Abstract
Background
The effectiveness of second‐line palliative chemotherapy in patients with recurrent/metastatic osteosarcoma is not well defined. Several small studies (6–19 patients) have reported on ifosfamide as second‐line treatment. In this study we report our single‐center experience with second‐line ifosfamide monotherapy in patients treated for recurrent/metastatic osteosarcoma.
Methods
A chart review was conducted of all patients with osteosarcoma treated with ifosfamide from 1978 until 2017. Until 1997 a 5 g/m2 regimen was used, and from 1997 onwards a 9 g/m2 regimen was used. Overall survival (OS) from start of ifosfamide was the primary endpoint. Progression‐free survival (PFS) from start of treatment was also studied. To assess difference in survival between groups the log rank test was applied. To investigate the effect of ifosfamide dose and World Health Organization performance status (PS) a Cox proportional hazard regression model was estimated.
Results
Sixty‐two patients were selected with recurrent/metastatic osteosarcoma treated with second‐line ifosfamide monotherapy (dose of 5 g/m2, n = 26; 9 g/m2, n = 36). OS was significantly better in univariate analysis for 9 g/m2 compared with 5 g/m2 (10.9 months [95% confidence interval (CI), 9.3–12.6] vs. 6.7 months [95% CI, 5.9–7.6], respectively) and for PS (median OS PS 0, 13.0 months [95% CI, 2.3–23.8]; PS 1, 8.2 months [95% CI, 5.4–11.1]; PS ≥2, 6.2 months [95% CI, 2.2–10.3]; and unknown PS, 5.4 months [95% CI, 2.2–8.5]). In multivariate analysis only PS showed a significant difference. No difference in PFS was found between 5 and 9 g/m2 ifosfamide treatment or PS.
Conclusion
This study suggests that ifosfamide is an effective second‐line treatment for patients with recurrent/metastatic osteosarcoma.
Implications for Practice
Ifosfamide monotherapy is commonly used as second‐line treatment in osteosarcoma, although large series to support this are lacking. This retrospective study reports overall and progression‐free survival for regimens with 5 g/m2 and with 9 g/m2. This study was unable to show a significant difference in survival between 5 and 9 g/m2 but showed an important impact of World Health Organization performance status on overall survival. This study sets a standard and reference for comparison with the multiple phase II studies under development.
Keywords: Osteosarcoma, Chemotherapy, Ifosfamide, Survival, Metastasis
Short abstract
The effectiveness of second‐line palliative chemotherapy for recurrent or metastatic osteosarcoma has not been determined. This article reports the Leiden University Medical Center experience with ifosfamide monotherapy as palliative treatment in patients with osteosarcoma.
Introduction
Osteosarcoma is the most common primary bone malignancy but remains a rare tumor affecting predominantly adolescents and patients of advanced age. The primary treatment of high‐grade osteosarcoma in case of local disease or local disease with pulmonary metastases is perioperative chemotherapy combined with surgery. The 3‐year event‐free survival is approximately 60%–70% 1, 2, 3, 4. The current first‐line treatment in most of the Western world is combination chemotherapy consisting of methotrexate, doxorubicin, and cisplatin. The EURAMOS study tried to improve cure rate in patients with a poor pathological response to the first cycles of chemotherapy by the addition of ifosfamide and etoposide to the perioperative regimen of methotrexate, doxorubicin, and cisplatin, but this study failed to reach its primary endpoint 5. At present, more than 40% of all patients with osteosarcoma continue to develop local recurrent or distant metastatic disease after primary treatment. These patients may still be cured when recurrence or metastases are limited and amenable to surgery 6, 7.
When cure is no longer possible, different palliative chemotherapeutic regimens are available, for example, ifosfamide, etoposide, ifosfamide/etoposide, and gemcitabine/docetaxel 8, 9, 10, 11, 12, 13, 14. These treatment regimens were studied in small, single‐arm studies and case series. No randomized phase II or III trials are available. Reported outcomes of these treatments are poor with response rates of 17% for gemcitabine/docetaxel, one out of eight patients for etoposide, and a higher response rate of 48%–59% for ifosfamide/etoposide, but as first‐line treatment 8, 9, 10, 11. Progression‐free survival (PFS) for gemcitabine/docetaxel was 3.5 months 11. Ifosfamide as second‐line treatment was studied in several small studies (between 6 and 19 patients per study) studying varying ifosfamide doses, ranging from 5 g/m2 in 1 day to a total of 14 g/m2 continuously over 7 days 12, 13, 14, 15. None of these studies reported the overall survival (OS) and/or PFS. Overall response rates varied between 24% and 44%.
A retrospective analysis of seven Children's Oncology Group studies, all with inactive drugs (according to the study criteria), by Lagmay et al. showed an event‐free survival (which is usually called PFS) of 12% at 4 months, which can be used as reference for new single‐arm studies 16. In a recently published small randomized phase II study including 43 patients, regorafenib was shown to have an 8‐week PFS of 65% versus 0% for the placebo group 17.
This study reports the Leiden University Medical Center experience with ifosfamide monotherapy as palliative treatment in patients with osteosarcoma. It also studies (to the authors’ knowledge for the first time in literature) whether the currently used dose of 9 g/m2 over 3 days continuously is better than the previously used 5 g/m2 as bolus infusion.
Materials and Methods
Patients
From our hospital cancer registry, all patients treated palliatively with ifosfamide monotherapy for an osteosarcoma were selected. Sixty‐two patients were identified, of whom two were excluded (one actually had a salivary gland tumor, and one had a uterine leiomyosarcoma treated as osteosarcoma). Three additional patients had the outdated diagnosis malignant fibrous histiocytoma. All three patients with so‐called malignant fibrous histiocytoma of bone had pathology review before study entry by an expert bone tumor pathologist (J.V.M.G.B.). For two patients, osteoid deposition by tumor cells was focally observed, and the diagnosis was changed to high‐grade osteosarcoma, and these two patients were included. The third patient was diagnosed with undifferentiated pleomorphic sarcoma after revision and was excluded. Patients were grouped based on their actual primary ifosfamide dose being ≤6 g/m2 (hereafter called intended dose 5 g/m2) or >6 g/m2 (called intended dose 9 g/m2). In our reference center ifosfamide 9 g/m2 was introduced in 1997. Before 1997, 5 g/m2 was used. The cutoff of 6 g/m2 was chosen because this cutoff takes into account a little overdosing in the 5 g/m2 dosed patients and a small dose reduction in patients with an intended dose of 9 g/m2.
For all of these patients a chart review was conducted. Data was collected on age and sex, date of primary diagnosis, primary localization, histological subtype, primary treatment, primary intent of treatment (palliative or curative), metastases at diagnosis, localization of metastases at diagnosis, date of recurrence, date of start ifosfamide, planned dose of ifosfamide, actual primary dose of ifosfamide given, number of cycles of ifosfamide, given dose of ifosfamide, dates of response evaluation, outcomes of response evaluations, and treatment after progression on ifosfamide.
Endpoints
The primary endpoint was OS, calculated as the time between start of ifosfamide and death. Secondary endpoints were PFS, calculated as time between start of ifosfamide and first documented progression and overall response rate, that is, complete remission, partial remission, stable disease, and progressive disease according to RECIST version 1.1, or in case of only clinical evaluation, clinical benefit. Covariates studied were histological subtype, World Health Organization (WHO) performance status (PS) at start of ifosfamide treatment, and time between primary diagnosis and start of ifosfamide treatment. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical Analysis
Statistical analysis was performed with IBM Statistical Package for Social Sciences (SPSS) Statistics version 24. Categorical data were summarized by frequencies and percentages; continuous variables were summarized by median and overall range. These were presented according to the two different groups. The characteristics were compared with a χ2 test for categorical variables and a Mann‐Whitney U test for the continuous variables. A χ2 test was used to compare response rates between the two treatment groups. Kaplan‐Meier's methodology was used to estimate OS and PFS. Univariate and multivariate Cox proportional hazard regression model was estimated to investigate the effect of prognostic factors on OS and PFS.
To compare with existing literature, 8‐week and 3‐month PFS and 9‐ and 12‐month OS were estimated by using Kaplan‐Meier's methodology.
The Medical Ethics Committee provided us a waiver for informed consent (registration number C14.167).
Results
Patients
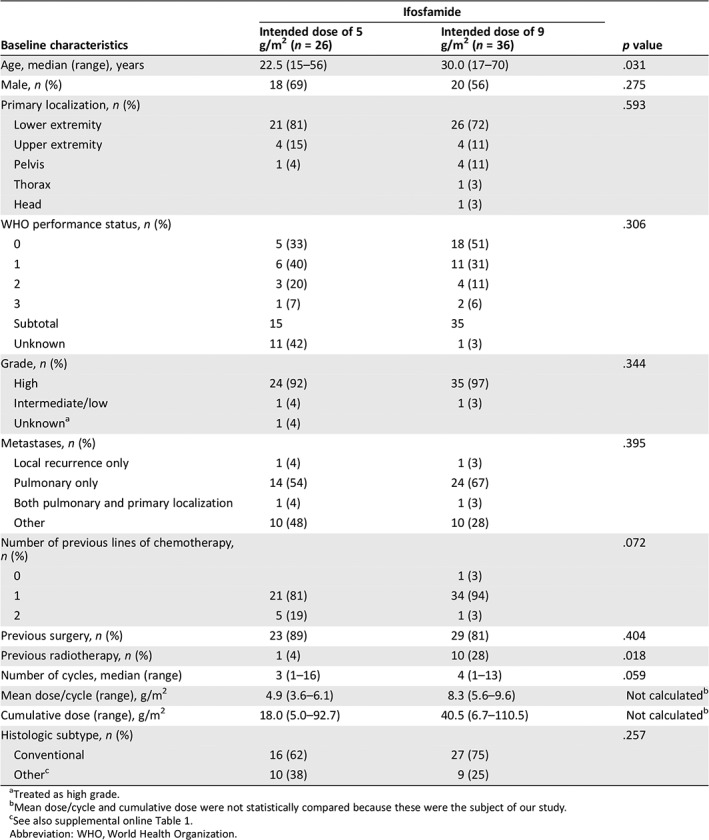
In total, 62 patients treated with palliative ifosfamide treatment for osteosarcoma were identified from our hospital cancer registry. Thirty‐six patients had a primary intended dose of 9 g/m2 ifosfamide, and the other 26 had an intended dose of 5 g/m2. Table 1 shows the characteristics of the included patients. Patients with an intended dose of 9 g/m2 were older (30.0 [17–70] years vs. 22.5 [15–56] years), received more radiotherapy before start of ifosfamide, and received more cycles of ifosfamide treatment (median three vs. four cycles). As expected, mean cycle dose and cumulative dose of ifosfamide was higher in patients with an intended dose of 9 g/m2. Only one patient (9 g/m2) was pretreated with a regimen containing ifosfamide (supplemental online Table 2). More patients were treated with chemotherapy or other treatment options after the end of ifosfamide in the dose 9 g/m2 group (21 vs. 12 patients; supplemental online Tables 4 and 5).
Table 1.
Baseline characteristics
| Baseline characteristics | Ifosfamide | p value | |
|---|---|---|---|
| Intended dose of 5 g/m2 (n = 26) | Intended dose of 9 g/m2 (n = 36) | ||
| Age, median (range), years | 22.5 (15–56) | 30.0 (17–70) | .031 |
| Male, n (%) | 18 (69) | 20 (56) | .275 |
| Primary localization, n (%) | .593 | ||
| Lower extremity | 21 (81) | 26 (72) | |
| Upper extremity | 4 (15) | 4 (11) | |
| Pelvis | 1 (4) | 4 (11) | |
| Thorax | 1 (3) | ||
| Head | 1 (3) | ||
| WHO performance status, n (%) | .306 | ||
| 0 | 5 (33) | 18 (51) | |
| 1 | 6 (40) | 11 (31) | |
| 2 | 3 (20) | 4 (11) | |
| 3 | 1 (7) | 2 (6) | |
| Subtotal | 15 | 35 | |
| Unknown | 11 (42) | 1 (3) | |
| Grade, n (%) | .344 | ||
| High | 24 (92) | 35 (97) | |
| Intermediate/low | 1 (4) | 1 (3) | |
| Unknowna | 1 (4) | ||
| Metastases, n (%) | .395 | ||
| Local recurrence only | 1 (4) | 1 (3) | |
| Pulmonary only | 14 (54) | 24 (67) | |
| Both pulmonary and primary localization | 1 (4) | 1 (3) | |
| Other | 10 (48) | 10 (28) | |
| Number of previous lines of chemotherapy, n (%) | .072 | ||
| 0 | 1 (3) | ||
| 1 | 21 (81) | 34 (94) | |
| 2 | 5 (19) | 1 (3) | |
| Previous surgery, n (%) | 23 (89) | 29 (81) | .404 |
| Previous radiotherapy, n (%) | 1 (4) | 10 (28) | .018 |
| Number of cycles, median (range) | 3 (1–16) | 4 (1–13) | .059 |
| Mean dose/cycle (range), g/m2 | 4.9 (3.6–6.1) | 8.3 (5.6–9.6) | Not calculatedb |
| Cumulative dose (range), g/m2 | 18.0 (5.0–92.7) | 40.5 (6.7–110.5) | Not calculatedb |
| Histologic subtype, n (%) | .257 | ||
| Conventional | 16 (62) | 27 (75) | |
| Otherc | 10 (38) | 9 (25) | |
Treated as high grade.
Mean dose/cycle and cumulative dose were not statistically compared because these were the subject of our study.
See also supplemental online Table 1.
Abbreviation: WHO, World Health Organization.
Overall Survival
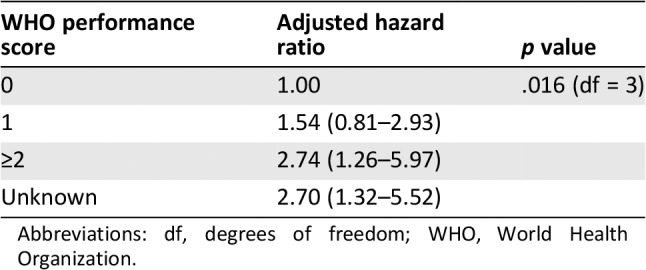
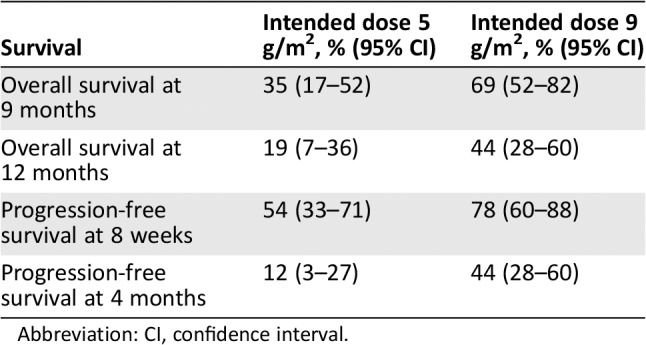
Median OS was 9.1 months (95% confidence interval [CI], 7.8–10.4) after start of ifosfamide (this is also the median follow‐up of the patients because all patients died; Fig. 1A). The OS was significantly different between the intended dose of 5 g/m2 and 9 g/m2 (p = .046). For the intended dose of 5 g/m2 the median OS was 6.7 months (95% CI, 5.9–7.6) versus 10.9 months (95% CI, 9.3–12.6) for the intended dose of 9 g/m2 (Fig. 1B). The OS was also significantly different between PS 0, 1, and ≥2 (p = .012) with a median OS for PS 0 of 13.0 months (95% CI, 2.3–23.8), for PS 1 of 8.2 months (95% CI, 5.4–11.1), for PS ≥2 of 6.2 months (95% CI, 2.2–10.3), and for PS unknown of 5.4 months (95% CI, 2.2–8.5; Fig. 1C). In multivariate analysis only PS was statistically significant associated to OS (Table 2). The 9‐ and 12‐month OS were estimated to compare with other studies (Table 3).
Figure 1.
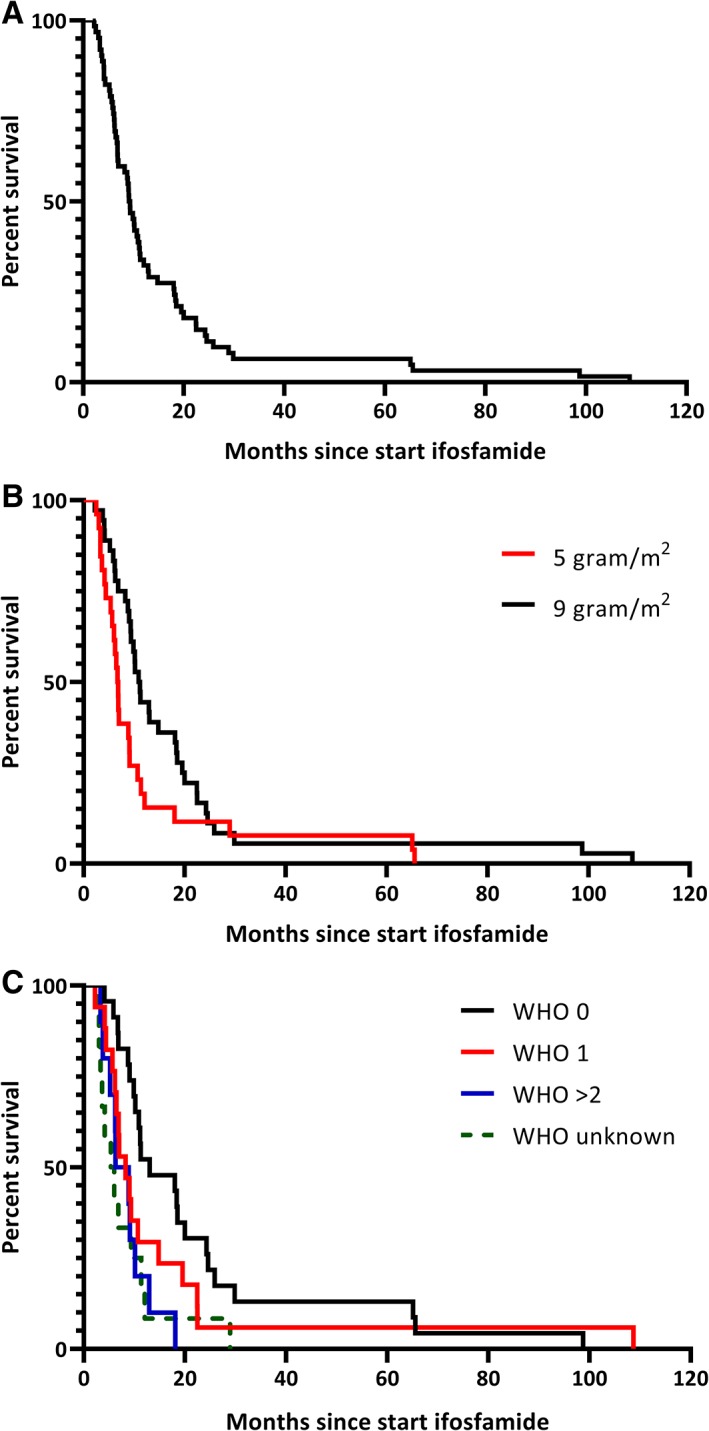
Overall survival. (A): All patients. (B): Split based on dose. (C): Split based on WHO performance score.Abbreviation: WHO, World Health Organization.
Table 2.
Multivariate analysis for prognostic factors for overall survival
| WHO performance score | Adjusted hazard ratio | p value |
|---|---|---|
| 0 | 1.00 | .016 (df = 3) |
| 1 | 1.54 (0.81–2.93) | |
| ≥2 | 2.74 (1.26–5.97) | |
| Unknown | 2.70 (1.32–5.52) |
Abbreviations: df, degrees of freedom; WHO, World Health Organization.
Table 3.
Overall survival and progression‐free survival percentages at specific time points
| Survival | Intended dose 5 g/m2, % (95% CI) | Intended dose 9 g/m2, % (95% CI) |
|---|---|---|
| Overall survival at 9 months | 35 (17–52) | 69 (52–82) |
| Overall survival at 12 months | 19 (7–36) | 44 (28–60) |
| Progression‐free survival at 8 weeks | 54 (33–71) | 78 (60–88) |
| Progression‐free survival at 4 months | 12 (3–27) | 44 (28–60) |
Abbreviation: CI, confidence interval.
Progression‐Free Survival
The overall median PFS was 2.6 months (95% CI, 2.2–3.0) after start of ifosfamide (Fig. 2A). The PFS showed a trend toward a better PFS for patients treated with an intended dose of 9 g/m2 (p = .098; Fig. 2B). For the intended dose of 5 g/m2 the median PFS was 2.1 months (95% CI, 1.3–2.9) versus 3.8 months (95% CI, 2.2–5.4) for the intended dose of 9 g/m2 (Fig. 2B). The PFS did not differ between the WHO performance states (Fig. 2C). The results of multivariate analysis resembled the univariate analysis. The 8‐week and 3‐month PFS were estimated to compare with other studies (Table 3).
Figure 2.
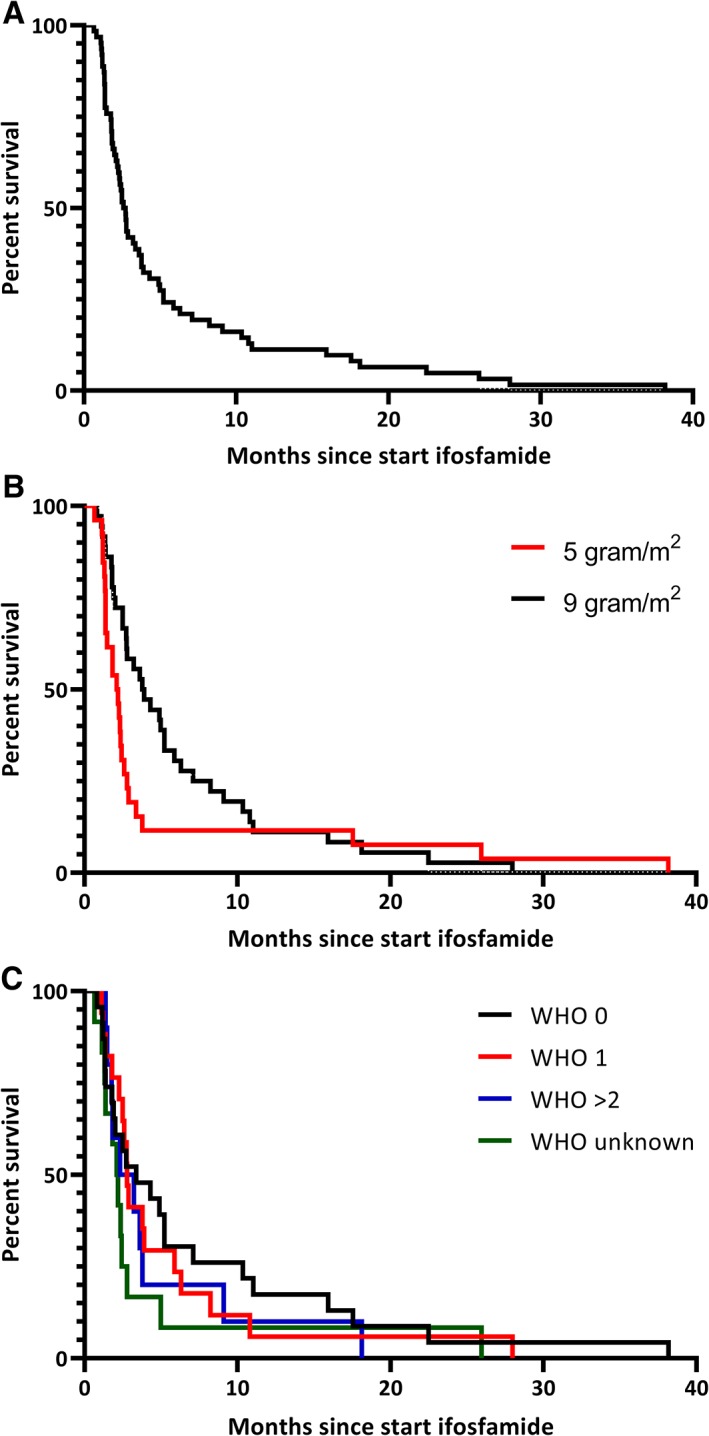
Progression‐free survival. (A): All patients. (B): Split based on dose. (C): Split based on WHO performance score.Abbreviation: WHO, World Health Organization.
Overall Response Rate
Best overall responses are reported in Table 4. Significantly more patients had at least stable disease (78% vs. 42%) when treated with ifosfamide 9 g/m2 (p = .005). Overall response rate did not differ significantly (36% vs. 25%, p = .27).
Table 4.
Best overall response
| Best response, n (%) | Total, n (%) | ||||
|---|---|---|---|---|---|
| CR, PR, clinical benefit | SD | PD | Not evaluable | ||
| Intended dose | |||||
| 5 g/m2 | 6 (23) | 5 (19) | 15 (58) | 0 (0) | 26 (100) |
| 9 g/m2 | 13 (36) | 15 (42) | 7 (19) | 1 (3) | 36 (100) |
| Total | 19 (31) | 20 (32) | 22 (35) | 1 (2) | 62 (100) |
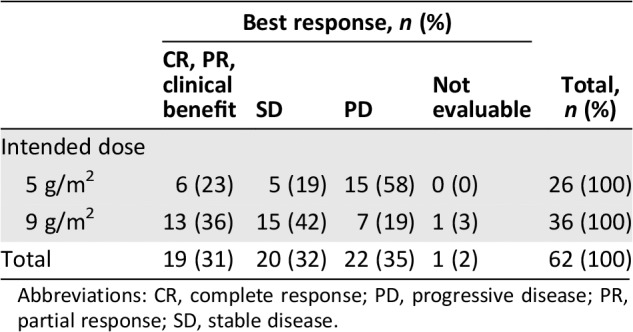
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Toxicity
As this is a retrospective study, toxicity was based on the reported toxicity in both the medical and nursing charts. This data is available in supplemental online Table 5.
Discussion
This study is the largest study reporting the outcomes of ifosfamide monotherapy in the palliative treatment of patients with osteosarcoma. Until now, only small studies with 6 to 19 patients reported outcome for patients treated with ifosfamide monotherapy. This study shows that PS is an important prognostic factor for overall survival of osteosarcoma, but it was unfortunately not possible to detect a significant difference between 9 g/m2 and 5 g/m2.
To our knowledge, this is the first study to report both OS and PFS of patients treated with second‐line or later‐line ifosfamide chemotherapy for locally advanced or metastatic osteosarcoma. The overall response rate in this study (intended dose 5 g/m2, 23%; 9 g/m2, 36%) is comparable to earlier reports on ifosfamide monotherapy in these patients 12, 13, 14, 15, 18. Additionally, an American Society of Clinical Oncology 2015 abstract reported results of ifosfamide 15 g/m2 continuously over 5 days 18. The overall response percentage was 22%, OS at 1 year was 60%, and PFS at 6 months was 53%. Compared with these data, our study reports probably a lower 1‐year OS and 6‐month PFS in a patient population with worse PS and older age, but their results were not yet published 18. In the French REGOBONE study, a placebo group was included with an 8‐week PFS of 0% and in the regorafenib arm 65% 17. In our study, ifosfamide showed an 8‐week PFS of 54% (95% CI, 33–71) for 5 g/m2 and 78% (95% CI, 60–88) for 9 g/m2, suggesting a higher PFS for ifosfamide 9 g/m2 compared with regorafenib. The 4‐month PFS of ifosfamide 9 g/m2 also compares favorably with the 4‐month PFS defined in the retrospective study of the Children's Oncology Group (44% [95% CI, 28–60] vs. 12% [95% CI, 6–19]) 16. Also, an increase in clinical benefit rate is shown when comparing 5 g/m2 with 9 g/m2.
One of the important results of this study is the prognostic impact of PS. This was already shown for some other tumors, for example, soft tissue sarcoma 19.
This study is limited by its retrospective character, the long study period, and no routine monitoring interval of computed tomography scan. All patients underwent computed tomography scan at least every two to three cycles. We made an effort to report the toxicity of ifosfamide therapy, but this is probably an underestimation of the real toxicity and is probably also biased. The toxicity of ifosfamide monotherapy is well known. The long study period and the different time periods the study groups were treated in (5 g/m2 until 1997 and 9 g/m2 from 1997 until now) hamper the study because supportive care has improved during the years and this could cause a difference in OS. Also, the improvements in imaging could result in an earlier diagnosis of recurrent disease and therefore a suggested improvement in OS. Although in univariate analysis OS was better for 9 g/m2 and there was a trend toward a better PFS for patients treated with 9 g/m2, no significant impact of the dose on OS was found in multivariate analysis. This study is still underpowered because of the still small number of patients included, but other causes are also present. At baseline, there were differences in the number of pretreated patients between both groups (higher number of patients with two lines of chemotherapy in the 5 g/m2 group). This could have an impact on the results in several ways: selecting for patients with a more indolent disease and a more chemosensitive tumor or creating more chemoresistant tumors. It is not possible to determine what the most dominant effect is. Also, PS was slightly imbalanced at baseline, favoring the 9 g/m2 group, which could improve the OS in this patient group. Due to the still small patient group it is not possible to distinguish the effect of PS and ifosfamide dose. Although it was the largest series, the small number of patients did not allow us to detect differences in for example histologic subtypes of osteosarcoma. Lastly, we could not compare the outcomes of ifosfamide treatment with an untreated patient cohort of the Leiden University Medical Center. Although an untreated patient group exists, most of these patients had a reason for which they were not treated, and the comparison would result in a biased study. However, we did compare the data with the placebo arm of the REGOBONE study and with the retrospective study of the Children's Oncology Group, showing that ifosfamide is an effective treatment (as reported above) 16, 17.
Conclusion
This is the largest study, to date, reporting data on OS and PFS of ifosfamide monotherapy as palliative treatment of osteosarcoma. Results of this study suggest that ifosfamide is an effective second‐line treatment for patients with recurrent osteosarcoma.
Author Contributions
Conception/design: Arie Jan Verschoor, Hans Gelderblom
Provision of study material or patients: Arie Jan Verschoor, P.D. Sander Dijkstra, Michiel A.J. van de Sande, Judith V.M.G. Bovée, Hans Gelderblom
Collection and/or assembly of data: Arie Jan Verschoor, Hans Gelderblom
Data analysis and interpretation: Arie Jan Verschoor, Hans Gelderblom, Marta Fiocco, Judith V.M.G. Bovée
Manuscript writing: Arie Jan Verschoor, Frank M. Speetjens, P.D. Sander Dijkstra, Marta Fiocco, Michiel A.J. van de Sande, Judith V.M.G. Bovée, Hans Gelderblom
Final approval of manuscript: Arie Jan Verschoor, Frank M. Speetjens, P.D. Sander Dijkstra, Marta Fiocco, Michiel A.J. van de Sande, Judith V.M.G. Bovée, Hans Gelderblom
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bielack SS, Kempf‐Bielack B, Delling G et al. Prognostic factors in high‐grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–790. [DOI] [PubMed] [Google Scholar]
- 2. Meyers PA, Schwartz CL, Krailo MD et al.; Children's Oncology Group. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children's Oncology Group. J Clin Oncol 2008;26:633–638. [DOI] [PubMed] [Google Scholar]
- 3. Smeland S, Bruland OS, Hjorth L et al. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow‐up of 4 years. Acta Orthop 2011;82:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis IJ, Nooij MA, Whelan J et al.; European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99:112–128. [DOI] [PubMed] [Google Scholar]
- 5. Marina NM, Smeland S, Bielack SS et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high‐grade osteosarcoma (EURAMOS‐1): An open‐label, international, randomised controlled trial. Lancet Oncol 2016;17:1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kempf‐Bielack B, Bielack SS, Jürgens H et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol 2005;23:559–568. [DOI] [PubMed] [Google Scholar]
- 7. Buddingh EP, Anninga JK, Versteegh MI et al. Prognostic factors in pulmonary metastasized high‐grade osteosarcoma. Pediatr Blood Cancer 2010;54:216–221. [DOI] [PubMed] [Google Scholar]
- 8. Gentet JC, Brunat‐Mentigny M, Demaille MC et al. Ifosfamide and etoposide in childhood osteosarcoma. A phase II study of the French Society of Paediatric Oncology. Eur J Cancer 1997;33:232–237. [DOI] [PubMed] [Google Scholar]
- 9. Goorin AM, Harris MB, Bernstein M et al. Phase II/III trial of etoposide and high‐dose ifosfamide in newly diagnosed metastatic osteosarcoma: A Pediatric Oncology Group trial. J Clin Oncol 2002;20:426–433. [DOI] [PubMed] [Google Scholar]
- 10. Kebudi R, Görgün O, Ayan I. Oral etoposide for recurrent/progressive sarcomas of childhood. Pediatr Blood Cancer 2004;42:320–324. [DOI] [PubMed] [Google Scholar]
- 11. Palmerini E, Jones RL, Marchesi E et al. Gemcitabine and docetaxel in relapsed and unresectable high‐grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer 2016;16:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel SR, Vadhan‐Raj S, Papadopolous N et al. High‐dose ifosfamide in bone and soft tissue sarcomas: Results of phase II and pilot studies–dose‐response and schedule dependence. J Clin Oncol 1997;15:2378–2384. [DOI] [PubMed] [Google Scholar]
- 13. Antman KH, Ryan L, Elias A et al. Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J Clin Oncol 1989;7:126–131. [DOI] [PubMed] [Google Scholar]
- 14. Marti C, Kroner T, Remagen W et al. High‐dose ifosfamide in advanced osteosarcoma. Cancer Treat Rep 1985;69:115–117. [PubMed] [Google Scholar]
- 15. Lee SH, Chang MH, Baek KK et al. High‐dose ifosfamide as second‐ or third‐line chemotherapy in refractory bone and soft tissue sarcoma patients. Oncology 2011;80:257–261. [DOI] [PubMed] [Google Scholar]
- 16. Lagmay JP, Krailo MD, Dang H et al. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children's Cancer Group, Pediatric Oncology Group, and Children's Oncology Group: Learning from the past to move forward. J Clin Oncol 2016;34:3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duffaud F, Mir O, Boudou‐Rouquette P et al.; French Sarcoma Group. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non‐comparative, randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet Oncol 2019;20:120–133. [DOI] [PubMed] [Google Scholar]
- 18. Palmerini E, Picci P, Marchesi E et al. High dose ifosfamide in metastatic high‐grade osteosarcoma, after failure of standard multimodal chemotherapy. J Clin Oncol 2015;33(suppl 15):10527A. [Google Scholar]
- 19. Van Glabbeke M, van Oosterom AT, Oosterhuis JW et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline‐containing first‐line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group study. J Clin Oncol 1999;17:150–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables


