Abstract
Diarrhoeal diseases collectively constitute a serious public health challenge globally, especially as the leading cause of death in children (after respiratory diseases). Childhood diarrhoea affecting children under the age of five accounts for approximately 63% of the global burden. Accurate and timely detection of the aetiology of these diseases is very crucial; but conventional methods, apart from being laborious and time-consuming, often fail to identify difficult-to-culture pathogens. The aetiological agent of an average of up to 40% of cases of diarrhoea cannot be identified. This review gives an overview of the recent trends in the epidemiology and treatment of diarrhoea and aims at highlighting the potentials of metagenomics technique as a diagnostic method for enteric infections.
Keywords: Microbiology, Molecular biology, Medical microbiology, Microbiology epidemiology, Diarrhoea, Epidemiology, Therapy, Diagnostics, Metagenomics, Childhood
Microbiology; Molecular biology; Medical microbiology; Microbiology epidemiology; Diarrhoea; Epidemiology; Therapy; Diagnostics; Metagenomics; Childhood.
1. Introduction
World health organization defines diarrhoea as the passage of three or more loose or liquid stools per day (or more frequent passage than is normal for the individual (WHO, 2007). Worldwide, diarrhoeal diseases are reported as the leading cause of mortality among children aged five years and below (UNICEF, 2018). In some parts of the world, they account for higher mortality rates than all other causes combined (Petri et al., 2008; UNICEF, 2018). Childhood diarrhoea affecting children five years old and below accounts for approximately 63% of the global diarrhoea burden (Walker et al., 2012; Zhang et al., 2016), and is the second significant cause of infant mortality in developing nations (Platts-Mills et al., 2015; Kotloff, 2017) where poor sanitation and insufficient potable water supply are key factors (Chakravarty et al., 2017; Squire and Ryan, 2017). In Africa, Asia, and South America, diarrhoea accounts for one in eight deaths among children younger than 5 years per annum (Keddy et al., 2016; Kotloff, 2017) and an estimated 16% of child deaths in Nigeria annually (Charyeva et al., 2015). In Ogun State, South-West Nigeria, diarrhoea is one of the three diseases (the others being typhoid fever and cholera) which together are the second most prevalent water-related disease (Omole et al., 2015).
Acute diarrhoea, the passage of stools with abnormal consistency and frequency in a day (e.g. more than three times) which lasts for less than two weeks, is a syndrome that is frequently not subject to differential diagnosis in medical practice (Petri et al., 2008; Zhang et al., 2016). Bacterial diarrhoeal cases are the most prevalent of all diarrhoeal cases around the globe (Zhang et al., 2018). Commonly reported enteric bacterial diarrhoeal diseases and the causative agents are botulism (Clostridium botulinum), Campylobacter gastroenteritis (Campylobacter jejuni), cholera (Vibrio cholerae), Escherichia coli gastroenteritis, Salmonellosis (various Salmonella serovars), Shigellosis (Shigella spp.), and Staphylococcal food poisoning (Staphylococcus aureus enterotoxins) (Humphries and Linscott, 2015; Tarr et al., 2018). Children below five years of age have the most at risk from foodborne pathogens, including Shiga toxin-producing Escherichia coli O157, Campylobacter, Shigella, Yersinia, Salmonella, and Cryptosporidium (Liu et al., 2014a, Liu et al., 2014b; Kotloff, 2017). In the past few decades, the awareness in handwashing has tremendously reduced the burden of diarrhoea caused by enteric bacteria and protozoans, yet, it has less impact on diarrhoea caused by viruses (Tagbo et al., 2019).
The mouth is the typical portal of entry for gastrointestinal pathogens, which are ingested alongside contaminated food and water (Rodulfo et al., 2012). Also, they are acquired via contact with diarrhoeic animals and their contaminated environments or with the faecal matter of a diarrhoeic person (Humphries and Linscott, 2015; Squire and Ryan, 2017). While in their gastrointestinal habitat, these pathogens, through a variety of pathological mechanisms by which they could be typed, trigger the over secretion of fluid in the lumen of the small intestine associated with electrolyte imbalance, and eventual diarrhoea (Humphries and Linscott, 2015; Crawford et al., 2017). In spite of the huge burden of diarrhoea, it is preventable with modern science and public health intervention (Centres for Disease Control and Prevention, 2016a, Centres for Disease Control and Prevention, 2016b). In some cases, diarrhoea may be self-limiting. In severe infections, however, antibiotics may be prescribed to prevent possible death. The resulting risk, however, is antibiotic resistance, which is an important public health threat towards the treatment of diarrhoea (Centres for Disease Control and Prevention, 2013). The continuous surveillance of antimicrobial resistance is an epidemiological strategy at tracking new and emerging resistances to some of the last-line antibiotics.
Accurate diagnosis of diarrhoeal pathogens is necessary for surveillance, prevention, and control of diarrhoea (Ranjbar et al., 2014; Tarr et al., 2018). Traditional, phenotypic tests such as Gram staining, bacteriological culture and recording of colonial characteristics, and biochemical tests form the mainstay of laboratory diagnosis in less developed countries (Liu et al., 2014a, Liu et al., 2014b). However, such tests take longer turnaround time to identify slow-growing bacteria, resulting in delayed treatment of patients (Khan and Jahan, 2017; Maciel and Leite, 2018). In many other cases, the results of these tests, even when considered in concert, are false negatives (Miller et al., 2013).
Conventional epidemiological typing methods which include biotyping, antibiogram, and serotyping are quite useful in describing temporal epidemiological studies (MacCannell, 2013). However, aetiological agents of approximately 40% of gastroenteritis cases go undetected by these methods, complicating diagnosis and treatment (Finkbeiner et al., 2008; Khan and Jahan, 2017).
Molecular approaches have increasingly brought to light significant viral, parasitic, and bacterial enteric pathogens and also their virulent traits (Zhou et al., 2016). Most molecular techniques employ polymerase chain reaction (PCR) to detect deoxyribonucleic acid (DNA) in a sample (Chang et al., 2013). One of the tools in molecular epidemiology is microbial typing. Microbial typing helps to diagnose the aetiology and the route of transmission of infection, identify virulent and resistant strains and evaluate the impact of control measures of infectious diseases (Ranjbar et al., 2014). Next-generation sequencing (NGS), a technique which quickly and extensively sequences a mixed population of DNA or ribonucleic acid (RNA) genomes have enhanced the study of infectious disease epidemiology (Platts-Mills et al., 2013). Metagenomics, which is the technique that directly sequences and analyses the total nucleic acids isolated from a sample, without culture, has a promising prospect in the field of infectious disease diagnosis (Decuypere et al., 2016).
Globally, researchers are already utilizing metagenomics in the aetiology and antimicrobial resistance surveillance of diarrhoea (Table 1). Conversely, most of the studies on diarrhoea aetiology in Sub-Saharan Africa has focused more on the prevalence, antimicrobial profile, and risk factors (Godana and Mengistie, 2013; Akingbade et al., 2013; Chiyangi et al., 2017). A few studies have reported the molecular characterisation of enteric pathogens isolated from diarrhoea patients (Ifeanyi et al., 2014; Tian et al., 2016). This review aims at highlighting recent trends in the epidemiology and treatment of enteric infections and gives an overview of the recent molecular methods of diagnosis. In furtherance, the potentials of metagenomics as a diagnostic method for diarrhoea infections will be highlighted.
Table 1.
Application of metagenomics in diarrhoea diagnosis.
| Country | References | Metagenomic method | Age (year) | Sample size (n) | Type of organisms detected |
| Australia | Finkbeiner et al. (2008) | Micro-mass sequencing | 0–5 | 12 | Viruses |
| Japan | Nakamura et al. (2008) | 454 Roche | 34 | 1 | Bacteria |
| Bangladesh | Smits et al. (2012) | 454 Roche | All | 105 | Viruses |
| Germany | Loman et al., 2013 | Deep Amplicon sequencing | n/a | 45 | Bacteria |
| Bangladesh | Monira et al. (2013) | 454 parallel sequencing | 2–3 | 9 | Bacteria |
| Australia | Holtz et al. (2014) | 454 Titanium platform | 0–5 | 8 | Viruses |
| Nicaragua | Becker-Dreps et al. (2015) | 16S rRNA amplicon sequencing | 0–5 | 66 | Bacteria |
| Coted’Ivoire | Schneeberger et al. (2016) | Shotgun | All | 4 | Multi-pathogens |
| U.S. A | Youmans et al. (2015) | 16S rRNA | Adults | 99 | Bacteria and viruses |
| China | Sun et al. (2016) | Deep amplicon sequencing | 0–5 | 7 | Viruses |
| U.S. A | Zhou et al. (2016) | Shotgun/WGS | 0–18 | 22 | Multi-pathogens |
| Israel | Braun et al. (2017) | I6S rRNA amplicon sequencing | All | 196 | Bacteria |
| Denmark | Joensen et al. (2017) | Deep amplicon sequencing | 0–95 | 58 | Bacteria |
| Bangladesh | Kieser et al. (2018) | 16S rRNA amplicon sequencing | 0–2 | 71 | Bacteria |
| Ethiopia | Aiemjoy et al. (2019) | RT-PCR/Illumina Miseq | 0–5 | 269 | Viruses |
| China | Liu et al. (2019) | 16S rDNA amplicon sequencing | 0–1 | 20 | Bacteria |
| Cameroun | Yinda et al. (2019) | Deep amplicon sequencing | 0–89 | 221 | Viruses |
2. Epidemiology of diarrhoea
2.1. Severity and trend of diarrhoea among children
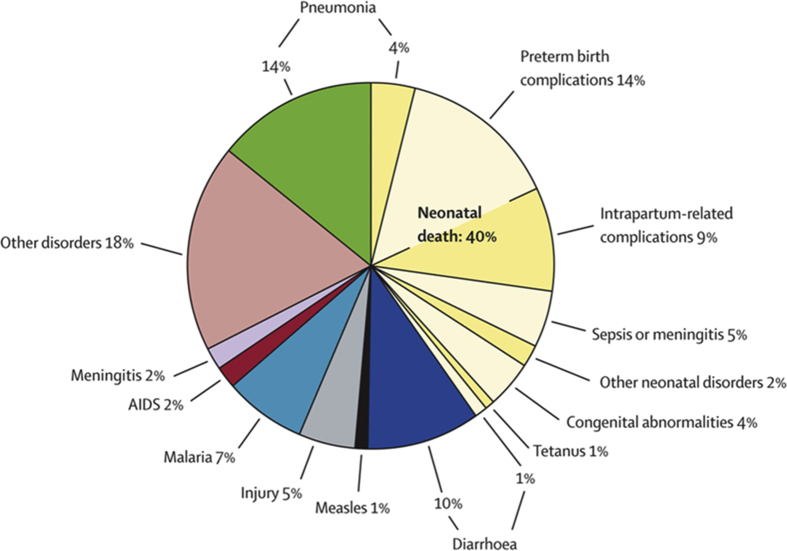
The four major infectious diseases that cause death in children under the age of 5 are pneumonia, diarrhoeal diseases, malaria and measles (Figure 1). However, 25% and 31% of the overall diarrhoeal burden has been attributed to diarrhoea among children below the age of 5 in Africa and Asia respectively (Walker et al., 2012), especially Sub-Saharan Africa which has the highest death rate (Figure 2). Despite the global decline in the diarrhoea mortality rate from 11% in 2010 to 9% in 2015, it is still the second cause of mortality among children under five years of age (Liu et al., 2012; Liu et al., 2016a, Liu et al., 2016b).
Figure 1.
Causes of child deaths worldwide; Source: Liu et al. (2012)..
Figure 2.
Data showing the high rate of diarrhoea morbidity among children below 5 years in Nigeria and some Asian Countries. Source: Computed by the Researcher with data from https://data.unicef.org/topic/child-health/diarrhoeal-disease/Accessed 17th February 2019.
2.2. Types of diarrhoea
-
i.
Acute watery diarrhoea
Acute watery diarrhoea often presents with sudden development of an unusual frequent stooling of mostly fluid. Other signs are vomiting, fever, nausea and abdominal pain (Dipasquale et al., 2018). In the gastrointestinal tract, absorption of over 90% of the physiologic net fluid takes place in the proximal small intestine. The pathogenic mechanism that leads to diarrhoea occurs when enteric pathogens attack the proximal small intestine (Thiagarajah et al., 2015). Acute watery diarrhoea is often caused by enterotoxin-secreting bacteria such as enterotoxigenic Escherichia coli (ETEC), and Vibrio cholerae, which cause fluid loss without cellular injury (Willey et al., 2013). Viruses such as rotaviruses and calciviruses that damage the intestinal epithelium also cause fluid loss. Besides, they have more tendency to cause fever, vomiting and watery stools without blood and mucus (Tagbo et al., 2019). Usually, cases of watery diarrhoea run an acute but brief (1–3 days) self-limiting duration.
-
ii.
Dysentery
As defined by World Health Organization, dysentery is bloody diarrhoea, i.e. any diarrhoeal episode in which the loose or watery stools contain visible red blood (UNICEF and WHO, 2009) Dysentery is most often caused by Shigella species (bacillary dysentery) or Entamoeba hystolytica (amoebic dysentery). Dysentery starts with the sudden onset of repeated stooling. However, unlike acute watery diarrhoea, stools are often smaller in quantity and are characterized by blood and pus. Thus, it is also referred to as acute bloody diarrhoea. Dysentery usually presents with fever, tenesmus, abdominal pain, and cramps; vomiting occurs less often (Wang et al., 2019). Inflammation of the colon (the part of the large intestine that extends from the cecum to the rectum) due to infection by one of a number of enteric pathogens leads to dysentery. The main cause of dysentery in children is the Shigellae (Tickell et al., 2017). Campylobacter jejuni and enteroinvasive E. coli or salmonellae of many serotypes being relatively less frequent causes. Entamoeba histolytica seldom causes dysentery in young children (Khan and Jahan, 2017: Delfino Vubil et al., 2018). Dysentery usually requires antimicrobial therapy (Williams and Berkley, 2018).
-
iii.
Persistent Diarrhoea
Persistent diarrhoea is acute as well as prolonged (at least 14 days) rather than brief also referred to as chronic diarrhoea (Centres for Disease Control and Prevention, 2016a, Centres for Disease Control and Prevention, 2016b). The case may begin with the passage of frequent watery or bloody stool but last for a long period thus causing loss of weight. It accounts for less than ten percent of all diarrhoea but is responsible for 30 to 50 percent of death caused by diarrhoea (Black, 1993). Persistent diarrhoea has different causes which are either infectious or non-infectious. The infectious causes include intestinal parasites (Cryptosporidium, Cyclospora, E. histolytica, Giardia, Microsporidia), bacteria (Aeromonas, Campylobacter, C. difficile, E. coli, Plesiomonas, Salmonella, Shigella), and viruses (norovirus, rotavirus). While the non-infectious causes include altered immune function, disorders of the pancreas, medications (antibiotics), heritable metabolic disorders (enzyme deficiency), intolerance to some food products (gluten, lactose), intestinal disorders, disorders o the thyroid, and reduced blood flow to the intestines (Centres for Disease Control and Prevention, 2016a, Centres for Disease Control and Prevention, 2016b; Holtman et al., 2016; Spitz et al., 2016; El-Chammas et al., 2017). Major organisms that are responsible for persistent diarrhoea are enteroaggregative E. coli, Shigella, and Cryptosporidium (DuPont, 2016). The pathogenesis of persistent diarrhoea could be multifactorial and fundamentally based on continuous damage to the mucosal linings o the intestines due to several infections with different pathogens (Giannattasio et al., 2016). Malnutrition also increases the likelihood of death in children with persistent diarrhoea. Evidence-based studies have established a strong relationship between chronic diarrhoea and HIV-positive patients in developing countries (Agholi et al., 2013; Gebremedhin et al., 2013; Kumurya and Gwarzo, 2013; Rostami et al., 2014).
2.3. Aetiology of diarrhoeal diseases
The aetiology of diarrhoea may be infectious or non-infectious. In non-infectious diarrhoea cases, factors such as food intolerances (lactose and gluten), intestinal complications (irritable bowel syndrome, ulcerative colitis, Crohn's disease, and celiac disease), and reactions to drugs (Humphries and Linscott, 2015).
Before the late 1960s, less than 20% of enteric infection symptoms could be linked to a specific aetiological agent by any known diagnostic method (Ahmad et al., 2010). Diarrhoeal diseases are caused by several viral, bacterial, and protozoan species (Platts-Mills et al., 2015; Zhang et al., 2016). Co-infections by a spectrum of enteric pathogens is the norm in diarrhoeal diseases (Serrano and Millán, 2014; Becker et al., 2015). Rotaviruses and diarrhoeagenic E. coli (DEC) are the most reported enteropathogens globally, with the DEC being particularly important in resource low countries (Onanuga et al., 2014).
Evidence based studies from Sudan (Adam et al., 2018), Burkina Faso (Bonkoungou et al.; 2013; Ouedraogo et al., 2016), China (Zhang et al., 2016), Nigeria (Enitan et al., 2019), and other endemic regions reveals that a significant amount of diarrhoea episodes in children are caused by enteric viruses. Rotaviruses, Noroviruses, Adenoviruses, Bocavirusese and Calciviruses have been implicated in childhood diarrhoea (Aktaş et al., 2019). However, recent reports show rotaviruses as the major cause of fatal cases among children below 5 years old (Gatinu et al., 2016; Nnukwu et al., 2017; Giri et al., 2019). Moreover, group A rotavirus, in particular, is the prominent aetiological agent that is responsible for infantile gastroenteritis globally, causing an estimated 20% of diarrhoea-related deaths in children below the age of five. Low-income countries and those which have no running RVA vaccination programmes are particularly affected by the rotavirus group A (RVA) diarrhoea (Gatinu et al., 2016; Zhang et al., 2016; Crawford et al., 2017).
Diarrhoeagenic E. coli (DEC) has been grouped into six pathotypes based on their pathogenic processes and clinical features. These subtypes are enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC). DEC pathotypes have also been further categorised based on their virulence mechanisms (Yu et al., 2015; Zhang et al., 2016; Thakur et al., 2018). Also, there are important regional variations in the prevalence of the different DEC pathotypes. DEC pathotypes from different locations around the globe are genetically diverse (Yu et al., 2018).
In outbreak situations, Vibrio cholerae, Escherichia coli and Clostridium difficile have been reported as the causative agents of diarrhoea in Bangkok (Dalsgaard et al., 1999), China (Scheutz et al., 2011; Escher et al., 2014), Yemen (Camacho et al., 2018; Weill et al., 2019), Iran (Tajeddin et al., 2019) and Nigeria (Eko et al., 1994; Lawoyin et al., 1999; Usman et al., 2005; Elimian et al., 2019). The role of viruses as agents of diarrhoea outbreaks among children cannot be overemphasized. Astroviruses in Bankok (Sirinavin et al., 2006), Noroviruses in China (Rogers et al., 2019), and Rotaviruses in India and Botswana (Barman et al., 2006; Erick, 2020) have been implicated in childhood diarrhoea outbreaks.
The parasitic diarrhoeal diseases of public health importance are Amebiasis (Entamoeba histolytica), Cryptosporidiosis (Cryptosporidium spp) and Giardiasis (Giardia lamblia). The two predominant causes that have been reported are Cryptosporidium and Giardia, of which Cryptosporidium is the more medically important across the world (Kotloff et al., 2013; Squire and Ryan, 2017; Troeger et al., 2017). However, studies from China (Yu et al., 2019), Ghana (Nkrumah and Nguah, 2011), Nigeria (Ighogboja and Ikeh, 1997; Akingbade et al., 2013), Kenya (Mbae et al., 2013) and Buzigi and Uganda, 2015 have detected other enteric parasites such as G. duodenalis, E. bieneusi, Schistosoma mansoni and C. cayetanensis among diarrhoeic children, though at very low proportion when compared to bacteria and viruses.
2.4. Mode of transmission of diarrhoea
Diarrhoea-causing pathogens are usually spread via the faecal-oral route through the consumption of food or fluid infected with faecal matter, or direct contact with infected stools (Pires et al., 2015; Kirk et al., 2017).
2.5. Risk factors for diarrhoea in children
-
i.
Duration of breastfeeding
Breastfeeding has a huge impact on the health status of children. A significant correlation exists between breastfeeding and diarrhoea episodes (Godana and Mengistie, 2013; Siregar et al., 2018). Sub-optimal breastfeeding increases the risk of developing diarrhoea because breast milk can confer proper functioning of the gut immune system in infants, both for a short- and long-term duration (Bartick et al., 2017; Ogbo et al., 2018). Also, breast milk decreases a newborn's risk of contracting gut diseases because it is made up of antibodies, immunoglobulin A (IgA), which confer protection against pathogenic bacteria and harmonize the activity of white blood cells (Willey et al., 2013; Hennet and Borsig, 2016).
In Africa, the rate of breastfeeding in Nigeria is lower than that in Uganda, Ethiopia, and Tanzania (Ogbo et al., 2017). This would mean that there might be a higher risk of infants presenting with diarrhoea in Nigeria than in the other countries of comparison (Figure 2). Infants should be given breast milk within the first hour after birth and breastfeeding should be exclusively practised during the first six months before feeding with supplementary foods (WHO, 2002). The initiation of supplementary food before the end of the first six months increases the risk of contamination, especially in the less developed countries where potable water and basic sanitation is lacking (Desmennu et al., 2017).
-
ii.
The Source of drinking water
Consumption of contaminated water is a viable means of transmission of diarrhoea-causing pathogens. Contamination may occur at the water source, during storage by unhygienic packaging, or during mealtimes through contact with unwashed hands or exposure (Pires et al., 2015; Wasihun et al., 2018). The availability of potable water may not necessarily be taken for granted in every part of the world; but the challenge of insufficient or outright lack of potable water is worse in less developed countries, thus predisposing their populations to a higher burden of diarrhoeal diseases (Omole et al., 2015). Unhygienic handling of drinking water is also an attributable factor for diarrhoea in children (Oloruntoba et al., 2014; Chakravarty et al., 2017).
-
iii.
Hygiene and Sanitation
Non-adherence of mothers/caregivers to the practice of handwashing predisposes their children/wards to diarrhoea (Chakravarty et al., 2017). Infant feeding bottles, which can be easily contaminated with faecal bacteria may, in turn, contaminate milk held in them, and milk encourages bacteria growth if not consumed immediately (Oloruntoba et al., 2014; Joshua et al., 2016; Wasihun et al., 2018). Also, unhygienic sewage disposal and the use of crude toilet facilities may predispose children to diarrhoea (Kapwata et al., 2018).
-
iv.
Age
Globally, the prevalence of diarrhoea has been reported to be higher in children than in adults, especially among children below five years (Walker et al., 2013; Charyeva et al., 2015; Zhang et al., 2016). Diarrhoea incidence among children has been reported to be highest in the age group below 24 months and declines with an increase in age (Walker et al., 2012; Akinnibosun and Nwafor, 2015).
-
v.
The Level of Maternal Educational
The impact of the educational level of mothers in the health status of their children is important because it is directly related to their awareness levels. Mothers without formal education are more likely to have children who will suffer diarrhoeal diseases when compared to educated mothers (Desmennu et al., 2017). Moreover, because access to formal education is limited in rural areas where more males than females go to school, there is a higher rate of uneducated mothers in such locations thus increasing the risk of children having diarrhoea in rural environments (Jolaiya Tolu et al., 2016).
Apart from the factors listed above, vitamin A deficiency (Elalfy et al., 2014; Stevens et al., 2015), zinc deficiency (Walker et al., 2013; Troeger et al., 2020), childhood wasting (Mokomane et al., 2018; Troeger et al., 2018), low use of ORS (Charyeva et al., 2015), mother's handwashing (Abuzerr et al., 2019; Alemayehu et al., 2020), childhood stunting (Larsen et al., 2017) and low rotavirus vaccine coverage (Troeger et al., 2020) have been indicated as risk for childhood diarrhoea in developing countries.
3. Approaches to diarrhoeal diseases diagnosis
The polymicrobial nature of diarrhoea aetiology requires the medical microbiologist to harness the differential diagnosis towards establishing the causative agent of the disease (Panchalingam et al., 2012; Humphries and Linscott, 2015). Accurate detection of the etiologic agent is a very important step for diarrhoeal disease surveillance and control activities (Tarr et al., 2018).
3.1. Conventional diagnostics
The traditional approach to the diagnosis of infectious diarrhoea includes Gram staining and microscopic examination, plate culture (and records of morphological characteristics), toxin assay, antigen-antibody assay, and biochemical testing (Ahmad et al., 2010). They are still being routinely used in clinical laboratories to detect and identify enteric pathogens (Liu et al., 2011). They have good sensitivity and specificity (Platts-Mills et al., 2013), but are quite laborious (Loman et al., 2013), because they must include a battery of methods to detect a spectrum of potential viruses, bacteria, and parasites (Chang et al., 2013). Also, time of identification takes longer, a factor which may threaten the chance of proper antibiotic and supportive therapy and ultimately of survival for a patient in a critical condition.
Laboratory diagnosis that is culture-based often produce low yield for enteropathogens and this may hamper antibiotic therapy (Forbes et al., 2017). Although stool microscopy for parasite detection is broadly used, it is, however, insensitive, time-consuming, and requires equipment and training (Panchalingam et al., 2012; DuPont, 2016). Moreover, bacteria referred to as ‘viable but not culturable’ (VBNC), as well as, ‘difficult to culture’ (DTC), are often missed out of the identification process. It has been estimated that only about 1% of bacteria are culturable (Allan, 2014).
3.2. Molecular diagnosis of diarrhoeal diseases
Molecular diagnostics, which generally utilise the amplification of DNA or RNA, are increasingly relevant in infectious disease diagnosis. Molecular epidemiology uses the genetic sequence of microorganisms and their hosts to describe the disease patterns as well as gain insight into possible gene function and origin by describing the distribution of genes or gene according to person, place, and time characteristics. Microbial DNA can be detected in all types of samples. The two approaches to molecular detection of microbes are the culture-based and culture-independent methods.
Culture-dependent molecular methods require that microbes are grown in culture media in the laboratory under optimum growth requirements before DNA is extracted for further molecular processing. On the other hand, culture-independent molecular methods do not require microbes to be cultivated on culture plates for DNA isolation. Rather, DNA is extracted directly from samples (Gosalbes et al., 2012; Loman et al., 2013).
3.3. Polymerase chain reaction
Polymerase chain reaction (PCR) is by far the most widely used method for nucleic acid amplification. Among several biomarkers for pathogen detection, nucleic acids are the ultimate. Molecular targets for many enteropathogens are known. Some researchers have utilised multiplex PCR (Liu et al., 2011; Onanuga et al., 2014), array singleplex PCR (Liu et al., 2014a, Liu et al., 2014b; Platts-Mills et al., 2013), and quantitative PCR (DuPont, 2016) in diarrhoeal research.
3.4. Metagenomics
Metagenomics, which is the culture-independent genomic analysis of microbial communities, has emerged as an influential new research tool in microbiology over the last twenty years (Allan, 2014). There are basically two approaches, the deep amplicon sequencing (DAS) or metagenome shotgun sequencing (MSS) (Miller et al., 2013). In diarrhoea diagnosis, the Bacterial DAS strategy typically entails the use of universal primers such as 16S rRNA (Miller et al., 2013; Decuypere et al., 2016). On the other hand, shotgun sequencing utilizes the process of randomly breaking up DNA sequences into many small pieces and then rearranging them by targeting regions of overlap, thus generating sequencing data that are naturally immune to primer bias (Hao and Chen, 2012). The shotgun approach has a wider coverage in terms of application in microbial community studies.
Other molecular biology techniques deal with the extraction of genomic DNA from an individual organism or cell from a pure isolate, whereas, metagenomics focuses on the direct investigation of total genomic DNA from clinical specimens. Rich phylogenetic information can be harnessed from amplicons of either or both of the two variant regions V3 and/or V6 of 16S ribosomal DNA (rDNA) of the bacteria in samples when pyro-sequenced (Miller et al., 2013).
Unlike the DAS, shotgun metagenomic sequencing approach can potentially detect all of the microbes present in a sample, despite their kingdom of origin, by sequencing all the nucleic acids extracted from a specimen (Zhou et al., 2016). Therefore, its application in the analysis of the diversity and the metabolic potential of microbial communities is indispensable, especially as only a few microorganisms in nature are culturable (Padmanabhan et al., 2013; Loman et al., 2013). Shotgun metagenomics has the potential to detect completely novel genes (Fredricks, 2013). Apart from the detection of enteric pathogens, shotgun metagenomics has other applications in diarrhoea epidemiology. It can be used to investigate the phylogenetic diversity of microbial genes or gene products, antimicrobial resistance genes, and virulence genes (Hu et al., 2013; Allan, 2014; Zhou et al., 2016; Braun et al., 2017).
Recent reports from around the world suggest the potential of metagenomics as a useful tool in diarrhoea diagnosis (Becker-Dreps et al., 2015; Sun et al., 2016; Kieser et al., 2018; Aiemjoy et al., 2019). Bender and Dien Bard (2018), predict a rising frequency in the application of metagenomic techniques in paediatric medicine. Enteric pathogens, including viruses, bacteria, and protozoans have been identified using different metagenomics methods such as micro-mass sequencing, pyro-sequencing, amplicon sequencing, shotgun sequencing, and whole-genome sequencing (Table 1). Despite the many advantages of metagenomics in pathogen detection, the major drawback is the enormous data that are often generated which require in-depth bioinformatics software application.
3.5. Bioinformatics
A very crucial part of metagenomics technique is the application of bioinformatics tools, especially for data analysis. The application of gene sequencing in epidemiology brings about huge data (Padmanabhan et al., 2013). Bioinformatics is the discipline that focuses on the development of ways to use computer software's to characterise molecular components of living things. In the past few decades, molecular epidemiology focused on strain typing for outbreak and surveillance investigation. The ability to analyse genetic sequences from microbes has transformed the molecular epidemiology of infectious diseases. Therefore, modern molecular epidemiology relies on new phylogenetic methods that enable the analysis of genetic data to estimate epidemiologic parameters and link epidemic processes to pathogen evolution (Foxman and Goldberg, 2010; Gosalbes et al., 2012).
Recent studies reveal the significance of bioinformatics tools in the diagnosis of clinically challenging cases such as asymptomatic clinical presentations (Bodian et al., 2017; Pathak et al., 2019). Besides, there are pieces of evidence showing that bioinformatics is a very reliable epidemiological tool during disease and antimicrobial resistance surveillance (Bessoff et al., 2013; Gardner and Hall, 2013; Kaiser et al., 2016; Yodmeeklin et al., 2017). Also, with bioinformatics applications, scientists are already discovering new drug targets for the treatment of diarrhoeal diseases (Allan, 2014; Ugboko et al., 2019).
4. Treatment and management of diarrhoeal diseases
The common treatment options for diarrhoeal diseases are fluid replacement and antimicrobial therapy. Fluid replacement therapy also referred to as oral rehydration therapy (ORT) is especially necessary for young children (Iannotti et al., 2015; Bruzzese et al., 2018). In Nigeria, cereal-based oral therapies and home-made fluids have proven to be effective in diarrhoea management (Peter and Umar, 2018).
In some instances, diarrhoea cases may be self-limiting. However, during severe cases of infection (persistent diarrhoea or dysentery), antimicrobial agents are required (Breurec et al., 2016). The first line antimicrobial agents for childhood diarrhoea therapy are co-trimoxazole and metronidazole which could be administered empirically. Others include penicillin, erythromycin, amoxycillin, ampicillin, cefuroxime, ceftriaxone, tetracycline, chloramphenicol, and ampicillin/cloxacillin, azithromycin, ciprofloxacin, and rifaximin (Udoh and Meremikwu, 2017; Bruzzese et al., 2018). Howteerakul et al. (2004), reported co-trimoxazole, norfloxacin, colistin sulfate, and nalidixic acid as the frequently recommended antibiotics for childhood therapy in Thailand. A study in Nigeria showed that metronidazole is the most prescribed antibiotic, followed by co-trimoxazole and gentamycin (Udoh and Meremikwu, 2017). Parenteral treatment with ceftriaxone or ciprofloxacinis recommended for severe diarrhoea cases (Bruzzese et al., 2018). Childhood diarrhoea caused by Shigella is responsible for most cases of mortality in non-bloody diarrhoea (Liu et al., 2016a, Liu et al., 2016b) and morbidity in moderate diarrhoea (Anderson et al., 2019), among children under five years in developing countries.
However, the emergence/re-emergence of antibiotic-resistant strains of enteric pathogens is becoming a huge threat (Willey et al., 2013). Worldwide, there are reports on multidrug-resistant strains of enteropathogens isolated from stools of children under five years (Elsherif et al., 2016). For instance, multidrug resistant Shigella spp has been reported in Ethiopia (Gebreegziabher et al., 2018), Mozambique (Vubil et al., 2018), and Nigeria (Ajayi et al., 2019).
Other treatment options include immunotherapy which is an alternative treatment option (Thu et al., 2017; Nagata et al., 2018; Zhao et al., 2019), fortified nutrition such as iron fortification and zinc replacement therapy (Paganini et al., 2016; Van Der Kam et al., 2016; Wessells et al., 2018), lactose-free diet (Iannotti et al., 2015), probiotics, though is limited awareness in Nigeria (Ajanya et al., 2018; Mokomane et al., 2018; Efunshile et al., 2019), and faecal microbiota transplantation which has been used mostly in Clostridium difficile-associated diarrhoea (Austin et al., 2014; Colman and Rubin, 2014; Barnes and Park, 2017). Oral zinc therapy could help reduce the severity and duration of diarrhoea in children above six months but may not have significant effect in children under six months of age (Lazzerini and Wanzira, 2016). Besides, Zinc absorption during diarrhoea may be reduced and vomiting may be a possible side effect (Ogunlesi et al., 2017; Somji et al., 2019). In Bangladesh, a combination of ORS, zinc and vitamin A supplementation was very effective at reducing death in children caused by diarrhoea (Billah et al., 2019).
4.1. Prevention and management of diarrhoeal infections
An adequate supply of potable water, sanitation, and vaccination are the main means of preventing diarrhoeal infections (Desmennu et al., 2017).
-
a)
Improved Water Supply and Sanitation
Acute diarrhoeal diseases can be prevented with a variety of measures focused on limiting the spread of organisms within the community and from person to person. Diarrhoeal diseases spread by the faecal-oral route. Therefore, handwashing is considered a key barrier to the transmission of enteric pathogens (Wolf et al., 2018). Studies from the developing world and from the U.S. and Australia childcare settings estimated 42%–47% reduction in the risk of diarrhoeal diseases by handwashing with soap (Bennett et al., 2014). Therefore, effective handwashing with soap should be practised by parents and caregivers before food preparation, before feeding their children and after leaving the toilet (Oloruntoba et al., 2014; Centres for Disease Control and Prevention, 2016a, Centres for Disease Control and Prevention, 2016b).
Globally, over 1 billion people lack access to improved drinking water supplies (Willey et al., 2013) and over 2.4 billion live without adequate sanitation (Darvesh et al., 2017). Strategies to improve the microbial quality of drinking water can be applied at the source or in the household. Water source strategy includes protected wells, boreholes and public tap stands (Chakravarty et al., 2017). Household strategies include improved water storage or approaches for treating water, such as chlorination, solar disinfection, filtration, or combined flocculation and disinfection (Darvesh et al., 2017).
-
b)
Vaccines
Immunization has reduced the burden of diarrhoea in children. However, there are limited vaccines which protect against few and specific pathogens. There are two enteric infectious agents for which vaccines have been licenced for use at the present time, they include rotavirus and V. cholerae O1((Preidis et al., 2011).
4.2. Rotavirus vaccine
Rotavirus vaccination has been very effective in reducing the hospitalization and death rate caused by diarrhoea in Africa. Studies by Das et al. (2013), showed commendable reduction in rates of death (74%) and hospitalization (47–57%) caused by rotavirus in childhood diarrhoea. There are two types: a pentavalent rotavirus vaccine recommended for routine use in infants with three doses given at two, three, and six months respectively; and a monovalent rotavirus vaccine recommended as an alternative of two doses given at two and four months (Shah et al., 2017; Weldegebriel et al., 2018; Mwenda et al., 2018). Rotarix and RotaTeq manufactured in Belgium and New Jersey respectively have been effective in the prevention of diarrhoea (Mokomane et al., 2018; Soares-Weiser et al., 2019). Rotavac and BRV- PV produced in India have also proved to be promising. BRV-PV, is a heat-stable, live, oral bovine rotavirus pentavalent vaccine (given at 6, 10, and 14 weeks), which has undergone trial in Niger with a 66.7% efficacy against infantile diarrhoea caused by rotavirus (Isanaka et al., 2017; Seck et al., 2017).
4.3. The cholera vaccine
The two oral cholera vaccines that are available for use and prequalified by the WHO are the oral, whole-cell, killed V. cholerae O1 vaccine supplemented with the B subunit of cholera toxin and the O139 vaccine without supplemented B with names as Dukoral, Shanchol, Euvichol, and mORC-Vax (Riddle et al., 2018; Boeckmann et al., 2019). These vaccines are given in two or three doses depending on age and produce protective efficacy against the moderate and severe disease of between 60% -85%, for up to two to three years after vaccination. They are less effective in children below two years and produce a shorter duration of protection in these individuals (Leung et al., 2012). A field trial of the oral cholera vaccine was done in Mozambique, where two doses of Dukoral showed 78% protective efficacy against disease in a cholera outbreak.
4.4. New enteric vaccines
Recent trends show evidence of novel enteric vaccines. These include ETEC, Shigella, and norovirus vaccines. In the past two decades, research has been ongoing in the area of vaccine development using several methods which include live attenuated, killed whole-cell, and subunit techniques. Some of the new vaccines are in the preclinical and clinical stage of development (Bourgeois et al., 2016; Mani et al., 2016; Tennant et al., 2016; Mokomane et al., 2018).
5. Conclusion
Diarrhoeal diseases remain an existential threat to global public health, especially to the children of ages five and below in low- and middle-income nations of the world, where access and the cost of quality healthcare remain below par and beyond affordable respectively. Treatment of childhood diarrhoeal diseases should be done within shorter turnaround times than ever before, given the need to reduce mortality due to them, especially during epidemic outbreaks. Conventional culture techniques, time-tested as they are, remain limited in offering rapid diagnosis, especially as a fair proportion of diarrhoeal pathogens are difficult-to-culture and yet-to-be-identified. Metagenomics and bioinformatics, in particular, offer great potentials in achieving a rapid diagnosis and epidemiological surveillance of diarrhoeal diseases, which in turn will lead to the outcome of rapid management, treatment, prevention, and control of diarrhoeal diseases.
Declarations
Author contribution statement
Harriet U. Ugboko; Obinna C. Nwinyi; Solomon U. Oranusi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; John O. Oyewale: Contributed reagents, materials, analysis tools or data; Harriet U. Ugboko: Wrote the paper.
Funding statement
This work was supported by Covenant University, who defrayed the cost of the article processing charge.
Competing interest statement
The authors declare no conflict of interest
Additional information
No additional information is available for this paper.
References
- Abuzerr S., Nasseri S., Yunesian M., Hadi M., Zinszer K., Mahvi A.H., Mohammed S.H. Water, sanitation, and hygiene risk factors of acute diarrhea among children under five years in the Gaza Strip. J. Water, Sanit. Hyg. Dev. 2020;10(1):111–123. [Google Scholar]
- Adam M.A., Wang J., Enan K.A., Shen H., Wang H., El Hussein A.R. Molecular survey of viral and bacterial causes of childhood diarrhea in Khartoum state, Sudan. Front. Microbiol. 2018;9:112. doi: 10.3389/fmicb.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agholi M., Hatam G.R., Motazedian M.H. HIV/AIDS-associated opportunistic protozoal diarrhea. AIDS Res. Hum. Retrovir. 2013;29(1):35–41. doi: 10.1089/aid.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Drew W.L., Plorde J.J. fifth ed. McGraw-Hill Companies; USA: 2010. Sherris Medical Microbiology; pp. 929–931. [Google Scholar]
- Aiemjoy K., Altan E., Aragie S., Fry D.M., Phan T.G., Deng X. Viral species richness and composition in young children with loose or watery stool in Ethiopia. BMC Infect. Dis. 2019;19(1):53. doi: 10.1186/s12879-019-3674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajanya B.U., Attah F., Mahmud M.E., Owolabi B.I., Adetoro R.O., Adeniyi K.A., Oyibo-Usman K.A. Therapeutic potency of probiotics in the treatment of gastrointestinal parasites. J. Publ. Health Dent. 2018;1(2):22–30. [Google Scholar]
- Ajayi O.I., Ojo D.A., Akinduti P.A., Akintokun A.K., Akinrotoye K.P. Prevalence and antibiotic resistance profiles of serotypes of Shigella species isolated from community children in Odeda local government, Ogun state. J. Environ. Treat. Tech. 2019;7(3):270–281. [Google Scholar]
- Akingbade O.A., Akinjinmi A.A., Ezechukwu U.S., Okerentugba P.O., Okonko I.O. Prevalence of intestinal parasites among children with diarrhoea in Abeokuta, Ogun State, Nigeria. Researcher. 2013;5(9):66–73. [Google Scholar]
- Akinnibosun F.I., Nwafor F.C. Prevalence of diarrhoea and antibiotic susceptibility test in children below 5 years at University of Benin Teaching Hospital, Nigeria. Int. Res. J. Publ. Environ. Health. 2015;2(4):49–55. [Google Scholar]
- Aktaş O., Aydin H., Timurkan M.O. A molecular study on the prevalence and coinfections of Rotavirus, Norovirus, Astrovirus and Adenovirus in children with gastroenteritis. Minerva Pediatr. 2019;71(5):431–437. doi: 10.23736/S0026-4946.16.04304-X. [DOI] [PubMed] [Google Scholar]
- Alemayehu B., Ayele B.T., Kloos H., Ambelu A. Individual and community-level risk factors in under-five children diarrhea among agro-ecological zones in southwestern Ethiopia. Int. J. Hyg. Environ. Health. 2020;224:113447. doi: 10.1016/j.ijheh.2019.113447. [DOI] [PubMed] [Google Scholar]
- Allan E. Metagenomics: unrestricted access to microbial communities. Virulence. 2014;5(3):397–398. doi: 10.4161/viru.28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.D., IV, Bagamian K.H., Muhib F., Amaya M.P., Laytner L.A., Wierzba T., Rheingans R. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Global Health. 2019;7(3):e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin M., Mellow M., Tierney W.M. Fecal microbiota transplantation in the treatment of Clostridium difficile infections. Am. J. Med. 2014;127(6):479–483. doi: 10.1016/j.amjmed.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Barman P., Ghosh S., Samajdar S., Mitra U., Dutta P., Bhattacharya S.K. RT-PCR based diagnosis revealed importance of human group B rotavirus infection in childhood diarrhoea. J. Clin. Virol. 2006;36(3):222–227. doi: 10.1016/j.jcv.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Barnes D., Park K.T. Donor considerations in fecal microbiota transplantation. Curr. Gastroenterol. Rep. 2017;19(3):10. doi: 10.1007/s11894-017-0548-y. [DOI] [PubMed] [Google Scholar]
- Bartick M.C., Jegier B.J., Green B.D., Schwarz E.B., Reinhold A.G., Stuebe A.M. Disparities in breastfeeding: impact on maternal and child health outcomes and costs. J. Pediatr. 2017;181:49–55. doi: 10.1016/j.jpeds.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Becker S.L., Chatigre J.K., Gohou J.P., Coulibaly J.T., Leuppi R., Polman K. Combined stool-based multiplex PCR and microscopy for enhanced pathogen detection in patients with persistent diarrhoea and asymptomatic controls from Côte d’Ivoire. Clin. Microbiol. Infect. 2015;21(6) doi: 10.1016/j.cmi.2015.02.016. 591-e1. [DOI] [PubMed] [Google Scholar]
- Becker-Dreps S., Allali I., Monteagudo A., Vilchez S., Hudgens M.G., Rogawski E.T. Gut microbiome composition in young Nicaraguan children during diarrhea episodes and recovery. Am. J. Trop. Med. Hyg. 2015;93(6):1187–1193. doi: 10.4269/ajtmh.15-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J.M., Dien Bard J. Metagenomics in pediatrics: using a shotgun approach to diagnose infections. Curr. Opin. Pediatr. 2018;30(1):125–130. doi: 10.1097/MOP.0000000000000577. [DOI] [PubMed] [Google Scholar]
- Bennett J.E., Dolin R., Blaser M.J. eighth ed. Vol. 1. Elsevier Health Sciences. Canada; 2014. (Principles and Practice of Infectious Diseases). [Google Scholar]
- Bessoff K., Sateriale A., Lee K.K., Huston C.D. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob. Agents Chemother. 2013;57(4):1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah S.M., Raihana S., Ali N.B., Iqbal A., Rahman M.M., Khan A.N.S. Bangladesh: a success case in combating childhood diarrhoea. J. Global Health. 2019;9(2) doi: 10.7189/jogh.09.020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.E. Persistent diarrhea in children in developing countries. Pediatr. Infect. Dis. J. 1993;12:751–761. doi: 10.1097/00006454-199309000-00010. [DOI] [PubMed] [Google Scholar]
- Bodian D.L., Vilboux T., Hourigan S.K., Jenevein C.L., Mani H., Kent K.C. Genomic analysis of an infant with intractable diarrhea and dilated cardiomyopathy. Mol. Case Stud. 2017;3(6):a002055. doi: 10.1101/mcs.a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann M., Roux T., Robinson M., Areal A., Durusu D., Wernecke B. Climate change and control of diarrhoeal diseases in South Africa: priorities for action Connections between temperature and diarrhoeal disease. SAMJ: S. Afr. Med. J. 2019;109(6):359–361. doi: 10.7196/SAMJ.2019.v109i6.14075. [DOI] [PubMed] [Google Scholar]
- Bonkoungou I.J.O., Haukka K., Österblad M., Hakanen A.J., Traoré A.S., Barro N., Siitonen A. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 2013;13(1):36. doi: 10.1186/1471-2431-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois A.L., Wierzba T.F., Walker R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34(26):2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- Braun T., Di Segni A., BenShoshan M., Asaf R., Squires J.E., Barhom S.F. Fecal microbial characterization of hospitalized patients with suspected infectious diarrhea shows significant dysbiosis. Sci. Rep. 2017;7(1):1088. doi: 10.1038/s41598-017-01217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breurec S., Vanel N., Bata P., Chartier L., Farra A., Favennec L. Etiology and epidemiology of diarrhoea in hospitalized children from low income country: a matched case-control study in Central African Republic. PLoS Neglected Trop. Dis. 2016;10(1) doi: 10.1371/journal.pntd.0004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzese E., Giannattasio A., Guarino A. Antibiotic treatment of acute gastroenteritis in children. F1000Research. 2018;7:193–196. doi: 10.12688/f1000research.12328.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzigi E., Uganda K. Prevalence of intestinal parasites, and its association with severe acute malnutrition related diarrhoea. J Biol. Agric. Healthc. 2015;5(2) [Google Scholar]
- Camacho A., Bouhenia M., Alyusfi R., Alkohlani A., Naji M.A.M., de Radiguès X. Cholera epidemic in Yemen, 2016–18: an analysis of surveillance data. Lancet Global Health. 2018;6(6):e680–e690. doi: 10.1016/S2214-109X(18)30230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centres for Disease Control and Prevention . Vol. 2013. 2013. pp. 36–37. (Antibiotic Resistance Threats in the United States. Atlanta, GA). p. 7. [Google Scholar]
- Centres for Disease Control and Prevention . 2016. Water, Sanitation, and Environmentally-Related Hygiene.https://www.cdc.gov/healthywater/hygiene/disease/chronic_diarrhea.html [Google Scholar]
- Centres for Disease Control and Prevention . 2016. National Centre for Emerging and Zoonotic Infectious Diseases (NCEZID). Division of Foodborne, Waterborne, and Environmental Diseases.https://www.cdc.gov/ncezid/dfwed/edeb/index.html [Google Scholar]
- Chakravarty I., Bhattacharya A., Das S.K. Water, sanitation and hygiene: the unfinished agenda in the world health organization south-east Asia region. WHO S. East Asia J. Public Health. 2017;6(2):22. doi: 10.4103/2224-3151.213787. [DOI] [PubMed] [Google Scholar]
- Chang S.S., Hsieh W.H., Liu T.S., Lee S.H., Wang C.H., Chou H.C. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis–a systemic review and meta-analysis. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charyeva Z., Cannon M., Oguntunde O., Garba A.M., Sambisa W., Bassi A.P. Reducing the burden of diarrhea among children under five years old: lessons learned from oral rehydration therapy corner program implementation in Northern Nigeria. J. Health Popul. Nutr. 2015;34(1):4. doi: 10.1186/s41043-015-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyangi H., Muma J.B., Malama S., Manyahi J., Abade A., Kwenda G., Matee M.I. Identification and antimicrobial resistance patterns of bacterial enteropathogens from children aged 0–59 months at the University Teaching Hospital, Lusaka, Zambia: a prospective cross-sectional study. BMC Infect. Dis. 2017;17(1):117. doi: 10.1186/s12879-017-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R.J., Rubin D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohn's Colitis. 2014;8(12):1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S.E., Ramani S., Tate J.E., Parashar U.D., Svensson L., Hagbom M. Rotavirus infection. Nat. Rev. Dis. Prim. 2017;3:17083. doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard A., Forslund A., Bodhidatta L., Serichantalergs O., Pitarangsi C., Pang L. A high proportion of Vibrio cholerae strains isolated from children with diarrhoea in Bangkok, Thailand are multiple antibiotic resistant and belong to heterogenous non-O1, non-O139 O-serotypes. Epidemiol. Infect. 1999;122(2):217–226. doi: 10.1017/s0950268899002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvesh N., Das J.K., Vaivada T., Gaffey M.F., Rasanathan K., Bhutta Z.A. Water, sanitation and hygiene interventions for acute childhood diarrhea: a systematic review to provide estimates for the Lives Saved Tool. BMC Publ. Health. 2017;17(4):776. doi: 10.1186/s12889-017-4746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J.K., Tripathi A., Ali A., Hassan A., Dojosoeandy C., Bhutta Z.A. Vaccines for the prevention of diarrhea due to cholera, shigella, ETEC and rotavirus. BMC Publ. Health. 2013;13(3):S11. doi: 10.1186/1471-2458-13-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere S., Meehan C.J., Van Puyvelde S., De Block T., Maltha J., Palpouguini L. Diagnosis of bacterial bloodstream infections: a 16S metagenomics approach. PLoS Neglected Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino Vubil S.A., Quintò L., Ballesté-Delpierre C., Nhampossa T., Kotloff K., Levine M.M. Clinical features, risk factors, and impact of antibiotic treatment of diarrhoea caused by Shigella in children less than 5 years in Manhiça District, rural Mozambique. Infect. Drug Resist. 2018;11:2095. doi: 10.2147/IDR.S177579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmennu A.T., Oluwasanu M.M., John-Akinola Y.O., Oladunni O., Adebowale S.A. Maternal education and diarrhoea among children aged 0-24 months in Nigeria. Afr. J. Reprod. Health. 2017;21(3):27–36. doi: 10.29063/ajrh2017/v21i3.2. [DOI] [PubMed] [Google Scholar]
- Dipasquale V., Corica D., Gramaglia S.M., Valenti S., Romano C. Gastrointestinal symptoms in children: primary care and specialist interface. Int. J. Clin. Pract. 2018;72(6) doi: 10.1111/ijcp.13093. [DOI] [PubMed] [Google Scholar]
- DuPont H.L. Persistent diarrhoea: a clinical review. Jama. 2016;315(24):2712–2723. doi: 10.1001/jama.2016.7833. [DOI] [PubMed] [Google Scholar]
- Efunshile A.M., Ezeanosike O., Nwangwu C.C., König B., Jokelainen P., Robertson L.J. Apparent overuse of antibiotics in the management of watery diarrhoea in children in Abakaliki, Nigeria. BMC Infect. Dis. 2019;19(1):275. doi: 10.1186/s12879-019-3899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eko F.O., Udo S.M., Antia-Obong O.E. Epidemiology and spectrum of vibrio diarrheas in the lower cross river basin of Nigeria. Cent. Eur. J. Publ. Health. 1994;2(1):37–41. [PubMed] [Google Scholar]
- Elalfy M.S., Elagouza I.A., Ibrahim F.A., AbdElmessieh S.K., Gadallah M. Intracranial haemorrhage is linked to late onset vitamin K deficiency in infants aged 2–24 weeks. Acta Paediatr. 2014;103(6):e273–e276. doi: 10.1111/apa.12598. [DOI] [PubMed] [Google Scholar]
- El-Chammas K., Williams S.E., Miranda A. Disaccharidase deficiencies in children with chronic abdominal pain. J. Parenter. Enteral Nutr. 2017;41(3):463–469. doi: 10.1177/0148607115594675. [DOI] [PubMed] [Google Scholar]
- Elimian K.O., Musah A., Mezue S., Oyebanji O., Yennan S., Jinadu A. Descriptive epidemiology of cholera outbreak in Nigeria, January–November, 2018: implications for the global roadmap strategy. BMC Publ. Health. 2019;19(1):1264. doi: 10.1186/s12889-019-7559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsherif R.H., Ismail D.K., El-Kholy Y.S., Gohar N.M., Elnagdy S.M., Elkraly O.A. Integron-mediated multidrug resistance in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolated from fecal specimens in Egypt. J. Egypt. Publ. Health Assoc. 2016;91(2):73–79. doi: 10.1097/01.EPX.0000483165.56114.d8. [DOI] [PubMed] [Google Scholar]
- Enitan S.S., Ihongbe J.C., Ochei J.O., Oluremi A.S., Ajulibe G.E. Detection of rotavirus and adenovirus Co-infection among apparently healthy school aged children in Ilishan-remo community of Ogun state, Nigeria. Asian J. Pediatr. Res. 2019:1–12. [Google Scholar]
- Erick P. Botswana: country report on children’s environmental health. Rev. Environ. Health. 2020 doi: 10.1515/reveh-2019-0092. [DOI] [PubMed] [Google Scholar]
- Escher M., Scavia G., Morabito S., Tozzoli R., Maugliani A., Cantoni S. A severe foodborne outbreak of diarrhoea linked to a canteen in Italy caused by enteroinvasive Escherichia coli, an uncommon agent. Epidemiol. Infect. 2014;142(12):2559–2566. doi: 10.1017/S0950268814000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Allred A.F., Tarr P.I., Klein E.J., Kirkwood C.D., Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008;4(2) doi: 10.1371/journal.ppat.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes J.D., Knox N.C., Ronholm J., Pagotto F., Reimer A. Metagenomics: the next culture-independent game changer. Front. Microbiol. 2017;8:1069. doi: 10.3389/fmicb.2017.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B., Goldberg D. Why the human microbiome project should motivate epidemiologists to learn ecology. Epidemiology (Cambridge, Mass.) 2010;21(6):757. doi: 10.1097/EDE.0b013e3181f4e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks D.N. John Wiley & Sons; 2013. The Human Microbiota: How Microbial Communities Affect Health and Disease. [Google Scholar]
- Gardner S.N., Hall B.G. When whole-genome alignments just won't work: kSNP v2 software for alignment-free SNP discovery and phylogenetics of hundreds of microbial genomes. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0081760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatinu B.W., Kiulia N.M., Nyachieo A., Macharia W., Nyangao J., Irimu G. Clinical features associated with group A rotavirus in children presenting with acute diarrhoea at Kenyatta national hospital, Nairobi, Kenya. J. Virol. Emerg. Dis. 2016;2(1) [Google Scholar]
- Gebreegziabher G., Asrat D., Hagos T. Isolation and antimicrobial susceptibility profile of Shigella and Salmonella species from children with acute diarrhoea in Mekelle Hospital and Semen Health Center, Ethiopia. Ethiop. J. Health Sci. 2018;28(2):197–206. doi: 10.4314/ejhs.v28i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin A., Gebremariam S., Haile F., Weldearegawi B., Decotelli C. Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort study. BMC Publ. Health. 2013;13(1):1047. doi: 10.1186/1471-2458-13-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio A., Guarino A., Vecchio A.L. Management of children with prolonged diarrhoea. F1000Research. 2016;5 doi: 10.12688/f1000research.7469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S., Nair N.P., Mathew A., Manohar B., Simon A., Singh T. Rotavirus gastroenteritis in Indian children< 5 years hospitalized for diarrhoea, 2012 to 2016. BMC Publ. Health. 2019;19(1):69. doi: 10.1186/s12889-019-6406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godana W., Mengistie B. Determinants of acute diarrhoea among children under five years of age in Derashe District, Southern Ethiopia. Rural Rem. Health. 2013;13(3) [PubMed] [Google Scholar]
- Gosalbes M.J., Abellan J.J., Durban A., Pérez-Cobas A.E., Latorre A., Moya A. Metagenomics of human microbiome: beyond 16s rDNA. Clin. Microbiol. Infect. 2012;18:47–49. doi: 10.1111/j.1469-0691.2012.03865.x. [DOI] [PubMed] [Google Scholar]
- Hao X., Chen T. OTU analysis using metagenomic shotgun sequencing data. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0049785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet T., Borsig L. Breastfed at tiffany's. Trends Biochem. Sci. 2016;41(6):508–518. doi: 10.1016/j.tibs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Holtman G.A., Kranenberg J.J., Blanker M.H., Ott A., Lisman-van Leeuwen Y., Berger M.Y. Dientamoeba fragilis colonization is not associated with gastrointestinal symptoms in children at primary care level. Fam. Pract. 2016 doi: 10.1093/fampra/cmw111. cmw111. [DOI] [PubMed] [Google Scholar]
- Holtz L.R., Cao S., Zhao G., Bauer I.K., Denno D.M., Klein E.J. Geographic variation in the eukaryotic virome of human diarrhea. Virology. 2014;468:556–564. doi: 10.1016/j.virol.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howteerakul N., Higginbotham N., Dibley M.J. 2004. Antimicrobial Use in Children under Five Years with Diarrhea in a central Region Province, Thailand. [PubMed] [Google Scholar]
- Hu Y., Yang X., Qin J., Lu N., Cheng G., Wu N. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013;4:2151. doi: 10.1038/ncomms3151. [DOI] [PubMed] [Google Scholar]
- Humphries R.M., Linscott A.J. Laboratory diagnosis of bacterial gastroenteritis. Clin. Microbiol. Rev. 2015;28(1):3–31. doi: 10.1128/CMR.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti L.L., Trehan I., Clitheroe K.L., Manary M.J. Diagnosis and treatment of severely malnourished children with diarrhoea. J. Paediatr. Child Health. 2015;51(4):387–395. doi: 10.1111/jpc.12711. [DOI] [PubMed] [Google Scholar]
- Ifeanyi C.I.C., Bassey B.E., Ikeneche N.F., Al-Gallas N. Molecular characterization and antibiotic resistance of Salmonella in children with acute gastroenteritis in Abuja, Nigeria. J. Infect. Dev. Crties. 2014;8(6):712–719. doi: 10.3855/jidc.4185. [DOI] [PubMed] [Google Scholar]
- Ighogboja I.S., Ikeh E.I. Parasitic agents in childhood diarrhoea and malnutrition. W. Afr. J. Med. 1997;16(1):36–39. [PubMed] [Google Scholar]
- Isanaka S., Guindo O., Langendorf C., Matar Seck A., Plikaytis B.D., Sayinzoga-Makombe N. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N. Engl. J. Med. 2017;376(12):1121–1130. doi: 10.1056/NEJMoa1609462. [DOI] [PubMed] [Google Scholar]
- Joensen K.G., Engsbro A.Ø., Lukjancenko O., Kaas R.S., Lund O., Westh H., Aarestrup F.M. Evaluating next-generation sequencing for direct clinical diagnostics in diarrhoeal disease. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36(7):1325–1338. doi: 10.1007/s10096-017-2947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolaiya Tolu F., Smith Stella I., Coker Akitoye O. Knowledge and assessment of parents on diarrhoea and its management in Lagos, Nigeria. Int. J. Health Sci. Res. 2016;6(7):138–143. [Google Scholar]
- Joshua I.A., Biji B.D., Gobir A.A., Aliyu A.A., Onyemocho A., Nmadu A.G. Social characteristics and risk factors for diseases among internally displaced persons: a study of stefano's foundation camp in Jos, Nigeria. Arch. Med. Surg. 2016;1(2):42. [Google Scholar]
- Kaiser P., Regoes R.R., Hardt W.D. Bacterial Persistence. Humana Press; New York, NY: 2016. Population dynamics analysis of ciprofloxacin-persistent S. Typhimurium cells in a mouse model for Salmonella diarrhoea; pp. 189–203. [DOI] [PubMed] [Google Scholar]
- Kapwata T., Mathee A., le Roux W., Wright C. Diarrhoeal disease in relation to possible household risk factors in South African Villages. Int. J. Environ. Res. Publ. Health. 2018;15(8):1665. doi: 10.3390/ijerph15081665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddy K.H., Smith A.M., Page N.A. GEMS extend understanding of childhood diarrhoea. Lancet. 2016;388(10051):1252–1254. doi: 10.1016/S0140-6736(16)31664-6. [DOI] [PubMed] [Google Scholar]
- Khan N.T., Jahan N. Prevalence of E. Histolytica associated dysentery in children in satellite town, quetta. Epidemiology (Sunnyvale) 2017;7(290) 2161-1165. [Google Scholar]
- Kieser S., Sarker S.A., Sakwinska O., Foata F., Sultana S., Khan Z. Bangladeshi children with acute diarrhoea show faecal microbiomes with increased Streptococcus abundance, irrespective of diarrhoea aetiology. Environ. Microbiol. 2018;20(6):2256–2269. doi: 10.1111/1462-2920.14274. [DOI] [PubMed] [Google Scholar]
- Kirk M.D., Angulo F.J., Havelaar A.H., Black R.E. Diarrhoeal disease in children due to contaminated food. Bull. World Health Organ. 2017;95(3):233. doi: 10.2471/BLT.16.173229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L. The burden and aetiology of diarrheal illness in developing countries. Pediatr. Clin. 2017;64(4):799–814. doi: 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kumurya A.S., Gwarzo M.Y. Cryptosporidiosis in HIV infected patients with diarrhoea in Kano state, North-western Nigeria. J. AIDS HIV Res. 2013;5(8):301–305. [Google Scholar]
- Larsen D.A., Grisham T., Slawsky E., Narine L. An individual-level meta-analysis assessing the impact of community-level sanitation access on child stunting, anemia, and diarrhea: evidence from DHS and MICS surveys. PLoS Neglected Trop. Dis. 2017;11(6) doi: 10.1371/journal.pntd.0005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoyin T.O., Ogunbodede N.A., Olumide E.A.A., Onadeko M.O. Outbreak of cholera in Ibadan, Nigeria. Eur. J. Epidemiol. 1999;15(4):365–368. doi: 10.1023/a:1007547117763. [DOI] [PubMed] [Google Scholar]
- Lazzerini M., Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst. Rev. 2016;12(12) doi: 10.1002/14651858.CD005436.pub5. CD005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D.T., Rahman M.A., Mohasin M., Patel S.M., Aktar A., Khanam F. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin. Vaccine Immunol. 2012;19(5):690–698. doi: 10.1128/CVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Guo M., Jiang Y., Cao Y., Qian Q., He X. Diagnosing and tracing the pathogens of infantile infectious diarrhea by amplicon sequencing. Gut Pathog. 2019;11(1):12. doi: 10.1186/s13099-019-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Shen Y., Yin J., Yuan Z., Jiang Y., Xu Y. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infect. Dis. 2014;14(1):25. doi: 10.1186/1471-2334-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Kabir F., Manneh J., Lertsethtakarn P., Begum S., Gratz J. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect. Dis. 2014;14(8):716–724. doi: 10.1016/S1473-3099(14)70808-4. [DOI] [PubMed] [Google Scholar]
- Liu J., Kibiki G., Maro V., Maro A., Kumburu H., Swai N. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. J. Clin. Virol. 2011;50(4):308–313. doi: 10.1016/j.jcv.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Platts-Mills J.A., Juma J., Kabir F., Nkeze J., Okoi C. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman N.J., Constantinidou C., Christner M., Rohde H., Chan J.Z.M., Quick J. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli O104: H4. Jama. 2013;309(14):1502–1510. doi: 10.1001/jama.2013.3231. [DOI] [PubMed] [Google Scholar]
- MacCannell D. Bacterial strain typing. Clin. Lab. Med. 2013;33(3):629–650. doi: 10.1016/j.cll.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Maciel I.A., Leite J.P.G. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Global Health. 2018;6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S., Wierzba T., Walker R.I. Status of vaccine research and development for Shigella. Vaccine. 2016;34(26):2887–2894. doi: 10.1016/j.vaccine.2016.02.075. [DOI] [PubMed] [Google Scholar]
- Mbae C.K., Nokes D.J., Mulinge E., Nyambura J., Waruru A., Kariuki S. Intestinal parasitic infections in children presenting with diarrhoea in outpatient and inpatient settings in an informal settlement of Nairobi, Kenya. BMC Infect. Dis. 2013;13(1):243. doi: 10.1186/1471-2334-13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.R., Montoya V., Gardy J.L., Patrick D.M., Tang P. Metagenomics for pathogen detection in public health. Genome Med. 2013;5(9):81. doi: 10.1186/gm485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokomane M., Kasvosve I., Melo E.D., Pernica J.M., Goldfarb D.M. The global problem of childhood diarrhoeal diseases: emerging strategies in prevention and management. Therapeut. Adv. Infect. Dis. 2018;5(1):29–43. doi: 10.1177/2049936117744429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monira S., Nakamura S., Gotoh K., Izutsu K., Watanabe H., Alam N.H. Metagenomic profile of gut microbiota in children during cholera and recovery. Gut Pathog. 2013;5(1):1. doi: 10.1186/1757-4749-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenda J.M., Parashar U.D., Cohen A.L., Tate J.E. Impact of rotavirus vaccines in Sub-Saharan African countries. Vaccine. 2018;36(47):7119–7123. doi: 10.1016/j.vaccine.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Yamamoto T., Kadowaki M. Long-term effect of combined therapy of oral immunotherapy and Japanese traditional medicine kakkonto on food allergy mice. J. Allergy Clin. Immunol. 2018;141(2):AB242. [Google Scholar]
- Nakamura S., Maeda N., Miron I.M., Yoh M., Izutsu K., Kataoka C. Metagenomic diagnosis of bacterial infections. Emerg. Infect. Dis. 2008;14(11):1784. doi: 10.3201/eid1411.080589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkrumah B., Nguah S.B. Giardia lamblia: a major parasitic cause of childhood diarrhoea in patients attending a district hospital in Ghana. Parasites Vectors. 2011;4(1):163. doi: 10.1186/1756-3305-4-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnukwu S.E., Utsalo S.J., Oyero O.G., Ntemgwa M., Ayukekbong J.A. Point-of-care diagnosis and risk factors of infantile, rotavirus-associated diarrhoea in Calabar, Nigeria. Afr. J. Lab. Med. 2017;6(1):1–5. doi: 10.4102/ajlm.v6i1.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbo F.A., Eastwood J., Page A., Efe-Aluta O., Anago-Amanze C., Kadiri E.A. The impact of sociodemographic and health-service factors on breast-feeding in sub-Saharan African countries with high diarrhoea mortality. Publ. Health Nutr. 2017;20(17):3109–3119. doi: 10.1017/S1368980017002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbo F.A., Nguyen H., Naz S., Agho K.E., Page A. The association between infant and young child feeding practices and diarrhoea in Tanzanian children. Trop. Med. Health. 2018;46(1):2. doi: 10.1186/s41182-018-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunlesi T.A., Olowonyo M.T., Runsewe-Abiodun T.I. Pre-hospital use of oral rehydration therapy and zinc and the risk of dehydration in childhood diarrhoea. J. Adv. Med. Med. Res. 2017:1–8. [Google Scholar]
- Oloruntoba E.O., Folarin T.B., Ayede A.I. Hygiene and sanitation risk factors of diarrhoeal disease among under-five children in Ibadan, Nigeria. Afr. Health Sci. 2014;14(4):1001–1011. doi: 10.4314/ahs.v14i4.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omole D.O., Emenike P.C., Tenebe I.T., Akinde A.O., Badejo A.A. An assessment of water related diseases in a Nigerian community. Res. J. Appl. Sci. Eng. Technol. 2015;10(7):776–781. [Google Scholar]
- Onanuga A., Igbeneghu O., Lamikanra A. A study of the prevalence of diarrhoeagenic Escherichia coli in children from Gwagwalada, Federal Capital Territory, Nigeria. Pan Afr. Med. J. 2014;17:146. doi: 10.11604/pamj.2014.17.146.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo N., Kaplon J., Bonkoungou I.J.O., Traore A.S., Pothier P., Barro N., Ambert-Balay K. Prevalence and genetic diversity of enteric viruses in children with diarrhea in Ouagadougou, Burkina Faso. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0153652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan R., Mishra A.K., Raoult D., Fournier P.E. Genomics and metagenomics in medical microbiology. J. Microbiol. Methods. 2013;95(3):415–424. doi: 10.1016/j.mimet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Paganini D., Uyoga M.A., Zimmermann M.B. Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhoea. Nutrients. 2016;8(8):494. doi: 10.3390/nu8080494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchalingam S., Antonio M., Hossain A., Mandomando I., Ochieng B., Oundo J. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin. Infect. Dis. 2012;55(suppl_4):S294–S302. doi: 10.1093/cid/cis754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S.J., Mueller J.L., Okamoto K., Das B., Hertecant J., Greenhalgh L. EPCAM mutation update: variants associated with congenital tufting enteropathy and Lynch syndrome. Hum. Mutat. 2019;40(2):142–161. doi: 10.1002/humu.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter A.K., Umar U. Combating diarrhoea in Nigeria: the way forward. J. Microbiol. Exp. 2018;6(4):191–197. [Google Scholar]
- Petri W.A., Jr., Miller M., Binder H.J., Levine M.M., Dillingham R., Guerrant R.L. Enteric infections, diarrhoea, and their impact on function and development. J. Clin. Invest. 2008;118(4):1277. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires S.M., Fischer-Walker C.L., Lanata C.F., Devleesschauwer B., Hall A.J., Kirk M.D. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills J.A., Babji S., Bodhidatta L., Gratz J., Haque R., Havt A. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Global Health. 2015;3(9):e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills J.A., Liu J., Houpt E.R. New concepts in diagnostics for infectious diarrhoea. Mucosal Immunol. 2013;6(5):876. doi: 10.1038/mi.2013.50. [DOI] [PubMed] [Google Scholar]
- Preidis G.A., Hill C., Guerrant R.L., Ramakrishna B.S., Tannock G.W., Versalovic J. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology. 2011;140(1):8–14. doi: 10.1053/j.gastro.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar R., Karami A., Farshad S., Giammanco G., Mammina C. Typing methods used in the molecular epidemiology of microbial pathogens: a how-to guide. New Microbiol. 2014;37(1):1–15. [PubMed] [Google Scholar]
- Riddle M.S., Chen W.H., Kirkwood C.D., MacLennan C.A. Update on vaccines for enteric pathogens. Clin. Microbiol. Infect. 2018;24(10):1039–1045. doi: 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Rodulfo H., Donato M.D., Luiggi J., Michelli E., Millán A., Michelli M. Molecular characterization of Salmonella strains in individuals with acute diarrhoea syndrome in the State of Sucre, Venezuela. Rev. Soc. Bras. Med. Trop. 2012;45(3):329–333. doi: 10.1590/s0037-86822012000300010. [DOI] [PubMed] [Google Scholar]
- Rogers B.R., Holmes C.W., Hull M., Westmoreland D., Celma C., Beard S. Persistent norovirus outbreaks in a hospital setting–The role of environmental contamination. J. Infect. 2019;79(3):277–287. doi: 10.1016/j.jinf.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Rostami M.N., Nikmanesh B., Haghi-Ashtiani M.T., Monajemzadeh M., Douraghi M., Ghalavand Z., Kashi L. Isospora belli associated recurrent diarrhea in a child with AIDS. J. Parasit. Dis. 2014;38(4):444–446. doi: 10.1007/s12639-013-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheutz F., Nielsen E.M., Frimodt-Møller J., Boisen N., Morabito S., Tozzoli R. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104: H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill. 2011;16(24):19889. doi: 10.2807/ese.16.24.19889-en. [DOI] [PubMed] [Google Scholar]
- Schneeberger P.H., Becker S.L., Pothier J.F., Duffy B., N'Goran E.K., Beuret C. Metagenomic diagnostics for the simultaneous detection of multiple pathogens in human stool specimens from Cote d'Ivoire: a proof-of-concept study. Infect. Genet. Evol. 2016;40:389–397. doi: 10.1016/j.meegid.2015.08.044. [DOI] [PubMed] [Google Scholar]
- Seck A.M., Isanaka S., Guindo O., Langendorf C., Plikaytis B.D., Sayinzoga-Makombe N. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger: a randomised, controlled trial. F1000Research. 2017:6. doi: 10.1056/NEJMoa1609462. [DOI] [PubMed] [Google Scholar]
- Serrano E., Millán J. What is the price of neglecting parasite groups when assessing the cost of co-infection? Epidemiol. Infect. 2014;142(7):1533–1540. doi: 10.1017/S0950268813002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.P., Tate J.E., Mwenda J.M., Steele A.D., Parashar U.D. Estimated reductions in hospitalizations and deaths from childhood diarrhoea following implementation of rotavirus vaccination in Africa. Expet Rev. Vaccine. 2017;16(10):987–995. doi: 10.1080/14760584.2017.1371595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siregar A.Y., Pitriyan P., Walters D. The annual cost of not breastfeeding in Indonesia: the economic burden of treating diarrhoea and respiratory disease among children (< 24mo) due to not breastfeeding according to recommendation. Int. Breastfeed. J. 2018;13(1):10. doi: 10.1186/s13006-018-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirinavin S., Techasaensiri C., Okascharoen C., Nuntnarumit P., Tonsuttakul S., Pongsuwan Y. Neonatal astrovirus gastroenteritis during an inborn nursery outbreak. J. Hosp. Infect. 2006;64(2):196–197. doi: 10.1016/j.jhin.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Smits S.L., Rahman M., Schapendonk C.M., van Leeuwen M., Faruque A.S., Haagmans B.L. Calicivirus from novel Recovirus genogroup in human diarrhea, Bangladesh. Emerg. Infect. Dis. 2012;18(7):1192. doi: 10.3201/eid1807.120344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Weiser K., Bergman H., Henschke N., Pitan F., Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst. Rev. 2019;10 doi: 10.1002/14651858.CD008521.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somji S.S., Dhingra P., Dhingra U., Dutta A., Devi P., Kumar J. Effect of dose reduction of supplemental zinc for childhood diarrhoea: study protocol for a double-masked, randomised controlled trial in India and Tanzania. BMJ Paediatr. Open. 2019;3(1) doi: 10.1136/bmjpo-2019-000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz M.A., Nguyen M.A., Roche S., Heron B., Milh M., De Lonlay P. Vol. 31. Springer; Berlin, Heidelberg: 2016. Chronic diarrhoea in L-amino acid decarboxylase (AADC) deficiency: a prominent clinical finding among a series of ten French patients; pp. 85–93. (JIMD Reports). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire S.A., Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasites Vectors. 2017;10(1):195. doi: 10.1186/s13071-017-2111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G.A., Bennett J.E., Hennocq Q., Lu Y., De-Regil L.M., Rogers L. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Global Health. 2015;3(9):e528–e536. doi: 10.1016/S2214-109X(15)00039-X. [DOI] [PubMed] [Google Scholar]
- Sun G., Zang Q., Gu Y., Niu G., Ding C., Zhang P. Viral metagenomics analysis of picobirnavirus-positive faeces from children with sporadic diarrhoea in China. Arch. Virol. 2016;161(4):971–975. doi: 10.1007/s00705-015-2726-2. [DOI] [PubMed] [Google Scholar]
- Tagbo B.N., Chukwubike C.M., Ifeyinwa R.I.R., Ani E.O. Adenovirus and rotavirus associated diarrhoea in fewer than 5 children from enugu rural communities, South east Nigeria. World J. Vaccine. 2019;9(3):71. [Google Scholar]
- Tajeddin E., Hasani Z., Ganji L., Gholam Mostafaei F.S., Azimirad M., Torabi P. Shiga toxin-producing bacteria as emerging enteric pathogens associated with outbreaks of foodborne illness in the Islamic Republic of Iran. East. Mediterr. Health J. 2019;25 doi: 10.26719/emhj.19.102. [DOI] [PubMed] [Google Scholar]
- Tarr G.A., Chui L., Lee B.E., Pang X.L., Ali S., Nettel-Aguirre A. Performance of stool-testing recommendations for acute gastroenteritis when used to identify children with 9 potential bacterial enteropathogens. Clin. Infect. Dis. 2018 doi: 10.1093/cid/ciy1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant S.M., Steele A.D., Pasetti M.F. Highlights of the 8th international conference on vaccines for enteric diseases: the Scottish encounter to defeat diarrheal diseases. Clin. Vaccine Immunol. 2016;23(4):272–281. doi: 10.1128/CVI.00082-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur N., Jain S., Changotra H., Shrivastava R., Kumar Y., Grover N., Vashistt J. Molecular characterization of diarrheagenic Escherichia coli pathotypes: association of virulent genes, serogroups, and antibiotic resistance among moderate-to-severe diarrhea patients. J. Clin. Lab. Anal. 2018;32(5) doi: 10.1002/jcla.22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah J.R., Donowitz M., Verkman A.S. Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 2015;12(8):446. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu H.M., Myat T.W., Win M.M., Thant K.Z., Rahman S., Umeda K. Chicken egg yolk antibodies (IgY) for prophylaxis and treatment of rotavirus diarrhea in human and animal neonates: a concise review. Kor. J. Food Sci. Anim. Resour. 2017;37(1):1. doi: 10.5851/kosfa.2017.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Zhu X., Chen Z., Liu W., Li S., Yu W. Characteristics of bacterial pathogens associated with acute diarrhea in children under 5 years of age: a hospital-based cross-sectional study. BMC Infect. Dis. 2016;16(1):253. doi: 10.1186/s12879-016-1603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickell K.D., Brander R.L., Atlas H.E., Pernica J.M., Walson J.L., Pavlinac P.B. Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis. Lancet Global Health. 2017;5(12):e1235–e1248. doi: 10.1016/S2214-109X(17)30392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C.E., Khalil I.A., Blacker B.F., Biehl M.H., Albertson S.B., Zimsen S.R. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2020;20(1):37–59. doi: 10.1016/S1473-3099(19)30401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C., Blacker B.F., Khalil I.A., Rao P.C., Cao S., Zimsen S.R. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger C., Forouzanfar M., Rao P.C., Khalil I., Brown A., Reiner R.C., Jr. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017;17(9):909–948. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udoh E.E., Meremikwu M.M. Antibiotic prescriptions in the case management of acute watery diarrhea in under-fives. Int. J. Contemp. Pediatr. 2017;4(3):691–695. [Google Scholar]
- Ugboko H.U., Nwinyi C.O., Oranusi S.U., Fatoki T.H., Akinduti P.A., Enibukun J.M. In silico screening and analysis of broad-spectrum molecular targets and lead compounds for childhood diarrhoea therapy. Bioinf. Biol. Insights. 2019;12:1–11. doi: 10.1177/1177932219884297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF. WHO . The United Nations Children’s Fund (UNICEF). World Health Organization (WHO); New York: 2009. Why Children Are Still Dying and what Can Be Done. [Google Scholar]
- United Nations International Children’s Emergency Fund 2018. http://www.data.unicef.org/topic/child-health/diarrhoeal-disease/
- Usman A., Sarkinfada F., Mufunda J., Nyarango P., Mansur K., Daiyabu T.M. Recurrent cholera epidemics in Kano-northern Nigeria. Cent. Afr. J. Med. 2005;51(3-4):34–38. [PubMed] [Google Scholar]
- Van Der Kam S., Salse-Ubach N., Roll S., Swarthout T., Gayton-Toyoshima S., Jiya N.M. Effect of short-term supplementation with ready-to-use therapeutic food or micronutrients for children after illness for prevention of malnutrition: a randomised controlled trial in Nigeria. PLoS Med. 2016;13(2) doi: 10.1371/journal.pmed.1001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vubil D., Balleste-Delpierre C., Mabunda R., Acácio S., Garrine M., Nhampossa T. Antibiotic resistance and molecular characterization of shigella isolates recovered from children aged less than 5 years in Manhiça, Southern Mozambique. Int. J. Antimicrob. Agents. 2018;51(6):881–887. doi: 10.1016/j.ijantimicag.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Walker C.L.F., Perin J., Aryee M.J., Boschi-Pinto C., Black R.E. Diarrhoea incidence in low-and middle-income countries in 1990 and 2010: a systematic review. BMC Publ. Health. 2012;12(1):220. doi: 10.1186/1471-2458-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.L.F., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Sun H., Qi L., Shi X.H., Zhou Y., Min K.Y. The surveillance of Yersiniosis among children in central area of Beijing from 2011 to 2018. Zhonghua yu fang yi xue za zhi [Chin. J.Prev. Med.] 2019;53(10):1027–1031. doi: 10.3760/cma.j.issn.0253-9624.2019.10.014. [DOI] [PubMed] [Google Scholar]
- Wasihun A.G., Dejene T.A., Teferi M., Marugán J., Negash L., Yemane D., McGuigan K.G. Risk factors for diarrhoea and malnutrition among children under the age of 5 years in the Tigray Region of Northern Ethiopia. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill F.X., Domman D., Njamkepo E., Almesbahi A.A., Naji M., Nasher S.S. Genomic insights into the 2016–2017 cholera epidemic in Yemen. Nature. 2019;565(7738):230–233. doi: 10.1038/s41586-018-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldegebriel G., Mwenda J.M., Chakauya J., Daniel F., Masresha B., Parashar U.D., Tate J.E. Impact of rotavirus vaccine on rotavirus diarrhoea in countries of East and Southern Africa. Vaccine. 2018;36(47):7124–7130. doi: 10.1016/j.vaccine.2017.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells K.R., Brown K.H., Kounnavong S., Barffour M.A., Hinnouho G.M., Sayasone S. Comparison of two forms of daily preventive zinc supplementation versus therapeutic zinc supplementation for diarrhoea on young children’s physical growth and risk of infection: study design and rationale for a randomized controlled trial. BMC Nutrition. 2018;4(1):39. doi: 10.1186/s40795-018-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey J., Sherwood L., Wolverton C. ninth ed. McGraw-Hill; New York: 2013. Prescott’s Microbiology; pp. 51–84. [Google Scholar]
- Williams P.C., Berkley J.A. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr. Int. Child Health. 2018;38(sup1):S50–S65. doi: 10.1080/20469047.2017.1409454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J., Hunter P.R., Freeman M.C., Cumming O., Clasen T., Bartram J. Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: updated meta-analysis and meta-regression. Trop. Med. Int. Health. 2018;23(5):508–525. doi: 10.1111/tmi.13051. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2002. Infant and Young Child Nutrition: Global Strategy on Infant and Young Child Feeding. Geneva, Switzerland.http://apps.who.int/gb/archive/pdf_files/WHA55/ea5515.pdf?ua=1 Available at: [Google Scholar]
- World Health Organization . 2007. Diarrhoeal Disease.https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease [Google Scholar]
- Yinda C.K., Vanhulle E., Conceição-Neto N., Beller L., Deboutte W., Shi C. Gut virome analysis of Cameroonians reveals high diversity of enteric viruses, including potential interspecies transmitted viruses. mSphere. 2019;4(1) doi: 10.1128/mSphere.00585-18. e00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodmeeklin A., Khamrin P., Chuchaona W., Kumthip K., Kongkaew A., Vachirachewin R. Analysis of complete genome sequences of G9P [19] rotavirus strains from human and piglet with diarrhea provides evidence for whole-genome interspecies transmission of nonreassorted porcine rotavirus. Infect. Genet. Evol. 2017;47:99–108. doi: 10.1016/j.meegid.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Youmans B.P., Ajami N.J., Jiang Z.D., Campbell F., Wadsworth W.D., Petrosino J.F. Characterization of the human gut microbiome during travelers' diarrhea. Gut Microb. 2015;6(2):110–119. doi: 10.1080/19490976.2015.1019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Chen X., Zheng S., Han D., Wang Y., Wang R. Prevalence and genetic diversity of human diarrhoeagenic Escherichia coli isolates by multilocus sequence typing. Int. J. Infect. Dis. 2018;67:7–13. doi: 10.1016/j.ijid.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Yu F., Li D., Chang Y., Wu Y., Guo Z., Jia L. Molecular characterization of three intestinal protozoans in hospitalized children with different disease backgrounds in Zhengzhou, central China. Parasites Vectors. 2019;12(1):543. doi: 10.1186/s13071-019-3800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Jing H., Lai S., Xu W., Li M., Wu J. Etiology of diarrhea among children under the age five in China: results from a five-year surveillance. J. Infect. 2015;71(1):19–27. doi: 10.1016/j.jinf.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.X., Zhou Y.M., Tian L.G., Chen J.X., Tinoco-Torres R., Serrano E. Antibiotic resistance and molecular characterization of diarrheagenic Escherichia coli and non-typhoidal Salmonella strains isolated from infections in Southwest China. Infect. Dis. Poverty. 2018;7(1):53. doi: 10.1186/s40249-018-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.X., Zhou Y.M., Xu W., Tian L.G., Chen J.X., Chen S.H. Impact of co-infections with enteric pathogens on children suffering from acute diarrhoea in southwest China. Infect. Dis. Poverty. 2016;5(1):64. doi: 10.1186/s40249-016-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Torabi B., Duncan L., Ke D., Cohen C.G., Ben-Shoshan M., Mazer B.D. Serological profile of children undergone cow's milk oral immunotherapy. J. Allergy Clin. Immunol. 2019;143(2):AB253. [Google Scholar]
- Zhou Y., Wylie K.M., El Feghaly R.E., Mihindukulasuriya K.A., Elward G.A. Metagenomic approach for identification of the pathogens associated with diarrhoea in stool specimens. J. Clin. Microbiol. 2016;54(2):368–375. doi: 10.1128/JCM.01965-15. [DOI] [PMC free article] [PubMed] [Google Scholar]