Abstract
Lessons Learned
Weekly treatment with 5‐fluorouracil and cisplatin, concurrent with radiotherapy, achieved promising response rates in patients with postoperative recurrent esophageal squamous cell carcinoma.
Superior toxicity results were also found.
Background
Concurrent chemoradiotherapy (CCRT) is one of the treatment strategies for patients with esophageal squamous cell carcinoma (ESCC) with postoperative locoregional recurrence. However, the once every 3 weeks chemotherapy regimen causes a high incidence of toxicity. The aim of this study was to evaluate the efficacy and toxicity of weekly 5‐fluorouracil (5‐FU) and cisplatin concurrent with radiotherapy in postoperative locoregional recurrent ESCC.
Materials and Methods
Patients received four weekly chemotherapy cycles of cisplatin (25 mg/m2, day 1) plus 5‐FU (1,176 mg/m2, day 1–3), and concurrent with radiotherapy (50.4–60 Gy). The primary endpoint was objective response rate (ORR). Secondary objectives were toxicity, disease control rate (DCR), progression‐free survival (PFS), and overall survival (OS).
Results
Between January 2013 and December 2015, 48 patients were enrolled. The ORR was 68.8% (12 patients with complete response, 21 patients with partial response), with DCR 68.8%. No treatment‐related grade 4 adverse events occurred. Grade 3 hematologic toxicities were observed in eight (17%) patients. Grade 3 vomiting or esophagitis occurred in four (8%) patients each. The median PFS and OS were 13.94 months (95% confidence interval [CI], 0.75–51.05) and 27.43 months (95% CI, 5.278–49.58; Fig. 1).
Conclusion
Weekly 5‐FU and cisplatin concurrent with radiotherapy achieved a promising response rate and improved toxicity in patients with postoperative locoregional recurrent ESCC.
Discussion
ESCC is highly prevalent in China, which accounts for more than 90% of the overall esophageal carcinoma population. Compared with traditional single treatment therapies, multimodal approaches that include esophagectomy have significantly prolonged the survival of early or locally advanced stage ESCC; however, a large number of patients develop recurrent disease within 1 to 2 years after surgery, and the optimal treatment strategy has not been established. The standard management for locally advanced esophageal cancer is CCRT, and promising results have been observed with CCRT in postoperative recurrent ESCC 1, 2.
Despite the greater survival advantage obtained from CCRT compared with radiotherapy or chemotherapy alone, greater toxicities were observed too. Combination fluorouracil and cisplatin, a commonly used regimen in ESCC, had a complication rate of approximately 70%, the effect being that one‐third of patients did not finish the planned treatment 3. Thus, there is a need to explore a modified chemotherapy regimen that is not inferior in efficacy but favorable in toxicity.
Fluorouracil combined with cisplatin (PF) is the most commonly used regimen in ESCC. We previously reported in a retrospective analysis of a small cohort that weekly cisplatin or nedaplatin with 5‐fluorouracil (5‐FU), administered concurrently with radiotherapy, was well tolerated for recurrent ESCC after surgery, which suggested that the weekly regimen is a potential alternative to the traditional 3‐weeks internal regimen 4. Based on these findings, we conducted this phase II trial and tried to explore the safety and efficacy of the modified weekly cisplatin and 5‐FU regimen concurrent with radiation in postoperative recurrent ESCC.
This phase II study found expected rates of toxicity, mostly grade 1 or 2; grade 3 or higher hematological toxicities were observed in only 17% (n = 8) of patients. Moreover, only four (8%) and four (8%) patients presented with grade 3 vomiting or esophagitis, respectively, a reduction compared with the traditional 3‐weekly regimen. Only eight patients (17%) did not finish the planned treatment, relative to an expected one‐third proportion in the every‐3‐week regimen. A broad range of overall survival rates has been previously reported for CCRT for recurrent ESCC, with the median overall survival ranging from 7 to 43 months and the 2‐year OS rate ranging from 15% to 51.3%. We obtained satisfactory response rates of 29.8% and 59.7% in the 3‐year PFS and OS, respectively, with median PFS and OS rates of 13.94 months and 27.43 months, respectively, which was not inferior to previous studies using the PF regimen (Fig. 1).
Figure 1.
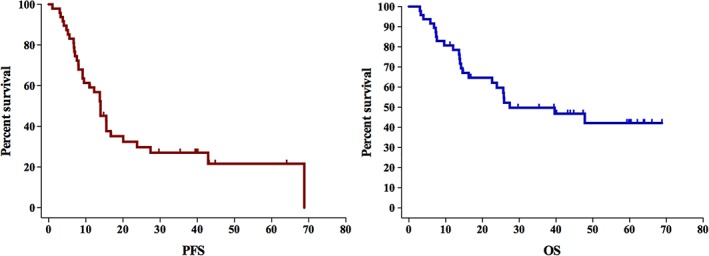
Overall survival (right) and progression‐free survival (left) of the whole cohort. As shown in the Kaplan‐Meier curves, the 1‐, 2‐, and 3‐year PFS rates for all patients were 56.9%, 29.8%, and 27%, respectively. The 1‐, 2‐, and 3‐year OS rates were 78.5%, 59.7%, and 49.7%, respectively. The median PFS was 13.94 months (95% confidence interval [CI], 0.75–51.05), and the median OS was 27.43 months (95% CI, 5.278–49.58).Abbreviations: OS, overall survival; PFS, progression‐free survival.
In conclusion, the weekly PF regimen achieved a relatively safe toxicity profile, with a satisfactory disease control rate in patients with postoperative locoregional recurrences of ESCC. A future phase III trial is warranted to confirm the efficacy and safety of the weekly regimen.
Trial Information
| Disease | Esophageal cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of study | Phase II, single arm |
| Primary Endpoint | Overall response rate |
| Secondary Endpoint | Toxicity |
| Secondary Endpoint | Disease control rate |
| Secondary Endpoint | Progression‐free survival |
| Secondary Endpoint | Overall survival |
| Additional Details of Endpoints or Study Design | |
| This study was conducted according to a protocol approved by the Sun Yat‐Sen University Cancer Center Human Research Ethical Committee. Informed consent was obtained from each enrolled patients prior to enrollment in the study. | |
| Eligibility Criteria: | |
| All enrolled patients had histopathologically confirmed ESCC. The inclusion criteria were as following: (a) Eastern Cooperative Oncology Group (ECOG) performance status scores from 0–2; (b) age from 18 to 75 years; (c) R0 resection for primary cancer with two‐incision esophagectomy or three‐incision esophagectomy; and (d) local postoperative recurrence comprehensively confirmed by endoscopy, ultrasonography, computed tomography (CT), physical examination, and/or biopsy. Local recurrence was defined as recurrence at anastomosis with or without regional lymph nodes or regional lymph nodes alone; (a) no distant metastasis; (b) no previous radiotheraphy (RT); and (c) no serious cardiac, liver, pulmonary, or renal disease. | |
| Study Design: | |
| Radiotherapy regimen: All patients received three‐dimensional conformal radiation therapy, intensity‐modulated radiation therapy, or Tomo treatment plan conducted by Pinnacle or Monacle planning system. A linear accelerator (6MV) was used as the X‐ray source. The prescribed total dose was from 50.4 to 60 Gy and the daily fractional dose was 2.0 Gy administered 5 days per week. Modification of the total dose was allowed according to individuals' target volume planning. The immobilization and simulation were performed according to the standard protocol established in our department. For treatment planning, briefly, the gross tumor volume (GTV) included the recurrent tumor or lymph nodes present in previous CT scans, positron emission tomography‐CT, or endoscopy. The clinical target volume (CTV) comprised the anastomosis, supraclavicular, and regional lymph nodes. PTV1 was defined as the GTV plus a 0.5‐cm margin, and PTV2 was defined as the CTV plus a 0.5‐cm margin in all directions, respectively. The spinal cord dose was not to exceed 46.0 Gy. Doses to normal lung tissue were calculated by dose‐volume histograms,with a mean lung dose less than 17 Gy and V20 less than 30%. | |
| Concurrent chemotherapy regimen: Continuous infusion cisplatin 25mg/m2 over 2h on day 1 and 5‐FU 1176/m2 over 72h on days 1–3 repeated weekly for 4 weeks. If grade 3 or greater toxicities occurred, the discontinuation or dose‐reduced chemotherapy will be considered. The treatment protocol schedule is shown in Figure 2. The primary endpoint was tumor response, and the secondary endpoints were survival rate and toxicity. | |
| Response, Toxicity, and Survival: During concurrent chemoradiotherapy, complete blood count and serum chemistry profile were examined each week to monitor for adverse events. Barium‐swallow examination and contrast‐enhanced CT scan with or without endoscopy were performed for response assessment at two months after the CCRT. RECIST criteria were used to determine response. A senior radiologist and a radiation oncologist independently evaluated the tumor response by a comprehensive review of the CT scan and endoscopy. The clinical response rate was defined as the proportion of patients with a complete response (CR) or partial response (PR). All patients were monitored regularly for acute and chronic toxicities during treatment and during follow‐up, which were graded according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE 3.0). After the CCRT, patients were first followed up at 2 months, then every 3 months for the first year, every 6 months for 2 years, and then annually to record the progression of the disease and survival. PFS was defined as the date from treatment to tumor progression or death. OS was measured from treatment to date of death by any cause. | |
| Statistical analysis: The SPSS 22.0 software program was used for analyses. The Kaplan‐Meier method was used to analyze the PFS and OS rate. The quantitative results are expressed as medians and ranges. Statistical comparisons between the two groups were performed with χ2 statistics. A value of p < .05 was considered to be significant. | |
| Investigator's Analysis | Active and should be pursued further |
Figure 2.
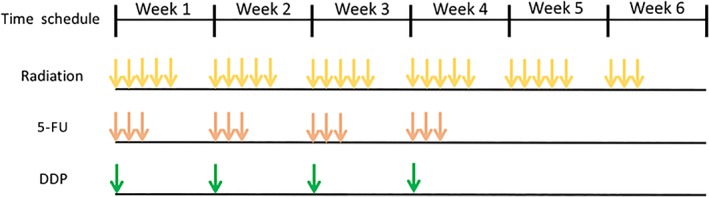
A total dose of 50.4–60 Gy radiation was daily administered at 2.0 Gy for 5 days per week. Concomitant chemotherapy is the continuous infusion of DDP 25 mg/m2/2 hours on day 1 and 5‐FU 1176/m2/72 hours on day 1–3 by repeated weekly for 4 weeks.Abbreviations: DDP, cisplatin; 5‐FU, 5‐fluorouracil.
Drug Information
| Drug 1 | |
| Generic/Working Name | 5‐fluorouracil |
| Trade Name | Fluorouracil injection |
| Company Name | Shanghai Xudong Haipu Pharmaceutical Co., Ltd |
| Drug Type | Small molecule |
| Drug Class | Antimetabolite |
| Dose | 1,176 mg/m2 |
| Route | Continuous intravenous infusion |
| Schedule of Administration | Days 1–3, weekly for 4 weeks |
| Drug 2 | |
| Generic/Working Name | Cisplatin |
| Trade Name | Nuoxin |
| Company Name | Hansoh Phamar |
| Drug Type | Small molecule |
| Drug Class | Platinum compound |
| Dose | 25 mg/m2 |
| Route | Intravenous |
| Schedule of Administration | Day 1, weekly for four weeks |
Patient Characteristics
| Number of Patients, Male | 37 |
| Number of Patients, Female | 11 |
| Stage (TNM, Postoperative) | |
| I | 2 (4.2) |
| II | 18 (37.5) |
| III | 28 (58.3) |
| Age | Median (range): 59 (44–75) |
| Number of Prior Systemic Therapies | Median: 1 |
| Performance Status: ECOG |
0 — 0 1 — 40 2 — 8 3 — 0 Unknown — 0 |
| Other | Consistent with the epidemiology of ESCC, the majority of enrolled patients were male (37/77.1%). The median age was 59 years (range, 44–75). Among these patients, 2 (4.2%), 18 (37.5%), and 28 (58.3%) were diagnosed with primary stage I, II, and III ESCC, respectively, after surgery, with a median of 14 months (2–133) relapse‐free survival after surgery. Most (31/64.6%) patients had only regional lymph node recurrence, whereas 11 (24.1%) patients had only anastomotic recurrence, and 6 (12.5%) patients had recurrence at both sites. |
| Additional Clinical Characteristics of Patients | n (%) |
| Smoking | |
| No | 20 (41.7) |
| Yes | 28 (58.3) |
| Drinking | |
| Constantly | 18 (37.5) |
| Never or seldom | 30 (62.5) |
| Tumor location | |
| Upper | 6 (12.5) |
| Middle | 31 (64.4) |
| Lower | 9 (18.8) |
| Multiple | 4 (8.3) |
| Primary radical surgery | |
| Two‐incision oesophagectomy | 18 (47.9) |
| Three‐incision oesophagectomy | 30 (52.1) |
| Postoperative complications | |
| No | 39 (81.3) |
| Anastomotic fistula | 6 (13) |
| Tracheoesophageal fistula | 2 (4) |
| Chylothorax | 1 (2) |
| RFS | |
| Median | 14 (2–133) |
| ≥12 m | 26 (47.9) |
| <12 m | 22 (52.1) |
| Recurrence pattern | |
| Anastomisis | 11 (22.9) |
| Regional lymph nodes | 31 (64.6) |
| Anastomisis combined with regional lymph nodes | 6 (12.5) |
| Abbreviation: RFS, relapse free survival. | |
| Cancer Types or Histologic Subtypes | Esophageal squamous cell carcinoma, 48 |
Primary Assessment Method
| Title | Final assessment |
| Number of Patients Screened | 97 |
| Number of Patients Enrolled | 48 |
| Number of Patients Evaluable for Toxicity | 48 |
| Number of Patients Evaluated for Efficacy | 48 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 12 (25%) |
| Response Assessment PR | n = 21 (43.8%) |
| Response Assessment SD | n = 7 (14.6%) |
| Response Assessment PD | n = 8 (16.7%) |
| Response Assessment OTHER | n = 0 (0%) |
| (Median) Duration Assessments PFS | 13.94 months, CI: 0.75–51.05 |
| (Median) Duration Assessments OS | 27.43 months, CI: 5.278–49.58 |
| Outcome Notes | The median prescribed radiation dose was 56 Gy (50.4–60 Gy), with 1.8–2.3 Gy per fraction. Twelve patients had a complete response (12/48, 25%), 21 patients had a partial response (21/48, 43.8%), and the other 7 patients had stable disease (7/48, 14.6%), yielding an ORR (CR + PR) and DCR (CR + PR + SD) at 68.8% and 83.3%, respectively (Table 1). |
| The median follow‐up was 29.5 months (range, 3.2–68). At the time of this analysis, 24 patients had died of cancer or any other cause, 5 patients were alive with the recurrent tumor, and the remaining 19 patients had no evidence of disease progression. As shown in the Kaplan‐Meier curves, the 1‐, 2‐, and 3‐year PFS rates for all patients were 56.9%, 29.8%, and 27%, respectively. The 1‐, 2‐, and 3‐year OS rates are 78.5%, 59.7%, and 49.7%, respectively. The median PFS was 13.94 months (95% CI, 0.75–51.05) and the median OS was 27.43 months (95% CI, 5.278–49.58; Fig. 2). Analysis of the prognostic factor identified only treatment response to be a significant predictor for PFS (hazard ratio, 4.859; 95% CI, 1.610–14.669, p = .005; Table 2). No clinical characteristic was found to be associated with the OS. |
Table 1.
Treatment information and tumor response (n = 48)
| Variable | n (%) |
|---|---|
| Radiotherapy techniques | |
| 3D‐CRT | 14 (29.2) |
| IMRT | 33 (68.8) |
| Tomo | 1 (2.1) |
| Radiation dose (Gy) | |
| Median (range) | 56 (50.4–60) |
| Tumor response | |
| CR | 12 (25) |
| PR | 21 (43.7) |
| SD | 7 (14.6) |
| PD | 8 (16.7) |

Abbreviations: 3D‐CRT, three‐dimensional conformal radiation therapy; CR, complete response; IMRT, intensity‐modulated radiation therapy; PD, progression disease; PR, partial response; SD, stable disease.
Table 2.
Univariate analysis of prognostic factors for PFS (n = 48)
| Variable | HR (95% CI) | p value |
|---|---|---|
| Gender (male vs. female) | 1.055 (0.456‐2.439) | .9 |
| Age (<60 vs. ≥60) | 0.554 (0.274‐1.120) | .1 |
| Smoking history (no vs. yes) | 1.287 (0.639‐2.594) | .479 |
| Drinking history (no vs. yes) | 1.665 (0.838‐3.309) | .146 |
| TNM stage (postoperative; I–II vs. III) | 0.645 (0.325‐1.280) | .21 |
| RFS (<12 m vs. ≥12 m) | 0.876 (0.441‐1.741) | .707 |
| Radiation dose (≤60 vs. >60) | 0.609 (0.299‐1.240) | .172 |
| Response | ||
| CR | reference | |
| PR | 1.253 (0.477‐3.290) | .647 |
| SD | 2.154 (0.640‐7.253) | .215 |
| PD | 4.859 (1.610‐14.669) | .005 |
| Recurrence pattern | ||
| Anastomosis | Reference | |
| Regional lymph nodes | 1.788 (0.729‐4.389) | .204 |
| Anastomosis with regional lymph nodes | 1.211 (0.302‐4.854) | .787 |
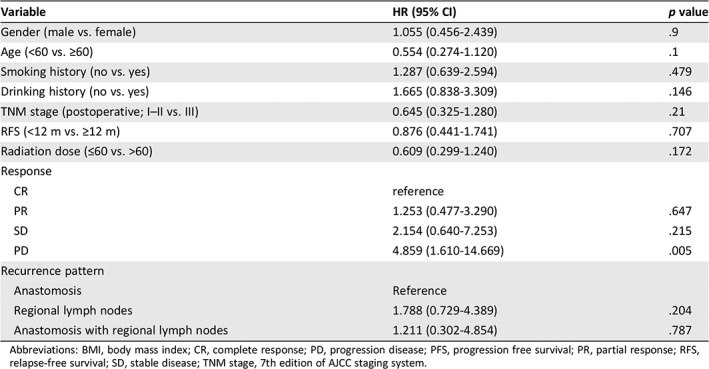
Abbreviations: BMI, body mass index; CR, complete response; PD, progression disease; PFS, progression free survival; PR, partial response; RFS, relapse‐free survival; SD, stable disease; TNM stage, 7th edition of AJCC staging system.
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
| White blood cell decreased | 26% | 19% | 42% | 13% | 0% | 0% | 74% |
| Platelet count decreased | 58% | 19% | 21% | 2% | 0% | 0% | 42% |
| Anemia | 41% | 40% | 17% | 2% | 0% | 0% | 59% |
| Vomiting | 13% | 69% | 10% | 8% | 0% | 0% | 87% |
| Esophagitis | 11% | 58% | 23% | 8% | 0% | 0% | 89% |
| ALT, serum glutamic pyruvic transaminase | 87% | 13% | 0% | 0% | 0% | 0% | 13% |
| AST, serum glutamic oxaloacetic transaminase | 83% | 15% | 0% | 2% | 0% | 0% | 17% |
| Pneumonitis/pulmonary infiltrates | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Cardiac arrhythmia—atrial fibrillation | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
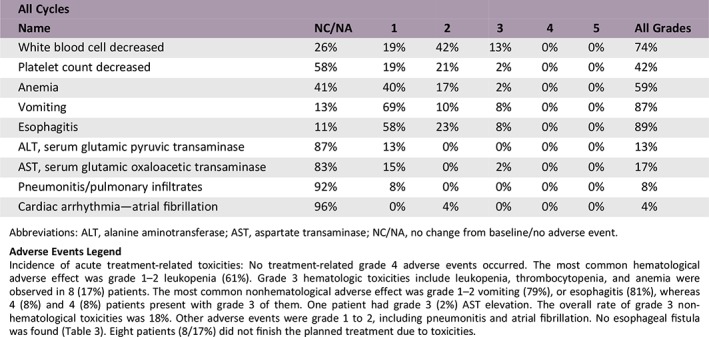
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; NC/NA, no change from baseline/no adverse event.
Adverse Events Legend
Incidence of acute treatment‐related toxicities: No treatment‐related grade 4 adverse events occurred. The most common hematological adverse effect was grade 1–2 leukopenia (61%). Grade 3 hematologic toxicities include leukopenia, thrombocytopenia, and anemia were observed in 8 (17%) patients. The most common nonhematological adverse effect was grade 1–2 vomiting (79%), or esophagitis (81%), whereas 4 (8%) and 4 (8%) patients present with grade 3 of them. One patient had grade 3 (2%) AST elevation. The overall rate of grade 3 nonhematological toxicities was 18%. Other adverse events were grade 1 to 2, including pneumonitis and atrial fibrillation. No esophageal fistula was found (Table 3). Eight patients (8/17%) did not finish the planned treatment due to toxicities.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Definitive esophagectomy with or without neoadjuvant chemoradiotherapy is the main therapeutic approach for early or locally advanced stage esophageal squamous cell carcinoma (ESCC). Despite the promising survival achieved by comprehensive multidisciplinary modalities, disease recurrence after curative esophagectomy is frequent. Locoregional relapse, defined as the recurrence of local or regional lymph nodes, is the most common failure pattern, which accounts for 22%–59.7% of patients with recurrent disease 5, 6, 7, 8. The recurrence of regional lymph nodes accounted for more than the local anastomotic recurrence, which was also observed in this study 7, 9.
Substantial evidence suggested that patients with postoperative recurrence of ESCC who received multimodal therapy would have longer survival compared with patients with best supportive care 10, 11. Despite several therapeutic modalities including salvage surgery, radiation therapy, chemotherapy, and concurrent chemoradiotherapy (CCRT), a consensus treatment approach has not been established. Individual therapy is decided by physicians based on patients’ clinical condition and treatment history. Salvage resection has been proven to prolong survival in patients with recurrence of particularly solitary neck lymph nodes, however, only a small portion of patients are eligible for salvage resection. Hiyoshi et al. reported that only ~14% of patients have a chance to undergo re‐resection 12. It was also recommended that repeat surgery be followed by radiation with or without chemotherapy to further improve the overall survival 12, 13. Thus, multiple disciplines including radiotheraphy (RT), chemotherapy, or CCRT are generally used in a majority of recurrent cases.
Instead of RT alone, CCRT has been recommended as the standard management for locally advanced or unresectable esophageal cancer without distant metastasis. However, whether CCRT is superior to radiation alone for postoperative recurrent esophageal cancer has not yet been determined. Recently, several studies have indicated that in comparison to RT alone, CCRT achieves a better response and longer survival. A retrospective study suggested that concurrent RT with two or more cycles of 5‐fluorouracil (5‐FU) plus cisplatin chemotherapy achieved a better objective response rate (97% vs. 76%). Furthermore, the median overall survival (OS) in CCRT was 17 months (95% CI, 13.61–20.39), which was significantly longer than that in the RT alone group (9 months, 95% CI, 6.96–11.04; p < .05). As expected, the acute toxicities were more frequent in CCRT groups 14. Similar results were reported in a phase II study that compared RT alone with RT plus weekly cisplatin CCRT for recurrent ESCC 15. Thus, CCRT was a promising approach for accomplishing disease control.
Fluorouracil combined with cisplatin (PF) is one of the recommended definitive concurrent chemotherapy regimens for ESCC in the National Comprehensive Cancer Network guidelines 16. The recommended standard regimen consists of cisplatin 75–100 mg/m2 on day 1 and 5‐FU 750–1,000 mg/m2 intravenous continuous infusion over 24 hours daily on days 1–4, repeated every 28 days for 2–4 cycles 17, 18. It is also the most commonly reported concurrent chemotherapy regimen for recurrent ESCC 1, 2, 14, 19, 20. Despite the survival advantage in CCRT, there was a higher toxicity rate was observed. According to previous studies, the grade 3–4 adverse event occurred in around 30%–40% of patients. We reviewed data from published studies and summarized the results in Table 3 1, 2, 4, 14, 19. Generally, 21%–36% of patients would have developed grade 3–4 hematological toxicities including leukopenia, anemia, and thrombocytopenia during treatment. Concerning the nonhematological adverse events, vomiting and nausea occurred in 7%–52% of patients.
Table 3.
The published studies of PF regimen concurrent with radiotherapy in postoperative recurrent ESCC
| Reference | Trial type | n | Groups | Chemotherapy regimen | Radiation dose, Gy | ORR, % | mPFS | mOS | Survival rate | Hematological toxicities | Nonhematological toxicities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jingu et al., 2006 2 | Phase II | 30 | RT+PF (31) | Nedaplatin: 70 mg/m2/2 h; 5‐FU: 500 mg/m2/24 h D1–5; 3 wk; 2 cycles | 60 | 73.70 | NA | 39 | 1‐y OS: 60.6%; 3‐y OS: 56.3% 1‐y PFS: 53.4%; 3‐y PFS:35.6% | Grade 3–4: 10 (33.3%) | Grade 3–4: 3 (10%) |
| Lu et al., 2010 14 | Retrospective | 73 | RT (42) | NA | 60 | 76 | NA | 9 | 1‐y OS: 33.8%; 3‐y OS: 0% | 0 | 0 |
| RT+PF (31) | 5‐FU: 750 mg/m2 on D1–5; cisplatin: 20 mg/m2 on D1–5 or 40 mg/m2 on D1–3; ≥2 cycles | 97 | 17 (p = .049) | 1‐y OS: 62.5%; 3‐y OS: 10.5% | Grade 3–4: 9 (29%) | Grade 3–4: 16 (52%) | |||||
| Bao et al., 2013 1 | Retrospective | 83 | RT+PF (39) |
1. Cisplatin: 60 mg/m2 on D1, 29; 5‐FU: 300 mg/m2 on D1–3 and 29–31; 2 cycles; 2. Cisplatin: 30 mg/m2 with 5‐FU: 500 mg/m2 on D1–5 and 29–33; 2 cycles; |
60 (56–68) | 75.9 | NA | 43 | 3‐y OS: 43.3% | Grade 3–4: 6 (23.1%) | Grade 3 vomiting: 8 (30.8%); grade 3 esophagitis: 2 (5.1%) |
| RT+TP (44) |
1. Docetaxel: 60 mg/m2, and cisplatin: 80 mg/m2 on D1,29: 2 cycles; 2. Docetaxel: 30 mg/m2 on D1; cisplatin: 30 mg/m2 on D1; weekly for 4–6 wk (n = 15) |
3‐y OS: 59.2% | Grade 3–4: 11 (36.7%) | Grade 3 vomiting: 10 (33.3%); grade 3 esophatitis: 3 (6.8%) | |||||||
| Zhang et al., 2012 19 | Retrospective | 50 | RT+PF (22) | 5‐FU: 500 mg/m2 on D1–D5; cisplatin: 75 mg/m2 on D1; 4 wk; 1–2 cycles | 60 (50.4–64) | 72.7 | 9.8 | 9.8 | NA | Grade 3–4: 7 (31.8%) | Grade 3–4: 6 (27.3%) |
| RT+TP (28) | Paclitaxel: 135 mg/m2 on D1; cisplatin: 75 mg/m2 on D1; 3 wk; 1–2 cycles | 71.7 | 9.8 | 16.3 (p = .012) | NA | Grade 3–4: 6 (21.4%) | Grade 3–4: 8 (28.6%) | ||||
| Zhang et al., 2015 4 | Retrospective | 27 | RT+CF (13) | Cisplatin: 25 mg/m2 on D1, 5‐FU: 300 mg/d on D 1–3, weekly for 4 wk | 56 (46.0–60.0) | 70.4 | 16.4 | 26 | 1‐y OS: 88.9%; 2‐y OS: 60.2%; 3‐y OS: 43.3% | Grade 3: 3 (21.4%) | Grade 3: 3 (7.1%) |
| RT+NF (14) | Nedaplatin: 25 mg/m2 on D1, 5‐FU: 300 mg/d on D1–3, weekly for 4 wk |
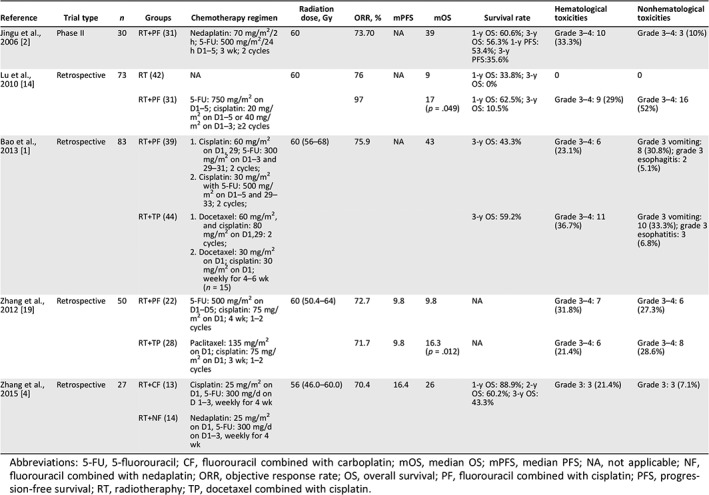
Abbreviations: 5‐FU, 5‐fluorouracil; CF, fluorouracil combined with carboplatin; mOS, median OS; mPFS, median PFS; NA, not applicable; NF, fluorouracil combined with nedaplatin; ORR, objective response rate; OS, overall survival; PF, fluorouracil combined with cisplatin; PFS, progression‐free survival; RT, radiotheraphy; TP, docetaxel combined with cisplatin.
Because of the accompanying postsurgery complication or the poor performance status after surgery, the compliance to regular CCRT for patients with recurrent disease is usually worse than the patients without previous treatment. Also, high toxicity rate is correlated with poor quality of life and compliance in patients. Thus, emerging studies have been conducted trying to modify the traditional PF regimen to reduce adverse events. Daily low‐dose platinum‐based regimens, which originated from the clinical practice in non‐small‐cell lung cancer, is an alternative option and frequently administered for patients with ESCC, especially in Japan 21, 22. The modified daily low‐dose regimen increased the efficiency by promoting radiation sensitivity but with a reduction in toxicity compared with regular concurrent chemotherapy. One study suggested that continuous infusion of 200 mg/m2 5‐FU combined with 4 mg/m2 cisplatin with concurrent radiotherapy for ESCC achieved an 8‐year OS and progressionfree survival (PFS) at 63.4% and 49.8%. The incidence of grade ≥ 3 adverse events was lower, in which the commonly observed adverse event was leukopenia (22%) 23. However, a phase III study comparing the low‐dose by to standard‐dose chemotherapy for unresectable ESCC indicated no difference in toxicities.
Thus, new approaches that are equivalent in effectiveness but safer warrant to be further developed. The modified regimen with the weekly schedule of chemotherapy have shown promise. Weekly cisplatin‐based CCRT has been applied in head and neck cancer and cervical cancer 24, 25, 26. The chemotherapy regimens of ESCC are similar to that in head and neck cancer, suggesting that the weekly PF regimen might be a promising approach in ESCC. Jeong et al. reported that weekly 5‐FU (1,000 mg/m2) and cisplatin (30mg/m2) based on definitive CCRT is tolerable and showed noninferiority with conventional PF in CCRT for untreated esophageal cancer 27. We previously reported on a retrospective analysis of the safety and effectiveness of weekly 5‐FU with cisplatin to replace the traditional regimen 4. The overall grade 3 treatment‐related hematological and nonhematological toxicities were 21.4% and 7.1%, which were similar to the daily low‐dose regimen but lower than regular PF chemotherapy.
In the current study, we aimed to explore the safety and survival of the modified weekly PF regimen in a phase II study and found expected rates of toxicity, with the majority of them being grade 1 or 2; grade 3 or higher hematological toxicities were only observed in 17% of patients. Eight patients (8/17%) did not finish the planned treatment, which was less than in previous studies. We obtained satisfactory response rates in the 3‐year PFS and OS of 29.8% and 59.7%, with a median PFS and OS of 13.94 and 27.43 months, respectively, which was comparable to previous studies using the PF regimen. Previously reported overall survival is quite varied across studies, with the median overall survival time ranging from 7 to 43 months and the 2‐year OS rate ranging from 15% to 51.3% (Table 3). Bao et al. reported a median OS of 43.0 months, which was relatively longer than other studies. Indeed, more than half of the patients in this study received the TP regimen, which might explain the advantage in OS obtained with the whole cohort, in concordance with a report suggesting that TP was superior to PF in ESCC 1.
Compared with the traditional 3‐weekly schedule of PF chemotherapy, the weekly regimen achieved a relatively safe toxicity profile but a comparable disease control rate in patients with postoperative locoregional recurrence of ESCC. A future phase III trial with a large sample size may confirm the efficacy and safety of weekly regimen in these patients.
Disclosures
The authors indicated no financial relationships.
Figure and Tables
Acknowledgments
This work was supported by Guangdong Esophageal Cancer Institute Science and Technology Program (No.Q201807).
Footnotes
- Sponsor: Sun Yat‐sen University Cancer Center
- Principal Investigator: Yujia Zhu
- IRB Approved: Yes
Contributor Information
Yonghong Hu, Email: huyh@sysucc.org.cn.
Yujia Zhu, Email: zhuyuj@sysucc.org.cn.
References
- 1. Bao Y, Liu S, Zhou Q et al. Three‐dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: Clinical efficacy and failure pattern. Radiat Oncol 2013;8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jingu K, Nemoto K, Matsushita H et al. Results of radiation therapy combined with nedaplatin (cis‐diammine‐glycoplatinum) and 5‐fluorouracil for postoperative locoregional recurrent esophageal cancer. BMC Cancer 2006;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy T, Galais MP, Raoul JL et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): Final results of a randomised, phase 2/3 trial. Lancet Oncol 2014;15:305–314. [DOI] [PubMed] [Google Scholar]
- 4. Zhang WW, Zhu YJ, Yang H et al. Concurrent radiotherapy and weekly chemotherapy of 5‐fluorouracil and platinum agents for postoperative locoregional recurrence of oesophageal squamous cell carcinoma. Sci Rep 2015;5:8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamashita K, Watanabe M, Mine S et al. Patterns and outcomes of recurrent esophageal cancer after curative esophagectomy. World J Surg 2017;41:2337–2344. [DOI] [PubMed] [Google Scholar]
- 6. Lou F, Sima CS, Adusumilli PS et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol 2013;8:1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyata H, Yamasaki M, Kurokawa Y et al. Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Ann Surg Oncol 2011;18:3353–3361. [DOI] [PubMed] [Google Scholar]
- 8. Nakajima Y, Kawada K, Tokairin Y et al. Prognostic factors for post‐recurrence survival in patients with thoracic esophageal squamous cell carcinoma after curative resection. Dig Surg 2016;33:136–145. [DOI] [PubMed] [Google Scholar]
- 9. Doki Y, Ishikawa O, Takachi K et al. Association of the primary tumor location with the site of tumor recurrence after curative resection of thoracic esophageal carcinoma. World J Surg 2005;29:700–707. [DOI] [PubMed] [Google Scholar]
- 10. Hsu PK, Wang BY, Huang CS et al. Prognostic factors for post‐recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg 2011;15:558–565. [DOI] [PubMed] [Google Scholar]
- 11. Sugiyama M, Morita M, Yoshida R et al. Patterns and time of recurrence after complete resection of esophageal cancer. Surg Today 2012;42:752–758. [DOI] [PubMed] [Google Scholar]
- 12. Hiyoshi Y, Morita M, Kawano H et al. Clinical significance of surgical resection for the recurrence of esophageal cancer after radical esophagectomy. Ann Surg Oncol 2015;22:240–246. [DOI] [PubMed] [Google Scholar]
- 13. Motoyama S, Kitamura M, Saito R et al. Outcome and treatment strategy for mid‐ and lower‐thoracic esophageal cancer recurring locally in the lymph nodes of the neck. World J Surg 2006;30:191–198. [DOI] [PubMed] [Google Scholar]
- 14. Lu JC, Kong C, Tao H. Radiotherapy with or without concurrent chemotherapy for lymph node recurrence after radical surgery of thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2010;78:710–714. [DOI] [PubMed] [Google Scholar]
- 15. Ma DY, Tan BX, Liu M et al. Concurrent three‐dimensional conformal radiotherapy and chemotherapy for postoperative recurrence of mediastinal lymph node metastases in patients with esophageal squamous cell carcinoma: A phase 2 single‐institution study. Radiat Oncol 2014;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ajani JA, D'Amico TA, Bentrem DJ et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:855–883. [DOI] [PubMed] [Google Scholar]
- 17. Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002;20:1167–1174. [DOI] [PubMed] [Google Scholar]
- 18. Herskovic A, Martz K, al‐Sarraf M et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–1598. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Peng F, Li N et al. Salvage concurrent radio‐chemotherapy for post‐operative local recurrence of squamous‐cell esophageal cancer. Radiat Oncol 2012;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jingu K, Matsushita H, Takeda K et al. Long‐term results of radiotherapy combined with nedaplatin and 5‐fluorouracil for postoperative loco‐regional recurrent esophageal cancer: Update on a phase II study. BMC Cancer 2012;12:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakai K, Inakoshi H, Sueyama H et al. Concurrent radiotherapy and chemotherapy with protracted continuous infusion of 5‐fluorouracil in inoperable esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 1995;31:921–927. [DOI] [PubMed] [Google Scholar]
- 22. van Harskamp G, Boven E, Vermorken JB et al. Phase II trial of combined radiotherapy and daily low‐dose cisplatin for inoperable, locally advanced non‐small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1987;13:1735–1738. [DOI] [PubMed] [Google Scholar]
- 23. Kumabe A, Fukada J, Kota R et al. Long‐term results of concurrent chemoradiotherapy with daily‐low‐dose continuous infusion of 5‐fluorouracil and cisplatin (LDFP) for stage I‐II esophageal carcinoma. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 24. Guan J, Zhang Y, Li Q et al. A meta‐analysis of weekly cisplatin versus three weekly cisplatin chemotherapy plus concurrent radiotherapy (CRT) for advanced head and neck cancer (HNC). Oncotarget 2016;7:70185–70193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu HJ, Yang CC, Wang LW et al. Modified weekly cisplatin‐based chemotherapy is acceptable in postoperative concurrent chemoradiotherapy for locally advanced head and neck cancer. Biomed Res Int 2015;2015:307576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen X, Zou H, Li H et al. Weekly versus triweekly cisplatin‐based chemotherapy concurrent with radiotherapy in the treatment of cervical cancer: A meta‐analysis. Int J Gynecol Cancer 2017;27:344–349. [DOI] [PubMed] [Google Scholar]
- 27. Jeong JW, Yang JH, Ro SM et al. The efficacy and feasibility of weekly 5‐FU and cisplatin based radical concurrent chemoradiation therapy(CRT) in patients with esophageal cancer. 2017;35:169a. [Google Scholar]


