Abstract
Background
Although an increasing number of treatments have become available for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP‐NETs), there remains little consensus on treatment sequence and its impact on health care resource use (HRU). We sought to describe treatment patterns and HRU, in a cohort of patients with metastatic GEP‐NETs treated at a tertiary referral center in the U.S.
Materials and Methods
We identified patients with a well‐differentiated, metastatic GEP‐NET evaluated at Dana‐Farber Cancer Institute between July 2003 and May 2015. For these patients, we describe the sequence of treatment regimens received for their disease, together with associated HRU.
Results
We identified 682 patients with advanced GEP‐NETs. Of these patients, 597 (87.0%) initiated ≥1 treatment over the follow‐up period. The mean age at diagnosis was 58.5 years, 50.2% were men, and 94.0% were white. A total of 83.1% initiated a somatostatin analog (SSA) as their first‐line treatment, with 55% and 31% of patients continuing with second‐ and third‐line therapies. A total of 31.2% of patients with SSAs underwent dose escalation to above standard dose. In this setting, patients with pancreatic neuroendocrine tumors were more commonly treated with cytotoxic regimens than other NET tumor types and also had higher HRU.
Conclusion
Our study suggests that, at a tertiary referral center, patients with advanced NETs commonly received multiple courses of treatments. Our data suggest a clear preference for use of SSAs as a first‐line treatment for patients with advanced NETs, with SSAs commonly escalated and continued throughout the course of treatment in combination with other regimens.
Implications for Practice
The current study demonstrates the common use of somatostatin analog as a first‐line therapy for patients with advanced gastroenteropancreatic neuroendocrine tumors as well as the incorporation of multiple different treatment regimens in the treatment course of patients with this disease.
Keywords: Neuroendocrine tumors, Treatment patterns, Somatostatin analogs, Health resource use
Short abstract
This article focuses on treatment of gastroenteropancreatic neuroendocrine tumors (GEP‐NETs) and health resource use among patients with advanced GEP‐NETs treated at a tertiary referral center in the United States.
Introduction
The incidence of gastroenteropancreatic neuroendocrine tumors (GEP‐NETs) is increasing worldwide; the age‐adjusted incidence has increased 3.6‐fold in the U.S. and between 3.8‐ and 4.8‐fold in Europe 1. In recent years, a number of new treatments have become available for patients with advanced NETs. Somatostatin analogs (SSAs), such as octreotide and lanreotide, remain a mainstay of treatment in this setting. Both agents have been shown to ameliorate symptoms of hormone secretion 2 and have also been shown to slow tumor progression, as reported in two clinical trials, PROMID 3 and CLARINET 4. Although symptoms can be effectively managed with SSAs at standard doses for most patients, some patients continue to experience symptoms owing to lack of effectiveness or loss of benefit over time. For patients with carcinoid syndrome inadequately controlled by an SSA, treatment with telotristat ethyl may be an option in this scenario. SSA doses may also be escalated to treat symptoms, and several studies have reported symptom improvement after upward titration of octreotide long‐acting release (LAR) 5, 6.
Although the role of SSAs is well recognized in treating metastatic GEP‐NETs, there is less consensus on the sequence of treatments following progression on SSAs. Everolimus was approved for the treatment of pancreatic neuroendocrine tumors (pNETs) in 2011, and for treatment of other NETs in 2016, based on randomized studies in both indications showing improvements in progression‐free survival 7, 8. In 2011, sunitinib was also shown to improve progression‐free survival in advanced pNETs and was approved for this indication 9. Treatment with cytotoxic therapies is also a potential treatment option for both pNET and non‐pNET neuroendocrine tumors 10. Streptozotocin has been evaluated as a treatment for both advanced pNET and non‐pNET tumors and was approved for use in advanced pNETs. More recently, temozolomide‐based combination therapy has been increasingly used, particularly in pNETs, and the combination of temozolomide and capecitabine was recently shown to improve both progression‐free and overall survival in a randomized study of advanced pNETs, compared with temozolomide monotherapy 11. Finally, 177 Lu‐Dotatate therapy was recently approved for the treatment of advanced neuroendocrine tumors based on the results of a randomized study in patients with advanced midgut NETs, which demonstrated improved progression‐free survival compared with treatment with octreotide alone 12 and a long‐term study in patients with GEP‐ and bronchial NETs showing good response rates with 29‐month progression‐free survival and 63‐month overall survival 13.
To date, there is little consensus and few data on treatment sequence beyond SSAs. Similarly, data on health resource use (HRU) among patients with advanced NETs are poorly understood. We sought to better understand GEP‐NET treatment sequences and HRU among patients with advanced GEP‐NETs treated at a tertiary referral center in the U.S.
Materials and Methods
Data Sources
We identified patients with a confirmed diagnosis of GEP‐NET (excluding small cell lung cancer) who were recruited to a prospective clinical database and biobanking study between July 2003 and May 2015 at Dana‐Farber Cancer Institute (DFCI). Approximately 95% of patients approached agreed to participate and provided informed consent. Data on demographics, medical history, staging, and treatments were collected at enrollment, and staging and treatments were updated every 4 months for the first year and yearly thereafter. Data (current and historical) were compiled from a number of sources, including the research database, institutional electronic medical records (EMRs) from DFCI and partner hospitals, and/or by contacting the patients’ other providers, thereby capturing treatments received outside of DFCI.
We linked the research database to patients’ EMRs from DFCI and Partners’ network. EMRs from DFCI contained data on outpatient visits, laboratory tests, and dispensed medications from DFCI's outpatient pharmacy. EMRs from the Partners’ network contained data on hospitalizations, outpatient visits, procedures, diagnostic and imaging tests, laboratory tests, and transfusions.
Study Population
We included patients diagnosed with a well‐differentiated, metastatic GEP‐NET between July 2003 and May 2015. The index date was defined as the metastatic GEP‐NET diagnosis date or the date of patient consent, whichever occurred later. To ensure that patients were receiving at least some of their GEP‐NET‐related care at DFCI, we restricted the population to patients who were seen at least twice and yearly at DFCI. For the HRU analysis, we also excluded patients with all visits separated by less than 7 days.
Treatment Sequence
Information about lines of treatment were derived from the research database. Treatments were categorized into the following categories: SSAs, cytotoxic therapies, sunitinib, everolimus, interferon, investigational, or other therapies. Therapies were considered investigational if they were not indicated for GEP‐NET or if non‐SSA treatments were used in combination (e.g., cytotoxic treatment and sunitinib). Three GEP‐NET treatment lines were defined. The first line of treatment was defined as any treatment initiated <30 days prior to or the first treatment strategy initiated after metastatic GEP‐NET diagnosis; the second line was defined as addition of another treatment while on the first‐line treatment or initiation of a new treatment after discontinuation of the first‐line treatment. This same approach was also used to define the third treatment line. Initiation of an additional treatment within 90 days of SSA initiation was categorized as SSA combination therapy. Unless there was evidence of SSA discontinuation, we assume SSA treatment continued indefinitely.
SSA Dose Escalation
Data on dosing of long‐acting SSAs (octreotide LAR or lanreotide) were obtained from DFCI's outpatient pharmacy dispensation records. The dispensation frequency of SSAs was categorized into weeks, and dispensations ±3 days were considered part of the same week. A standard monthly dosing regimen was estimated as (dose/number of weeks between dispensation) × 4. Dose escalations were defined as ≥2 consecutive increases in monthly SSA dosing regimens above the recommended dosing levels (i.e., >30 mg per month for octreotide LAR and >120 mg per month for lanreotide), compared with the last two consecutive SSA monthly regimens. If a patient had >2 increases in monthly SSA doses, the patient was considered to have had one dose escalation. The follow‐up period was defined as the period between the first and last dispensations; patients with >6 weeks between dispensations were considered lost to follow‐up, but these patients could re‐enter the cohort if SSA dispensations were subsequently observed.
Health Resource Use
HRU was evaluated using combined data from the research database and EMRs from DFCI and the Partners’ network. We categorized HRU into the following categories: outpatient visits, inpatient visits, laboratory tests, transfusions, diagnostic and imaging tests, and radiology. Patients were considered to have a gap in follow‐up if the time between outpatient visits exceeded 3 months on treatment and 9 months off treatment, with treatment status assessed using the research database. Patients lost to follow‐up could re‐enter the cohort if HRU was subsequently observed.
Statistical Analysis
We report patient demographic and disease severity at baseline. We report treatment strategies stratified by treatment line as well as by treatment line and primary tumor location. We describe patterns of SSA dose escalations by reporting their frequency, type of escalation (i.e., increase in dose or frequency), and regimens from and to which doses were escalated. We report the proportions of patients using each type of HRU and mean HRU per patient‐year (PY) and SD overall and stratified by primary tumor location. To protect patient confidentiality, we do not report results in which the cell size is ≤11. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Clinical Characteristics of Patients
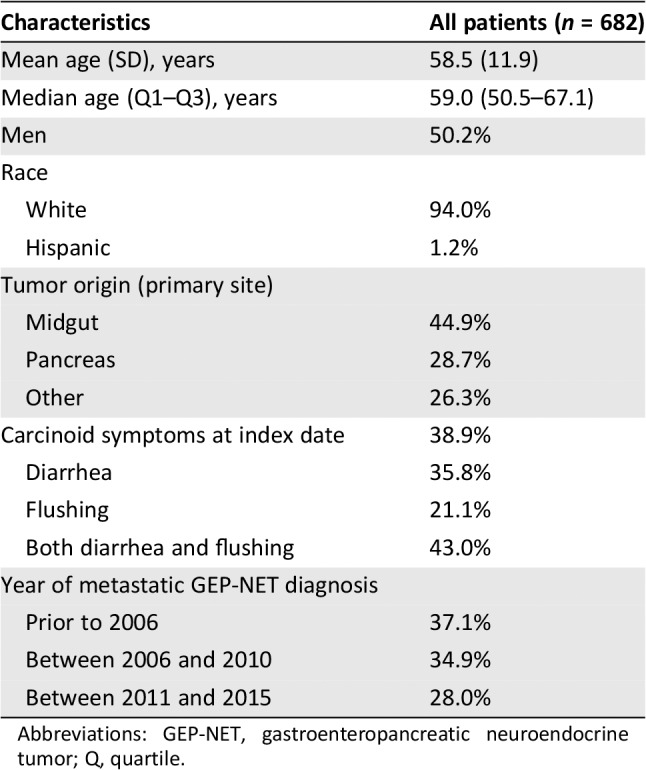
Six hundred eighty‐two patients with metastatic, well‐differentiated GEP‐NETs who were receiving at least some oncology care at DFCI were identified. Mean age at diagnosis of metastatic disease was 58.5 years (SD: 11.9), 50.2% were men, and 94.0% were white (Table 1). The most common primary tumor locations were midgut and pancreas, accounting for 44.9% and 28.7% of cases, respectively. Carcinoid syndrome symptoms were present in 38.9% of patients, of whom 35.8% had diarrhea only, 21.1% had flushing only, and 43.0% had both. A total of 37.1% of patients were diagnosed with metastatic disease prior to 2006, 34.9% between 2006 and 2010, and 28.0% between 2011 and 2015.
Table 1.
Demographic and clinical characteristics of patients
| Characteristics | All patients (n = 682) |
|---|---|
| Mean age (SD), years | 58.5 (11.9) |
| Median age (Q1–Q3), years | 59.0 (50.5–67.1) |
| Men | 50.2% |
| Race | |
| White | 94.0% |
| Hispanic | 1.2% |
| Tumor origin (primary site) | |
| Midgut | 44.9% |
| Pancreas | 28.7% |
| Other | 26.3% |
| Carcinoid symptoms at index date | 38.9% |
| Diarrhea | 35.8% |
| Flushing | 21.1% |
| Both diarrhea and flushing | 43.0% |
| Year of metastatic GEP‐NET diagnosis | |
| Prior to 2006 | 37.1% |
| Between 2006 and 2010 | 34.9% |
| Between 2011 and 2015 | 28.0% |
Abbreviations: GEP‐NET, gastroenteropancreatic neuroendocrine tumor; Q, quartile.
Treatment Patterns
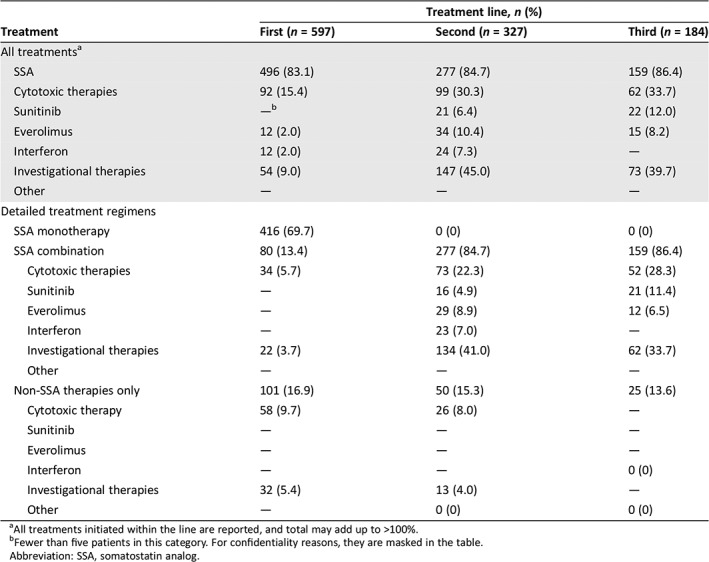
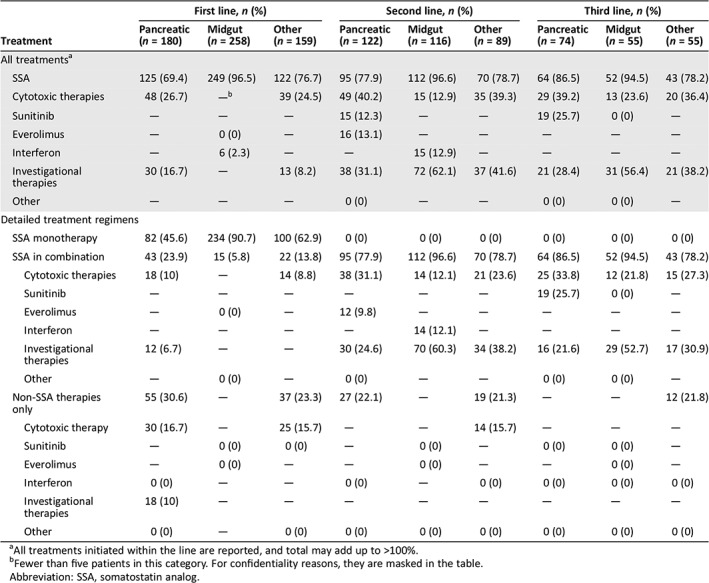
A total of 87.5% of patients (597/682) initiated at least one type of treatment (Table 2). The most commonly used first‐line treatment regimen for metastatic GEP‐NET was SSA monotherapy (69.7%). SSA combination therapy was used in 13.4% of patients in the first line, and cytotoxic chemotherapy without SSA in 9.7% of patients. In the first‐line setting, cytotoxic chemotherapy was most commonly used in patients with pNETs, either alone (16.7%) or in combination with an SSA (10.0%; Table 3). In contrast, cytotoxic chemotherapy was seldom used as first‐line therapy among patients with midgut NETs. In other NETs, cytotoxic chemotherapy and investigational therapies were used in the first line in 24.5% and 8.2% of patients, respectively.
Table 2.
Overall treatment strategy distribution, by treatment line
| Treatment | Treatment line, n (%) | ||
|---|---|---|---|
| First (n = 597) | Second (n = 327) | Third (n = 184) | |
| All treatmentsa | |||
| SSA | 496 (83.1) | 277 (84.7) | 159 (86.4) |
| Cytotoxic therapies | 92 (15.4) | 99 (30.3) | 62 (33.7) |
| Sunitinib | —b | 21 (6.4) | 22 (12.0) |
| Everolimus | 12 (2.0) | 34 (10.4) | 15 (8.2) |
| Interferon | 12 (2.0) | 24 (7.3) | — |
| Investigational therapies | 54 (9.0) | 147 (45.0) | 73 (39.7) |
| Other | — | — | — |
| Detailed treatment regimens | |||
| SSA monotherapy | 416 (69.7) | 0 (0) | 0 (0) |
| SSA combination | 80 (13.4) | 277 (84.7) | 159 (86.4) |
| Cytotoxic therapies | 34 (5.7) | 73 (22.3) | 52 (28.3) |
| Sunitinib | — | 16 (4.9) | 21 (11.4) |
| Everolimus | — | 29 (8.9) | 12 (6.5) |
| Interferon | — | 23 (7.0) | — |
| Investigational therapies | 22 (3.7) | 134 (41.0) | 62 (33.7) |
| Other | — | — | — |
| Non‐SSA therapies only | 101 (16.9) | 50 (15.3) | 25 (13.6) |
| Cytotoxic therapy | 58 (9.7) | 26 (8.0) | — |
| Sunitinib | — | — | — |
| Everolimus | — | — | — |
| Interferon | — | — | 0 (0) |
| Investigational therapies | 32 (5.4) | 13 (4.0) | — |
| Other | — | 0 (0) | 0 (0) |
All treatments initiated within the line are reported, and total may add up to >100%.
Fewer than five patients in this category. For confidentiality reasons, they are masked in the table.
Abbreviation: SSA, somatostatin analog.
Table 3.
Distribution of SSA treatment strategies, by treatment line and primary tumor location
| Treatment | First line, n (%) | Second line, n (%) | Third line, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pancreatic | Midgut | Other | Pancreatic | Midgut | Other | Pancreatic | Midgut | Other | |
| (n = 180) | (n = 258) | (n = 159) | (n = 122) | (n = 116) | (n = 89) | (n = 74) | (n = 55) | (n = 55) | |
| All treatmentsa | |||||||||
| SSA | 125 (69.4) | 249 (96.5) | 122 (76.7) | 95 (77.9) | 112 (96.6) | 70 (78.7) | 64 (86.5) | 52 (94.5) | 43 (78.2) |
| Cytotoxic therapies | 48 (26.7) | —b | 39 (24.5) | 49 (40.2) | 15 (12.9) | 35 (39.3) | 29 (39.2) | 13 (23.6) | 20 (36.4) |
| Sunitinib | — | — | — | 15 (12.3) | — | — | 19 (25.7) | 0 (0) | — |
| Everolimus | — | 0 (0) | — | 16 (13.1) | — | — | — | — | — |
| Interferon | — | 6 (2.3) | — | — | 15 (12.9) | — | — | — | — |
| Investigational therapies | 30 (16.7) | — | 13 (8.2) | 38 (31.1) | 72 (62.1) | 37 (41.6) | 21 (28.4) | 31 (56.4) | 21 (38.2) |
| Other | — | — | — | 0 (0) | — | — | 0 (0) | 0 (0) | — |
| Detailed treatment regimens | |||||||||
| SSA monotherapy | 82 (45.6) | 234 (90.7) | 100 (62.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SSA in combination | 43 (23.9) | 15 (5.8) | 22 (13.8) | 95 (77.9) | 112 (96.6) | 70 (78.7) | 64 (86.5) | 52 (94.5) | 43 (78.2) |
| Cytotoxic therapies | 18 (10) | — | 14 (8.8) | 38 (31.1) | 14 (12.1) | 21 (23.6) | 25 (33.8) | 12 (21.8) | 15 (27.3) |
| Sunitinib | — | — | — | — | — | — | 19 (25.7) | 0 (0) | — |
| Everolimus | — | 0 (0) | — | 12 (9.8) | — | — | — | — | — |
| Interferon | — | — | — | — | 14 (12.1) | — | — | — | — |
| Investigational therapies | 12 (6.7) | — | — | 30 (24.6) | 70 (60.3) | 34 (38.2) | 16 (21.6) | 29 (52.7) | 17 (30.9) |
| Other | — | 0 (0) | — | 0 (0) | — | — | 0 (0) | 0 (0) | — |
| Non‐SSA therapies only | 55 (30.6) | — | 37 (23.3) | 27 (22.1) | — | 19 (21.3) | — | — | 12 (21.8) |
| Cytotoxic therapy | 30 (16.7) | — | 25 (15.7) | — | — | 14 (15.7) | — | — | — |
| Sunitinib | — | 0 (0) | 0 (0) | — | 0 (0) | — | 0 (0) | 0 (0) | — |
| Everolimus | — | 0 (0) | — | — | 0 (0) | — | — | 0 (0) | — |
| Interferon | 0 (0) | — | — | 0 (0) | — | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Investigational therapies | 18 (10) | — | — | — | — | — | — | — | — |
| Other | 0 (0) | — | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
All treatments initiated within the line are reported, and total may add up to >100%.
Fewer than five patients in this category. For confidentiality reasons, they are masked in the table.
Abbreviation: SSA, somatostatin analog.
Of the 597 patients who received at least one treatment regimen, 327 (54.8%) received second‐line therapy (Table 2). In the second line, continuation of SSA therapy was common, with 84.7% of patients receiving treatment with an SSA combined with another agent. Cytotoxic chemotherapy was the second most commonly used agent in second‐line therapy overall (30.3%). In the second line, cytotoxic chemotherapy was used in 40.2% of patients with pNETs, 12.9% of patients with midgut NETs, and 39.3% of patients with other NETs (Table 3). The use of investigational agents in the second‐line setting was 45.0% (147/327) overall, with 31.1% of patients with pNETs, 62.1% of patients with midgut NETs, and 41.6% of patients with other NETs receiving investigational therapy.
A total of 30.8% of patients who received at least one treatment regimen received third‐line therapy (184/597). Overall, 33.7% (62/184) received cytotoxic chemotherapy, generally with continuation of SSAs. A total of 39.7% of patients overall (73/184) received treatment with an investigational agent, 28.4% of patients with pNETs, 56.4% of patients with midgut NETs, and 38.2% of patients with other NETs.
The overall use of the molecularly targeted agents everolimus and sunitinib was relatively low. Sunitinib and everolimus were used as standard therapy in only 2.0% and by <11 patients in the first line, and 10.4% and 6.4% of patients in the second line, respectively (Table 2). In the second and third line, use of everolimus and sunitinib was more common in pNETs than in midgut NETs (Table 3). Of note, however, is that these two agents were approved in 2011; prior to 2011, fewer than 11 patients received everolimus or sunitinib as first‐line therapy and only 15 received them in the second line. After 2011, 13 patients received these agents in the first line and 40 in the second line.
Use of Somatostatin Analogs
We identified 340 patients dispensed SSAs by DFCI's outpatient pharmacy. Of note, lanreotide was not approved until 2017 to treat gastrointestinal NETs, and at the time of the analysis, <10 dispensations of lanreotide were observed. Patients were treated with octreotide LAR for a mean of 1,083 days (SD: 973). We observed 195 dose escalations of octreotide LAR above the standard dose of 30 mg per month, in a total of 31.2% (106/340) of patients. In 41.0% of dose escalations, we observed an increase in dose, in 56.9% an increase in dispensation frequency, and in 2.1% an increase in both dose and dispensation frequency. The most common dosing regimen to which patients were escalated was 40 mg per month (39.5%) and 53 mg per month (reflecting 40 mg every 3 weeks; 43.1%). Escalation to monthly octreotide doses of 60 mg per month or greater was not common.
Health Resource Use
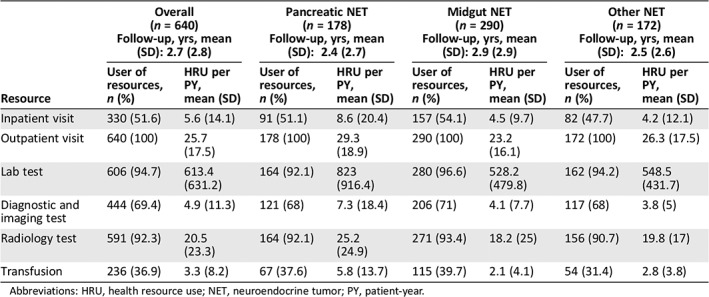
A total of 640 out of 682 patients were included in the HRU analysis. The average follow‐up time was 2.7 years (SD: 2.8). Although 257 patients (40.2%) had a least one gap in follow‐up, the majority had just one (80.2%) or two gaps (14.8%).
On average, patients had 25.7 outpatient visits, 5.6 inpatients visits, and 20.5 radiology tests per PY. Approximately 50% of patients were hospitalized during the follow‐up period (Table 4). Overall, laboratory tests accounted for the greatest component of HRU (613.4 PY). Rates of HRU tended to be higher (approximately 1.5–2 times higher) for patients with pNETs compared with patients with midgut or other NETs.
Table 4.
Health resource use, stratified by primary tumor location (n = 640)
| Overall (n = 640)Follow‐up, yrs, mean (SD): 2.7 (2.8) | Pancreatic NET (n = 178)Follow‐up, yrs, mean (SD): 2.4 (2.7) | Midgut NET (n = 290)Follow‐up, yrs, mean (SD): 2.9 (2.9) | Other NET (n = 172)Follow‐up, yrs, mean (SD): 2.5 (2.6) | |||||
|---|---|---|---|---|---|---|---|---|
| Resource | User of resources, n (%) | HRU per PY, mean (SD) | User of resources, n (%) | HRU per PY, mean (SD) | User of resources, n (%) | HRU per PY, mean (SD) | User of resources, n (%) | HRU per PY, mean (SD) |
| Inpatient visit | 330 (51.6) | 5.6 (14.1) | 91 (51.1) | 8.6 (20.4) | 157 (54.1) | 4.5 (9.7) | 82 (47.7) | 4.2 (12.1) |
| Outpatient visit | 640 (100) | 25.7 (17.5) | 178 (100) | 29.3 (18.9) | 290 (100) | 23.2 (16.1) | 172 (100) | 26.3 (17.5) |
| Lab test | 606 (94.7) | 613.4 (631.2) | 164 (92.1) | 823 (916.4) | 280 (96.6) | 528.2 (479.8) | 162 (94.2) | 548.5 (431.7) |
| Diagnostic and imaging test | 444 (69.4) | 4.9 (11.3) | 121 (68) | 7.3 (18.4) | 206 (71) | 4.1 (7.7) | 117 (68) | 3.8 (5) |
| Radiology test | 591 (92.3) | 20.5 (23.3) | 164 (92.1) | 25.2 (24.9) | 271 (93.4) | 18.2 (25) | 156 (90.7) | 19.8 (17) |
| Transfusion | 236 (36.9) | 3.3 (8.2) | 67 (37.6) | 5.8 (13.7) | 115 (39.7) | 2.1 (4.1) | 54 (31.4) | 2.8 (3.8) |
Abbreviations: HRU, health resource use; NET, neuroendocrine tumor; PY, patient‐year.
Discussion
This work represents one of the few available studies evaluating treatment sequence and HRU among patients with metastatic NETs. As anticipated, use of SSAs was common, with 83.1% of patients receiving treatment with an SSA in the first line. Our observations differ to some extent from prior studies, including an evaluation of a U.S. claims database 2009–2014, which found that among the patients with gastrointestinal NETs, 62% started SSA monotherapy in the first line 14. A second study using a U.S. oncology network EMR database reported that 77% of patients with metastatic GEP‐NETs initiated the SSA treatment in the first line 15. The more common use of SSAs in our population may reflect more rapid adoption of SSAs for use in tumor control, given the time horizon of our study and the availability of the PROMID 3 and CLARINET 4 data during the study period.
A second observation in our study is that SSAs were commonly continued throughout the treatment course. Overall, 257 patients continued treatment with SSAs beyond first‐line therapy, generally in combination with a second agent. Continuation of SSA therapy is generally necessary in patients with functional tumors, for continued control of symptoms of hormonal hypersecretion. A recent study also suggests that continued use of SSAs after treatment progression by transitioning patients from octreotide to lanreotide may result in disease stabilization 16.
We additionally found that dose escalation of SSAs in our cohort was relatively common. Approximately one third of patients received above‐standard doses of SSAs. Although we did not explore the reasons for dose escalation in this study, other studies have reported that the most common reason that higher‐than‐recommended doses of SSAs are used is for symptom control 5, 6, 17.
Cytotoxic chemotherapy was the second most commonly used treatment regimen in our analysis. Prior studies have demonstrated that cytotoxic chemotherapy is generally more effective in patients with pNETs than in other types of NET, and more common use of cytotoxic agents was observed in the pNET cohort 18, 19. Use of investigational agents was also high across all NET subtypes, particularly in the second‐ and third‐line settings. This high use of investigational agents likely reflects, in part, the fact that our data are drawn from an academic tertiary referral center. An additional observation, however, is that use of currently approved agents such as everolimus and sunitinib is relatively low. This likely reflects the fact that approval of these agents did not occur until 2011 and later, whereas our database reflects treatment patterns dating back to 2003. Lutathera (lutetium Lu 177 dotatate) was not approved for use in North America until 2017 and therefore is not included in this analysis.
Our analysis of HRU data revealed that diagnostic testing, including both laboratory testing and radiologic imaging, made up a high proportion of HRU in this patient population. Our observation that HRU is higher in patients with pNETs is consistent with the often more aggressive course of these malignancies, which was reflected in a study demonstrating that well‐differentiated pNETs have worse overall survival compared with the other types of NETs 20.
The findings from this study must be interpreted in the context of certain limitations. For the treatment patterns analysis, we assumed that patients continued SSA treatment unless there was evidence of discontinuation, which may have overestimated true SSA exposure. For the SSA dose escalation analysis, it also was not possible to explore dose escalations among patients not dispensed SSAs at DFCI. From our data, we were not able to determine the reasons for a change in treatment regimen or in dose escalation. We may have underestimated true HRU, as care received outside of DFCI or the Partners’ network would not be captured. Lastly, the results from this study are based on the experience from a single tertiary center in the northeastern U.S. and may not be generalizable to other centers or countries.
Conclusion
Results from this study suggest a clear preference for the use of an SSA as a first‐line treatment for patients with advanced NETs; in many cases, SSA treatment is continued throughout the course of treatment for patients. Escalation of SSA dose is also common, presumably for improved symptom control. Patients with advanced NETs commonly receive multiple courses of treatment incorporating both cytotoxic and molecularly targeted therapies, with patients who have advanced pNETs more commonly treated with cytotoxic regimens. Patients with pNETs also have higher HRU, consistent with a more aggressive clinical course. Our study suggests that, at a tertiary referral center, patients with advanced NETs receive multiple different treatment regimens; this pattern is likely to continue as new options become available.
Author Contributions
Conception/design: Jessica J. Jalbert, Roman Casciano, Jie Meng, Lauren K. Brais, Sonia J. Pulgar, Anthony Berthon, Jerome Dinet, Matthew H. Kulke
Provision of study material or patients: Lauren K. Brais, Matthew H. Kulke
Collection and/or assembly of data: Lauren K. Brais, Matthew H. Kulke
Data analysis and interpretation: Jessica J. Jalbert, Roman Casciano, Jie Meng, Sonia J. Pulgar, Anthony Berthon, Jerome Dinet, Matthew H. Kulke
Manuscript writing: Jessica J. Jalbert, Jie Meng, Sonia J. Pulgar, Matthew H. Kulke
Final approval of manuscript: Jessica J. Jalbert, Roman Casciano, Jie Meng, Lauren K. Brais, Sonia J. Pulgar, Anthony Berthon, Jerome Dinet, Matthew H. Kulke
Disclosures
Jie Meng: Analytica Laser Inc. (C/A, E); Roman Casciano: Analytica Laser Inc. (C/A, E), Ipsen (RF); Jessica J. Jalbert: Analytica Laser Inc. (E, OI); Sonia J. Pulgar: Ipsen Biopharmaceuticals (E), Ipsen (OI); Anthony Berthon: Ipsen Biopharmaceuticals (E); Jerome Dinet: Ipsen Biopharmaceuticals (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was funded by Ipsen. We thank all patients involved in the research as well as their caregivers, care team, and investigators and research staff in participating institutions. M.H.K. is currently affiliated with the Boston University School of Medicine and Boston Medical Center, Boston, MA. S.J.P is currently affiliated with Seattle Genetics, Bothell, WA.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Jie Meng, Email: jie.meng@certara.com.
Matthew H. Kulke, Email: Matthew.Kulke@bmc.org.
References
- 1. Fraenkel M, Kim M, Faggiano A et al. Incidence of gastroenteropancreatic neuroendocrine tumors: A systematic review of the literature. Endocr Relat Cancer 2014;21:R153–R163. [DOI] [PubMed] [Google Scholar]
- 2. Strosberg JR, Halfdanarson TR, Bellizzi AM et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017;46:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rinke A, Muller HH, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 4. Caplin ME, Pavel M, Cwikla JB et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 5. Strosberg J, Weber J, Feldman M et al. Above‐label doses of octreotide‐LAR in patients with metastatic small intestinal carcinoid tumors. Gastrointest Cancer Res 2013;6:81–85. [PMC free article] [PubMed] [Google Scholar]
- 6. Strosberg JR, Benson AB, Huynh L et al. Clinical benefits of above‐standard dose of octreotide LAR in patients with neuroendocrine tumors for control of carcinoid syndrome symptoms: A multicenter retrospective chart review study. The Oncologist 2014;19:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao JC, Shah MH, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao JC, Fazio N, Singh S et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): A randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raymond E, Dahan L, Raoul JL et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–513. [DOI] [PubMed] [Google Scholar]
- 10. Krug S, Gress TM, Michl P et al. The role of cytotoxic chemotherapy in advanced pancreatic neuroendocrine tumors. Digestion 2017;96:67–75. [DOI] [PubMed] [Google Scholar]
- 11. Ramirez RA, Beyer DT, Chauhan A et al. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. The Oncologist 2016;21:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strosberg J, El‐Haddad G, Wolin E et al. Phase 3 trial of 177Lu‐dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brabander T, van der Zwan WA, Teunissen JM et al. Long‐term efficacy, survival, and safety of [(177)Lu‐DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 2017;23:4617–4624. [DOI] [PubMed] [Google Scholar]
- 14. Benson AB III, Broder MS, Cai B et al. Real‐world treatment patterns of gastrointestinal neuroendocrine tumors: A claims database analysis. World J Gastroenterol 2017;23:6128–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiao X, Pulgar S, Boyd M et al. Treatment patterns and clinical outcomes in patients with metastatic gastroenteropancreatic neuroendocrine tumors treated in the community practice setting in the United States. Pancreas 2018;47:173–182. [DOI] [PubMed] [Google Scholar]
- 16. Saif MW, Parikh R, Ray D et al. Medical record review of transition to lanreotide following octreotide for neuroendocrine tumors. J Gastrointest Oncol 2019;10:674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao JC, Phan AT, Chang DZ et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low‐ to intermediate‐grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol 2008;26:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner NC, Strauss SJ, Sarker D et al. Chemotherapy with 5‐fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer 2010;102:1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strosberg JR, Fine RL, Choi J et al. First‐line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]


