Abstract
Background
In the absence of randomized controlled trials, real‐world evidence may aid practitioners in optimizing the selection of therapy for patients with cancer. The study's aim was to determine real‐word use, as well as compare effectiveness, of single‐agent and combination chemotherapy as palliative treatment for female patients with metastatic breast cancer (mBC).
Materials and Methods
Using administrative claims data from the Symphony Health's Integrated Oncology Dataverse, female patients with mBC treated with at least one chemotherapy‐only treatment (COT) between January 1, 2013, and December 31, 2017 were selected. The frequency of use of single‐agent versus combination chemotherapy overall and by line of therapy (LOT) was calculated whereas effectiveness was measured using time to next treatment (TNT).
Results
A total of 12,381 patients with mBC were identified, and 3,777 (31%) received at least one line of COT. Of the 5,586 observed LOTs among the 3,777 patients, 66.5% were single‐agent and 33.5% combination chemotherapy. Combination chemotherapy was most frequently used in first‐line (45%) and least frequently in fifth‐line (16%). Across all LOTs, median TNT was significantly longer for single‐agent versus combination chemotherapy (5.3 months vs. 4.1 months, p < .0001). Comparison of median TNT by LOT showed significance in third‐line and greater but not in first‐line or second‐line. Among single agents, the median TNT for patients receiving capecitabine was longest in comparison to all other single agents.
Conclusions
The frequency of combination COT use, particularly in first‐line, warrants further research given published guideline recommendations. The observed TNT difference favoring single‐agent treatment in later lines supports guideline recommendations. Variance between single‐agent preference and observed TNT was noteworthy.
Implications for Practice
Although published data from evidence‐ and consensus‐based guidelines recommend single‐agent over combination chemotherapy, the extensive list of agents available for use and a gap in the comparative effectiveness research of these agents have resulted in significant variances in patterns of care. The aim of this study was to assess real‐world treatment patterns and their effectiveness during palliative therapy of metastatic breast cancer. The objective was to understand when and how chemotherapy‐only treatment is used in metastatic breast cancer and whether comparative effectiveness analysis supports the observed patterns of care.
Keywords: Breast cancer, Disease progression, Treatment outcomes, Comparative effectiveness research
Short abstract
This article analyzes the use of palliative chemotherapy in metastatic breast cancer, in aggregate and by cancer subtype, to better understand the effectiveness of single‐agent versus combination chemotherapy among patients in the real‐world setting.
Introduction
Despite a spate of recent drug approvals with novel mechanisms of action, traditional cytotoxic chemotherapy remains a frequent and guideline‐recommended practice in the treatment of metastatic breast cancer (mBC) 1, 2 Although hormonal, targeted, and immuno‐oncology (IO) drugs have become recommended first‐line (1L) therapy in pathologically defined subsets of mBC, multiple lines of chemotherapy‐only treatment (COT) remain common for a considerable number of patients with mBC. These patients are typically symptomatic, are “triple‐negative” (estrogen, progesterone, and human epidermal growth factor receptor‐2 [HER2]/neu negative), have progressive bone and/or visceral disease, or have become refractory to endocrine therapy 3. The use of combination chemotherapy to rapidly reduce cancer burden may be warranted in a minority of patients experiencing significant and/or life‐threatening symptoms; however, sequential single‐agent chemotherapy is the guideline‐recommended standard of care because of lower risk of toxicity, better quality of life, and noninferiority in survival 4.
Randomized controlled trials (RCTs) have demonstrated superiority of novel targeted therapies in 1L over traditional cytotoxic chemotherapy across all patients with mBC with specific mutations or markers including HER2 targeted drugs for HER2‐positive (HER2+) patients and cyclin‐dependent kinase (CDK) 4/6 inhibitors in combination with aromatase inhibitors (and fulvestrant) for hormone receptor‐positive (HR+) patients 5, 6. Most recently, programmed cell death ligand 1 (PD‐L1) inhibitors in combination with chemotherapy have demonstrated superiority for patients with triple‐negative mBC (TNmBC) 7. A similar standardized approach to treatment selection following 1L disease progression has not been derived by clinical trial or consensus. Such a task is complicated by the more than 60 approved single‐agent and combination regimens listed as treatment options for palliative intent chemotherapy of mBC 8. The pivotal RCTs leading to U.S. Food and Drug Administration approvals of this extensive list of single and combination agents evaluated a variety of clinical endpoints in heterogeneous populations of patients with mBC (e.g., extent of prior therapy) 9, 10, 11, 12, 13. The result of this gap in evidence‐based medicine (EBM) is that providers must rely on their personal experience and training, without an objective referee, to make treatment selections. This in turn contributes to significant variances in patterns of care and sequencing of palliative therapy of mBC.
All stakeholders (patients, providers, and payers) espouse preference for EBM when determining a treatment approach. EBM can result in better outcomes, including increased efficacy, lower toxicity, higher quality of life, and reduced health care costs 14, 15, 16. Outside of RCTs, comparative effectiveness research using real‐world data to inform EBM has particular resonance in the current period of transition to a value‐based care paradigm. Our objective was to analyze the contemporaneous use of palliative chemotherapy in mBC, in aggregate and by mBC subtype, to understand use of single‐agent versus combination chemotherapy, specific drug and regimen preferences, and the effectiveness of respective single agents to each other and versus combination chemotherapy across all lines of palliative therapy among real‐world female patients with mBC.
Materials and Methods
Administrative Claims Database
We conducted a retrospective cohort study using payer claims data from the Symphony Health's Integrated Dataverse. The database integrates data from physician practices, pharmacies, and hospitals for a broad longitudinal view of a patient's disease history, treatment patterns, and health care resource use. Initially, any woman with a diagnosis of mBC receiving at least one single‐agent or combination chemotherapy between January 1, 2013, and December 31, 2017, was selected. The database followed patients through October 31, 2018, providing at least 10 months of follow‐up for all patients. More specifically, the following rules were applied to identify the analytical study cohort: (a) female; (b) a minimum of one nondiagnostic claim for breast cancer (International Statistical Classification of Diseases and Related Health Problems [ICD]‐9/10 codes: 174.x, D05.x, D24.x, D48.6x, C50.x); (c) a minimum of one claim for distant metastases (ICD‐9/10 codes: 196.1–196.2, 196.5, 196.8, 197.0–197.8, 198.0–198.1, 198.3–198.8, 198.82, 198.89, or 199.0, C77.x, C78.x, C79.x); (d) continuous medical and/or pharmacy claims 6 months pre‐ and 6 months postdiagnosis; and (e) use of single‐agent or combination chemotherapy in at least one line of therapy (LOT). Patients with a claim for any other primary malignancy prior to the date of initial breast cancer diagnosis and those with a claims activity gap more than 24 months after the initiation of any line of chemotherapy were excluded from analysis, as they may have received care outside of the database catchment area.
All regimens prescribed after the diagnosis of metastatic disease were considered. LOT determination was made by identifying the first claim for any systemic therapy after the metastatic diagnosis (i.e., 1L). Neoadjuvant and/or adjuvant therapies were not considered a LOT. Claims for drugs occurring within 30 days of each other were considered combination therapy. Only regimens containing cytotoxic chemotherapy drugs were considered COT; a patient prescribed a cytotoxic chemotherapy in combination with any targeted therapy was not considered COT. The LOT was increased if there was an addition of a new agent (after 30 days), discontinuation of an agent in a regimen for greater than 60 days, or a gap (no claims for the agent or agents) of more than 90 days.
Analyses
Only patients who had received at least one line of COT were included in analysis. Based on all observed regimens used for each patient, the patient was classified into one of four mutually exclusive mBC subtypes: HR+/HER2+, HR+/HER2‐negative (HER2−), HR−/HER2+, and TNmBC. Patients were classified as HR+/HER2− if at any time they received an aromatase inhibitor, selective estrogen receptor modulator, estrogen antagonist, luteinizing hormone‐releasing hormone analog, CDK 4/6 inhibitor, and/or mammalian target of rapamycin. Patients were described as HR−/HER2+ if at any time they received a selectively targeted HER2 receptor pathway antagonist. Patients were described as HR+/HER2+ if they received both agents previously described as HR+ or HER2+ independently or in combination in any LOT. Patients described as TNmBC never received any HR+ or HER2+ agents in any LOT.
The effectiveness of chemotherapy regimens was assessed using time to next treatment (TNT) as a proxy for progression‐free survival (PFS) 17. TNT was defined from the first claim for a regimen in any LOT until the first claim for the next LOT minus one day. TNT was calculated using the Kaplan‐Meier method to account for variable follow‐up. If no next LOT was observed, the patient was censored on their last date of treatment in the observed line. As death or a date of death cannot be determined in administrative claims databases, patients who may have died and therefore did not receive a subsequent LOT were considered censored on their last date of treatment in the LOT.
Results
Study Cohort Characteristics
A total of 39,790 patients with any claim for breast cancer during the index period from January 1, 2013, to December 31, 2017, were identified. Of those, 12,381 had a claim for distant metastatic disease and initiated any systemic therapy. Of these, 3,777 (30.5%) received at least one COT in any LOT. The remaining 8,604 patients (69.5%) had received only endocrine or a targeted agent or chemotherapy in combination with these agents across all observed LOTs during the follow‐up period and were excluded from this analysis.
Of those patients who had received at least one COT LOT, 3,777 patients received 1L, 2,433 received second‐line (2L), and 1,431 received third‐line (3L) or greater. The majority (54%) were classified as TNmBC, with an additional 35% classified as HR+/HER2−, 7% as HR+/HER2+, and 4% as HR−/HER2+. Among all patients in the study cohort, the mean age at mBC diagnosis was 59.9 years (SD = 12.0), and the majority (63%) had commercial or employer‐sponsored health plans at the time of diagnosis (followed by Medicare [24%] and Medicaid [6%]) and resided in the Western U.S. (33%, with 27% from the South, 22% from the Northeast, and 18% from the Midwest). The mean follow‐up duration from mBC diagnosis was 25.8 months (SD = 18.2). The median number of LOTs received was 2 and the mean was 2.3 (SD = 1.4); 35.6% had received only 1L during the follow‐up period, 26.5% received 1L and 2L, and 17.2% received 3L or greater. A total of 5,586 individual COT LOTs were counted during the follow‐up period among the 3,777 patients. Of the 3,777, a total of 3,013 (79.8%) received COT in 1L.
Treatment Patterns
Across all 5,586 unique COT LOTs, 66.5% were single‐agent and 33.5% were combination chemotherapy (Table 1). The lowest proportion of single‐agent chemotherapy use was 55.0% in 1L, whereas in LOTs 2–5, at least 79.7% of COT was single‐agent. Only in the sixth line (97 patients) was this trend reversed, with 62.2% of patients receiving single‐agent chemotherapy among those who received COT. By mBC subtype, the proportion of single‐agent compared with combination chemotherapy across all observed LOTs was highest in the TNmBC group, at 68.1%, and lowest in the HR−/HER2+ group, at 61.7% (Table 1). Combination chemotherapy was most frequently used in 1L among HR+/HER2+ patients at 63.7% compared with 61.3% among HR+/HER2−, 55.4% among HR−/HER2+, and 37.1% among patients with TNmBC.
Table 1.
Proportion of patients receiving single‐agent and combination chemotherapy (considering COT) by LOT and metastatic breast cancer subtype
| LOTs in the metastatic setting | Single chemo, n (% of COT) | Combo chemo, n (% of COT) |
|---|---|---|
| All patients | ||
| Total LOTs | 3,712 (66.5) | 1,874 (33.5) |
| 1L | 1,658 (55.0) | 1,355 (45.0) |
| 2L | 1,030 (79.7) | 263 (20.3) |
| 3L | 506 (83.1) | 103 (16.9) |
| 4L | 274 (80.8) | 65 (19.2) |
| 5L | 147 (83.5) | 29 (16.5) |
| 6L | 97 (62.2) | 59 (37.8) |
| TNmBC | ||
| Total LOTs | 2,100 (68.1) | 982 (31.9) |
| 1L | 1,276 (62.8) | 755 (37.1) |
| 2L | 527 (77.6) | 152 (22.4) |
| 3L+ | 297 (79.8) | 75 (20.1) |
| HR+/HER2− | ||
| Total LOTs | 1,253 (64.3) | 696 (35.7) |
| 1L | 312 (38.7) | 494 (61.3) |
| 2L | 407 (83.6) | 80 (16.4) |
| 3L+ | 534 (81.4) | 122 (18.6) |
| HR+/HER2+ | ||
| Total LOTs | 230 (66.5) | 116 (33.5) |
| 1L | 37 (36.3) | 65 (63.7) |
| 2L | 47 (79.7) | 12 (20.3) |
| 3L+ | 146 (78.9) | 39 (21.1) |
| HR−/HER2+ | ||
| Total LOTs | 129 (61.7) | 80 (38.3) |
| 1L | 33 (44.6) | 41 (55.4) |
| 2L | 49 (72.1) | 19 (27.9) |
| 3L+ | 47 (70.1) | 20 (29.9) |
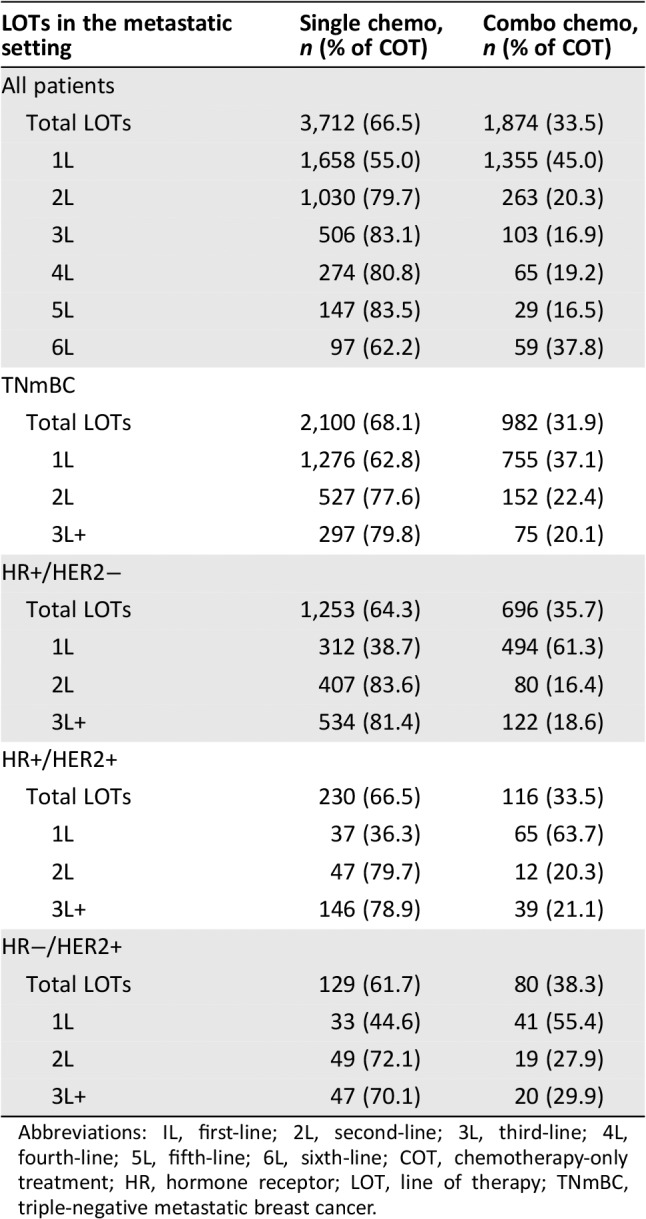
Abbreviations: IL, first‐line; 2L, second‐line; 3L, third‐line; 4L, fourth‐line; 5L, fifth‐line; 6L, sixth‐line; COT, chemotherapy‐only treatment; HR, hormone receptor; LOT, line of therapy; TNmBC, triple‐negative metastatic breast cancer.
The most frequently prescribed single‐agent (of all single‐agent LOTs) was capecitabine (23.7%), followed by eribulin (12.6%), nab‐paclitaxel (12.5%), gemcitabine (9.9%), and paclitaxel (9.7%; Table 2). Capecitabine was the most commonly prescribed single‐agent of choice in 1L through fifth‐line, with nab‐paclitaxel second most common in 1L, paclitaxel in 2L, and eribulin in 3L or greater. In the sixth line, eribulin was the most frequently prescribed (17%). For combination chemotherapy, cyclophosphamide plus doxorubicin (AC) was the most common in 1L (37.2%; for all chemotherapy in 1L), followed by carboplatin plus gemcitabine (16.1%) and docetaxel and cyclophosphamide (TC) (14.3%). In 2L and 3L and greater, carboplatin plus gemcitabine was the most commonly prescribed regimen (27.8% in 2L and 25.0% in 3L or greater).
Table 2.
Top five most frequent single‐agent and combination chemotherapy of COT across all LOTs and by individual LOTs
| All COT LOTs | 1L | 2L | 3L and greater | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single regimen | % | Combo regimen | % | Single regimen | % | Combo regimen | % | Single regimen | % | Combo regimen | % | Single regimen | % | Combo regimen | % |
| All patients | |||||||||||||||
| cape | 23.7 | AC | 31.2 | cape | 27.9 | AC | 37.2 | cape | 20.8 | carbo+gem | 27.8 | cape | 19.9 | carbo+gem | 25.0 |
| eribulin | 12.6 | carbo+gem | 18.9 | nab pac | 12.4 | carbo+gem | 16.1 | pac | 18.6 | AC | 21.7 | eribulin | 17.6 | AC | 9.4 |
| nab pac | 12.5 | TC | 11.0 | carbo | 10.4 | TC | 14.3 | eribulin | 13.6 | cyclo+fluor+MTX | 5.3 | nab pac | 12.7 | cyclo+fluor+MTX | 7.0 |
| gem | 9.9 | carbo+pac | 5.9 | gem | 10.1 | carbo+pac | 7.3 | nab pac | 12.5 | carbo+docet | 4.6 | gem | 11.6 | carbo+docet | 3.9 |
| pac | 9.7 | cyclo+fluor+MTX | 4.3 | pac | 9.5 | cyclo+fluor+MTX | 3.6 | gem | 7.9 | carbo+pac | 4.2 | lipo doxo | 11.5 | carbo+nab pac | 3.5 |
| TNmBC | |||||||||||||||
| cape | 20.0 | AC | 21.5 | cape | 28.1 | carbo+gem | 26.8 | cape | 21.1 | carbo+gem | 36.5 | eribulin | 20.9 | carbo+gem | 33.3 |
| eribulin | 13.2 | carbo+gem | 27.4 | nab pac | 11.5 | AC | 24.4 | eribulin | 17.2 | AC | 17.1 | cape | 12.5 | cyclo+fluor+MTX | 10.7 |
| nab pac | 10.8 | TC | 6.8 | carbo | 11.4 | carbo+pac | 9.8 | nab pac | 14.1 | carbo+docet | 4.4 | gem | 11.8 | AC | 9.3 |
| gem | 9.7 | carbo+pac | 7.9 | gem | 10.7 | TC | 8.5 | pac | 9.8 | cyclo+fluor+MTX | 4.4 | nab pac | 10.8 | carbo+docet | 5.3 |
| pac | 6.5 | cyclo+fluor+MTX | 4.0 | eribulin | 10.6 | carbo+docet | 3.7 | gem | 9.1 | carbo+pac | 3.9 | vin | 10.4 | carbo+nab pac | 2.7 |
| HR+/HER2− | |||||||||||||||
| cape | 26.2 | AC | 40.2 | cape | 26.1 | AC | 51.6 | pac | 44.9 | AC | 32.3 | cape | 19.7 | carbo+gem | 26.2 |
| eribulin | 11.5 | TC | 17.8 | pac | 20.2 | TC | 23.6 | cape | 16.1 | carbo+gem | 11.3 | eribulin | 17.0 | cyclo+fluor+MTX | 8.2 |
| nab pac | 14.7 | carbo+gem | 9.0 | nab pac | 15.2 | TC + doxo | 4.4 | nab pac | 8.7 | carbo+pac | 6.5 | lipo doxo | 13.7 | AC | 6.6 |
| gem | 9.8 | cyclo+fluor+MTX | 5.6 | gem | 7.5 | cyclo+fluor+MTX | 4.4 | docet | 5.9 | carbo + nab pac | 6.5 | nab pac | 13.1 | carbo+nab pac | 5.7 |
| pac | 17.1 | carbo+pac | 3.3 | docet | 7.1 | carbo+pac | 3.5 | lipo doxo | 4.7 | cyclo+fluor+MTX | 6.5 | gem | 11.4 | carbo+docet | 5.0 |
| HR+/HER2− | |||||||||||||||
| cape | 44.0 | AC | 59.6 | cape | 40.7 | AC | 73.8 | cape | 45.0 | Cyclo+epiru+fluor | 50.0 | cape | 32.9 | carbo+gem | 12.8 |
| eribulin | 11.4 | carbo+gem | 5.6 | gem | 14.8 | TC | 9.5 | pac | 20.0 | carbo+nab pac | 12.5 | nab pac | 18.5 | AC | 12.8 |
| nab pac | 20.2 | TC | 9.0 | nab pac | 14.8 | carbo+pac | 4.8 | nab pac | 10.0 | TC + doxo | 12.5 | eribulin | 13.7 | carbo+docet | <1 |
| gem | 14.0 | carbo+pac | 3.4 | docet | 11.1 | cape + ixabepilone | 2.4 | carbo | 5.0 | AC | 12.5 | gem | 13.7 | gem+nab pac | <1 |
| pac | 5.2 | carbo+docet | 3.4 | pac | 7.4 | carbo+docet | 2.4 | docet | 5.0 | gem+vin | 12.5 | lipo doxo | 8.2 | nab pac | <1 |
| HR−/HER2+ | |||||||||||||||
| cape | 41.8 | AC | 45.2 | cape | 30.3 | AC | 53.7 | cape | 39.1 | AC | 41.7 | cape | 29.8 | AC | 20.0 |
| eribulin | 14.6 | carbo+gem | 13.7 | pac | 18.2 | carbo+pac | 12.2 | lipo doxo | 13.0 | carbo+docet | 25.0 | eribulin | 14.9 | carbo+gem | 10.0 |
| nab pac | 11.7 | TC | 5.5 | nab pac | 15.2 | carbo+gem | 9.8 | docet | 8.7 | cyclo+fluor+MTX | 25.0 | lipo doxo | 10.6 | cape+nab pac | 10.0 |
| gem | 6.8 | carbo+pac | 6.8 | carbo | 12.1 | TC | 9.8 | pac | 8.7 | cape+eribulin | 12.5 | gem | 6.4 | cisplatin+gem | 5.0 |
| pac | 8.7 | cyclo+fluor+MTX | 2.7 | lipo doxo | 6.1 | Cyclo+epiru | 4.9 | nab pac | 8.7 | cisplatin+gem | 0.6 | vin | 6.4 | carbo+lipo doxo | <1 |
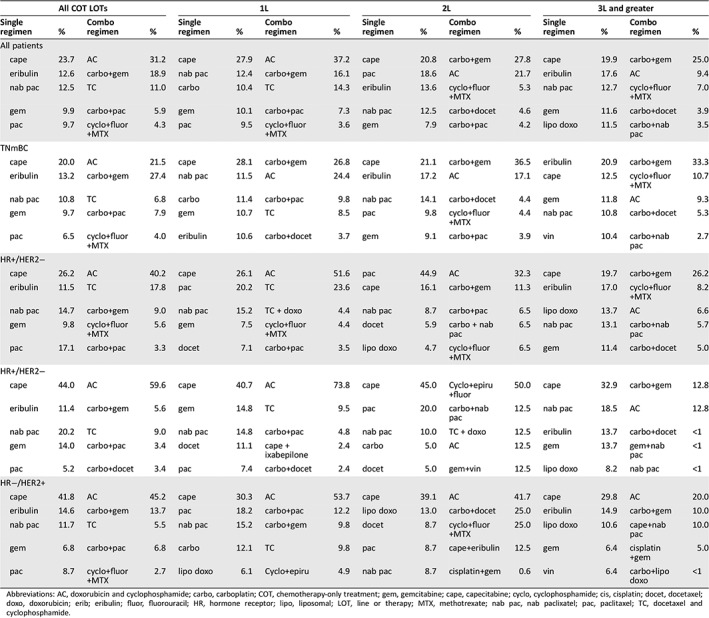
Abbreviations: AC, doxorubicin and cyclophosphamide; carbo, carboplatin; COT, chemotherapy‐only treatment; gem, gemcitabine; cape, capecitabine; cyclo, cyclophosphamide; cis, cisplatin; docet, docetaxel; doxo, doxorubicin; erib; eribulin; fluor, fluorouracil; HR, hormone receptor; lipo, liposomal; LOT, line or therapy; MTX, methotrexate; nab pac, nab paclixatel; pac, paclitaxel; TC, docetaxel and cyclophosphamide.
Time to Next Treatment
Across all LOTs, the median TNT for single agents was 5.3 months (95% confidence interval [CI], 5.1–5.6), significantly longer (p < .0001) than combination therapy at 4.1 months (95% CI, 3.9–4.4; Fig. 1). When comparing TNT by LOT, the median TNT was significantly longer for single‐agent in 1L but not 2L. By regimen, the median TNT for capecitabine was 8.4 months (95% CI, 7.4–9.5) compared with 4.8 months for eribulin (95% CI, 4.4–5.3) and 5.3 months for nab‐paclitaxel (95% CI, 4.6–6.1). The median TNT for capecitabine was significantly longer in comparison to each of these single agents in each LOT and to all combination regimens in each LOT (p < .001).
Figure 1.
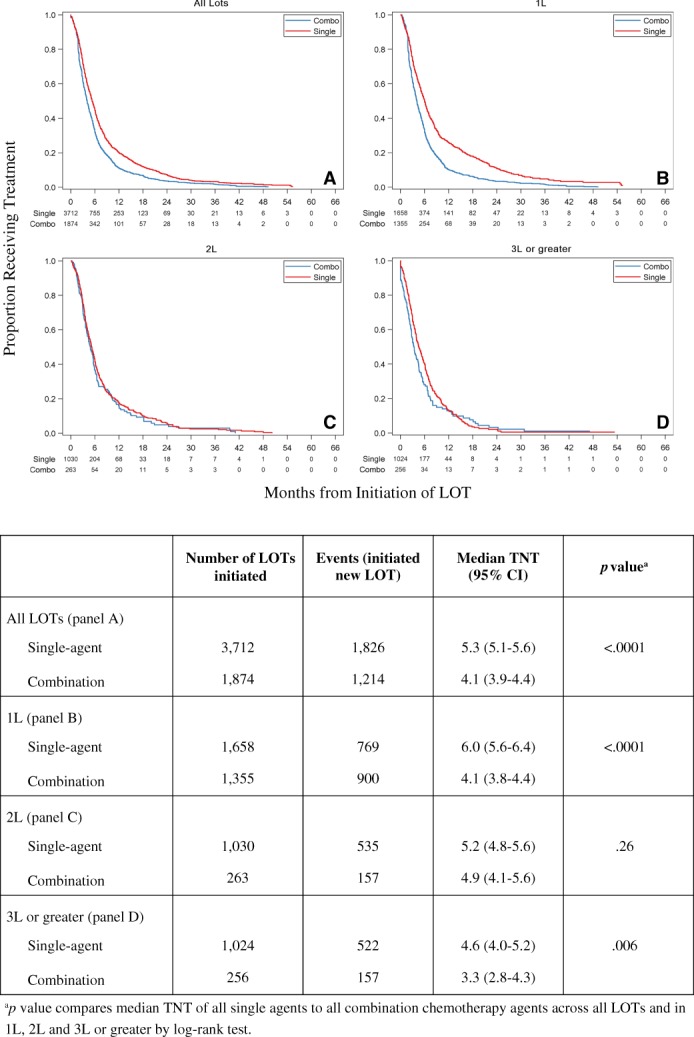
Time to next treatment (months) from the start of a LOT across single and combination chemotherapy agents using Kaplan‐Meier estimates.Abbreviations: 1L, first‐line; 2L, second‐line; 3L, third‐line; LOT, line of therapy.
TNT in 3L or greater was compared to illustrate differences by regimen in the most common setting in which cytotoxic chemotherapy is used (Table 3). The median TNT for the most frequently prescribed agents in 3L or greater were (in order of frequency of use): capecitabine = 6.2 months (95% CI, 5.6–7.1); eribulin = 3.9 months (95% CI, 3.3–4.6); nab‐paclitaxel = 6.2 (95% CI, 4.8–7.4); gemcitabine = 3.6 months (95% CI, 2.8–4.8); and liposomal doxorubicin = 3.9 months (95% CI, 3.4–6.2). Comparing these agents, capecitabine and nab‐paclitaxel demonstrated a significantly longer median TNT when compared with the aggregate of the remaining single agents (p < .041 and p < .049, respectively; Table 3). Similarly, these were the only two agents that demonstrated significance when compared with 3L or greater combination therapies in which the median TNT was 3.3 months (95% CI, 2.8–4.3; p < .001 and p < .002, respectively).
Table 3.
Median TNT (95% CI) in months for the most frequently received chemotherapy agents in 3L or greater compared with all other single agents received in 3L or greater
| Chemotherapy agents | 3L and greater regimen (left‐hand column) | All other 3L and greater single‐agent chemotherapya | |
|---|---|---|---|
| TNT (95% CI) | TNT (95% CI) | p valueb | |
| Capecitabine | 6.2 (5.6–7.1) | 4.4 (3.9–4.8) | .041 |
| Eribulin | 3.9 (3.3–4.6) | 4.8 (3.1–5.7) | .110 |
| Nab‐paclitaxel | 6.2 (4.8–7.4) | 4.4 (3.9–4.9) | .049 |
| Gemcitabine | 3.6 (2.8–4.8) | 4.8 (4.3–5.3) | .144 |
| Liposomal doxorubicin | 3.9 (3.4–6.2) | 4.6 (4.1–5.2) | .898 |
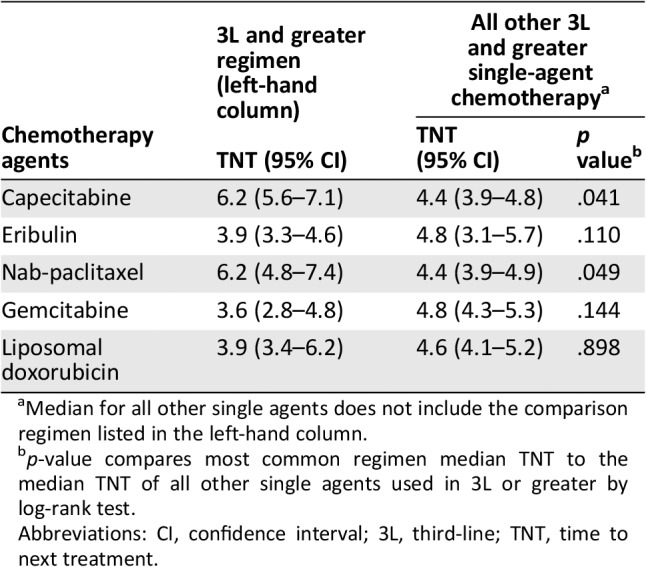
Median for all other single agents does not include the comparison regimen listed in the left‐hand column.
p‐value compares most common regimen median TNT to the median TNT of all other single agents used in 3L or greater by log‐rank test.
Abbreviations: CI, confidence interval; 3L, third‐line; TNT, time to next treatment.
Discussion
The large number of drugs and regimens approved for mBC, the eras in which the pivotal clinical trials for those drugs and regimens were performed, the multiple mBC subtypes, and the fact that 85% of patients present with early stage disease and may have exposure to neoadjuvant and adjuvant chemotherapy result in mBC management having more complexity and variance than any other common cancer. Published guidelines have attempted to address this complexity and variance by recommending the vast majority of patients with mBC prescribed COT for disease refractory to targeted, hormonal, or IO drugs should consist of single‐agent, sequential treatment rather than combination drug management. More specifically, the American Society of Clinical Oncology (ASCO) guidelines (2014) state that endocrine therapy should be standard 1L treatment in HR+/HER2− advanced and/or metastatic disease, except for immediate life‐threatening disease 4. Or, if there is concern over endocrine resistance, single‐agent chemotherapy rather than combination therapy should be preferred. Regarding both HR+/HER2− and TNmBC, the guidelines state that combination regimens should only be considered for immediate life‐threatening disease. In regard to second‐ and later‐line therapy, the guidelines state sequential single‐agent chemotherapy may be of clinical benefit and should be offered as determined by previous treatments, toxicity, coexisting medical conditions, and patient choice, but no clear evidence exists for the superiority of any regimen 4, 8. We sought to explore how contemporaneous real‐world practice reflects this recommendation and to provide further evidence of the comparative effectiveness of single‐agent chemotherapy compared with combination chemotherapy and acknowledge the recent pace of innovation in mBC therapy development challenges any real‐world research. In the 5 years after the published 2014 ASCO guidelines, the recommended 1L treatments have changed dramatically with the introduction of checkpoint inhibitors and CDK 4/6 therapy, but not in the setting of relapsed and/or refractory mBC 8. Therefore, the above guidance is as relevant today as it was in 2014, and so are the insights generated by our analysis, particularly with patients with mBC receiving increasing LOTs.
Patterns of single‐agent sequencing are complex outside of clinical trials: physician bias and patient comorbidities, prior therapies, response and tolerance of prior therapy, preferences for quality of life or survival, as well as social determinants, all factor into treatment selection and sequencing. The research we conducted used a payer claims data set to establish patterns of care based upon billing information. Such real‐world data sources contain limited data useful for describing clinical factors critical to patient care. Therefore, we were unable to examine how the use of single versus combination chemotherapy was associated with disease severity. Despite this limitation, we observed that single agents represented 67% of all COTs prescribed and only 55% of COTs in 1L. It is interesting that with those factors in mind, the single agent used with the greatest frequency in our cohort, capecitabine, exceeds the combined use of the next two agents (nab‐paclitaxel and eribulin). Noteworthy is the fact that capecitabine is the only oral chemotherapy choice and has the lowest published rates of alopecia and neuropathy among approved single agents, factors often identified as the most important to patients when evaluating treatment options 18, 19. However, when considering the three approved taxanes together (paclitaxel, docetaxel, and nab‐paclitaxel) as a class, the combined prescribing frequency does exceed that of capecitabine, suggesting that taxanes are still the most frequently prescribed drugs as a class.
In terms of effectiveness as measured by TNT, which incorporates components of efficacy, toxicity, and patient preferences, we found no evidence of benefit from the use of combination chemotherapy over single agents across all observed LOTs. Although this effect may be in part attributable to the longer TNT in 1L of 6 months for single‐agent compared with 4.1 months for combination chemotherapy, this longer TNT was also observed in 3L or greater. This 3L or greater subgroup analysis provides greater clarity because the patients are more likely to be relapsed and refractory (not de novo metastatic) and not undergoing preplanned complex regimen sequencing eliminating many of the unmeasured clinical and treatment‐related effect modifiers when evaluating TNT across all LOTs. Moreover, our data showed a remarkably consistent TNT of 5.3 months across all LOTs, specifically in third‐, fourth‐, and fifth‐line therapy.
We acknowledge that comparing single‐agent to combination chemotherapy in mBC, particularly in early LOTs, is a complex undertaking, as standard sequenced therapies including the AC to paclitaxel sequence in the HR+/HER2− cohort and AC to paclitaxel plus trastuzumab in the HR−/HER2+ cohort include prespecified durations of therapy for the agents. In our analysis, AC was the most frequently prescribed 1L combination chemotherapy and often was followed by paclitaxel in 2L. When comparing TNT between single and combination chemotherapy in 2L specifically, in which the combination chemotherapy carboplatin plus gemcitabine was the most common, we observed no difference in median TNT (5.2 months single‐agent and 4.9 months combination chemotherapy; p = .26). In 3L or greater, in which sequence‐specific therapies are less likely, we observed a longer median TNT for single‐agent chemotherapy compared with combination chemotherapy (4.6 months vs. 3.3 months; p = .006). These results further support the recommendations promulgated by ASCO and the National Comprehensive Cancer Network (NCCN). Although we could not account for clinical differences in patients because of the lack of clinical detail (e.g., stage, tumor burden, and patient performance status), our findings suggest that there is no effectiveness benefit for combination chemotherapy in the real world outside of the clinical trial at the population level. Nonetheless, the prevalence of combination therapy in later lines (approximately 20%), irrespective of mBC subtype, is also worthy of comment. In both instances, this pattern of care is well outside published guideline recommendations.
Limitations
Despite an average 25 months of follow‐up after mBC diagnosis, the need for longer follow‐up is apparent in that only 17% (1,336 of 7,692) of those patients identified as HR+/HER2− received COT, despite the natural history of this disease, which eventually results in hormone refractory status. This is further supported by the fact that two‐thirds of the COT patients analyzed had not received treatment beyond 2L. Together, these data points suggest our analysis underestimates the full extent of COT in mBC treatment. Next, analyses based on claims data are limited by the lack of clinical details including stage, molecular subtype, and mortality, and as such, the severity of disease and its impact on TNT was unable to be considered. There may have been misclassification of molecular subtype of our patients which may impact our interpretation of treatment patterns in relation to guideline recommendations. Finally, we acknowledge that TNT is not a perfect proxy for PFS, but a wealth of research has found it directional 17.
Conclusion
Our research demonstrated that despite a dramatic expansion of the nontraditional cytotoxic drug arsenal used to treat mBC, traditional chemotherapy remains a significant contribution to patient care. Multiple evidence‐based guidelines have supported sequential single‐agent palliative chemotherapy for mBC, yet our research reveals both significant use of combination chemotherapy as well as its lower efficacy in later‐line therapy as measured by TNT. Two single agents, capecitabine and nab‐paclitaxel, stand out as the most commonly prescribed and most effective therapies. Further research is needed to better understand these prescribing preferences, their relationship with the clinical characteristics of the patient, and how these factors impact real‐world effectiveness.
Author Contributions
Conception/design: Bruce Feinberg, Jonathan Kish, Igoni Dokubo, Jeff Wojtynek, Ajeet Gajra, Kevin Lord
Provision of study material or patients: Bruce Feinberg, Jonathan Kish, Ajeet Gajra, Kevin Lord
Collection and/or assembly of data: Bruce Feinberg, Jonathan Kish, Ajeet Gajra, Kevin Lord
Data analysis and interpretation: Bruce Feinberg, Jonathan Kish, Igoni Dokubo, Jeff Wojtynek, Ajeet Gajra, Kevin Lord
Manuscript writing: Bruce Feinberg, Jonathan Kish, Igoni Dokubo, Jeff Wojtynek, Ajeet Gajra, Kevin Lord
Final approval of manuscript: Bruce Feinberg, Jonathan Kish, Igoni Dokubo, Jeff Wojtynek, Ajeet Gajra, Kevin Lord
Disclosures
Bruce Feinberg: Cardinal Health (E); Jonathan Kish: Cardinal Health (E); Igoni Dokubo: Athenex, Inc. (E); Jeff Wojtynek: Athenex, Inc. (E); Ajeet Gajra: Cardinal Health and ICON (E), AstraZeneca (C/A); Kevin Lord: Cardinal Health (E).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was sponsored by Athenex, Inc. and conducted by Cardinal Health.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol 2016;31:97–103. [DOI] [PubMed] [Google Scholar]
- 2. Feinberg B, Kish J, Dokubo I et al. Reports of the demise of chemotherapy have been greatly exaggerated. Am J Manag Care 2019;25:270–272. [PubMed] [Google Scholar]
- 3. Nabholtz JM, Falkson C, Campos D et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first‐line chemotherapy for metastatic breast cancer: Results of a randomized, multicenter, phase III trial. J Clin Oncol 2003;21:968–975. [DOI] [PubMed] [Google Scholar]
- 4. Partridge AH, Rumble RB, Carey LA et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2‐negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2014;32:3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Shaughnessy J, Petrakova K, Sonke GS et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2‐ advanced breast cancer in the randomized MONALEESA‐2 trial. Breast Cancer Res Treat 2018;168:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue K, Ninomiya J, Saito T et al. Eribulin, trastuzumab, and pertuzumab as first‐line therapy for patients with HER2‐positive metastatic breast cancer: A phase II, multicenter, collaborative, open‐label, single‐arm clinical trial. Invest New Drugs 2019;37:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. New Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (NCCN Evidence Blocks), Version 1.2018. 2018. Accessed March 1, 2019.
- 9. Joensuu H, Sailas L, Alanko T et al. Docetaxel versus docetaxel alternating with gemcitabine as treatments of advanced breast cancer: Final analysis of a randomised trial. Ann Oncol 2010;21:968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pivot X, Im SA, Guo M et al. Subgroup analysis of patients with HER2‐negative metastatic breast cancer in the second‐line setting from a phase 3, open‐label, randomized study of eribulin mesilate versus capecitabine. Breast Cancer 2018;25:370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albain KS, Nag SM, Calderillo‐Ruiz G et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 2008;26:3950–3957. [DOI] [PubMed] [Google Scholar]
- 12. Twelves C, Cortes J, Vahdat L et al. Efficacy of eribulin in women with metastatic breast cancer: A pooled analysis of two phase 3 studies. Breast Cancer Res Treat 2014;148:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaufman PA, Awada A, Twelves C et al. Phase III open‐label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015;33:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hakamies‐Blomqvist L, Luoma M, Sjöström J et al. Quality of life in patients with metastatic breast cancer receiving either docetaxel or sequential methotrexate and 5‐fluorouracil. A multicentre randomised phase III trial by the Scandinavian breast group. Eur J Cancer 2000;36:1411–1417. [DOI] [PubMed] [Google Scholar]
- 15. Liu J, Tu D, Dancey J et al. Quality of life analyses in a clinical trial of DPPE (tesmilifene) plus doxorubicin versus doxorubicin in patients with advanced or metastatic breast cancer: NCIC CTG Trial MA.19. Breast Cancer Res Treat 2006;100:263–271. [DOI] [PubMed] [Google Scholar]
- 16. Cortes J, Hudgens S, Twelves C et al. Health‐related quality of life in patients with locally advanced or metastatic breast cancer treated with eribulin mesylate or capecitabine in an open‐label randomized phase 3 trial. Breast Cancer Res Treat 2015;154:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blumenthal GM, Gong Y, Kehl K et al. Analysis of time to treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non‐small cell lung cancer. Ann Oncol 2019;30:830–838. [DOI] [PubMed] [Google Scholar]
- 18. Irwin E, Arnold A, Whelan TJ et al. Offering a choice between two adjuvant chemotherapy regimens: A pilot study to develop a decision aid for women with breast cancer. Patient Educ Couns 1999;37:283–291. [DOI] [PubMed] [Google Scholar]
- 19. Górnás M, Szczylik C. Oral treatment of metastatic breast cancer with capecitabine: What influences the decision‐making process? Eur J Cancer (Engl) 2010;19:131–136. [DOI] [PubMed] [Google Scholar]


