± SD baseline PNQ sensory scores were 2.5 ± 0.8 in the acupuncture group and 2.5 ± 0.9 in the control group (p = .97; Table 3), corresponding to moderate levels of sensory neuropathy. Mean ± SD motor scores were 1.5 ± 1.1 in the acupuncture group and 1.8 ± 1.1 in the control group (p = .41).
Table 3.
Acupuncture for chemotherapy‐induced peripheral neuropathy, results of a randomized controlled trial
| Measure, time points | Acupuncture | Usual care | p value | ||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||
| Primary outcome: PNQ summary sensory score (0–4) | |||||
| Baseline | 20 | 2.5 ± 9.8 | 18 | 2.5 ± 0.9 | .97 |
| Changes at week 8 | 16 | −1.0 ± 0.9 | 17 | −0.3 ± 0.6 | .01 |
| Primary outcome: PNQ summary motor score (0–4) | |||||
| Baseline | 20 | 1.5 ± 1.1 | 18 | 1.8 ± 1.1 | .41 |
| Changes at week 8 | 16 | −0.5 ± 1.2 | 17 | 0.1 ± 1.1 | .19 |
| Secondary outcome: FACT‐NTX summary score (0–44) | |||||
| Baseline | 20 | 25.0 ± 8.4 | 18 | 22.1 ± 9.4 | .40 |
| Changes at week 8 | 16 | 8.7 ± 8.9 | 17 | 1.2 ± 5.4 | .002 |
| Secondary outcome: BPI‐SF pain severity (0–10) | |||||
| Baseline | 20 | 3.9 ± 1.6 | 18 | 3.7 ± 2.0 | .43 |
| Changes at week 8 | 14 | −1.1 ± 1.7 | 17 | 0.3 ± 1.5 | .03 |
| Secondary outcome: BPI‐SF pain interference (0–10) | |||||
| Baseline | 20 | 3.7 ± 2.1 | 18 | 3.2 ± 2.1 | .46 |
| Changes at week 8 | 15 | −1.2 ± 1.9 | 17 | 0.6 ± 1.6 | .01 |
| Secondary outcome: BPI‐SF average pain (0–10) | |||||
| Baseline | 20 | 4.5 ± 1.9 | 18 | 4.1 ± 2.3 | .55 |
| Changes at week 8 | 14 | −1.5 ± 2.0 | 17 | 0.2 ± 1.4 | .01 |
| Secondary outcome: EORTC QLQ‐C30 global health status (0–100) | |||||
| Baseline | 20 | 57.5 ± 17.9 | 18 | 57.4 ± 21.2 | .87 |
| Changes at week 8 | 16 | 12.0 ± 15.2 | 17 | 2.0 ± 12.3 | .03 |
Abbreviations: BPI‐SF, Brief Pain Inventory—short form; EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality‐of‐Life Questionnaire Core 30; FACT‐NTX, Functional Assessment of Cancer Therapy—Neurotoxicity subscale; PNQ, Patient Neurotoxicity Questionnaire.
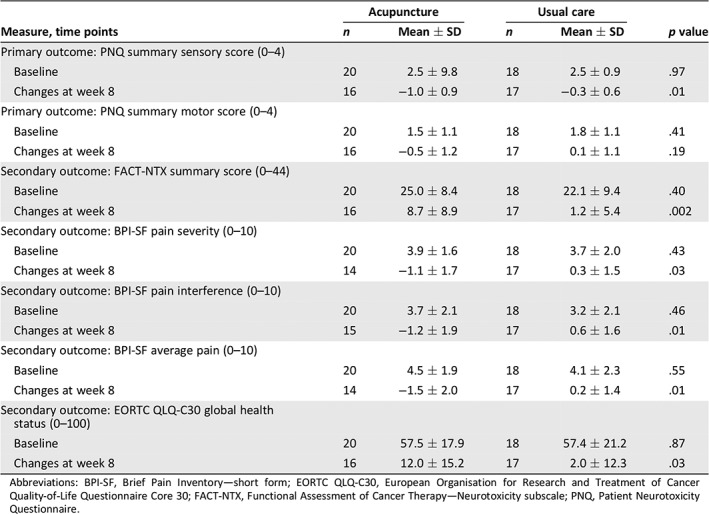
Over the initial 8‐week intervention period, the response rate (defined as a one‐unit change in sensory or motor score on the PNQ) in the acupuncture arm was 65% (95% confidence interval, 41%–85%) compared with 35% (95% confidence interval, 15%–59%) in the control arm (p = .11). The acupuncture intervention led to a significant reduction in CIPN sensory symptoms versus waitlist control (Fig. 3). The mean ± SD PNQ sensory score decreased by 1.0 ± 0.9 points between baseline and week 8 in the acupuncture group and by 0.3 ± 0.6 points in the control arm (p = .01). Mean PNQ motor scores were not significantly different in the two groups. At 8 weeks, 60% of the 20 participants assigned to the acupuncture group reported at least a one‐point improvement in PNQ sensory scores, and 35% reported at least a one‐point improvement in motor scores. Of the 20 participants assigned to the waitlist control group, 30% reported an improvement in sensory scores and 20% in motor scores.
Figure 3.
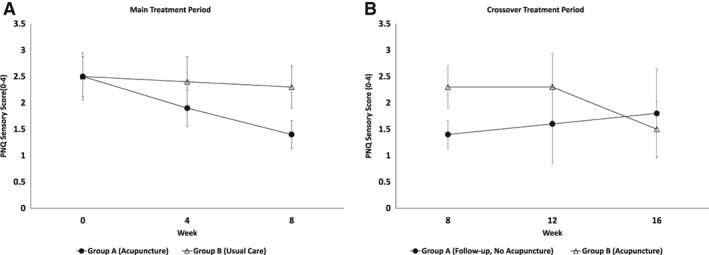
Changes in PNQ sensory scores between acupuncture and usual care in breast cancer with chemotherapy‐induced peripheral neuropathy during the main and crossover treatment periods. p = .01 at week 8; error bars represent 95% confidence intervals. Abbreviation: PNQ, Patient Neurotoxicity Questionnaire.
Secondary Outcomes
Feasibility
A total of 40 participants were randomized over the 2‐year enrollment period, meeting the planned sample size. Additionally, 87.5% of participants completed at least 80% of planned sessions, meeting the criterion established for feasibility.
CIPN‐Specific QOL
At 8 weeks, participants randomized to the acupuncture group had significant improvement in CIPN‐specific QOL compared with those in the control group (Fig. 4A). The mean ± SD change in the FACT‐NTX total score was 8.7 ± 8.9 in the acupuncture group versus 1.2 ± 5.4 in the usual care group (p = .002; Table 3), reflecting a 49.8% improvement from baseline in the acupuncture group versus 5.4% in the control group.
Figure 4.
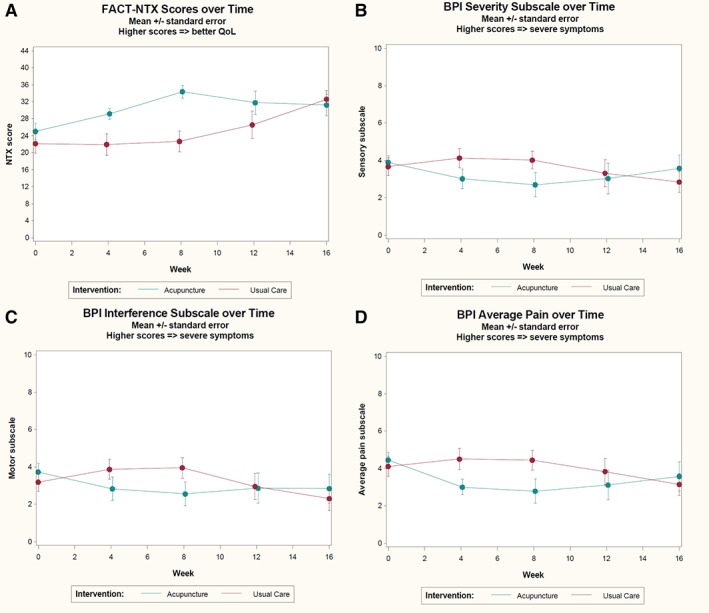
Changes of secondary outcomes in FACT‐NTX and BPI—short form scores between acupuncture and usual care groups during the main treatment period and crossover treatment periods. (A): FACT‐NTX scores; p = .002 at week 8. (B): BPI pain severity; p = .03 at week 8. (C): BPI pain interference; p = .01 at week 8. (D): BPI average pain; p = .01 at week 8. Abbreviations: BPI, Brief Pain Inventory; FACT‐NTX, Functional Assessment of Cancer Therapy—Neurotoxicity subscale; QoL, quality of life.
Neuropathic Pain and Medication Usage
At baseline, participants reported moderate level of neuropathic pain. Mean ± SD BPI‐SF pain severity scores were 3.9 ± 1.6 in the acupuncture group and 3.7 ± 2.0 in the control group (p = .55; Table 3). At 8 weeks, participants randomized to acupuncture reported significantly greater reductions in pain severity (p = .03), pain interference (p = .03), and average pain (p = .01) compared with participants randomized to the control group (Table 3; Figs. 4B, 4C, 4D). Use of medications to manage neuropathic symptoms did not change over time between baseline and week 8 or 16 in either study arm (Table 2).
Quality of Life
At baseline, participants in both groups reported similar scores in the global health status (GHS) subscale of QLQ‐C30 (acupuncture group: 57.5 ± 17.9, control group: 57.4 ± 21.2; Table 3). At week 8, participants randomized to acupuncture reported significantly improved GHS scores compared with participants in the control group (12.0 ± 15.2 vs. 2.0 ± 12.3 points; p = .03).
Assessments over Time
Figure 3 presents the PNQ sensory scores in the acupuncture and control groups over time. As described above, sensory scores improved significantly in the acupuncture group versus the control group during the initial 8‐week acupuncture intervention. Improvements in sensory scores in the immediate acupuncture group became apparent at week 4 of treatment. After discontinuation of acupuncture, sensory scores worsened gradually in the immediate intervention group, increasing by 0.4 points at 16 weeks.
Participants randomized to the control arm received a low‐intensity acupuncture intervention (twice weekly acupuncture in week 1, then weekly sessions over the following 7 weeks) between weeks 8 and 16. Over this time period, waitlist participants experienced a 0.8 ± 0.5 point improvement in PNQ sensory scores (from 2.3 ± 0.8 to 1.5 ± 0.9; p = .007). Improvements in sensory scores were not apparent until 4 weeks after acupuncture treatment. Similar patterns were seen in other secondary measures (Fig. 4).
Adverse Events
There were no serious adverse events reported in response to the acupuncture intervention in either the immediate acupuncture group or in the waitlist control group. Two participants (one in each group) reported mild reactions that were possibly related to the acupuncture: one developed grade 1 pruritis in the feet, and one developed grade 2 joint pain.
Discussion
In this randomized pilot trial of 40 women with persistent CIPN symptoms after completing chemotherapy for early breast cancer, an acupuncture intervention reduced sensory symptoms compared with a waitlist control. Acupuncture also led to clinically meaningful reductions in pain intensity and pain interference, as well as improvements in CIPN‐specific and general quality of life. The change scores of multiple outcomes, including PNQ, BPI‐SF, FACT‐NTX, and GHS, all exceeded the benchmark of half an SD as clinical significance 28, 29. Exploratory analyses suggested that a lower‐intensity acupuncture program also led to clinically meaningful reductions in sensory CIPN symptoms, but effects may have taken longer to occur. Furthermore, this pilot trial demonstrated that it is feasible and safe to recruit and retain breast cancer survivors with CIPN into a randomized controlled acupuncture trial. The acupuncture protocol used in the study demonstrated preliminary but promising results for breast cancer survivors with chronic, moderate CIPN.
A few other studies have evaluated the impact of acupuncture on CIPN symptoms in patients with breast and other cancer 30, 31. In a 2013 systematic review 31, Franconi et al. identified seven acupuncture trials for management or prevention of CIPN in patients with mixed cancer, including three randomized trials. One randomized controlled trial enrolled patients with mixed lung, breast, or ovarian cancer who developed CIPN after paclitaxel or oxaliplatin treatment (n = 64). Participants were randomized to receive acupuncture (ten sessions) versus daily adenosylcobalamin injections for 2 weeks. At the end of the trial, the patients in the acupuncture arm reported a response rate (one point or greater reduction in PNQ score) of 66.7% versus 40.0% in the adenosylcobalamin arm 27. Another recently published single‐arm trial (n = 27), assessing acupuncture's efficacy in preventing the escalation of grade II CIPN to grade III CIPN in patients with breast cancer undergoing paclitaxel chemotherapy 11, found that 26 out of 27 participants did not develop grade III CIPN. These early studies provide preliminary evidence that acupuncture may help to reduce CIPN symptoms, but additional work is clearly needed.
A number of pharmacological agents have also been evaluated as treatment for CIPN, including acetyl‐l‐carnitine, tricyclic antidepressants, and gabapentin 32. Results of these studies have been mixed. Smith et al. reported a randomized double‐blind crossover trial using duloxetine versus placebo for CIPN‐related neuropathic pain (n = 231) in a mixed group of cancer survivors 33. Participants in the duloxetine arm reported a significant reduction in BPI‐SF average pain compared with the placebo arm (mean change score, 1.06 vs. 0.34; p = .03) at the end of a 5‐week intervention. A recent review of 15 NCI‐sponsored clinical trials found that only duloxetine demonstrated therapeutic benefit for patients with CIPN 34. In keeping with these findings, guidelines from the American Society of Clinical Oncology and the Oncology Nursing Society conclude that a moderate level of evidence supports the use of duloxetine for management of CIPN but do not recommend other pharmacologic treatments to manage this condition, highlighting the need for more effective CIPN therapies 8, 35.
Despite these promising results, our randomized pilot study has several limitations. First, we compared the effect of an acupuncture intervention to a waitlist control on self‐reported CIPN symptoms, rather than including an active control group such as sham acupuncture. For this reason, we are not able to exclude the possibility that the placebo effect accounted for at least some of the improvement in CIPN symptoms seen with the acupuncture treatment. Second, our small sample size limits our ability to assess factors associated with improvements in both the intervention and control groups. Additionally, our intervention duration was relatively short (8 weeks), and we are thus not able to determine the duration of the effect of our intervention. Our trial also enrolled patients with CIPN at any point after the completion of chemotherapy treatment. Other trials have largely required that patients have persistent CIPN symptoms for at least 3–6 months after the completion of treatment. In our trial, the median time between study enrollment and the completion of chemotherapy was 14 months. Thus, most patients did have persistent neuropathy symptoms over time, but it is possible that improvements in patients enrolled closer to the completion of chemotherapy may have been independent of the acupuncture intervention. Additionally, because the majority of our enrolled participants had mild to moderate levels of CIPN, we are not able to determine the effectiveness of our intervention in patients with severe CIPN. Finally, our study only enrolled women with a history of early‐stage breast cancer who had completed adjuvant treatment with taxane‐based therapies, limiting the generalizability of these study findings.
Conclusion
This randomized pilot trial demonstrated that an 8‐week acupuncture intervention, versus usual care, led to clinically meaningful and statistically significant improvements in neuropathic sensory symptoms in breast cancer survivors with mild and moderate CIPN after the completion of chemotherapy. Given the significant, often long‐lasting, impact of CIPN on quality of life and functional status in breast cancer survivors, as well as the lack of effective treatments to mitigate CIPN symptoms in this and other cancer populations, this study could provide preliminary evidence laying the groundwork for a large‐scale trial testing the impact of acupuncture on CIPN symptoms, quality of life, and functional measures in cancer patients.
Author Contributions
Conception/design: Weidong Lu, Anita Giobbie‐Hurder, Im Hee Shin, Jennifer A. Ligibel
Provision of study material or patients: Weidong Lu, Rachel A. Freedman, Nancy U. Lin, Ann H. Partridge, David S. Rosenthal, Jennifer A. Ligibel
Collection and/or assembly of data: Weidong Lu, Anita Giobbie‐Hurder
Data analysis and interpretation: Weidong Lu, Anita Giobbie‐Hurder, Jennifer A. Ligibel
Manuscript writing: Weidong Lu, Anita Giobbie‐Hurder, Rachel A. Freedman, Im Hee Shin, Nancy U. Lin, Ann H. Partridge, David S. Rosenthal, Jennifer A. Ligibel
Final approval of manuscript: Weidong Lu, Jennifer A. Ligibel
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1
Acknowledgments
This study was supported by The Comprehensive and Integrative Medicine Institute (CIMI), South Korea (Grant Number: 090‐091‐3000‐3038‐301‐320‐01).
We thank the study acupuncturists: Zhi Ping Li, Grant Hou, Liying Wu, Joy Yue Zhang, Dongyan Yu, and research staff: Laura Shockro, Kelly Stecker, and Keelin A. O'Connor.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Seretny M, Currie GL, Sena ES et al. Incidence, prevalence, and predictors of chemotherapy‐induced peripheral neuropathy: A systematic review and meta‐analysis. Pain 2014;155:2461–2470. [DOI] [PubMed] [Google Scholar]
- 2. Bao T, Basal C, Seluzicki C et al. Long‐term chemotherapy‐induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016;159:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon NB, Danso MA, Alberico TA et al. The prevalence and pattern of chemotherapy‐induced peripheral neuropathy among women with breast cancer receiving care in a large community oncology practice. Qual Life Res 2017;26:2763–2772. [DOI] [PubMed] [Google Scholar]
- 4. Bandos H, Melnikow J, Rivera DR et al. Long‐term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG oncology/NSABP B‐30. J Natl Cancer Inst 2018;110:djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanabe Y, Hashimoto K, Shimizu C et al. Paclitaxel‐induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int J Clin Oncol 2013;18:132–138. [DOI] [PubMed] [Google Scholar]
- 6. Winters‐Stone KM, Horak F, Jacobs PG et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy‐induced peripheral neuropathy. J Clin Oncol 2017;35:2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolb NA, Smith AG, Singleton JR et al. The association of chemotherapy‐induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 2016;73:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hershman DL, Lacchetti C, Dworkin RH et al. Prevention and management of chemotherapy‐induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:1941–1967. [DOI] [PubMed] [Google Scholar]
- 9. Ezzo J, Vickers A, Richardson MA et al. Acupuncture‐point stimulation for chemotherapy‐induced nausea and vomiting. J Clin Oncol 2005;23:7188–7198. [DOI] [PubMed] [Google Scholar]
- 10. Hershman DL, Unger JM, Greenlee H et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early‐stage breast cancer: A randomized clinical trial. JAMA 2018;320:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao T, Seidman AD, Piulson L et al. A phase IIa trial of acupuncture to reduce chemotherapy‐induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer 2018;101:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia MK, Cohen L, Guo Y et al. Electroacupuncture for thalidomide/bortezomib‐induced peripheral neuropathy in multiple myeloma: A feasibility study. J Hematol Oncol 2014;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molassiotis A, Suen LKP, Cheng HL et al. A randomized assessor‐blinded wait‐list‐controlled trial to assess the effectiveness of acupuncture in the management of chemotherapy‐induced peripheral neuropathy. Integr Cancer Ther 2019;18:1534735419836501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han X, Wang L, Shi H et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy‐induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer 2017;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacPherson H, White A, Cummings M et al. Standards for reporting interventions in controlled trials of acupuncture: The STRICTA recommendations. J Altern Complement Med 2002;8:85–89. [DOI] [PubMed] [Google Scholar]
- 16. Shimozuma K, Ohashi Y, Takeuchi A et al. Feasibility and validity of the patient neurotoxicity questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N‐SAS BC 02. Support Care Cancer 2009;17:1483–1491. [DOI] [PubMed] [Google Scholar]
- 17. Hausheer FH, Schilsky RL, Bain S et al. Diagnosis, management, and evaluation of chemotherapy‐induced peripheral neuropathy. Semin Oncol 2006;33:15–49. [DOI] [PubMed] [Google Scholar]
- 18. Shimozuma K, Ohashi Y, Takeuchi A et al. Taxane‐induced peripheral neuropathy and health‐related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N‐SAS BC 02, a randomized clinical trial. Support Care Cancer 2012;20:3355–3364. [DOI] [PubMed] [Google Scholar]
- 19. McCrary JM, Goldstein D, Boyle F et al. Optimal clinical assessment strategies for chemotherapy‐induced peripheral neuropathy (CIPN): A systematic review and delphi survey. Support Care Cancer 2017;25:3485–3493. [DOI] [PubMed] [Google Scholar]
- 20. Kuroi K, Shimozuma K, Ohashi Y et al. Prospective assessment of chemotherapy‐induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP‐HOR 02 study). Support Care Cancer 2009;17:1071–1080. [DOI] [PubMed] [Google Scholar]
- 21. Cella D, Peterman A, Hudgens S et al. Measuring the side effects of taxane therapy in oncology: The Functional Assesment of Cancer Therapy‐Taxane (FACT‐Taxane). Cancer 2003;98:822–831. [DOI] [PubMed] [Google Scholar]
- 22. Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 23. Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore 1994;23:129–138. [PubMed] [Google Scholar]
- 24. Aaronson N, Ahmedzia S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 25. Osoba D, Zee B, Peter J et al. Psychometric properties and responsiveness of the EORTC Quality of Life Questionnaire (QLQ‐C30) in patients with breast, ovarian and lung cancer. Qual Life Res 1994;3:353–364. [DOI] [PubMed] [Google Scholar]
- 26. Cocks K, King MT, Velikova G et al. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur J Cancer 2008;44:1793–1798. [DOI] [PubMed] [Google Scholar]
- 27. Xu WR, Hua BJ, Hou W et al. Clinical randomized controlled study on acupuncture for treatment of peripheral neuropathy induced by chemotherapeutic drugs [in Chinese]. Zhongguo Zhen Jiu 2010;30:457–460. [PubMed] [Google Scholar]
- 28. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: The remarkable universality of half a standard deviation. Med Care 2003;41:582–592. [DOI] [PubMed] [Google Scholar]
- 29. Wong K, Zeng L, Zhang L et al. Minimal clinically important differences in the brief pain inventory in patients with bone metastases. Support Care Cancer 2013;21:1893–1899. [DOI] [PubMed] [Google Scholar]
- 30. Li K, Giustini D, Seely D. A systematic review of acupuncture for chemotherapy‐induced peripheral neuropathy. Curr Oncol 2019;26:e147–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franconi G, Manni L, Schroder S et al. A systematic review of experimental and clinical acupuncture in chemotherapy‐induced peripheral neuropathy. Evid Based Complement Alternat Med 2013;2013:516916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gewandter JS, Freeman R, Kitt RA et al. Chemotherapy‐induced peripheral neuropathy clinical trials: Review and recommendations. Neurology 2017;89:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith EML, Pang H, Cirrincione C et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy‐induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013;309:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Majithia N, Temkin SM, Ruddy KJ et al. National Cancer Institute‐supported chemotherapy‐induced peripheral neuropathy trials: Outcomes and lessons. Support Care Cancer 2016;24:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Visovsky C, Collins M, Abbott L et al. Putting evidence into practice: Evidence‐based interventions for chemotherapy‐induced peripheral neuropathy. Clin J Oncol Nurs 2007;11:901–913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1


