Abstract
One of the most critical challenges for the food packaging industry to overcome is the development of biodegradable coatings from renewable sources. In this work, purple yam starch (PYS), chitosan (CS), and glycerol were blended to obtain biodegradable films for characterization as intended food coatings. The films had a homogeneous surface, and the amount of CS highly influenced the film thickness. Infrared spectroscopy indicated hydrogen bond interactions between PYS and CS in the films. Thermogram data suggested that glycerol contributed to the thermal stability of the films, due to its greater interaction with CS than to the PYS. Finally, the application of a YS/CS film on apples for 4 weeks was able to preserve the fruit quality, as weight loss from the coated apple was significantly lower than the uncoated apple (p = 0.44, Dunnet's posthoc test). YS/CS films have great prospects in the food packaging industry as a new biodegradable coating.
Keywords: Food science, Food technology, Materials science, Yam, Dioscorea trifida, Starch, Chitosan, Films, Food coating
Food science, Food technology, Materials science, Yam, Dioscorea trifida, Starch, Chitosan, Films, Food coating.
1. Introduction
Biodegradable polymeric films offer an alternative for sustainable packaging and an increase in the shelf-life of foods. The development of biodegradable material from renewable sources and reduction in the use of plastic packages reinforce the goal of environmental preservation that is now expected from the packaging industry. Biopolymers are used as a matrix in several industries (Lawal et al., 2008) due to their abundance, low cost, biocompatibility, non-toxicity and the fact that they are a renewable source (Gutiérrez et al., 2015). Natural polymers, such as chitosan (Valencia-sullca, Atarés & Vargas, 2018), starches (Gutiérrez et al., 2015) and others (Galus and Kadzińska, 2015), have already been studied as biodegradable packages or edible thin layers to cover and extend the shelf-life of food products.
Chitosan is a linear natural biopolymer derived from the alkaline deacetylation of chitin (Van Den Broek, Knoop, Kappen and Boeriu, 2015). On account of its biocompatibility, good mechanical properties and antimicrobial activity, chitosan has been widely used as an edible film to coat foods (Elsabee and Abdou, 2013). These films were classified according to thickness as either edible thin films or coatings (<30 μm), or films and blends (>30 μm) (Van Den Broek, Knoop, Kappen and Boeriu, 2015). Some challenges related to water permeability and brittleness have been overcome with the addition of hydrophobic substances and plasticizers into chitosan-based films (Elsabee and Abdou, 2013). The combination of chitosan with other polysaccharides, such as starches, has also been reported to decrease the water permeability rate and increase the elongation values when compared to non-blended films (Elsabee and Abdou, 2013).
Yams, such as Dioscorea trifida, are considered an important cultivated root by the American indigenous people for its traditional use and consumption as a food (Pérez et al., 2011; Kay, 1987). Africa and the Americas are the biggest yam producers, at 96% and 3%, respectively. With a world production of 65.9 million tons reported in 2016, Nigeria and Brazil contribute heavily to this total (FAO, 2018). Yet yam (Dioscorea sp.) cultivation worldwide is lower than of cassava (Manihot esculenta), which is another starchy tuber. However, yams still have high socioeconomic importance in tropical countries, such as Brazil, especially the Northeast region (Reis et al., 2013). Given the excellent nutritional quality and energy of the yam (Lebot, 2009), its starch has some interesting functional and industrial properties, comparable to that of cereals (Trèche, 1998). Despite the significant yam production, its starches are poorly available on the market. Yam starches from different species have been characterized by some researchers (Jiang et al., 2012; Pérez et al., 2011) and used as films to coat perishable fruits (Mali and Grossmann, 2003), which was reported to enhance their shelf-life. As films, yam starch from D. trifida demonstrated higher Young's modulus and resistance values when compared to cassava, which indicates its great applicability as food packaging (Gutiérrez et al., 2015).
The production of films with polysaccharides is a sustainable alternative for increasing the shelf-life of fresh fruits. In addition, the development of edible films can contribute to conserving the environment by reducing the use of non-biodegradable polymers and improving bioeconomic food industries. Therefore, this study aimed to extract and physicochemically characterize an Amazonian purple yam (D. trifida) starch for the development of biodegradable films blended with chitosan, and their application in the preservation of fresh apples.
2. Materials and methods
2.1. Materials
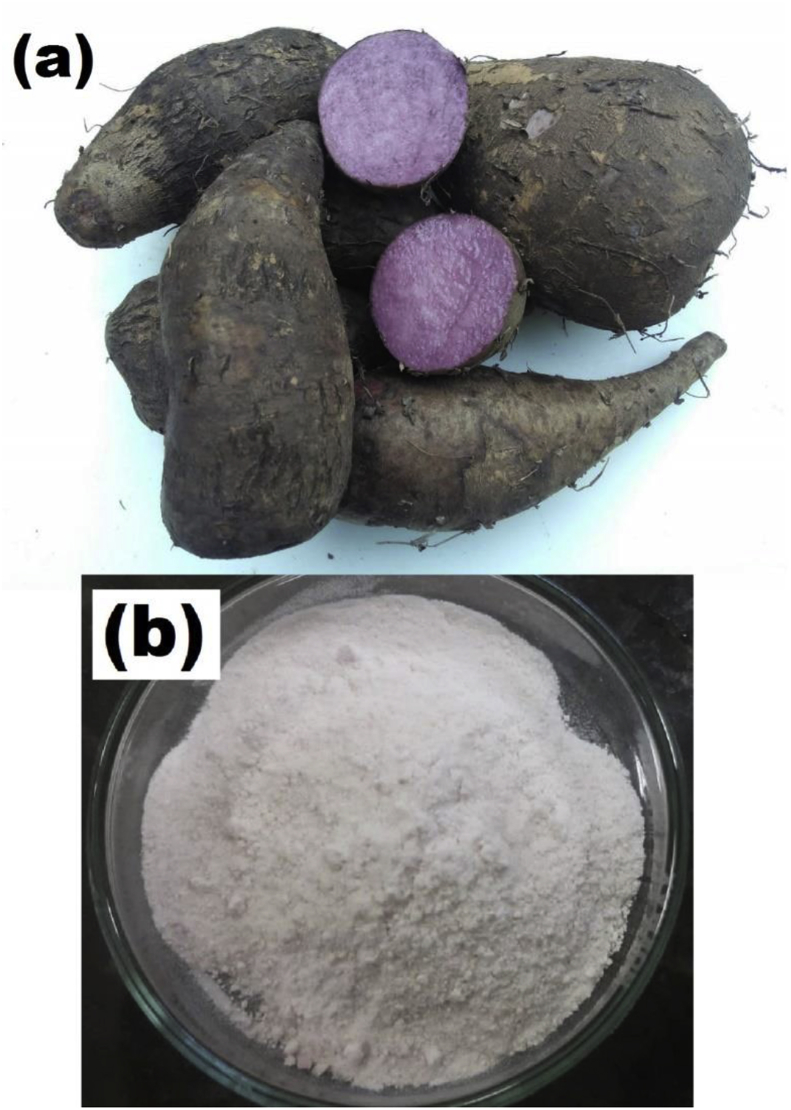
Flour and starch were extracted from the purple yam roots (Dioscorea trifida), a photo of which is shown in Figure 1(a), purchased from local farmers in Caapiranga city (Amazonas, Brazil). Chitosan (CS) (degree of deacetylation of 82% and Mw of 1 × 105 g/mol) (Polymar, Ceará, Brazil) and glycerol (Nuclear, São Paulo, Brazil) were purchased from the respective manufacturers, and the other chemicals used were analytical grade. Intact Fuji apple cultivars purchased from a local supermarket in the city of Manaus (Amazonas, Brazil) were used in the experiments for the application of the elaborated films. The yams and apples were selected based on uniformity in size and without any apparent ripeness. They were then washed with distilled water to remove physical dirt from the surface, and stored until futher analysis.
Figure 1.
Digital photographs of purple yam roots (a) and its extracted starch (b).
2.2. Extraction of the purple yam flour and starch
The purple yam flour (PYF) was obtained from the edible portion of the purple yams by milling and then air-drying at 105 °C until constant weight was achieved (Solab oven, model SL-100, São Paulo, Brazil). The PYF was stored at 8 °C for further analysis. The purple yam starch (PYS) was obtained using a methodology adapted from Ascheri et al. (2014), where the purple yam was milled, successively washed, decanted and filtrated at room temperature using a polyester mesh (40 cm × 40 cm width and 0.1 mm pore size) until the entire sample had visual characteristics of starch as shown in Figure 1(b). Subsequently, it was air-dried in a Solab oven at 105 °C until constant weight was attained and passed through a 48 mesh sieve (n° 50 with 0.297 mm opening) to homogenize the samples.
2.3. Preparation of purple yam starch/chitosan films
The PYS (2 g) was dissolved in 100 mL of distilled water and subsequently heated at 70 °C with constant magnetic stirring until complete starch gelatinization (20–30 min), then left to cool to room temperature. CS (0.5 and 1.0 g) were dissolved in 100 mL of 5.0% (v/v) acetic acid solution under magnetic stirring for 4 h at 25 °C. Then, the PYS and CS solutions were mixed to obtain the film-forming solutions. Glycerol, as a plasticizer, was added to these film-forming solutions at 2.0% (w/v) concentration under stirring for 20 min. These film-forming solutions at 0.0, 0.5 and 1.0% of CS were named YS/CS0, YS/CS0.5 and YS/CS1.0, respectively.
The films were prepared using the casting method. Aliquots (20 mL) of the film-forming solution were cast on glass plates (14 cm in diameter) and air-dried in a Solab oven at ≈50 °C until constant weight was attained. The dried films were conditioned in desiccators containing a modified atmosphere of saturated NaCl solution at a 75% relative humidity (RH) for 7 days, and then peeled off manually and properly stored at room temperature until the following analyses (Fakhouri et al., 2007). All the casted solutions were prepared in triplicate.
2.4. Application of the film coatings
The film-forming solutions were applied to the surface of the apples to investigate whether the films affect weight loss and internal appearance of the apples during the storage time, according to the adapted methodology of León-Zapata et al. (2015). The apples were washed and disinfected with a chlorine solution (500 ppm) for 10 min and dried at room temperature. The apples were dipped in the film-forming solutions, YS/CS0, YS/CS0.5 and YS/CS1.0, for 2 s, suspended, and dried in an incubator at a controlled temperature (25 ± 2 °C). Apples without any applied coating (WC) were used as a control group and placed in the same conditions as the coated apples. The apples were weighed every five days for the 30 days of storage. This analysis was carried out in duplicate for each group.
2.5. Proximate composition analysis of the raw materials
Moisture, protein, crude fibre, fat and ash contents of the PYF and PYS were analyzed according to the methods of Association of Official Analytical Chemists [AOAC] (2000). The carbohydrate content was obtained by the remaining difference.
2.6. Scanning electron microscopy
Shape and distribution of PYS granules, surface, and cross-section of the YS/CS films were observed by scanning electron microscopy (SEM). PYS powder and film pieces were sprinkled onto pieces of double-sided adhesive tape, attached to circular specimen stubs, coated with gold under 6 × 10−1 mbar ultrapure argon vacuum (Baltec, SCD 005 Sputter Coater, Balzers, Liechtenstein) for 150 s, examined with an accelerating voltage of 5 kV and photographed using a scanning electron microscope (TM3030 Plus, Hitachi, Tokyo, Japan).
2.7. Water-binding capacity, pH, gel clarity and crystallinity of PYS
The water-binding capacity (WBC) of PYS was determined using a method from Jiang et al. (2012) with modifications. A suspension of 5 g starch (dry weight) in 75 mL distilled water was magnetically stirred for 1 h and centrifuged at 5000 x g (Himac, CR22GII, Hitachi Koki Co. Ltd., Tokyo, Japan) for 10 min. Free water was removed from starch through decanting for 10 min, and the wet starch weight was measured. The WBC could be defined by Eq. (1):
| (1) |
where W0 is the initial weight of the dried PYS, and W1 is the weight of swollen PYS.
A 1% PYS aqueous solution was prepared with distilled water and tested. The pH was determined by a digital pH meter. The PYS gel clarity was measured using the methodology from Craig et al. (1989). The PYS solution was boiled with continuous mixing every 5 min for 30 min. After cooling to room temperature, the light transmittance percentage (T%) was measured at 650 nm in a spectrophotometer (model UV-1800, Shimadzu, Tokyo, Japan). Three quantifications per sample were made.
The crystallinity degree (CrD) of the PYS was analyzed through the X-ray diffractometer (Ultima IV, Rigaku Corporation, Tokyo, Japan) in the angular region 2° to 50° (2θ) at 0.05o per second (2θ) in continuous scanning mode with CuKα radiation generated at 40 kV and 20 mA. The CrD of the sample was calculated as the ratio of the crystalline area to the total area under diffraction peaks (peak deconvolutions were executed with Fytik®).
2.8. Moisture content, thickness and water vapor permeability of the films
The moisture in the films was gravimetrically determined. The films were dried in an air circulation oven at 50 °C until constant weight was attained. Samples were analyzed in triplicate, and results were expressed as the mean of g of water per 100 g of dried films ±SD.
The film thickness (L) [mm] was measured with a digital micrometer with a 0.001 mm resolution (PANTEC®, model 13101-25, Rio Grande do Sul, Brazil). Five measurements were taken on each testing sample at different points. The results were expressed as mean ± SD.
The water vapor permeability (WVP) of the films was determined gravimetrically based on the Standard Test Method for Water Vapour Transmission of Materials (ASTM, 2016). Film samples of 7 mm in diameter were tightly sealed to a permeation cell containing distilled water (RH = 100%) at a 3⁄4 level from the film. The permeation cells were weighed and then placed in desiccators containing granular anhydrous calcium chloride (RH = 2%), providing relative humidity gradients at 25 °C. The tests were carried out in quadruplicate for each film sample. The cells were periodically weighed for 14 days, and the WVP in g m−1 s−1 Pa−1 was obtained from Eq. (2).
| (2) |
where Δm is the weight change (g), L is the film thickness (m), A is the area (0.006 m2) exposed for a time Δt (s) to a partial water pressure Δp (Pa).
2.9. Infrared spectroscopy
Fourier transform infrared spectroscopy (FT-IR) was used to evaluate the molecular structure of PYS and to better understand the molecular interaction in the YS/CS films. The analyses were carried out from 4000 – 600 cm−1 by using a Frontier FT-IR/FIR spectrophotometer (model 98737, Perkin Elmer Spectrum, Connecticut, USA) equipped with a Universal Attenuated Total Reflectance (ATR) cell device. All spectra were registered by averaging 60 scans with a resolution of 4 cm−1 in transmission mode. CS and PYS were analyzed as potassium bromide (KBr) disks.
2.10. Thermogravimetric analysis
To investigate the thermal behavior of the PYS, CS and the films, thermogravimetric (TG) and differential thermal analyses (DTA) were carried out for the dried samples under nitrogen atmosphere on TGA Q-500 equipment (TA Instruments, New Castle, Delaware USA). Approximately 10 mg of the sample was heated from 25 °C to 700 °C at a 10 °C/min rate.
2.11. Statistical analysis
The experimental data were submitted to the analyses of variance (ANOVA). Significant differences between the average amounts were established at p ≤ 0.05. The Tukey's and Dunnett's posthoc tests were applied when necessary.
3. Results and discussion
3.1. Proximate composition analysis of the purple yam raw materials
Purple yams (D. trifida) were processed to obtain purple yam flour (PYF) and starch (PYS). The proximate composition of the PYF and PYS are shown in Table 1. PYF was high in moisture content, ash, protein, and fiber. There was a similar content of lipids, but a lower carbohydrate content than the PYS. The protein content in the PYF and PYS were lower than reported (5%–7%) by Pérez et al. (2011) for the same species. The 2% moisture content in the PYS is below the maximum value, 15%, allowed by the U.S. Food and Drug Administration (FDA), that establishes the standards for cereal flours and related products (FDA, 2011). Moisture content is an essential variable in the storage life of flours and starches, as a greater moisture content can lead to a great risk of microbial contamination. PYS presented minor amounts of protein, fat, and ash, which are similar to the composition values determined by Pérez et al. (2011). The ash content in PYS was also similar to the values (1–2%) determined by Jiang et al. (2012) in a different Dioscorea species. This proximate composition analysis shows that the PYS is suitable as an edible material.
Table 1.
Characterization of the purple yam flour (PYF), starch (PYS), and films based on PYS/chitosan (CS).
| Proximate composition | Flour (PYF) | Starch (PYS) |
|---|---|---|
| Moisture (g/100 g)∗ | 67 ± 1 | 2.3 ± 0.1 |
| Protein (%) | 1.6 ± 0.7 | 0.50 ± 0.01 |
| Ash (%) | 1.5 ± 0.2 | 0.007 ± 0.003 |
| Fat (%) | 0.08 ± 0.01 | 0.12 ± 0.01 |
| Crude Fibre (%) | 2.16 ± 0.05 | 1.6 ± 0.1 |
| Carbohydrate (%) | 27.4 ± 0.4 | 95.3 ± 0.1 |
| WBC (%) | - | 174 ± 37 |
| pH | - | 4.1 ± 0.1 |
| Gel clarity (%T) | - | 39.5 ± 0.1 |
| Films | Moisture content (g/100 g) | Film thickness (mm) | WVP × 10−10 (g m−1 s−1 Pa−1) |
|---|---|---|---|
| YS/CS0 | 77.4 ± 0.4a | 0.20 ± 0.01a | 1.31 ± 0.05a |
| YS/CS0.5 | 76.7 ± 0.4a | 0.22 ± 0.01a | 1.35 ± 0.06a |
| YS/CS1.0 | 76.1 ± 0.5b | 0.33 ± 0.04b | 1.94 ± 0.04b |
WBC, water-binding capacity. Results are expressed as mean ± SD. - Not determined. Different superscripts a, b letters in the same column indicate significantly different (p < 0.05) by Tukey's posthoc test.
Except for the moisture content, results are given on a dry basis (db).
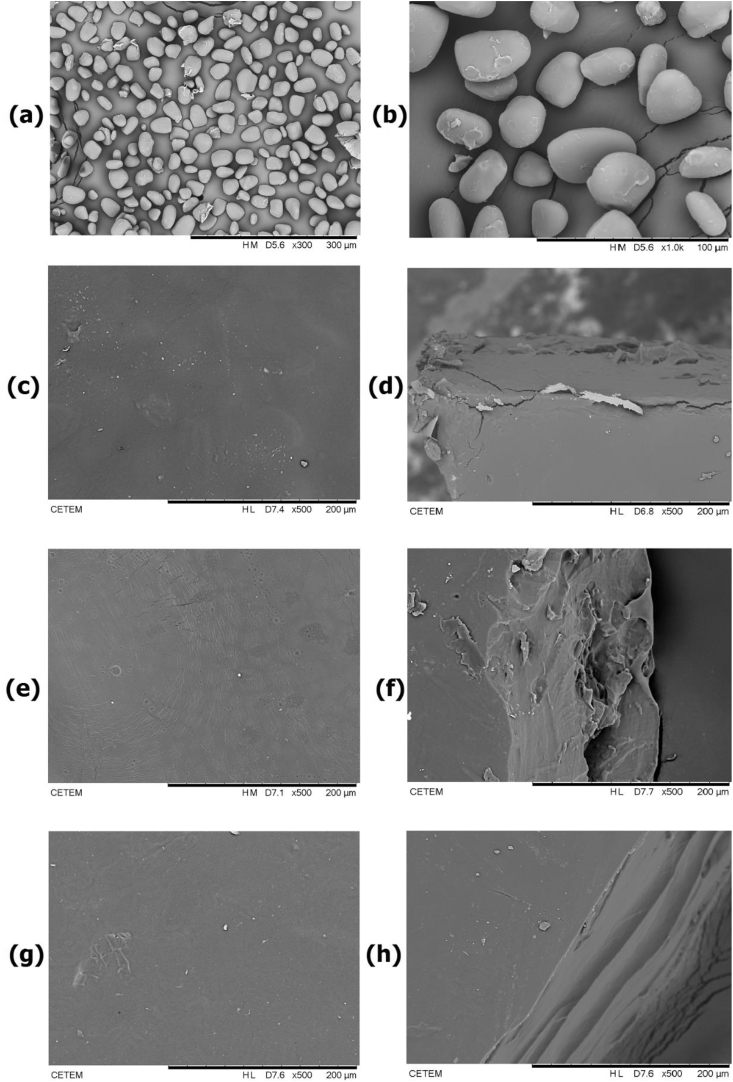
3.2. SEM of the PYS and YS/CS films
Film-forming solutions were prepared using PYS with either 0.0%, 0.5% or 1.0% of CS (YS/CS0, YS/CS0.5 and YS/CS1.0 respectively), and glycerol as a plasticizer. SEM microphotographs of PYS granules, as well as the surface and fractured cross-section of the YS/CS films are shown in Figure 2. The starch granules of the purple yam are large, oval, with compact and smooth surfaces, as can be visualized in Figure 2(a-b). Mao et al. (2018), Chen et al. (2017), and Pérez et al. (2011) also described a similar shape for starch granules of Dioscorea species in their investigations. The YS/CS films presented homogeneous surfaces without cracks or porosity, as shown in Figure 2(c, e, and g). The homogenous surface is related to the complete solubilization of the starch and chitosan in the filmogenic solutions and demonstrates the absence of starch granules after processing. In the cross-section of the fractured films, the film does not appear porous and no phase separation between CS, PYS, and glycerol could be observed, photographs of this are given in Figure 2(d, f, and h). However, some irregularities could be detected in these cross-sections due to the fracturing method applied. There were no apparent differences in the three films with different YS/CS compositions. The films were around 200 μm in thickness, as can also be visualized through the cross-sections in Figure 2. This result is in accordance with the thickness measurements given in Table 1. Gutiérrez et al. (2015) also found non-porous films produced from purple yam (D. trifida) and cassava (Manihot esculenta C.) starches (2.0% w/v) with glycerol (1.9% w/v). However, these authors observed the occurrence of strokes or lines in the films, which was related to a high amount of amylose in cassava starch. These features were not observed in any of the YS/CS films. Corn starch/chitosan/glycerol films studied by Bof et al. (2016) also presented a homogenous phase structure that was attributed to the compatibility and good miscibility of both polymers. Mali and Grossmann (2003) likewise found a similar structure of casted films formulated from yam starch and glycerol. For the authors, homogenous surfaces on films are a good indicator of their integrity and mechanical properties. Nevertheless, all YS/CS films presented a good structure, which is a positive indication of an active coating.
Figure 2.
SEM photographs of purple yam starch (PYS) granules with magnification of 300x (a) and of 1000x (b). SEM photographs of yam starch (YS)/chitosan (CS) films: surface of YS/CS0 (c) and its cross-section (d); surface of YS/CS0.5 (e) and its cross-section (f); surface of YS/CS1.0 (g) and its cross-section (h).
3.3. Water-binding capacity, pH, gel clarity and crystallinity of PYS
The results of water-binding capacity (WBC), pH, and gel clarity of the PYS are shown in Table 1. The WBC is related to the water absorption and the intensity of molecule association within the starch granules (Deepika et al., 2017). The WBC of the PYS was 174%, which is a much lower result than the value (436% and 556%) for other Dioscorea sp. starches reported by Deepika et al. (2013).
A 1% PYS aqueous solution had a slightly acid pH at 4.2. This is more acidic than the pH, around 6.3, found by Pérez et al. (2011) of the same yam species. It is known that the pH can influence the pasting properties of starches and a well-washed native starch at pH 6 may reveal high stability to heat treatment (Mali et al., 2003). Mali et al. (2003) suggested that using yam starch as a thickner would be better at slight acidic conditions, due to a greater viscosity.
The PYS solution exhibited a lower percentage of transmittance, 40%, which is an indication of an opaque and more viscous gel. This result could be due to the mild acidity of the PYS, since Mali et al. (2003) found a higher viscosity of yam starch paste at pH 5 when compared with cassava starch at pH 6. Other authors have reported a gel clarity ranging from 22% to 79% in purple yam starches (Pérez et al., 2011). Whereas the gel clarity of starches from different sources can be vastly different, for example, rice starch at 10% and potato starch at 80% (Sánchez et al., 2010). The clarity of starch gels is an essential feature for the viscosity and appearance of foods (Craig et al., 1989).
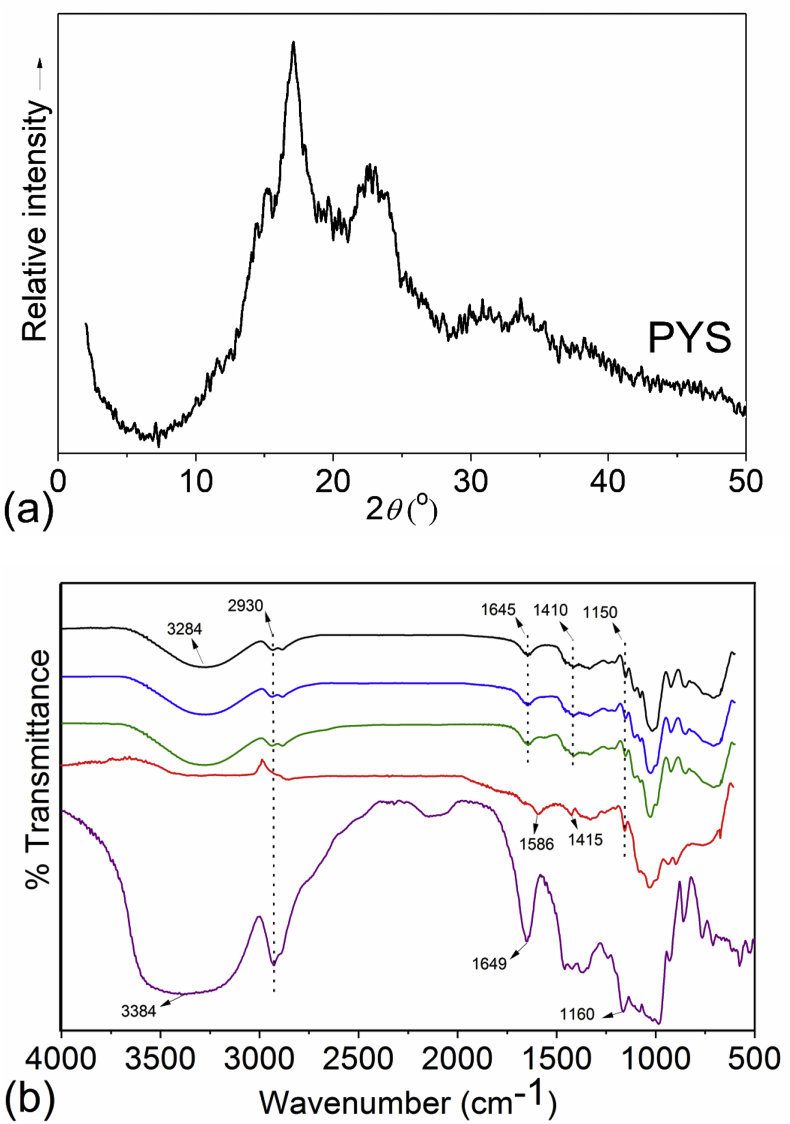
The crystallinity diffractogram of the PYS is shown in Figure 3(a). The PYS displayed diffraction peaks corresponding to the 2θ located at 15°, 17°, and 23°, with a pattern type A comparable to that found by Ascheri et al. (2014) for Pachyrhizus tuberosus starch. The crystallinity degree (CrD) of the PYS was computer-plotted using Fytik® and calculated as the ratio of the upper diffraction peak area (crystalline portion) to the total diffraction area. The amorphous section was taken as the lower area between a smooth curve below the crystalline portion and a linear baseline that connected the two points of intensity, 5° and 30° (2θ). The CrD of the PYS, 27.8%, was similar to the results (24%–35%) for Dioscorea sp. obtained by Jayakody et al. (2007).
Figure 3.
X-ray diffractogram (a) of purple yam starch (PYS) in the 2θ region of 2°–50° and FTIR spectra (b) of PYS (purple line), chitosan (red line), YS/CS0 (black line), YS/CS0.5 (blue line) and YS/CS1.0 (green line).
3.4. Moisture content, thickness and water vapor permeability of the films
The results of moisture content (MC), thickness, and water vapor permeability (WVP) of the films are listed in Table 1. The YS/CS films presented a high MC ranging from 76.1 g/100g to 77.4 g/100g. This result could be related to the hydrophilic nature of starch as compared to chitosan. The MC decreased significantly (p < 0.05) at a higher concentration of chitosan, due to a smaller amount of free hydrophilic groups of chitosan that can interact with water, as also observed by Thakur et al. (2017) in pea starch-chitosan films. In other studies with Dioscorea starch-glycerol films, Reis et al. (2013) found that MC can change depending on the concentration of starch and temperature applied and that glycerol concentration did not influence drying curves of the films.
The average thickness of the films ranged from 0.20 mm to 0.33 mm, with a significant increase with higher CS content (p < 0.05). This result is in accordance with the findings of Thakur et al. (2017), where a higher content of chitosan resulted in films with superior thickness. Different thickness values may also be explained due to the differences in formulation and film spreading procedure.
The WVP demonstrated that the permeability of the films significantly (p < 0.05) increases with a higher content of CS. It can be inferred that the WVP was highly influenced by the concentration of CS and the increase in film thickness. The WVP values were higher than those found by Rivero et al. (2016) for CS/glycerol films (1.08 ×10−10 g m−1 s−1Pa−1). The increase of WVP values may also be related to the high capacity of hydrophilic glycerol to interact with chitosan, facilitating the film permeation. Gutiérrez et al. (2015) found inferior WVP values (1.8 × 10−11 g m−1 s−1Pa−1) for cush-cush yam starch films with glycerol when compared to the cassava starch films (2.1 × 10−10 g m−1 s−1Pa−1).
3.5. Infrared spetroscopy of PYS, CS and YS/CS films
The FT-IR spectrum provided information on chemical groups and their vibrational state, relating to changes in the chemical composition of the raw materials (García-Salcedo, Torres-Vargas, del Real and Rodriguez-Garcia, 2018) and their interaction in the YS/CS films. The FT-IR spectrum of PYS, CS, and films are shown in Figure 3(b). The broad band at 3384 cm−1 in the PYS became less intense and changed the absorption peak to 3284 cm−1 in the YS/CS films. This effect can be an indication of the PYS and CS interaction through hydrogen bonds (Wu et al., 2019). The region at 2930 cm−1 is present in PYS and also in the YS/CS films, and can be related to C–H stretch (Kizil et al., 2002; Olsson and Salm, 2004). Bands at 1645 - 1649 cm−1 are assigned as the stretching vibration of the C=O band (amide I) (Dankar et al., 2018) and also as water molecules in the amorphous region (Kizil et al., 2002). The spectrum band at 1586 cm−1 can be associated to the stretching vibration of amide II in CS (Rivero et al., 2016). Bands between 1415 – 1410 cm−1 are linked to –CH2 bending and –COO stretch in CS and YS/CS films (Cael et al., 1975; Dankar et al., 2018). The bands at 1160–1150 cm−1 can be attributed to vibrations of the glucosidic C–O–C bond and the whole glucose ring of polysaccharides, which can present different modes of vibrations and bending conformations (Olsson and Salm, 2004). The region between ~1120 – 900cm-l is generally recognized in carbohydrates and polysaccharides, as the band intensity is related to the degree of the sample hydration (Cael et al., 1975).
3.6. TG and DTG analyses of PYS, CS, and the YS/CS films
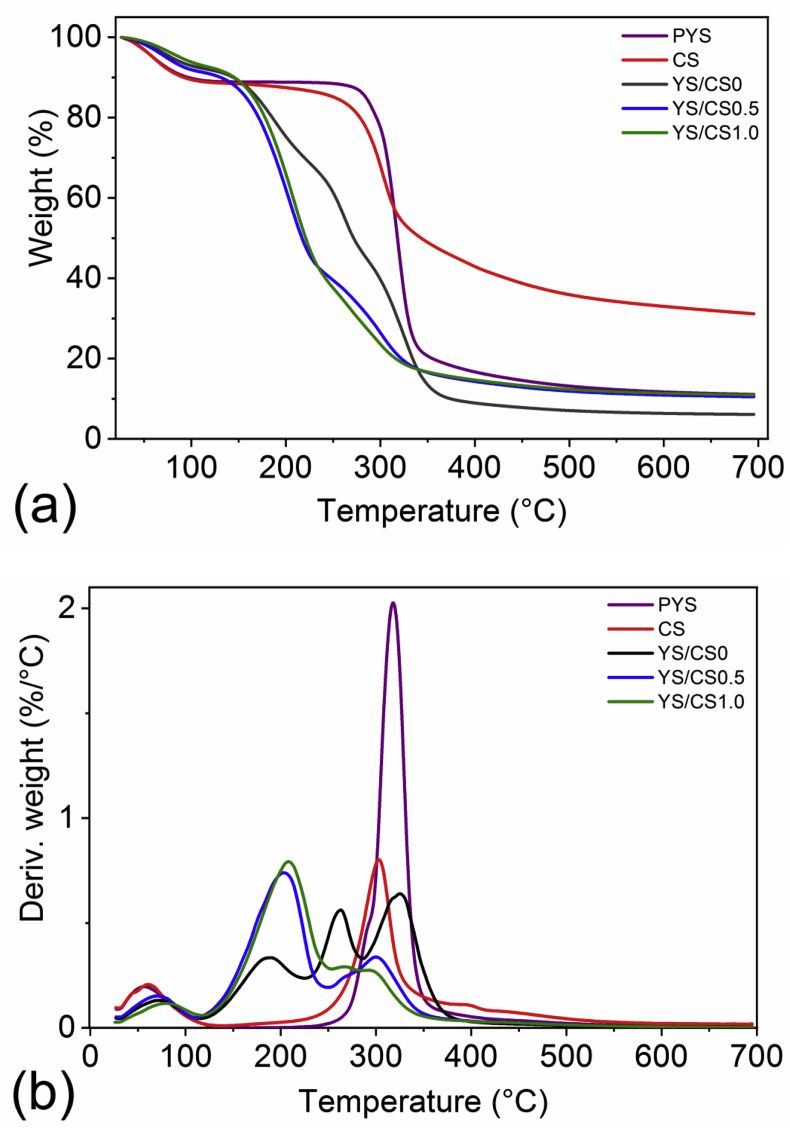
TG and its derivative (DTG) analyses were used to evaluate the thermal stability of PYS, CS, and the YS/CS films. The thermograms are shown in Figure 4. PYS and pure CS displayed the highest initial decomposition temperature (Tonset) when compared to the films, as can be visualized in Figure 4(a). This can be explained by the presence of plasticizer in the films since glycerol can interact with acetamide groups of CS and decrease the mobility/interactions between chains of the polymers (Rivero et al., 2016). The plasticizer acts by decreasing the Tg (glass transition temperature) of CS (Rivero et al., 2016), which also affects its Tonset. The thermal behavior of CS and PYS presented two decomposition stages. The first stage ranged from 25 °C to ~150 °C and can be attributed to the loss of adsorbed and bound water in the raw matter, with ~12% of weight loss (Bilbao-Sainz et al., 2017). The second stage had an initial decomposition temperature (Tonset) for CS and PYS between 275 °C and 300 °C. This second mass loss refers to saccharide degradation with the carbonization of the organic matter, followed by its complete oxidation until 700 °C (Bilbao-Sainz et al., 2017). The maximum degradation temperature (Tpeak) found for CS was 303 °C, a higher value when compared to the Tpeak, 275–288 °C determined by Bilbao-Sainz et al. (2017) in two different types of CS. Whilst Kumar et al. (2018) found a Tpeak of 284 °C for pure chitosan. The PYS presented a Tpeak of 318 °C. This is in accordance with the values (259–354 °C) reported by Lawal et al. (2008) in native starch from Dioscorea alata.
Figure 4.
TG (a) and DTG curves (b) of PYS (purple line), chitosan (red line), YS/CS0 (black line), YS/CS0.5 (blue line) and YS/CS1.0 (green line).
The YS/CS0.5 and YS/CS1.0 films exhibited three steps of mass loss. The first initial weight loss, until 100 °C, can be related to the loss of water (~10%). This behavior was also observed of corn starch/chitosan blended films in the studies from Bof et al. (2016). The second weight loss occurred around 200 °C, with Tonset of 165 °C and 170 °C for YS/CS0.5 and YS/CS1.0, respectively. The degradation of glycerol can be associated with this step (Valencia-Sullca et al., 2018). The Tpeak determined for YS/C0.5 and YS/C1.0 was 203 °C and 210 °C, respectively. These values are inferior to the Tpeak determined for cassava starch/chitosan/glycerol films in the work of Valencia-Sullca et al. (2018). This discrepancy could be due to the different film formulations. The third stage occurred between 250 and 350 °C, with the degradation of the organic matter until 700 °C.
The YS/CS0 film displayed one more step of mass loss and a greater Tpeak (324 °C) when compared to the other films. This difference could be associated with the absence of CS in the YS/CS0 since glycerol can have more affinity to interact with acetamide groups of CS than to the amylose from yam starch. This also can be explained by the weaker glycerol-starch interaction during gelatinization as a result of a stronger interaction of water-starch in the system, as described by Gutiérrez et al. (2015). To these authors, starch-based films derived from purple yam presented moisture plasticization when compared with films derived from cassava. The first stage of mass loss in the YS/CS0 is related to water loss (8% of weight loss). The second stage with Tonset at 155 °C and the third stage between 245 – 305 °C. These two steps could be related to the degradation of glycerol in the film, since its degradation temperature can range from 150 to 250 °C (Valencia-Sullca et al., 2018). The fourth and last stage, beyond 305 °C, is related to the degradation and carbonization of the starch in the film and all other organic matter.
3.7. Evaluation of fruits during film storage
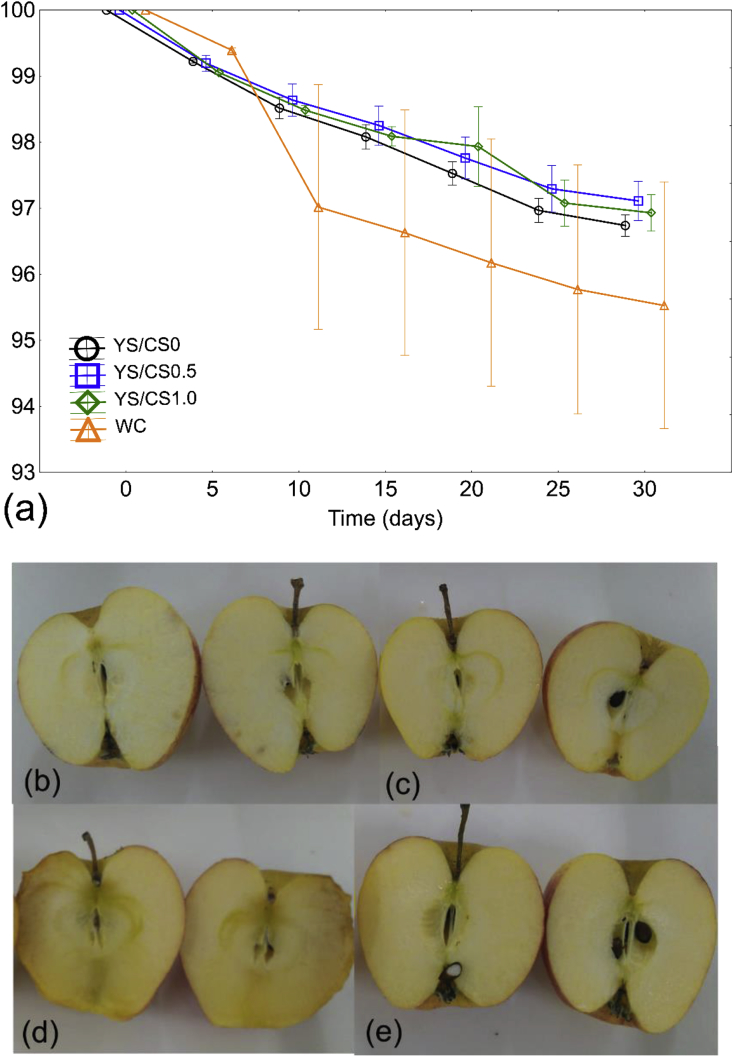
Finally, YS/CS films were assessed for their ability to preserve a perishable fruit. Weight reduction percentage over time for coated and control apples without coating (WC) is shown in Figure 5. In all treatments, fruit weight loss, which can be considered loss of quality, continued over the 4-week storage period as expected. This is due to post-harvest natural respiratory and transpiration processes. Application of physical barriers, such as coatings on the fruit surface, regulates selective permeability to O2, CO2, and water vapor, thereby delaying the natural physiological ripening process (Maftoonazad et al., 2008). Apples coated with YS/CS0.5 resulted in lower weight loss (2.89% ± 0.42), followed by YS/CS1.0 (3.07% ± 0.39) and YS/CS0 (3.34% ± 0.11). Whereas the WC control apples had the greatest weight loss of 4.47% ± 2.64. The weight loss was statistically evaluated by ANOVA to verify the interaction effects from the factors of time and the coating treatment of the apples. The interaction effect of the treatments for all samples did not show a significant difference (p = 0.998) on weight loss. This result is also similar to the studies from León-Zapata et al. (2015). These authors investigated the shelf-life of apples coated with candelilla wax and fermented extract of tarbush over 8 weeks of storage. They found that the weight loss of the apples coated with the edible film did not present significant differences from the control apples without the coating after this storage period.
Figure 5.
Weight loss of coated apples vs time (30 days) (a) and digital photographs of the internal appearance of the apples coated with YS/CS0 (b), YS/CS0.5 (c), YS/CS1.0 (d) and without coating (WC) (e).
In the present work, the results of weight loss were also statically evaluated by the Dunnett posthoc test as a complementary tool. The test was applied to verify significant differences between the WC control group and the film groups. The control group was only significantly different from the YS/CS0.5 group (p = 0.444).
After 30 days of controlled storage, the internal appearance of the apples was visually evaluated by cutting them in half and taking a photograph, shown in Figure 5(b-e). The apples coated with the YS/CS0.5 film had a better internal visual appearance when compared to the other samples. This result is in accordance with the minor weight loss found for the apples coated with YS/CS0.5. It is worth saying that this could also be correlated to a low water permeability rate (shown in Table 1) determined for this film when compared to the YS/CS1.0, since one of the many purposes in the use of coatings is to reduce the water vapor transportation by application of a solid network (León-Zapata et al., 2015).
4. Conclusion
Purple yam starch demonstrated good physical properties to be used as a matrix for edible films. Films of blended purple yam starch, chitosan, and glycerol were developed and characterized through several techniques. Their characteristics indicated that the films could be a feasible coating to food products and to improve the shelf-life of apples when applied for 4 weeks. This work not only has scientific significance but also socioeconomic importance for all tropical countries that produce this yam, as it is a prevalent food in these regions and can contribute to increasing its agro-industrial potentialities and its market value.
Declarations
Author contribution statement
Joice C. Martins da Costa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Karine S. L. Miki, Amanda da Silva Ramos: Performed the experiments; Analyzed and interpreted the data.
Bárbara E. Teixeira-Costa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Eloisa Mano Macromolecules Institute (IMA) and Mineral Technology Centre (CETEM) of the Federal University of Rio de Janeiro Federal University (UFRJ) for providing access to tools and facilities.
References
- AOAC . seventeenth ed. Association of Official Analytical Chemists; Washington, DC: 2000. Official Methods of Analysis. [Google Scholar]
- Ascheri J.L.R., Zamudio L.H.B., Carvalho C.W.P., Arevalo A.M., Fontoura L.M. Extraction and characterization of starch fractions of five phenotypes Pachyrhizus tuberosus (Lam.) Spreng. Food Nutr. Sci. 2014;5(October):1875–1885. doi: 10.4236/fns.2014.519200. [DOI] [Google Scholar]
- ASTM Standard test method for water vapor transmission of materials E96/E96M. Am. Soc. Test. Mater. 2016;14:1–10. doi: 10.1520/E0096_E0096M-16. West Conshohocken, US: ASTM International. [DOI] [Google Scholar]
- Bilbao-Sainz C., Chiou B. Sen, Williams T., Wood D., Du W.X., Sedej I., Ban Z., Rodov V., Poverenov E., Vinokur Y., McHugh T. Vitamin D-fortified chitosan films from mushroom waste. Carbohydr. Polym. 2017;167:97–104. doi: 10.1016/j.carbpol.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Bof M.J., Jiménez A., Locaso D.E., García M.A., Chiralt A. Grapefruit seed extract and lemon essential oil as active agents in corn starch–chitosan blend films. Food Bioprocess Technol. 2016;9:2033–2045. doi: 10.1007/s11947-016-1789-8. [DOI] [Google Scholar]
- Cael J.J., Koenig J.L., Blackwell J. Infrared and Raman spectroscopy of carbohydrates. Part VI: normal coordinate analysis of V-amylose. Biopolymers. 1975;14:1885–1903. doi: 10.1002/bip.1975.360140909. [DOI] [Google Scholar]
- Chen X., Li X., Mao X., Huang H., Wang T., Qu Z., Miao J., Gao W. Effects of drying processes on starch-related physicochemical properties, bioactive components and antioxidant properties of yam flours. Food Chem. 2017;224:224–232. doi: 10.1016/j.foodchem.2016.12.028. [DOI] [PubMed] [Google Scholar]
- Craig S.A.S., Maningat C.C., Seib P.A., Hoseney R.C. Starch paste clarity. Cereal Chem. 1989;66(3):173–182. [Google Scholar]
- Dankar I., Haddarah A., Omar F.E.L., Sepulcre F. Characterization of food additive-potato starch complexes by FTIR and X-ray diffraction. Food Chem. 2018;260(15):7–12. doi: 10.1016/j.foodchem.2018.03.138. [DOI] [PubMed] [Google Scholar]
- Deepika V., Kumar K.J., Anima P. Isolation and physicochemical characterization of sustained releasing starches from Dioscorea of Jharkhand. Int. J. Biol. Macromol. 2013;55:193–200. doi: 10.1016/j.ijbiomac.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Deepika V., Anima P., Hermenean A., Yáñez-Gascón M.J., Pérez-Sánchez H., Kumar K.J. Effect of dry heating and ionic gum on the physicochemical and release properties of starch from Dioscorea. Int. J. Biol. Macromol. 2017;95:557–563. doi: 10.1016/j.ijbiomac.2016.11.064. [DOI] [PubMed] [Google Scholar]
- Elsabee M.Z., Abdou E.S. Chitosan based edible films and coatings: a review. Mater. Sci. Eng. C. 2013;33(4):1819–1841. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- FAO FAOSTAT - crop statistics. Food and agriculture organization of the United Nations. 2018. http://www.fao.org/faostat/en/#data/QC/visualize
- FDA Part 137 - cereal flours and related products. In code of Federal Regulations of food and Drug administration (FDA) Department of Health and Human Services. 2011;21(B):436–451. https://www.gpo.gov/fdsys/pkg/CFR-2011-title21-vol2/pdf/CFR-2011-title21-vol2-part137.pdf Title 21 - Food and Drugs, Subchapter B - Food for Human Consumption. [Google Scholar]
- Fakhouri F.M., Fontes L.C.B., Gonçalves P.V.D.M., Milanez C.R., Steel C.J., Collares-Queiroz F.P. Filmes e coberturas comestíveis compostas à base de amidos nativos e gelatina na conservação e aceitação sensorial de uvas Crimson. Cienc. Tecnol. Aliment. 2007;27(2):369–375. doi: 10.1590/S0101-20612007000200027. [DOI] [Google Scholar]
- Galus S., Kadzińska J. Food Applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015;45(2):273–283. doi: 10.1016/j.tifs.2015.07.011. [DOI] [Google Scholar]
- García-Salcedo Á.J., Torres-Vargas O.L., del Real A., Contreras-Jiménez B., Rodriguez-Garcia M.E. Pasting, viscoelastic, and physicochemical properties of chia (Salvia hispanica L.) flour and mucilage. Food Struct. 2018;16:59–66. doi: 10.1016/j.foostr.2018.03.004. [DOI] [Google Scholar]
- Gutiérrez T.J., Morales N.J., Pérez E., Tapia M.S., Famá L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packag. Shelf-Life. 2015;3:1–8. doi: 10.1016/j.fpsl.2014.09.002. [DOI] [Google Scholar]
- Jayakody L., Hoover R., Liu Q., Donner E. Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp.) starches grown in Sri Lanka. Carbohydr. Polym. 2007;69(1):148–163. doi: 10.1016/j.carbpol.2006.09.024. [DOI] [Google Scholar]
- Jiang Q., Gao W., Li X., Xia Y., Wang H., Wu S., Huang L., Liu C., Xiao P. Characterizations of starches isolated from five different Dioscorea L. species. Food Hydrocolloids. 2012;29(1):35–41. doi: 10.1016/j.foodhyd.2012.01.011. [DOI] [Google Scholar]
- Kay D.E. TDRI Crops and Products Digest No. 2. second ed. Tropical development and research institute; London: 1987. Root crops. Revised by gooding EGB; p. 308.http://www.nzdl.org/ [Google Scholar]
- Kizil R., Irudayaraj J., Seetharaman K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002;50:3912–3918. doi: 10.1021/jf011652p. [DOI] [PubMed] [Google Scholar]
- Kumar D., Kumar P., Pandey J. Binary grafted chitosan film: synthesis, characterization, antibacterial activity and prospects for food packaging. Int. J. Biol. Macromol. 2018;115:341–348. doi: 10.1016/j.ijbiomac.2018.04.084. [DOI] [PubMed] [Google Scholar]
- Lawal O.S., Lechner M.D., Kulicke W.M. Single and multi-step carboxymethylation of water yam (Dioscorea alata) starch: synthesis and characterization. Int. J. Biol. Macromol. 2008;42(5):429–435. doi: 10.1016/j.ijbiomac.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Lebot V. MPG Biddles Ltd; London: 2009. Postharvest Quality and Marketing. in: Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids; pp. 265–275. [Google Scholar]
- León-Zapata M.A., Sáenz-Galindo A., Rojas-Molina R., Rodríguez-Herrera R., Jasso-Cantú D., Aguilar C.N. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packag. Shelf-Life. 2015;3:70–75. doi: 10.1016/j.fpsl.2015.01.001. [DOI] [Google Scholar]
- Maftoonazad N., Ramaswamy H.S., Marcotte M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008;43:951–957. doi: 10.1111/j.1365-2621.2006.01444.x. [DOI] [Google Scholar]
- Mali S., Ferrero C., Redigonda V., Beleia A.P., Grossmann M.V.E., Zaritzky N.E. Influence of pH and hydrocolloids addition on yam (Dioscorea alata) starch pastes stability. LWT - Food Sci. Technol. 2003;36(5):475–481. doi: 10.1016/S0023-6438(03)00043-4. [DOI] [Google Scholar]
- Mali S., Grossmann M.V.E. Effects of yam starch films on storability and quality of fresh strawberries (Fragaria ananassa) J. Agric. Food Chem. 2003;51(24):7005–7011. doi: 10.1021/jf034241c. [DOI] [PubMed] [Google Scholar]
- Mao X., Lu J., Huang H., Gao X., Zheng H., Chen Y., Li X., Gao W. Four types of winged yam (Dioscorea alata L.) resistant starches and their effects on ethanol-induced gastric injury in vivo. Food Hydrocolloids. 2018;85:21–29. doi: 10.1016/j.foodhyd.2018.06.036. [DOI] [Google Scholar]
- Olsson A., Salm L. The association of water to cellulose and hemicellulose in paper examined by FTIR spectroscopy. Carbohydr. Res. 2004;339:813–818. doi: 10.1016/j.carres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Pérez E., Gibert O., Rolland-Sabaté A., Jiménez Y., Sánchez T., Giraldo A., Pontoire B., Guilois S., Lahon M.C., Reynes M., Dufour D. Physicochemical, functional, and macromolecular properties of waxy yam starches discovered from “mapuey” (Dioscorea trifida) genotypes in the Venezuelan Amazon. J. Agric. Food Chem. 2011;59(1):263–273. doi: 10.1021/jf100418r. [DOI] [PubMed] [Google Scholar]
- Reis R.C., Côrrea P.C., Devilla I.A., Santos E.S., Ascheri D.P.R., Servulo A.C.O., Souza A.B.M. Drying of yam starch (Dioscorea ssp.) and glycerol filmogenic solutions at different temperatures. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2013;50(2):651–656. doi: 10.1016/j.lwt.2012.07.033. [DOI] [Google Scholar]
- Rivero S., Damonte L., Garcia M.A., Pinotti A. An insight into the role of glycerol in chitosan films. Food Biophys. 2016;11(2):117–127. doi: 10.1007/s11483-015-9421-4. [DOI] [Google Scholar]
- Sánchez T., Dufour D., Moreno I.X., Ceballos H. Comparison of pasting and gel stabilities of waxy and normal starches from potato, maize, and rice with those of a novel waxy cassava starch under thermal, chemical, and mechanical stress. J. Agric. Food Chem. 2010;58(8):5093–5099. doi: 10.1021/jf1001606. [DOI] [PubMed] [Google Scholar]
- Thakur R., Saberi B., Pristijono P., Stathopoulos C.E., Golding J.B., Scarlett C.J., Bowyer M., Vuong Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017;54(8):2270–2278. doi: 10.1007/s13197-017-2664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trèche S. Valeur nutritionnelle des ignames. In: Berthaud J., Bricas N., Marchand J.L., editors. Yam, Old Plant and Crop for the Future. Editions CIRAD; Montpellier: 1998. pp. 305–331. [Google Scholar]
- Valencia-Sullca C., Atarés L., Vargas M. Physical and antimicrobial properties of compression-molded cassava starch-chitosan films for meat Preservation. Food Bioprocess Technol. 2018;11:1339–1349. [Google Scholar]
- Van Den Broek L.A.M., Knoop R.J.I., Kappen F.H.J., Boeriu C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015;116:237–242. doi: 10.1016/j.carbpol.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Wu H., Lei Y., Lu J., Zhu R., Xiao D., Jiao C., Xia R., Zhang Z., Shen G., Liu Y., Li S., Li M. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocolloids. 2019;97:105208. doi: 10.1016/j.foodhyd.2019.105208. [DOI] [Google Scholar]