Abstract
Background
Owing to the rarity of this tumor, there is limited information about second‐line chemotherapy for patients with previously treated advanced thymic carcinoma.
Material and Methods
We performed a multi‐institutional, retrospective study named NEJ023 for patients with advanced thymic carcinoma. Patients without indications for curative treatment were treated with chemotherapy from 1995 to 2014 at 40 institutions in the North East Japan Study Group. Demographic and clinicopathologic characteristics, data on treatment methods, and outcomes of second‐line chemotherapy were obtained from medical records.
Results
In total, 191 patients were enrolled in this study. Second‐line chemotherapy included platinum‐based doublets in 57.6% of patients, other multidrug chemotherapy (e.g., cisplatin, doxorubicin, vincristine, and cyclophosphamide) in 13.6%, and monotherapy in 28.8%. The median follow‐up time was 50.5 months, and the median overall survival (OS) from the start of second‐line chemotherapy was 22.4 (95% confidence interval, 17.5‐26.7) months. The average response rate (RR) was 20.0% overall; it was 21.6% for patients treated with platinum‐based doublet chemotherapy, 13.6% for those treated with other multidrug chemotherapy, and 19.6% for those treated with single agent chemotherapy. There was no significant difference in OS between platinum‐based doublet chemotherapy, other multidrug chemotherapy, and monotherapy (the median OS was 22.4, 25.7, and 21.4 months, respectively).
Conclusion
The median OS was 22.4 months in patients with advanced thymic carcinoma treated with second‐line chemotherapy. There were no significant differences in RR and OS between monotherapy and multidrug chemotherapy in this study.
Implications for Practice
Owing to the rarity of this tumor, there is limited information about second‐line chemotherapy for patients with previously treated advanced thymic carcinoma. This is the largest data for those patients treated with second‐line chemotherapy. This study suggests there is no significant difference in efficacy between monotherapy and multidrug chemotherapy for previously treated advanced thymic carcinoma. This result can support the adequacy to select monotherapy as treatment of those patients.
Keywords: Thymic carcinoma, Second‐line chemotherapy, S‐1, Platinum‐based doublet chemotherapy
Short abstract
Thymic carcinoma is rare and highly progressive. This study aimed to evaluate the efficacy of second‐line chemotherapy for patients previously treated for advanced thymic carcinoma and to identify promising chemotherapeutic regimens for clinical practice and further investigation.
Introduction
Thymic carcinoma is a rare epithelial neoplasm with malignant cytologic features. It accounts for approximately 5%‐36% of thymic epithelial tumors 1, 2, 3. Thymic carcinoma is highly progressive and tends to metastasize and invade surrounding tissues more frequently than does thymoma 4. The prognosis of patients with thymic carcinoma is poor, with a 5‐year survival rate of only 30%–50% 5, 6.
Approximately half of patients with thymic carcinoma have advanced‐stage disease at initial diagnosis 5, 6, 7. Patients with advanced thymic carcinoma are usually treated with palliative chemotherapy or supportive care; however, there is little evidence in support of chemotherapy because of the rarity of this tumor. Furthermore, there are very few reports about second‐line chemotherapy for patients with previously treated advanced thymic carcinoma, and these are all from retrospective studies with small sample sizes (Table 1) 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21. Owing to the limited number of studies performed, it is difficult to select a chemotherapy regimen for these patients in clinical practice.
Table 1.
Previous reports for salvage chemotherapy in patients with thymic carcinoma
| Author | Year | Study design | Sample size | Regimen | RR, % | mPFS, mo | mOS, mo |
|---|---|---|---|---|---|---|---|
| Gbolahan | 2018 | Prospective | 11 | PEM | 9.0 | 2.9 | 9.8 |
| Okuma | 2016 | Retrospective | 14 | S‐1 | 42.9 | 8.1 | 30.0 |
| Bluthgen | 2016 | Retrospective | 15 | ETP | 13.0 | 4.0 | 13.0 |
| Okuma | 2015 | Retrospective | 11 | GEM | 36.4 | 4.3 | 28.5 |
| Liang | 2015 | Retrospective | 10 | PEM | 10.0 | 6.5 | 12.7 |
| Watanabe | 2015 | Retrospective | 13 | DTX | 31.0 | 5.5 | 24.0 |
| Palmieri | 2014 | Prospective | 8 | CG | 37.5 | 6.0 | N/A |
| Hirai | 2013 | Retrospective | 9 | AMR | 44.4 | 4.9 | 6.4 |
| Giaccone | 2009 | Prospective | 7 | Ima | 0.0 | 2.0 | 4.0 |
| Thomas | 2015 | Prospective | 23 | Sun | 26.0 | 7.2 | N/A |
| Zucali | 2017 | Prospective | 19 | Eve | 15.8 | 5.6 | 14.7 |
| Giaccone | 2016 | Prospective | 30 | Pembro | 24.0 | 36 wk | N/A |
| Cho | 2017 | Prospective | 26 | Pembro | 23.1 | 6.1 | N/A |
| Katsuya | 2019 | Prospective | 15 | Nivo | 0.0 | 3.8 | 14.1 |
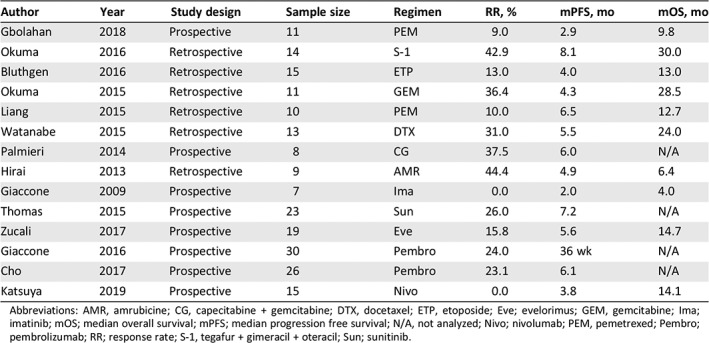
Abbreviations: AMR, amrubicine; CG, capecitabine + gemcitabine; DTX, docetaxel; ETP, etoposide; Eve; evelorimus; GEM, gemcitabine; Ima; imatinib; mOS; median overall survival; mPFS; median progression free survival; N/A, not analyzed; Nivo; nivolumab; PEM, pemetrexed; Pembro; pembrolizumab; RR; response rate; S‐1, tegafur + gimeracil + oteracil; Sun; sunitinib.
This study aimed to evaluate the efficacy of second‐line chemotherapy for patients with previously treated advanced thymic carcinoma and to identify promising chemotherapeutic regimens for clinical practice and further clinical investigation. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (identifier: UMIN000015649).
Materials and Methods
Study Cohort
Details of the study design and results regarding the efficacy of first‐line chemotherapy in patients with advanced thymic carcinoma have been published previously 22. In this observational multicenter study, we retrospectively reviewed the medical records of patients diagnosed and treated in Japan between April 1995 and March 2014. All institutions belonging to the North East Japan Study Group were invited to participate. Inclusion criteria for this study were (a) a histologic diagnosis of thymic carcinoma in each institution; (b) presence of advanced‐stage disease without indications for curative‐intent surgery or radiotherapy at diagnosis, or recurrent thymic carcinoma without indications for curative‐intent treatment; and (c) treatment with palliative‐intent chemotherapy.
Data Analysis
Data were initially obtained from 324 consecutive patients at 40 institutions. Thirty‐seven patients who did not meet eligibility requirements and one patient for whom data were missing were excluded from this analysis. Two hundred eighty‐six patients received first‐line chemotherapy. Among them, 95 did not receive second‐line chemotherapy. In total, 191 patients who received second‐line chemotherapy and were enrolled for this analysis (supplemental online Fig. 1). The institutional review boards of all participating institutions approved the protocol of this retrospective study.
The following details were extracted from the medical records: date of diagnosis, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, Masaoka‐Koga stage 23, World Health Organization (WHO) TNM stage (supplemental online Table 3) 24, 25, histology, date of death or last follow‐up, regimen of second‐line chemotherapy, duration of chemotherapy, and efficacy of chemotherapy. Furthermore, we collected the date of initiation and progressive disease of a part of chemotherapeutic regimens (carboplatin plus paclitaxel; cisplatin plus etoposide; carboplatin plus etoposide; cisplatin plus irinotecan; cisplatin plus docetaxel; cisplatin, doxorubicin, vincristine, and cyclophosphamide [ADOC]; cisplatin, doxorubicin, and cyclophosphamide [PAC]; S‐1 monotherapy; doxetaxel monotherapy; and amrubicin monotherapy). Histologic subtypes were determined based on the 2004 WHO classification in each institution 24. Response rate (RR) and progression‐free survival (PFS) of chemotherapy was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 26. If the patients with no measurable lesions were determined as noncomplete response and/or non‐progressive disease, they were categorized to stable disease in this study.
Statistical Analysis
All categorical variables were analyzed by Fisher's exact test, as applicable. All continuous variables were analyzed using the Student t test. The Kaplan‐Meier method was used to estimate overall survival (OS) and PFS curves. The log‐rank test was used to evaluate the differences among subgroups. A p value of <.05 was considered statistically significant. All analyses were performed using JMP 10 for Windows statistical software (SAS Institute Japan Inc., Tokyo, Japan).
OS was defined as the period between the start of second‐line chemotherapy and the date of death from any cause. PFS was defined as the period between the start of second‐line chemotherapy and the development of progressive disease or death from any cause.
Results
Patient Characteristics
The clinical characteristics of the 191 patients with advanced thymic carcinoma who received second‐line chemotherapy are shown in Table 2. The study population consisted of 137 men and 54 women, with a median age of 60 years (range, 13–83) at the start of second‐line chemotherapy. One hundred seventy‐three (90.6%) patients had an ECOG PS 0 or 1. One hundred twenty‐five (65.4%) patients were former or current smokers. The most frequent histologic subtype was squamous cell carcinoma (70.7%), followed by undifferentiated carcinoma (14.1%) and poorly differentiated neuroendocrine carcinoma (9.4%). Masaoka‐Koga stages III, IVa, and IVb were noted in 6 (3.1%), 54 (28.3%), and 95 (48.7%) patients, and WHO TNM stages III and IV were noted in 5 (2.6%) and 150 (78.5%) patients, respectively. Thirty‐six (18.8%) patients had postoperative recurrence.
Table 2.
Patient characteristics
| Characteristics | n (%) |
|---|---|
| Age, median (range), yr | 60 (13–83) |
| Sex, male/female | 137/54 (71.7/28.3) |
| ECOG PS | |
| 0 | 73 (38.2) |
| 1 | 100 (52.4) |
| 2 | 13 (6.8) |
| 3 | 1 (0.5) |
| Unknown | 4 (2.1) |
| Smoking status | |
| Never | 64 (33.5) |
| Former or current | 125 (65.4) |
| Unknown | 2 (1.0) |
| Histology | |
| Squamous cell carcinoma | 135 (70.7) |
| Undifferentiated carcinoma | 27 (14.1) |
| Lymphoepithelioma‐like carcinoma | 1 (0.5) |
| Adenocarcinoma | 2 (1.0) |
| Sarcomatoid carcinoma | 1 (0.5) |
| Basaloid carcinoma | 1 (0.5) |
| Papillary adenocarcinoma | 1 (0.5) |
| Poorly differentiated neuroendocrine carcinoma | 18 (9.4) |
| Well differentiated neuroendocrine carcinoma | 5 (2.6) |
| Staging | |
| Masaoka‐Koga staging | |
| Stage III | 6 (3.1) |
| Stage IVa | 54 (28.3) |
| Stage IVb | 95 (49.7) |
| Postoperative recurrence | 36 (18.8) |
| WHO TNM staging | |
| Stage III | 5 (2.6) |
| Stage IV | 150 (78.5) |
| Postoperative recurrence | 36 (18.8) |
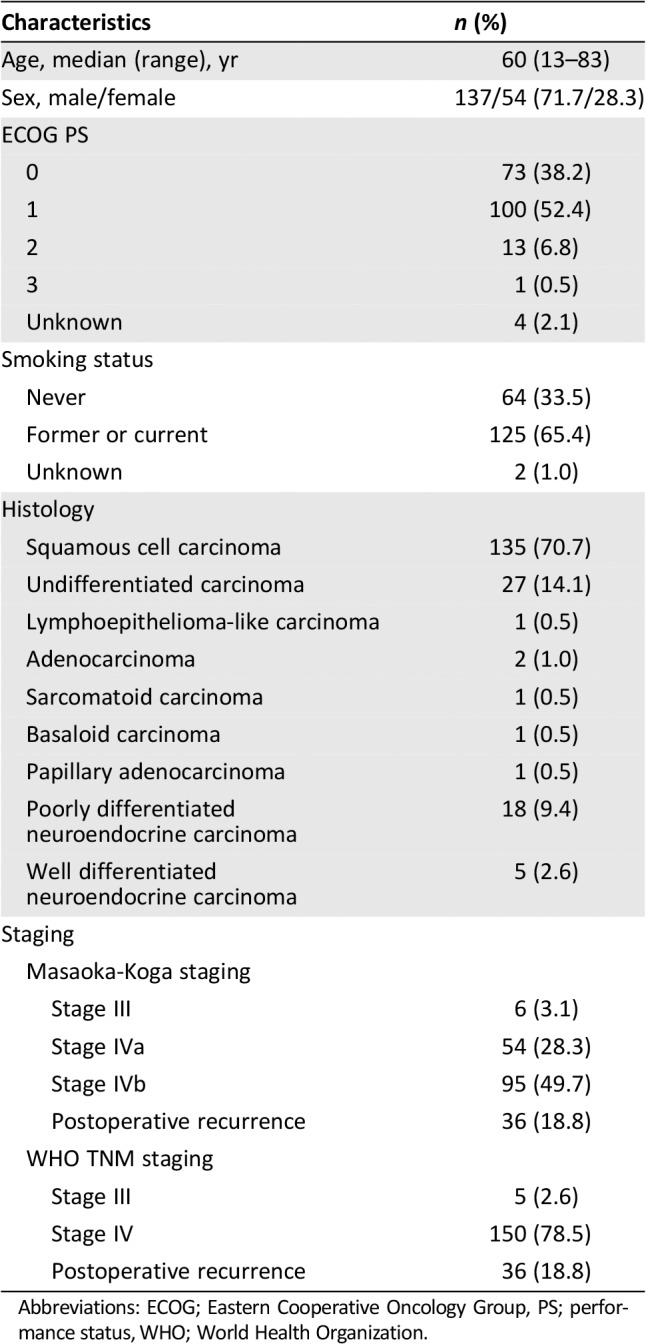
Abbreviations: ECOG; Eastern Cooperative Oncology Group, PS; performance status, WHO; World Health Organization.
Chemotherapy Regimens
The second‐line chemotherapy regimens are shown in Table 3. One hundred ten (57.6%) patients received treatment with platinum‐based doublets. The most popular platinum‐based doublet regimen was carboplatin plus paclitaxel (60 patients), followed by cisplatin plus etoposide (7 patients) and cisplatin pus irinotecan (6 patients). Other multidrug chemotherapies were administered to 26 (13.6%) patients. Of these patients, most received ADOC (17 patients). Fifty‐five (28.8%) patients received single agent chemotherapy as the second‐line treatment. The most popular regimen in single agent chemotherapy was S‐1 (tegafur, gimeracil, and oteracil; 18 patients), followed by docetaxel (13 patients) and amrubicin (9 patients). One hundred four patients (54.5%) were treated with third‐line or higher chemotherapy. The details after third‐line chemotherapy are shown in supplemental online Figure 2. The more chemotherapy the patients received, the better the survival outcome.
Table 3.
Regimens and efficacy of second‐line chemotherapy
| Regimen | Patients, n | RR, % | mPFS, mo | MST, mo |
|---|---|---|---|---|
| CBDCA+PTX | 60 | 21.2 | 6.9 | 25.3 |
| S‐1 | 18 | 38.9 | 8.3 | 21.4 |
| ADOC | 17 | 21.4 | 6.7 | 17.8 |
| DTX | 13 | 0.0 | 2.3 | 21.7 |
| AMR | 9 | 14.3 | 2.9 | 40.4 |
| GEM | 8 | 28.6 | N/A | 31.8 |
| CDDP+VP‐16 | 7 | 33.3 | 7.8 | 14.4 |
| CDDP+CPT‐11 | 6 | 33.3 | 6.9 | 45.9 |
| Other platinum doublet | 37 | 21.2 | N/A | 20.8 |
| Other multidrug chemotherapy | 9 | 0.0 | N/A | 30.2 |
| Other monotherapy | 7 | 15.4 | N/A | 16.8 |
| All regimens | 191 | 20.0 | N/A | 22.4 |
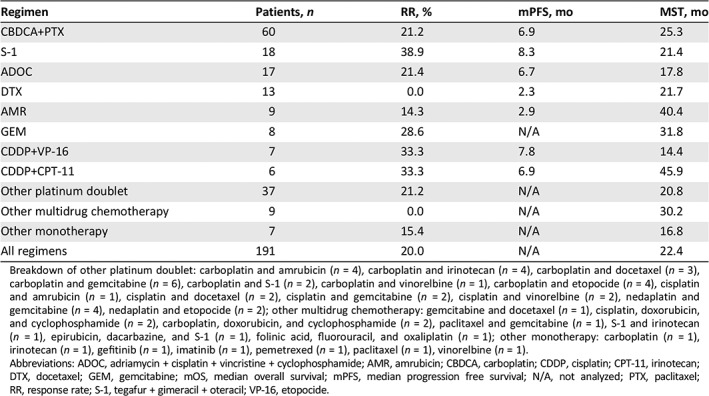
Breakdown of other platinum doublet: carboplatin and amrubicin (n = 4), carboplatin and irinotecan (n = 4), carboplatin and docetaxel (n = 3), carboplatin and gemcitabine (n = 6), carboplatin and S‐1 (n = 2), carboplatin and vinorelbine (n = 1), carboplatin and etopocide (n = 4), cisplatin and amrubicin (n = 1), cisplatin and docetaxel (n = 2), cisplatin and gemcitabine (n = 2), cisplatin and vinorelbine (n = 2), nedaplatin and gemcitabine (n = 4), nedaplatin and etopocide (n = 2); other multidrug chemotherapy: gemcitabine and docetaxel (n = 1), cisplatin, doxorubicin, and cyclophosphamide (n = 2), carboplatin, doxorubicin, and cyclophosphamide (n = 2), paclitaxel and gemcitabine (n = 1), S‐1 and irinotecan (n = 1), epirubicin, dacarbazine, and S‐1 (n = 1), folinic acid, fluorouracil, and oxaliplatin (n = 1); other monotherapy: carboplatin (n = 1), irinotecan (n = 1), gefitinib (n = 1), imatinib (n = 1), pemetrexed (n = 1), paclitaxel (n = 1), vinorelbine (n = 1).
Abbreviations: ADOC, adriamycin + cisplatin + vincristine + cyclophosphamide; AMR, amrubicin; CBDCA, carboplatin; CDDP, cisplatin; CPT‐11, irinotecan; DTX, docetaxel; GEM, gemcitabine; mOS, median overall survival; mPFS, median progression free survival; N/A, not analyzed; PTX, paclitaxel; RR, response rate; S‐1, tegafur + gimeracil + oteracil; VP‐16, etopocide.
Efficacy of Second‐Line Chemotherapy Regimens
The median follow‐up period was 50.5 months (95% confidence interval [CI], 36.5‐76.0 months; Kaplan‐Meier estimate). The efficacy of each regimen is shown in Table 3. The RR and median PFS were 21.2% and 6.9 months for patients treated with carboplatin plus paclitaxel, 38.9% and 8.3 months for those treated with S‐1 monotherapy, and 21.4% and 6.7 months for those treated with ADOC, respectively. S‐1 monotherapy conferred relatively good RR and PFS; however, there were no significant differences between the RR and PFS for carboplatin plus paclitaxel (RR, p = .149 and PFS, p = .060) and ADOC (RR, p = .285 and PFS, p = .231).
The comparison of OS among platinum‐based doublet chemotherapy, other multidrug chemotherapy, and monotherapy as second‐line regimens is shown in Table 4 and Figure 1. After stratification based on the type of regimen, the patient characteristics were well‐balanced with the exception of type of the first‐line chemotherapy regimen; these are summarized in supplemental online Table 1. The median OS for platinum‐based doublet chemotherapy, other multidrug chemotherapy, and monotherapy was 22.4, 25.7, and 21.4 months, respectively. There was no significant difference in OS between platinum‐based doublet and other multidrug chemotherapy or monotherapy (platinum‐based doublet versus other multidrug chemotherapy: hazard ratio [HR], 1.116; 95% CI, 0.667–1.789, p = .665; platinum‐based doublet versus monotherapy: HR, 1.193; 95% CI, 0.794‐1.766, p = .389). The results of univariate and multivariate analyses for OS are shown in supplemental online Table 4. The type of first‐line chemotherapy regimen was not related to OS from start of second‐line chemotherapy. In univariate analysis, sex, ECOG PS, and Masaoka‐Koga stage were significantly predictive for OS. In the multivariate analysis of these factors, the prognostic factors associated with good survival were ECOG PS (0‐1 vs. 2‐3: HR, 0.359; 95% CI, 0.225–0.599, p < .001) and Masaoka‐Koga stage (IVa vs. IVb: HR, 0.605; 95% CI, 0.386‐0.932, p = .022).
Table 4.
Comparison of efficacies between types of chemotherapy regimen
| Regimen | Patients, n | RR, % | MST (95%CI), mo | HR (95% CI) | p value |
|---|---|---|---|---|---|
| Platinum doublets | 110 | 21.6 | 22.4 (16.1–28.4) | 1.00 | |
| Other multidrug chemotherapy | 26 | 13.6 | 25.7 (11.5–37.0) | 1.116 (0.667–1.789) | .665 |
| Monotherapy | 55 | 19.6 | 21.4 (13.5–31.8) | 1.193 (0.794–1.766) | .389 |
| All regimens | 191 | 20.0 | 22.4 (17.5–26.7) |
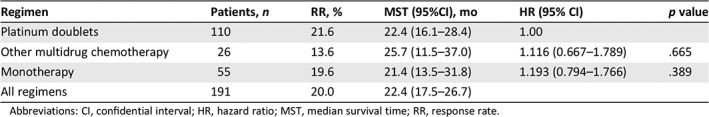
Abbreviations: CI, confidential interval; HR, hazard ratio; MST, median survival time; RR, response rate.
Figure 1.
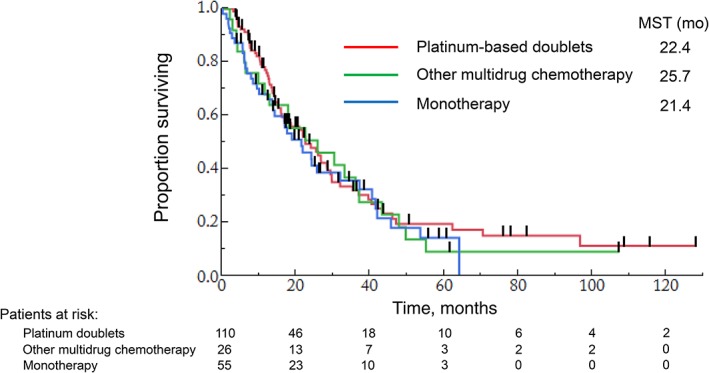
Overall survival in patients treated with each second‐line regimens.Abbreviation: MST, median survival time.
Sequence of First‐ and Second‐Line Chemotherapies
Treatment sequences of 191 patients are shown in Figure 2. In total, 114 patients were treated with platinum‐based doublets as the first‐line chemotherapy, 72 with other multidrug chemotherapy, and 5 with monotherapy. Among the 114 patients who received platinum‐based doublets as first‐line chemotherapy, about half were treated with platinum‐based doublets again, and 40 were treated with single agent chemotherapy in the second‐line setting. Among the 72 patients who received other multidrug chemotherapy as the first‐line therapy, about 70% were treated with platinum‐based doublets as the second‐line chemotherapy. The details of relationship between first and second‐line chemotherapy efficacy are shown in supplemental online Table 2.
Figure 2.
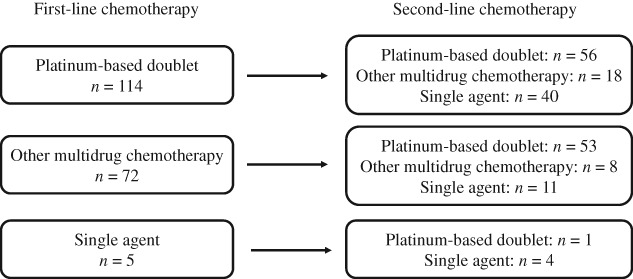
Sequence of first and second‐line chemotherapies.
Discussion
To our knowledge, this study is the largest retrospective analysis of second‐line chemotherapy for patients with advanced thymic carcinoma. The results show that there was no significant difference in OS among chemotherapeutic regimens, including platinum‐based doublet and monotherapy.
Previous results from studies on second‐line chemotherapy in patients with thymic carcinoma are summarized in Table 1. No comparative trial had been conducted, and the number of patients enrolled in these studies was very small. In the National Comprehensive Cancer Network guideline, several chemotherapy regimens (pemetrexed, paclitaxel, gemcitabine, etoposide, 5‐FU with leucovorin, and ifosfamide) are described as candidates for second‐line chemotherapy in patients with advanced thymic carcinoma. However, these regimens are not strongly recommended in this guideline, owing to limited evidence 27.
Unexpectedly, carboplatin plus paclitaxel was the most common regimen in the second‐line setting in this study. Even if patients received platinum‐based doublet or other multidrug chemotherapy as the first‐line treatment, platinum‐based doublets were often selected again as second‐line chemotherapy (Fig. 2). The cause of this selection is unclear, but the lack of evidence for monotherapy for patients with thymic carcinoma may be a contributing factor. In this study, there were no significant differences in efficacy between monotherapies and platinum‐based doublets used to treat previously treated advanced thymic carcinoma. However, the toxicities of platinum‐based doublets, especially bone marrow suppression and gastrointestinal toxicities, are reportedly more severe than those of monotherapies 8, 9, 10, 11, 12, 28, 29, 30. These data support the use of monotherapy as second‐line chemotherapy for patients with previously treated advanced thymic carcinoma.
In single agent chemotherapy, the most frequently investigated chemotherapeutic regimen is S‐1. S‐1 is an oral fluoropyrimidine agent containing the 5‐fluorouracil prodrug tegafur and two enzyme inhibitors, namely, 5‐chloro‐2, 4‐dihydroxypyridine and potassium oxonate, which can reduce the adverse effects of tegafur. This regimen conferred a relatively good RR and PFS in our study. Similarly, several previous reports support the efficacy of S‐1 9, 31. Currently, a prospective phase II trial to evaluate the efficacy of S‐1 for patients with previously treated advanced thymic carcinoma is ongoing (UMIN000010736). It has been demonstrated that the anticancer activity of S‐1 is related to the intratumoral expression of dihydropyrimidine dehydrogenase and thymidylate synthase in advanced gastric cancer 32. The combination of a relatively low expression of thymidylate synthase and high expression of orotate phosphoribosyltransferase suggests a better antitumor effect of 5‐FU drugs in thymic carcinoma than in lung carcinoma 33. Immunohistological examination of these enzymes in thymic cancer may be helpful in elucidating the pharmacological mechanisms of S‐1 action. Furthermore, docetaxel and amrubicin were selected as monotherapy regimens in this study but showed poor efficacy. However, previous reports showed promising effects of these regimens for previously treated thymic carcinoma 11, 12. Larger studies to evaluate efficacy of single agent chemotherapy for these patients are warranted.
Recently, several new treatment strategies for thymic carcinoma have been evaluated, including molecular targeted agents. Specifically, sunitinib and everolimus have been reported to have promising efficacies in thymic carcinoma. Sunitinib is a multikinase inhibitor that inhibits c‐Kit and platelet‐derived growth factor, and everolimus is a rapamycin analog that inhibits mammalian target of rapamycin. The RR and PFS for thymic carcinoma were 26% and 7.2 months with sunitinib 17 and 25% and 12.1 months with everolimus 18, respectively. Another new strategy is immunotherapy. Pembrolizumab is a humanized, monoclonal antibody designed to bind to programmed cell death 1 (PD‐1) and block the interaction between PD‐1 and its ligands. The RR and PFS of pembrolizumab were 19.2%–22.5% and 4.2–6.1 months, respectively, in patients with previously treated advanced thymic carcinoma 19, 20. These results are certainly promising; However, the cost of these agents differs. Molecular targeted agents and immunotherapies are more expensive than cytotoxic chemotherapy agents. In Japan, the treatment costs for a 6‐week course of sunitinib, everolimus, and pembrolizumab are 838,000 yen (approximately $7,800), 874,000 yen (approximately $8,100), and 1,458,000 yen (approximately $13,500), respectively. However, the cost for a 6‐week course of S‐1 monotherapy in 87,900 yen (approximately $818). In addition, no comparative studies and limited biomarker analysis has been conducted following these new treatments. Therefore, there are not enough data currently available to select an appropriate treatment for each patient. Further evaluations of new treatments are warranted, including cost‐benefit analyses and measurement of disease biomarkers.
There were several limitations to our study. First, this was a retrospective study. However, thymic carcinoma is a very rare disease, making it difficult to perform a prospective study, especially in the second‐line setting. To our knowledge, the sample size of our study is by far the largest among retrospective studies for second‐line chemotherapy of advanced thymic carcinoma. It is hoped that based on our study, an appropriate prospective study would be conducted in the future. Second, pathological reviews of samples were only conducted in each institution. Therefore, there may be some variability in pathological diagnosis due to difficulties in the pathological diagnosis for thymic epithelial tumors. The lack of a central pathology review is a weakness of this study. Further studies should include a central pathological review to evaluate the accuracy of diagnosis across institutions.
Conclusion
The second‐line chemotherapy regimens for advanced thymic carcinoma were platinum doublets in 57.6% of patients, monotherapy in 28.8%, and other multidrug chemotherapy in 13.6%. The median OS was 22.4 months in patients with advanced thymic carcinoma treated with second‐line chemotherapy. There were no significant differences in RR and OS between monotherapy and multidrug chemotherapy in this study.
Author Contributions
Conception/design: Ryo Ko, Takehito Shukuya, Kunihiko Kobayashi, Kazuhisa Takahashi
Provision of study material or patients: Kazunari Tateishi, Ryo Ko, Takehito Shukuya, Yusuke Okuma, Satoshi Watanabe, Shoichi Kuyama, Kyoko Murase, Yoko Tsukita, Hironori Ashinuma, Taku Nakagawa, Kazutsugu Uematsu, Mika Nakao, Yoshiaki Mori, Kyoichi Kaira, Atsuto Mouri, Takao Miyabayashi, Hiroyuki Sakashita, Yoko Matsumoto, Tomoyuki Tanigawa, Tomonobu Koizumi, Kunihiko Kobayashi, Toshihiro Nukiwa, Kazuhisa Takahashi
Collection and/or assembly of data: Kazunari Tateishi, Ryo Ko, Takehito Shukuya, Yusuke Okuma, Satoshi Watanabe, Shoichi Kuyama, Kyoko Murase, Yoko Tsukita, Hironori Ashinuma, Taku Nakagawa, Kazutsugu Uematsu, Mika Nakao, Yoshiaki Mori, Kyoichi Kaira, Atsuto Mouri, Takao Miyabayashi, Hiroyuki Sakashita, Yoko Matsumoto, Tomoyuki Tanigawa, Tomonobu Koizumi, Kunihiko Kobayashi, Toshihiro Nukiwa, Kazuhisa Takahashi
Data analysis and interpretation: Kazunari Tateishi, Ryo Ko, Takehito Shukuya, Satoshi Morita
Manuscript writing: Kazunari Tateishi, Ryo Ko, Takehito Shukuya
Final approval of manuscript: Kazunari Tateishi, Ryo Ko, Takehito Shukuya, Yusuke Okuma, Satoshi Watanabe, Shoichi Kuyama, Kyoko Murase, Yoko Tsukita, Hironori Ashinuma, Taku Nakagawa, Kazutsugu Uematsu, Mika Nakao, Yoshiaki Mori, Kyoichi Kaira, Atsuto Mouri, Takao Miyabayashi, Hiroyuki Sakashita, Yoko Matsumoto, Tomoyuki Tanigawa, Tomonobu Koizumi, Satoshi Morita, Kunihiko Kobayashi, Toshihiro Nukiwa, Kazuhisa Takahashi
Disclosures
Ryo Ko: Boehringer Ingelheim (RF), Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceutical, Ono Pharmaceutical, Chugai Pharmaceutical, Novartis, Merck Sharpe & Dohme (H); Takehito Shukuya: AstraZeneca, Eli Lilly & Co, Chugai Pharmaceutical (H); Yusuke Okuma: Takeda Oncology, Chugai Pharmaceutical (RF); Satoshi Watanabe: AstraZeneca, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb, Taiho Pharmaceutical, Boehringer Ingelheim (H); Yoko Tsukita: Chugai Pharmaceutical (H); Hironori Ashinuma: AstraZeneca, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb, Boehringer Ingelheim (H); Kazutsugu Uematsu: Boehringer Ingelheim, Taiho Pharmaceutical, Chugai Pharmaceutical, Novartis (RF), Boehringer Ingelheim, Taiho Pharmaceutical, Chugai Pharmaceutical, Novartis, AstraZeneca, Astellas, Kyorin Pharmaceutical, Eli Lilly & Co, Bristol‐Myers Squibb, Merck Sharpe & Dohme (H); Hiroyuki Sakashita: Ono Pharmaceutical, Bristol‐Myers Squibb, Boehringer Ingelheim, Chugai Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Merck Sharpe & Dohme (H); Kunihiko Kobayashi: AstraZeneca, Boehringer Ingelheim, Taiho Pharmaceutical (H); Kazuhisa Takahashi: Novartis, Boehringer Ingelheim, AstraZeneca, Novelpharma, Shionogi Pharma, Taiho Pharmaceutical, Tsumura & Co., GlaxoSmithKline, Kyowa Hakko Kirin, Astellas Pharma, Toyama Chemical Co., Torii Pharmaceutical, Nippon Shinyaku, Nipro Corporation (RF), Boehringer Ingelheim, AstraZeneca, Novelpharma, Shionogi Pharma, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Sumitomo Dainippon, Bristol‐Myers Squibb, Otsuka, Meiji Seika Kaisha (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Supplemental Figures
Acknowledgments
This study was funded by the Department of Respiratory Medicine, Juntendo University Graduate School of Medicine. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Ryo Ko, Email: rkou@juntendo.ac.jp.
Takehito Shukuya, Email: tshukuya@juntendo.ac.jp.
References
- 1. Wick MR, Scheithauer BW, Wiland LH et al. Primary thymic carcinomas. Am J Surg Pathol 1982;6:613–630. [DOI] [PubMed] [Google Scholar]
- 2. Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025–1032. [DOI] [PubMed] [Google Scholar]
- 3. Hsu CP, Chen CY, Chen CL et al. Thymic carcinoma. Ten years’ experience in twenty patients. J Thorac Cardiovasc Surg 1994;107:615–620. [PubMed] [Google Scholar]
- 4. Liu HC, Hsu WH, Chen YJ et al. Primary thymic carcinoma. Ann Thorac Surg 2002;73:1076–1081. [DOI] [PubMed] [Google Scholar]
- 5. Kondo K, Monden Y. Therapy for thymic epithelial tumors: A clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878–885. [DOI] [PubMed] [Google Scholar]
- 6. Weksler B, Dhupar R, Parikh V et al. Thymic carcinoma: A multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299–304. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa K, Toita T, Uno T et al. Treatment and prognosis of thymic carcinoma: A retrospective analysis of 40 cases. Cancer 2002;94:3115–3119. [DOI] [PubMed] [Google Scholar]
- 8. Okuma Y, Hosomi Y, Miyamoto S et al. Correlation between S‐1 treatment outcome and expression of biomarkers for refractory thymic carcinoma. BMC Cancer 2016;16:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okuma Y, Hosomi Y, Watanabe K et al. Gemcitabine in patients previously treated with platinum‐containing chemotherapy for refractory thymic carcinoma: Radiographic assessment using the RECIST criteria and the ITMIG recommendations. Int J Clin Oncol 2016;21:531–538. [DOI] [PubMed] [Google Scholar]
- 10. Liang Y, Padda SK, Riess JW et al. Pemetrexed in patients with thymic malignancies previously treated with chemotherapy. Lung Cancer 2015;87:34–38. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe N, Umemura S, Niho S et al. Docetaxel for platinum‐refractory advanced thymic carcinoma. Jpn J Clin Oncol 2015;45:665–669. [DOI] [PubMed] [Google Scholar]
- 12. Hirai F, Seto T, Yamanaka T et al. Amrubicin as second‐line and beyond treatment for platinum‐refractory advanced thymic carcinoma. Jpn J Clin Oncol 2013;43:1018–1022. [DOI] [PubMed] [Google Scholar]
- 13. Gbolahan OB, Porter RF, Salter JT et al. A phase II study of pemetrexed in patients with recurrent thymoma and thymic carcinoma. J Thorac Oncol 2018;13:1940–1948. [DOI] [PubMed] [Google Scholar]
- 14. Bluthgen MV, Boutros C, Fayard F et al. Activity and safety of oral etoposide in pretreated patients with metastatic or recurrent thymic epithelial tumors (TET): A single‐institution experience. Lung Cancer 2016;99:111–116. [DOI] [PubMed] [Google Scholar]
- 15. Palmieri G, Buonerba C, Ottaviano M et al. Capecitabine plus gemcitabine in thymic epithelial tumors: Final analysis a phase II trial. Future Oncol 2014;10:2141–2147. [DOI] [PubMed] [Google Scholar]
- 16. Giaccone G, Rajan A, Ruijter R et al. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol 2009;4:1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas A, Rajan A, Berman A et al. Sunitinib in patients with chemotherapy‐refractory thymoma and thymic carcinoma: An open‐label phase 2 trial. Lancet Oncol 2015;16:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zucali PA, De Pas T, Palmieri G et al. Phase II study of everolimus in patients with thymoma and thymic carcinoma previously treated with cisplatin‐based chemotherapy. J Clin Oncol 2018;36:342–349. [DOI] [PubMed] [Google Scholar]
- 19. Giaccone G, Kim C, Thompason J et al. Pembrolizumab in patients with thymic carcinoma: A single‐arm, sigle‐centre, phase 2 study. Lancet Oncol 2018;19:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho J, Kim HS, Ku BM et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: An open‐label phase II trial. J Clin Oncol 2019;37:2162–2170. [DOI] [PubMed] [Google Scholar]
- 21. Katsuya Y, Horinouchi H, Seto T et al. Single‐arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019;113:78–86. [DOI] [PubMed] [Google Scholar]
- 22. Ko R, Shukuya T, Okuma Y et al. Prognostic factors and efficacy of first‐line chemotherapy in patients with advanced thymic carcinoma: A retrospective analysis of 286 patients from NEJ023 study. The Oncologist 2018;23:1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Detterbeck FC, Nicholson AG, Kondo K et al. The Masaoka‐Koga stage classification for thymic malignancies: Clarification and definition of terms. J Thorac Oncol 2011;6(suppl 3):S1710–S1716. [DOI] [PubMed] [Google Scholar]
- 24. Travis WD, Brambilla E, Müller‐Hermelink HK et al. Tumours of the thymus In: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Third edition, volume 10 Lyon, France: World Health Organization Classification of Tumors, IARC Press, 2044:145–248. [Google Scholar]
- 25. Yamakawa Y, Masaoka A, Hashimoto T et al. A tentative tumor‐node‐metastasis classification of thymoma. Cancer 1991;68:1984–1987. [DOI] [PubMed] [Google Scholar]
- 26. Eisenhauser EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Rerevised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 27. NCCN Clinical Guidelines in Oncology . Thymomas and Thymic Carcinomas. February 16, 2016. Available at https://www.nccn.org. Accessed November 16, 2018.
- 28. Lemma GL, Lee JW, Aisner SC et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirai F, Yamanaka T, Taguchi K et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363–368. [DOI] [PubMed] [Google Scholar]
- 30. Inoue A, Sugawara S, Harada M et al. Phase II study of Amrubicin combined with carboplatin for thymic carcinoma and invasive thymoma: North Japan Lung Cancer Group Study 0803. J Thorac Oncol 2014;9:1805–1809. [DOI] [PubMed] [Google Scholar]
- 31. Koizumi T, Agatsuma T, Komatsu Y et al. Successful S‐1 monotherapy for chemorefractory thymic carcinoma. Anticancer Res 2011;31:299–301. [PubMed] [Google Scholar]
- 32. Takiuchi H, Kawabe S, Gotoh M et al. Thymidylate synthase gene expression in primary tumors predicts activity of s‐1‐based chemotherapy for advanced gastric cancer. Gastrointest Cancer Res 2007;1:171–176. [PMC free article] [PubMed] [Google Scholar]
- 33. Yokota K, Sasaki H, Okuda K et al. Expression of thymidylate synthase and orotate phosphoribosyltransferase in thymic carcinoma. Exp Ther Med 2012;4:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Supplemental Figures


