Abstract
Thymomas comprise a group of rare epithelial neoplasms of the anterior mediastinum. Whereas localized disease carries a favorable prognosis, the majority of patients with metastatic thymomas experience progression or recurrence over a 10‐year period. Although targeted therapies have become standard of care in many malignancies, no clinically actionable mutations have consistently been identified in metastatic thymomas. Here, we describe a patient with an aggressive thymoma complicated by extensive pleural metastases. Over a 16‐year period, she progressed on multiple treatment regimens. To identify additional treatment options, tissue from a pleural metastasis was sent for next‐generation sequencing, revealing mutations in BRCA2, tyrosine kinase 2, and SET domain containing 2. Based on supporting evidence for poly (ADP‐ribose) polymerase (PARP) inhibition in other BRCA‐mutated tumors, the patient was started on the PARP inhibitor olaparib. She derived significant clinical benefit from treatment, with imaging showing overall stabilization of her disease. Here, we review the genotyping results of her tumor and discuss the functional and clinical significance of the mutations in her cancer as well as implications for managing patients with advanced BRCA‐mutant thymomas.
Key Points
Targeted therapy has yet to enter the standard clinical management of metastatic thymomas.
Patients with BRCA2‐mutant thymomas may benefit from poly (ADP‐ribose) polymerase inhibition.
Short abstract
The case of a patient with aggressive thymoma is described, including the genotyping results of her tumor, the functional and clinical significance of the mutations in her cancer, and the implications for managing patients with advanced BRCA‐mutant thymomas.
Patient Story
A 60‐year‐old woman with a history of autoimmune hepatitis was diagnosed with both thymoma (World Health Organization type B) and papillary thyroid cancer in September 2003. At this time, she underwent resection of the thymoma as well as thyroidectomy followed by iodine‐131 therapy for her thyroid cancer. Subsequent positron emission tomography imaging revealed mediastinal lymphadenopathy that was treated with external beam radiation. Two years after diagnosis, imaging identified a solitary left pleural lesion pathologically confirmed to be metastatic thymoma. When the lesion began enlarging after another 2 years, she was treated with sandostatin LAR and prednisone. Over the next 10 years, she was treated with multiple regimens, including a combination of carboplatin and etoposide, single‐agent gemcitabine, CAP (cyclophosphamide, doxorubicin, and cisplatin), single‐agent pemetrexed, capecitabine, and single‐agent everolimus. She was also treated with the anti‐insulin‐like growth factor 1 receptor antibody cixutumumab on a clinical trial. Although cixutumumab halted significant progression for nearly 2 years, in each case she eventually progressed on treatment and ultimately developed extensive, bulky pleural metastases (Fig. 1).
Figure 1.
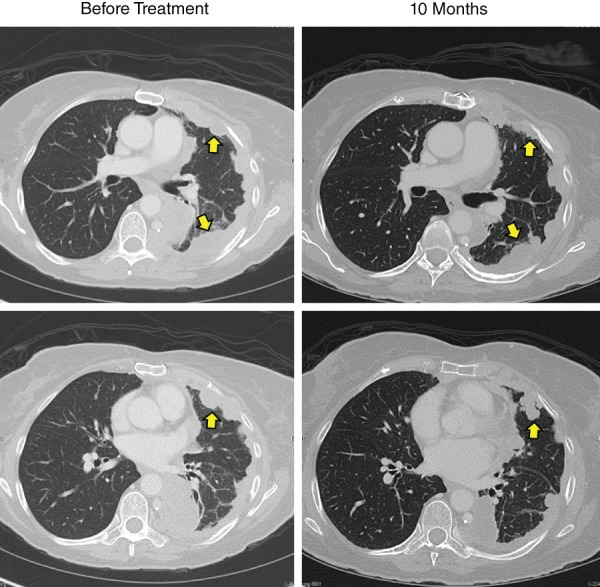
Computed tomography (CT) image of the chest before and after treatment. Baseline chest CT imaging showing extensive metastatic pleural‐based nodules on the left (arrows) from metastatic thymoma, and overall stable disease after 10 months on olaparib.
To identify additional treatment options, a sample of her tumor was sent for FoundationOne next‐generation sequencing (NGS), which identified mutations in BRCA2, tyrosine kinase 2 (TYK2), and SET domain containing 2 (SETD2). Although neither TYK2 nor SETD2 mutations are clinically actionable, cancers harboring inactivation mutations in BRCA1 or BRCA2 can respond to poly (ADP‐ribose) polymerase (PARP) inhibitors 1. Thus, the patient was started on olaparib and has remained on treatment for 14 months with overall stable disease. The patient had some reduction in tumor size (approximate of 20% reduction in the sum of the longest diameter for all target lesions); however, this did not meet RECIST criteria for a partial response (Fig. 1). Because of toxicity of nausea and fatigue, the patient has remained on dose‐reduced olaparib and has since been able to wean down her pain regimen for management of pleural‐based cancer pain.
Molecular Tumor Board
Precision medicine has become standard of care in several cancers. Tyrosine kinase inhibitors targeting the BCR‐ABL fusion protein have revolutionized the treatment of chronic myeloid leukemia 2, and many therapies targeting molecular alterations have made an impact in breast cancer 3, non‐small cell lung cancer, and others. Comprehensive genomic profiling is also changing the treatment paradigm toward molecularly targeted therapies, regardless of histology. For instance, a trial of 1,144 patients with any solid tumor evaluated in a large phase I program showed that patients harboring distinct molecular aberrations treated with a matched targeted therapy had significant improvements in overall response rates, time to treatment failure, and overall survival 4. Although a patient with thymic carcinoma overexpressing mutant KIT responded to imatinib 5, such approaches have yet to enter the management of metastatic thymomas. Although thymic epithelial tumors frequently harbor mutations in GTF2I, HRAS, NRAS, and TP53, none of these mutations are clinically actionable at this time 6. Similarly, although thymic epithelial tumors can be clustered into four genetic subtypes (38% GTF2I mutant, 33% T‐cell enriched, 21% chromosome unstable, 8% chromosome stable), these groupings have yet to influence clinical practice 7.
Genotyping Results and Interpretation of Molecular Results
The FoundationOne Heme (Foundation Medicine, Cambridge, MA) assay is recommended by the manufacturer for thymomas, and it uses whole‐genome shotgun library construction and hybrid‐capture NGS to provide a focused interrogation of 406 genes and 31 introns, 265 RNAs associated with gene fusions, and information regarding the tumor mutational burden and microsatellite instability status. Briefly, massively parallel sequencing is done using 50–200 ng of DNA from a formalin‐fixed paraffin‐embedded tissue specimen. Hybrid‐capture–selected libraries are sequenced to high uniform depth. FoundationOne sequences patient tissues to a median depth of approximately 500× unique coverage for DNA and RNA to an average of ∼6.9 million unique pairs (targeting >500× coverage by non–polymerase chain reaction duplicate read pairs, with >99% of exons at coverage >100×) using the Illumina HiSeq 2500 platform (Illumina, San Diego, CA, https://www.illumina.com). The panel identifies base substitutions, insertions and deletions, copy number alterations, and rearrangements 8. In our patient, the results revealed three distinct mutations: TYK2 V15A, SETD2 T1652fs*14, and BRCA2 K1800fs*16.
Although each of these genes has been investigated in other cancers, none has a clearly defined role in thymic cancers. For instance, TYK2 encodes for a nonreceptor JAK family tyrosine kinase with contradictory roles in inflammation. Although TYK2 is involved in interleukin (IL)‐12 and interferon signaling, it also acts downstream of IL‐10, and Tyk2 knockout mice are unable to fully generate or respond to IL‐10 9, 10. In cancer, the effects of TYK2 are similarly dichotomous. Although TKY2 is seemingly required for oncogenic fibroblast growth factor and epidermal growth factor signaling, TYK2 inhibition also appears to impede anticancer CD8 responses 11. Interestingly, in murine models of allografted thymoma cells, genetic deletion of TYK2 accelerated tumor development largely because of defects in CD8‐mediated cytotoxicity 12. Although interesting, the TYK2 V15A mutation has not been described in metastatic thymoma and its clinical significance is unknown.
SETD2 is a histone methyltransferase specific for lysine‐36 of histone H3, and it is generally considered a tumor suppressor gene 13. SETD2 is frequently altered in other malignancies, including approximately 30% of pediatric high‐grade gliomas and 15% of clear cell renal cell carcinomas 14, 15. In breast cancer, SETD2 is often downregulated, and lower SETD2 mRNA levels have been associated with both local and distant recurrences and poor survival 16. Similarly, in B‐cell acute lymphoblastic leukemia, loss of SETD2 is associated with chemotherapy resistance through loss of DNA damage recognition and impaired apoptotic signaling 17. Although the specific SETD2 T1652fs*14 frame‐shift mutation observed in our patient has not been described, it occurs in the coding region for the SET domain (AA 1561‐1667) and is likely analogous to the oncogenic SETD2 R1625C mutation 18. However, the clinical significance of this mutation is unclear at this time.
Finally, our patient's tumor harbored a BRCA2 K1800fs*16 frame‐shift mutation with presumptive loss of function. After learning this, our patient was referred to a hospital geneticist. A blood sample was sent for BRCA2 sequencing (±20 base pairs of adjacent intronic sequence) through Invitae, and this mutation was found to be germline, although the patient had no personal or family history of BRCA‐associated cancers. Whereas the role of BRCA2 in thymic cancers is largely unexplored, familial BRCA1 and BRCA2 mutations have been well described in breast and ovarian cancers. Although familial BRCA mutations drive only a relatively small percentage of these cancers, women with an inherited BRCA1 mutation have a lifetime risk of 65%–80% of developing breast cancer and 37%–62% for ovarian cancer. Similarly, women carrying familial BRCA2 mutations have a lifetime risk of 45%–85% for breast cancer and 11%–23% for ovarian cancer 19. BRCA1 and BRCA2 are established tumor suppressor genes with distinct but important roles in DNA damage response and repair pathways 20. Given the strong evidence supporting the use of PARP inhibitors in BRCA‐mutant ovarian cancers 1, our patient was started on olaparib.
Functional and Clinical Significance of the Specific Mutation in Thymic Cancer
Little is known regarding the role of BRCA mutations in thymic cancers. To date, there has only been one reported case of a BRCA1‐mutant metastatic thymoma, although this patient also developed thyroid and ovarian cancers during her lifetime 21. A similar report described a family with frequent thymomas, potentially attributed to a germline translocation of the BRCA2‐associated DNA repair protein RAD51B 22. Another family was reported to have high rates of BRCA‐linked breast/ovarian cancers and thymomas, although there was insufficient evidence to suggest a familial cancer syndrome caused by a single unifying mutation 23. Of note, one series of 72 patients with thymoma or thymic carcinoma identified 1 patient with an ataxia‐telangiectasia mutated (ATM) gene mutation, which can affect DNA repair mechanisms similar to BRCA mutations 24. Interestingly, deletion of ATM in vivo is associated with spontaneous thymoma formation, whereas restoration of ATM expression reverses this phenotype 25.
Despite these observations, the frequency of BRCA mutations in thymomas is unknown. We therefore evaluated the established The Cancer Genome Atlas (TCGA) genomic database for mutations in either BRCA1 or BRCA2 using cBioportal 26, 27. Of 123 thymoma patients, none had a BRCA1 mutation, and two (1.61%) had mutations in BRCA2 (Table 1). Of the observed BRCA2 mutations, one consisted of a single amino acid substitution of unknown significance (M3118V) at the oligonucleotide/oligosaccharide‐binding domain 3 (AA: 3052 – 3190). The second mutation was another substitution (R2336C) analogous to the oncogenic R2336H/P mutations 28, 29. Our patient displayed a frame‐shift mutation at the 1800 position (Fig. 2). While this particular mutation has not been characterized, several similar BRCA2 frame‐shift mutations have been identified in breast and ovarian cancers, many leading to missing or nonfunctional proteins 30. However, given the small sample size of the TCGA cohort, the overall rate of oncogenic BRCA mutations in patients with thymomas remains unclear. Given the efficacy of olaparib in our patient, PARP inhibition in BRCA‐mutated advanced thymomas warrants further investigation.
Table 1.
Frequency of BRCA mutations in breast, ovarian, and thymic cancers in genomic databases
| Data set | Sample size | BRCA1 mutation | BRCA2 mutation | Combined |
|---|---|---|---|---|
| Breast Cancer (MSK) | 1,918 | 1.30% | 3.18% | 4.38% |
| Breast Cancer (METABRIC) | 2,506 | 1.68% | 1.80% | 3.43% |
| Breast Cancer (TCGA) | 1,066 | 2.53% | 2.72% | 5.07% |
| Ovarian Cancer (TCGA) | 585 | 3.08% | 2.56% | 5.64% |
| Thymoma (TCGA) | 124 | 0% | 1.61% | 1.61% |
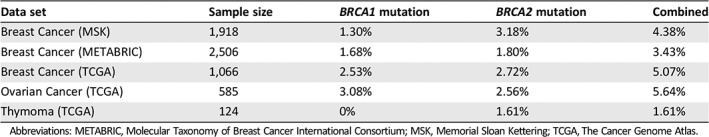
Abbreviations: METABRIC, Molecular Taxonomy of Breast Cancer International Consortium; MSK, Memorial Sloan Kettering; TCGA, The Cancer Genome Atlas.
Figure 2.
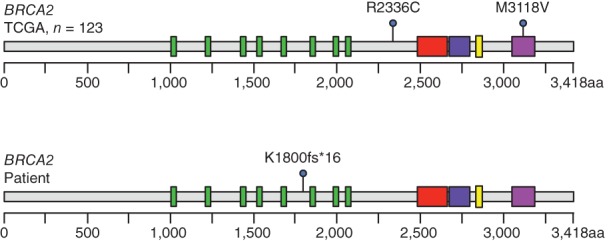
Known BRCA2 mutations in patients with thymomas. Using the TCGA genomic dataset, we evaluated the known mutations of BRCA1 and BRCA2 in 123 patients with thymomas. Two patients harbored point mutations leading to single amino acid substitutions either at R2236C or M3118V at the oligonucleotide/oligosaccharide‐binding, domain 3. In contrast, our patient had a frameshift mutation at the K1800 position, presumptively causing loss of function. Abbreviation: TCGA, The Cancer Genome Atlas.
Potential Strategies to Target the Pathway and Implications for Clinical Practice
The mechanistic link between BRCA and PARP inhibition is well established. In brief, PARP is critical for single‐strand break repair (SSBR) 31. As such, PARP inhibitors such as olaparib prevent SSBR, leading to double strand breaks (DSBs). As BRCA1 and BRCA2 play key roles in DSB repair, namely, through homologous recombination 32, the use of olaparib in BRCA‐mutant cancers leads to the accumulation of irreparable DSBs, ultimately causing cell death (Fig. 3). As discussed, this approach has shown significant efficacy in BRCA‐mutant ovarian and breast cancers. Olaparib became the first Food and Drug Administration (FDA)‐approved PARP inhibitor for patients with ovarian cancer with germline BRCA mutations in 2014. This was based on results from Study 19, a randomized, placebo‐controlled trial showing a statistically significant improvement in both progression‐free and overall survival 33. Three PARP inhibitors, olaparib, rucaparib, and niraparib, have now been FDA approved as maintenance therapy after platinum‐based chemotherapy in patients with ovarian cancer with either germline or somatic BRCA mutations or homologous recombination deficiency 34, 35, 36. Olaparib was also FDA approved in 2018 for germline BRCA‐mutated breast cancer based on the randomized, phase III OlympiAD trial showing an improved response rate (59.9% vs. 28.8%) and progression‐free survival (7.0 vs. 4.2 months) compared with standard therapy 37.
Figure 3.
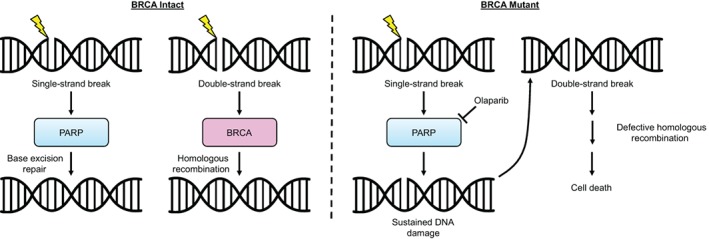
Schema describing the efficacy of PARP inhibition in BRCA‐deficient tumor cells. Under physiologic conditions, DNA damage causing single‐strand breaks (SSBs) leads to the rapid synthesis and recruitment of PARP. This promotes base excision repair, leading to repair of SSBs. In the case of double‐strand breaks (DSBs), BRCA proteins interact with a variety of additional factors to promote repair via homologous recombination. In the setting of BRCA deficiency, the repair of DSBs is significantly impaired. Therefore, when PARP is inhibited with olaparib, cells lack the ability to repair SSBs, leading to the accumulation of DSBs. With no means of repairing these DSBs, cells eventually undergo programmed cell death. Abbreviation: PARP, poly (ADP‐ribose) polymerase.
Additionally, emerging evidence suggests there are additional mutations that may be indicative of homologous recombination deficiencies. These include alterations in ATM, BARD1, BRIP1, CHEK2, FAAP20, FAN1, FANCE, FANCM, PALB2, POLQ, RAD51B, RAD51C, and RAD51D 38, 39, 40. Several of these mutations, namely, ATM and PALB2, have correlated with responsiveness to PARP inhibition in other malignancies including metastatic prostate cancer 41. Given these results, and those seen in our patient, PARP inhibition warrants further investigation in other malignancies with germline or somatic BRCA mutations and potentially other mutations in DNA repair pathways.
Patient Update
After 14 months on treatment, the patient reports that her pain is under control without new chest/abdominal pain or other related symptoms. The first 3 months on olaparib were complicated by nausea and fatigue, but after a dose adjustment, the patient is tolerating treatment well. Her most recent imaging demonstrates overall stable disease with no new pleural‐based nodules.
Glossary of Genomic Terms and Nomenclature
DSBs: double‐strand breaks
NGS: next‐generation sequencing
SSBs: single strand breaks
SSBR: single‐strand break repair
Author Contributions
Conception/design: Daniel R. Principe, Suneel D. Kamath, Hidayatullah G. Munshi, Nisha A. Mohindra
Provision of study material or patients: Daniel R. Principe, Suneel D. Kamath, Hidayatullah G. Munshi, Nisha A. Mohindra
Collection and/or assembly of data: Daniel R. Principe, Suneel D. Kamath, Hidayatullah G. Munshi, Nisha A. Mohindra
Data analysis and interpretation: Daniel R. Principe, Suneel D. Kamath, Hidayatullah G. Munshi, Nisha A. Mohindra
Manuscript writing: Daniel R. Principe, Suneel D. Kamath, Hidayatullah G. Munshi, Nisha A. Mohindra
Final approval of manuscript: Daniel R. Principe, Suneel D. Kamath, Hidayatullah G. Munshi, Nisha A. Mohindra
Disclosures
The authors indicated no financial relationships.
Acknowledgments
We thank our patient for giving us the permission to include her clinical information in this report, and we wish her well in her continued recovery. This work was supported by NIH F30CA236031 to D.R.P.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Konecny GE, Kristeleit RS. PARP inhibitors for BRCA1/2‐mutated and sporadic ovarian cancer: Current practice and future directions. Br J Cancer 2016;115:1157–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. An X, Tiwari AK, Sun Y et al. BCR‐ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: A review. Leuk Res 2010;34:1255–1268. [DOI] [PubMed] [Google Scholar]
- 3. Maximiano S, Magalhaes P, Guerreiro MP et al. Trastuzumab in the treatment of breast cancer. BioDrugs 2016;30:75–86. [DOI] [PubMed] [Google Scholar]
- 4. Tsimberidou AM, Iskander NG, Hong DS et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin Cancer Res 2012;18:6373–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strobel P, Hartmann M, Jakob A et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med 2004;350:2625–2626. [DOI] [PubMed] [Google Scholar]
- 6. Radovich M, Pickering CR, Felau I et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell 2018;33:244–258 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee HS, Jang HJ, Shah R et al. Genomic analysis of thymic epithelial tumors identifies novel subtypes associated with distinct clinical features. Clin Cancer Res 2017;23:4855–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He J, Abdel‐Wahab O, Nahas MK et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood 2016;127:3004–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw MH, Freeman GJ, Scott MF et al. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL‐10 signaling and promoting IFN‐gamma‐dependent IL‐10 reactivation. J Immunol 2006;176:7263–7271. [DOI] [PubMed] [Google Scholar]
- 10. Hashiguchi T, Oyamada A, Sakuraba K et al. Tyk2‐dependent bystander activation of conventional and nonconventional Th1 cell subsets contributes to innate host defense against Listeria monocytogenes infection. J Immunol 2014;192:4739–4747. [DOI] [PubMed] [Google Scholar]
- 11. Ubel C, Mousset S, Trufa D et al. Establishing the role of tyrosine kinase 2 in cancer. Oncoimmunology 2013;2:e22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simma O, Zebedin E, Neugebauer N et al. Identification of an indispensable role for tyrosine kinase 2 in CTL‐mediated tumor surveillance. Cancer Res 2009;69:203–211. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Duns G, Westers H et al. SETD2: An epigenetic modifier with tumor suppressor functionality. Oncotarget 2016;7:50719–50734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cancer Genome Atlas Research Network . Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fontebasso AM, Liu XY, Sturm D et al. Chromatin remodeling defects in pediatric and young adult glioblastoma: A tale of a variant histone 3 tail. Brain Pathol 2013;23:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al Sarakbi W, Sasi W, Jiang WG et al. The mRNA expression of SETD2 in human breast cancer: Correlation with clinico‐pathological parameters. BMC Cancer 2009;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mar BG, Chu SH, Kahn JD et al. SETD2 alterations impair DNA damage recognition and lead to resistance to chemotherapy in leukemia. Blood 2017;130:2631–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hacker KE, Fahey CC, Shinsky SA et al. Structure/function analysis of recurrent mutations in SETD2 protein reveals a critical and conserved role for a SET domain residue in maintaining protein stability and histone H3 Lys‐36 trimethylation. J Biol Chem 2016;291:21283–21295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balmana J, Diez O, Rubio IT et al. BRCA in breast cancer: ESMO clinical practice guidelines. Ann Oncol 2011;22(suppl 6):vi31–vi34. [DOI] [PubMed] [Google Scholar]
- 20. Roy R, Chun J, Powell SN. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat Rev Cancer 2011;12:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yi EJ, Park JH, Lee HW et al. BRCA1 gene mutation in thymic malignant melanoma. Ann Thorac Surg 2013;96:677–680. [DOI] [PubMed] [Google Scholar]
- 22. Nicodeme F, Geffroy S, Conti M et al. Familial occurrence of thymoma and autoimmune diseases with the constitutional translocation t(14;20)(q24.1;p12.3). Genes Chromosomes Cancer 2005;44:154–160. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Wang T, Wang W et al. Does familial breast cancer and thymoma suggest a cancer syndrome? A family perspective. Gene 2015;573:333–337. [DOI] [PubMed] [Google Scholar]
- 24. Enkner F, Pichlhofer B, Zaharie AT et al. Molecular profiling of thymoma and thymic carcinoma: Genetic differences and potential novel therapeutic targets. Pathol Oncol Res 2017;23:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Siena S, Campolo F, Gimmelli R et al. Atm reactivation reverses ataxia telangiectasia phenotypes in vivo. Cell Death Dis 2018;9:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cerami E, Gao J, Dogrusoz U et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao J, Aksoy BA, Dogrusoz U et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Claes K, Poppe B, Coene I et al. BRCA1 and BRCA2 germline mutation spectrum and frequencies in Belgian breast/ovarian cancer families. Br J Cancer 2004;90:1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laitman Y, Simeonov M, Herskovitz L et al. Recurrent germline mutations in BRCA1 and BRCA2 genes in high risk families in Israel. Breast Cancer Res Treat 2012;133:1153–1157. [DOI] [PubMed] [Google Scholar]
- 30. Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 2010;12:245–259. [DOI] [PubMed] [Google Scholar]
- 31. Fisher AE, Hochegger H, Takeda S et al. Poly(adp‐ribose) polymerase 1 accelerates single‐strand break repair in concert with poly(adp‐ribose) glycohydrolase. Mol Cell Biol 2007;27:5597–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, West SC. Distinct functions of BRCA1 and BRCA2 in double‐strand break repair. Breast Cancer Res 2002;4:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ledermann J, Harter P, Gourley C et al. Olaparib maintenance therapy in platinum‐sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382–1392. [DOI] [PubMed] [Google Scholar]
- 34. Moore K, Colombo N, Scambia G et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495–2505. [DOI] [PubMed] [Google Scholar]
- 35. Coleman RL, Oza AM, Lorusso D et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017;390:1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirza MR, Monk BJ, Herrstedt J et al. Niraparib maintenance therapy in platinum‐sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–2164. [DOI] [PubMed] [Google Scholar]
- 37. Robson M, Im SA, Senkus E et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–533. [DOI] [PubMed] [Google Scholar]
- 38. Hoppe MM, Sundar R, Tan DSP et al. Biomarkers for homologous recombination deficiency in cancer. J Natl Cancer Inst 2018;110:704–713. [DOI] [PubMed] [Google Scholar]
- 39. Riaz N, Blecua P, Lim RS et al. Pan‐cancer analysis of bi‐allelic alterations in homologous recombination DNA repair genes. Nat Commun 2017;8:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mateo J, Carreira S, Sandhu S et al. DNA‐repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]


