Abstract
Background
Although predictive value of immune‐related adverse events (irAEs) induced by immune checkpoint inhibitors (ICIs) have been suggested by several studies, their assessments were insufficient because patients were categorized only by the occurrence of irAEs. It has not been elucidated whether irAEs also play a significant role even in responders.
Materials and Methods
Between December 2015 and September 2018, 106 patients with advanced non‐small cell lung cancer treated with ICIs were enrolled in our prospective biomarker study. Twenty‐three of these were responders, defined as those with complete or partial response. We investigated the proportion of irAEs among overall and responders. For responders, progression‐free survival (PFS) and overall survival of ICIs were compared between those with and without irAEs. As an exploratory analysis, we measured 41 proteins from peripheral blood before and after ICI treatment.
Results
The proportion of irAEs was significantly higher in responders than nonresponders (65.2% vs. 19.3%, p < .01). Among responders, clinical characteristics did not differ regardless of the occurrence of irAEs. However, there was a significant difference in PFS among responders (irAE group 19.1 months vs. non‐irAE group 5.6 months; hazard ratio: 0.30 [95% confidence interval: 0.10–0.85]; p = .02). Of 41 protein analyses, fibroblast growth factor‐2 at baseline and monocyte chemoattractant protein fold change showed significant differences between them (p < .04).
Conclusion
Although this is a small sample–sized study, irAE might be a predictive factor of durable efficacy, even in patients who responded to ICIs. Investigation into the significance of irAEs in responders will contribute to the establishment of optimal administration of ICI.
Implications for Practice
Although the predictive value of immune‐related adverse events (irAEs) induced by immune checkpoint inhibitors (ICIs) has been suggested by several studies, it has not been elucidated whether irAEs also play a significant role even in responders. This study showed that more than 60% of responders had irAEs. It demonstrated the strong correlation between irAEs and efficacy even in responders. Investigation into the significance of irAEs in responders will contribute to the establishment of optimal administration of ICI.
Keywords: Immune‐related adverse event, Immune checkpoint inhibitor, Predictive marker
Short abstract
Immune‐related adverse events might be a predictive factor of efficacy, even I patients who respond to immune checkpoint inhibitor therapy. This study investigated the significance of immune‐related adverse events in patients with non‐small cell lung cancer who had complete or partial response after treatment with immune checkpoint inhibitors.
Introduction
Immune checkpoint inhibitors (ICIs) have become the standard treatment of advanced non‐small cell lung cancer (NSCLC) 1, 2, 3. ICIs sometimes achieve durable efficacy, which has never been experienced with any other chemotherapeutic drugs. Longer follow‐up data reported that the 3‐year survival rate of pretreated patients with NSCLC reached about 17% 4. On the other hand, some patients demonstrated immune‐related adverse events (irAEs) such as pneumonitis, thyroid hormone disorder, or colitis.
The predictive value of irAEs in efficacy has been suggested by several studies 5, 6, 7. However, their assessments were insufficient because patients were categorized only by the occurrence of irAEs. Thus, it has not been elucidated whether irAEs also play a significant role even in responders. Additionally, it may also be critical to explore the biomarkers of irAEs. Here, we report the result of a prospective biomarker study of patients who were treated with ICIs.
Material and Methods
Between December 2015 and September 2018, 106 patients with advanced NSCLC were treated with ICIs in our department and provided written informed consent to our prospective biomarker study, which prespecified the timing of blood collection and radiological evaluation. Administration of ICI and evaluation of efficacy and toxicity were determined by each investigator. Radiological evaluation was done every 6–8 weeks according to RECIST version 1.1. We defined responders as patients whose best response was either complete response (CR) or partial response (PR). We calculated the period from the day of ICI initiation to the time of progression or death from any cause as progression‐free survival (PFS). Overall survival (OS) was defined as the period from the day of ICI initiation to death or the latest visit. Toxicity was also judged by each physician followed by Common Terminology Criteria for Adverse Events version 4.0. Immune‐related adverse events were defined as previously described 8, having a potential immunological basis that required more frequent monitoring and potential intervention with immune suppression and/or endocrine replacement therapy. At the time of this analysis, ICI plus cytotoxic chemotherapy was not yet approved in Japan. Thus, all the patients in this study underwent ICI monotherapy (nivolumab, pembrolizumab, or atezolizumab).
In this study, 25 mL of peripheral blood was collected just before ICI treatment, at weeks 4–6, 8–12, and 24, and at the time of progression. Using peripheral blood, multiplexed serum proteins (supplemental online Table 1) were analyzed using Luminex 200 analyzer (Luminex, Austin, TX) with Milliplex MAP system (Millipore, Billerica, MA), and the number of circulating tumor cells was also detected using a microcavity array system. Programmed death‐ligand 1 (PD‐L1) immunohistochemistry was done using tumor tissue specimen with 22C3 pharmDx antibody (clone 22C3; Dako North America, Inc., Carpinteria, CA) in accordance with recommended methods. Results from the initial analysis were already published by Oyanagi et al. 9.
Regarding irAEs, we compared their proportion between responders and nonresponders using Fisher's exact test. Baseline characteristics of these two groups were compared using Fisher's exact test or chi‐square test. Among responders, PFS with ICIs was calculated by using the Kaplan‐Meier method and compared between those with and without irAEs by Cox proportional hazards regression. As an exploratory analysis of serum proteins, baseline values and fold changes were compared between responders with and without irAEs using Mann‐Whitney U test. To calculate fold changes, each values measured at the first time point (4–6 weeks of treatment) were divided by those at baseline. Statistical analyses were conducted with GraphPad Prism version 7.00 for Windows (GraphPad Software, San Diego, CA). If the p value was <.05, we considered the difference significant. For biomarker testing, we did not change the significance level because this was an exploratory analysis.
This study was approved by the institutional review board in our hospital and registered at the University Medical Hospital Information Network (UMIN) Clinical Trials Registry (UMIN000024414).
Results
Of 106 patients enrolled in this study, overall response rate was 21.7% (n = 23; 2 CR and 22 PR) and median PFS was 2.9 months. Median follow‐up time was 19.3 months. Characteristics of the responders are shown in Table 1. Median age was 69 years (range: 52–90). Male and smoker made up about 80% of the patients. In 10 patients, their tumors expressed PD‐L1 ≥ 50%, and 8 of them were chemo‐naïve. Regarding the ICIs administered, 11 patients were treated with pembrolizumab, 11 patients were treated with nivolumab, and 1 patient was treated with atezolizumab.
Table 1.
Patient characteristics
| Characteristics | Overall responders (n = 23) | Responders with irAEs (n = 15) | Responders without irAEs (n = 8) | p value |
|---|---|---|---|---|
| Age, years | .96 | |||
| Median (range) | 69 (52–90) | 69 (52–90) | 69.5 (57–85) | |
| Sex, n (%) | 1.00 | |||
| Male | 18 (78) | 12 (80) | 6 (75) | |
| Female | 5 (22) | 3 (20) | 2 (25) | |
| Smoking history, n (%) | 1.00 | |||
| Smoker | 17 (74) | 11 (73) | 6 (75) | |
| Non‐ or light smoker | 6 (26) | 4 (27) | 2 (25) | |
| ECOG PS, n (%) | .59 | |||
| 0–1 | 19 (83) | 13 (87) | 6 (75) | |
| 2 | 4 (17) | 2 (13) | 2 (25) | |
| Histology, n (%) | .12 | |||
| Non‐squamous cell carcinoma | 18 (78) | 10 (67) | 8 (100) | |
| EGFR mutated/wild‐type | 1/0 | 0/0 | 1/0 | |
| Squamous cell carcinoma | 5 (22) | 5 (33) | 0 | |
| PD‐L1 expression, n (%) | .72 | |||
| ≥ 50% | 10 (43) | 6 (40) | 4 (50) | |
| 1%–49% | 2 (9) | 1 (7) | 1 (13) | |
| < 1% | 3 (13) | 1 (7) | 2 (25) | |
| Unknown | 8 (35) | 7 (46) | 1 (13) | |
| No. of prior chemotherapeutic regimens, n (%) | 1.00 | |||
| 0 | 8 (35) | 5 (33) | 3 (38) | |
| ≥ 1 | 15 (65) | 10 (67) | 5 (62) | |
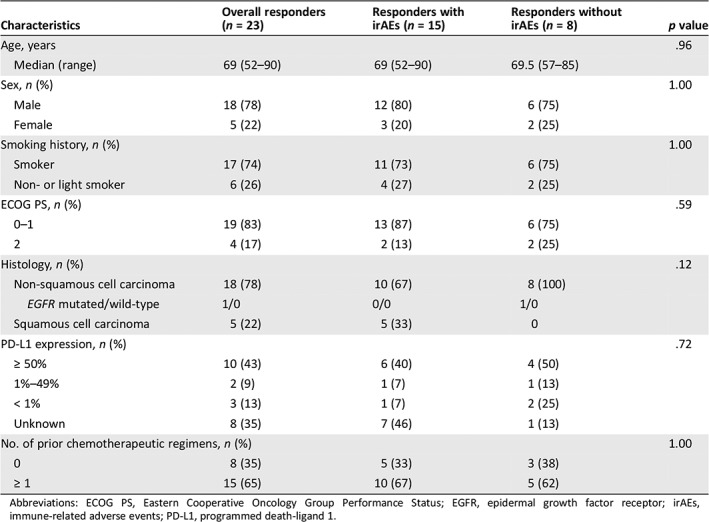
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; irAEs, immune‐related adverse events; PD‐L1, programmed death‐ligand 1.
Of 23 responders, 15 (65.2%) had at least one irAE (25 events in total). Among 83 nonresponders, 16 (19.3%) had at least one irAE (Fig. 1). These indicated that incidence of irAEs was significantly higher in responders (relative risk 7.85 [95% confidence interval (CI): 2.84–21.70]; p < .01). Among responders with irAEs, median number of ICI administration was 6 (range: 1–53). Median time from ICI treatment to irAE onset was 50 days (range: 1–692), and more than 70% of irAEs occurred within 3 months. Nine patients experienced multiple irAEs. Of 25 events, 4 were grade 3 (2 pneumonitis, 1 aspartate aminotransferase elevation, and 1 hyperthyroidism), but no one died as a result of irAEs. Two common irAEs were pneumonitis (n = 7) and hypothyroidism (n = 6).
Figure 1.
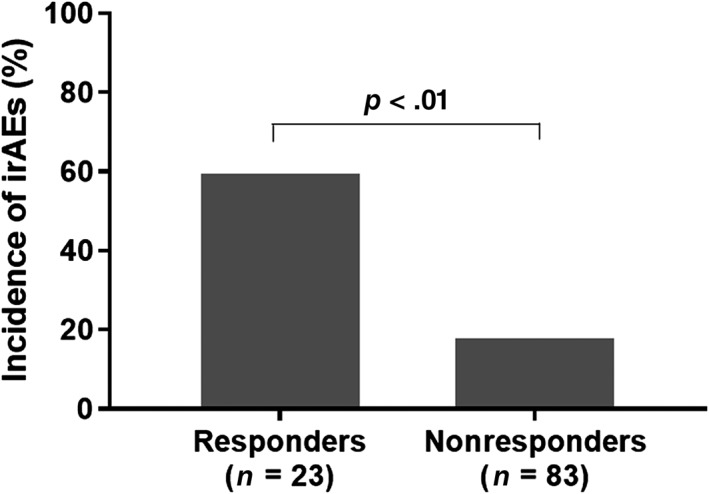
Proportion of those who had at least one immune‐related adverse event between responders (n = 23) and nonresponders (n = 83).Abbreviation: irAEs, immune‐related adverse events.
Between responders with or without irAEs, baseline characteristics were not different (Table 1). Among responders with irAEs, median PFS was 19.1 months (95% CI: 5.5 months to not reached), whereas that of non‐irAE responders was 5.6 months (95% CI: 1.6–9.9 months). PFS rate at 1 year was 53.3% in irAE responders and 12.5% in non‐irAE responders. Between these two groups, there was a statistically significant difference in PFS (hazard ratio [HR]: 0.30 [95% CI: 0.10–0.85]; p = .02; Fig. 2). Details of the clinical course among responders are shown in Figure 3. Among the irAE group, 11 patients (73.3%) discontinued ICI treatment because of irAEs, but their response was maintained for a long time (median: 16.8 months; range: 3.6–39.9 months). Regarding OS, responders with irAEs showed median OS of 27.8 months (95% CI: 10.5 months to not reached), whereas those without irAEs showed median OS of 16.1 months (95% CI: 6.8 months to not reached). There was not a significant difference between them (HR: 0.45 [95% CI: 0.11–1.88]; p = .25; Fig. 4).
Figure 2.
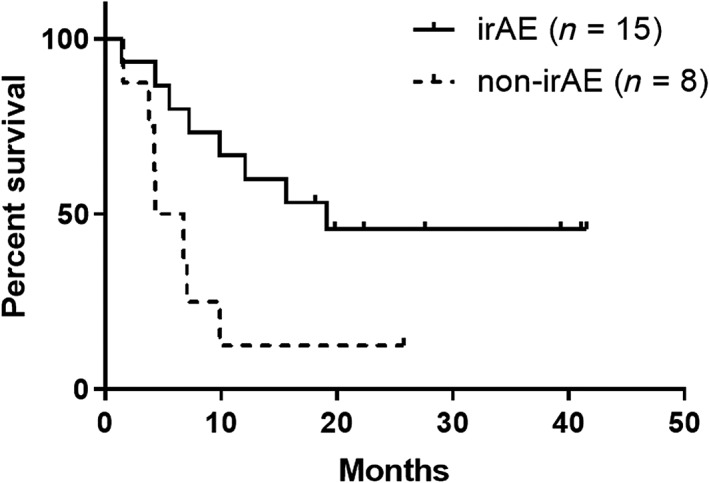
Kaplan–Meier curve of progression‐free survival (bold line: irAE group [n = 15]; dashed line: no‐irAE group [n = 8]).Abbreviation: irAE, immune‐related adverse event.
Figure 3.
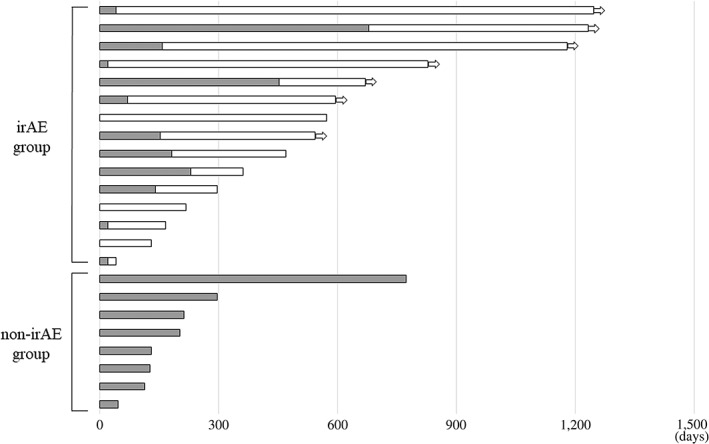
Swimmers’ plot of responders with and without irAEs (black bar: periods with immune checkpoint inhibitor [ICI] administered; white bar: periods without ICI). Arrow indicates response is ongoing.Abbreviation: irAE, immune‐related adverse event.
Figure 4.
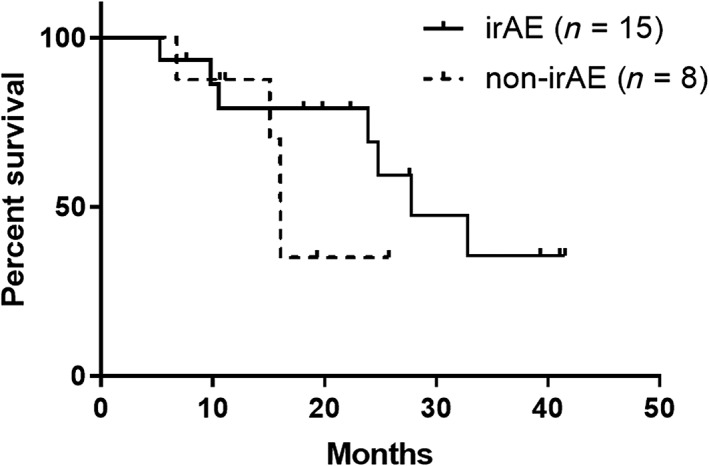
Kaplan‐Meier curve of overall survival (bold line: irAE group [n = 15]; dashed line: no‐irAE group [n = 8]).Abbreviation: irAE, immune‐related adverse event.
Regarding 41 proteins collected from peripheral blood, fibroblast growth factor‐2 at baseline and monocyte chemoattractant protein fold change showed significant differences between them (p < .04). Interferon gamma‐induced protein 10 and macrophage‐derived chemokine at baseline also showed marked, but not significant, difference (supplemental online Fig. 1).
Discussion
Although previous reports suggested that patients with irAEs were likely to benefit from ICIs, their assessment had several drawbacks. First, they did not assess the significance of responder without irAEs. In a phase III trial, about 6% of the patients completed 2 years of ICI treatment without severe toxicity, and most of them were responders 10. Second, they could not completely exclude nonresponders from the non‐irAE group. Thus, the predictive value of irAEs might be overestimated. Based on these, proper analysis with responders has been warranted. In this study, we initially demonstrated that responders had sevenfold higher incidence of irAEs than nonresponders. In addition, our study clearly showed that irAEs were strong predictive factors of efficacy (HR 0.30), even in responders. In other words, among those who achieved CR or PR in the early course of ICI treatment, PFS was eventually unsatisfactory (median 5.6 months) if they did not show irAEs.
In this context, to predict the onset of irAEs should be important. Similar to previous reports, we could not find any clinical features to predict irAEs. Most irAEs occurred within 3 months of ICI treatment, indicating that immune reaction during the early period may maximize the efficacy of ICIs. Regarding efficacy biomarker, several attempts have been reported. Mezuquita et al, reported that derived neutrophils/leukocytes ratio and lactate dehydrogenase were correlated with efficacy 11. As a predictive marker of safety, our analysis suggested some potential serum proteins. Of those, IP‐10 was also detected as a predictive marker of efficacy in the entire population 9. On the contrary, a recent report showed the importance of CXCL2 and MMP2 12. Although serum biomarker has more advantages in its convenience, cost‐effectiveness, and less invasiveness, these results should be interpreted cautiously because any definite mechanism between these proteins and clinical phenomenon has not been clearly shown. A validation study with a larger sample size is required to prove its significance.
Our study had several limitations. We could not assess the significance of severity and number of irAEs because of the small sample size. Second, assessment of antitumor response and irAE were dependent on each investigator. Nonetheless, our results are evidently consistent with those of previous studies. Third, we did not consider the influence of clinical characteristics on values of biomarkers even though some few patients received multiple lines of systemic treatment and had poor performance status. We speculate that these factors may influence but not have an impact on the difference between the arms, which were well balanced. We believe that our data among a properly enriched population may provide useful information into our practice.
Conclusion
Although this is a small sample–sized study, irAEs might be a predictive factor of durable efficacy, even in patients who responded to ICIs. Investigation into the significance of irAEs in this population will contribute to the establishment of optimal administration of ICI.
Author Contributions
Conception/design: Hiroaki Akamatsu, Eriko Murakami, Jun Oyanagi, Yasuhiro Koh, Nobuyuki Yamamoto
Provision of study material or patients: Hiroaki Akamatsu, Eriko Murakami, Jun Oyanagi, Takahiro Kaki, Eri Takase, Masanori Tanaka, Yuhei Harutani, Nao Yamagata, Yuka Okuda, Katsuyuki Furuta, Takeya Sugimoto, Shunsuke Teraoka, Atsushi Hayata, Nahomi Tokudome, Yuichi Ozawa
Collection and/or assembly of data: Hiroaki Akamatsu, Eriko Murakami, Jun Oyanagi, Yasuhiro Koh
Data analysis and interpretation: Hiroaki Akamatsu, Eriko Murakami, Jun Oyanagi, Yasuhiro Koh, Nobuyuki Yamamoto
Manuscript writing: Hiroaki Akamatsu, Eriko Murakami, Jun Oyanagi, Yasuhiro Koh
Final approval of manuscript: Hiroaki Akamatsu, Eriko Murakami, Jun Oyanagi, Ryota Shibaki, Takahiro Kaki, Eri Takase, Masanori Tanaka, Yuhei Harutani, Nao Yamagata, Yuka Okuda, Katsuyuki Furuta, Takeya Sugimoto, Shunsuke Teraoka, Atsushi Hayata, Nahomi Tokudome, Yuichi Ozawa, Keita Mori, Yasuhiro Koh, Nobuyuki Yamamoto
Disclosures
Hiroaki Akamatsu: Ono Pharmaceutical Co., Chugai Pharmaceutical Co. Ltd., Bristol‐Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Taiho Pharmaceutical Co. (H), Merck Sharp & Dohme (RF). Shunsuke Teraoka: Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. (H); Yasuhiro Koh: Merck Sharp & Dohme, Ono Pharmaceutical Co., Bristol‐Myers Squibb (RF), Chugai Pharmaceutical Co. Ltd. (H); Nobuyuki Yamamoto: Merck Sharp & Dohme, Ono Pharmaceutical Co., AstraZeneca, Chugai Pharmaceutical Co. Ltd. (SAB), Merck Sharp & Dohme, Chugai Pharmaceutical Co. Ltd. (RF), Merck Sharp & Dohme, Ono Pharmaceutical Co., AstraZeneca, Chugai Pharmaceutical Co. Ltd. (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table
Acknowledgments
We thank Benjamin Phillis at Wakayama Medical University for proofreading and editing. This work was supported by Grant‐in‐Aid for Scientific Research 18K07271 and Project for Cancer Research and Therapeutic Evolution (P‐CREATE) from the Japan Agency for Medical Research and Development, AMED (grant numbers 16cm0106418h0001 and 17cm0106418h0002).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vokes EE, Ready N, Felip E et al. Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959–965. [DOI] [PubMed] [Google Scholar]
- 5. Haratani K, Hayashi H, Chiba Y et al. Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol 2018;4:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato K, Akamatsu H, Murakami E et al. Correlation between immune‐related adverse events and efficacy in non‐small cell lung cancer treated with nivolumab. Lung Cancer 2018;115:71–74. [DOI] [PubMed] [Google Scholar]
- 7. Teraoka S, Fujimoto D, Morimoto T et al. Early immune‐related adverse events and association with outcome in advanced non‐small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol 2017;12:1798–1805. [DOI] [PubMed] [Google Scholar]
- 8. Weber JS, Hodi S, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–792. [DOI] [PubMed] [Google Scholar]
- 9. Oyanagi J, Koh Y, Sato K et al. Predictive value of serum protein levels in patients with advanced non‐small cell lung cancer treated with nivolumab. Lung Cancer 2019;132:107–113. [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS, Garon EB, Kim DW et al. KEYNOTE‐010: Durable clinical benefit in patients with previously treated, PD‐L1‐expressing NSCLC who completed pembrolizumab. J Thorac Oncol 2017;12(suppl 1):S254–S255. [Google Scholar]
- 11. Mezquita L, Auclin E, Ferrara R et al. Association of the Lung Immune Prognostic Index with immune checkpoint inhibitor outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol 2018;4:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuo N, Azuma K, Hattori S et al. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti‐PD‐1 inhibitor. Int J Cancer 2019;144:1170–1179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure
Supplemental Table


