Introduction
Pseudohypoparathyroidism (PHP) is characterized by hypocalcemia, hyperphosphatemia, and elevated PTH levels owing to the resistance of target tissue to PTH. PHP Ia is associated with the phenotype of Albright hereditary osteodystrophy, including short stature, obesity, round face, and brachydactyly. PHP Ia is caused by an inactivating mutation in the GNAS gene on the maternal allele, which encodes the α-subunit of the stimulatory guanine nucleotide-binding protein (1).
Pubertal development in male patients with PHP Ia has not been fully elucidated. Among female patients with PHP Ia, some have been observed to show delayed puberty due to resistance to LH and FSH (2). Although two boys with PHP Ia and peripheral precocious puberty have been reported (3), no reports have described PHP Ia with central precocious puberty.
Here, we report a boy with central precocious puberty and PHP Ia due to a novel GNAS mutation.
Case Report
The proband was an 11-yr-old boy, who was the first child of healthy Japanese parents. He was born after an uneventful pregnancy and delivery. Neither significant hypocalcemia nor precocious puberty were reported in first-degree relatives.
At 10 yr of age, he had afebrile convulsions for 5 min due to hypocalcemia (5.3 mg/dL, reference: 8.2–10.2 mg/dL). Hyperphosphatemia (11.1 mg/dL, reference: 2.5–4.6 mg/dL) and elevated serum intact PTH levels (363 pg/mL, reference: 10–65 pg/mL) were also detected. The serum 25-hydroxyvitamin D level was 16.2 ng/mL (reference: ≥ 20.0 ng/mL). His height was 138 cm (+ 0.2 SD), weight was 38.5 kg (+ 0.7 SD), face was rounded, and the fourth and fifth metacarpals were shortened. The Wechsler Intelligence Scale for Children-IV showed a Full Scale Intelligence Quotient of 61, indicating intellectual disability. We clinically diagnosed the patient as having PHP Ia.
On admission, at 10 yr and 1 mo of age, voice breaking was noticed. The right and left testicular volumes were 8 and 6 mL, respectively. The stretched penile length was 9.5 cm, and his genital hair development was characterized as Tanner stage II. A growth spurt occurred at 9 yr of age, and his bone age was assessed to be 13 yr, indicating accelerated growth and maturation. Testosterone (2.5 ng/mL, reference for adults: 1.42–9.23 ng/mL) and stimulated gonadotropin levels (LH: 18.0 mIU/mL; FSH: 7.0 mIU/mL) after administration of GnRH were consistent with central precocious puberty. No abnormal findings, except calcifications in the basal ganglia, were detected using magnetic resonance imaging of the head.
We started treatment with sc injection of leuprorelin to suppress pubertal progression. Leuprorelin treatment suppressed testosterone (0.074 ng/mL) and gonadotropin (LH: 0.6 mIU/mL; FSH: 0.4 mIU/mL) levels close to a prepubertal range.
Mutation Analysis
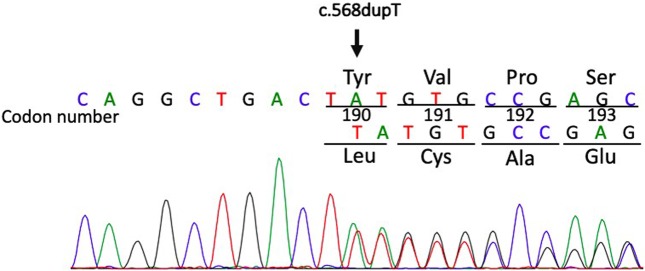
After receiving approval from the institutional review board of Yokohama Municipal Citizen’s Hospital and obtaining informed consent from the proband’s mother, we extracted genomic DNA from the peripheral blood samples of the proband. We amplified exons 2–13 and their splice sites in the GNAS gene and performed direct sequencing. A heterozygous frameshift variant, c.568dupT, p.Tyr190Leufs*20, in the GNAS gene (NM_000516; Fig. 1) was identified. This variant was not found in the Human Genetic Variation Database or the Exome Aggregation Consortium Database.
Fig. 1.
Partial sequence of exon 7 in the GNAS gene. This figure shows a chromatogram of the proband who had a heterozygous mutation, c.568dupT, p.Tyr190Leufs*20, denoted by an arrow.
Discussion
We identified a novel GNAS frameshift mutation in a boy clinically diagnosed as having PHP Ia with central precocious puberty. To the best of our knowledge, this is the first report of PHP Ia with central precocious puberty.
The association between inactive GNAS mutation and central precocious puberty is unclear. GNAS is expressed in many cell types but has not been reported to be involved in the gonadotropin inhibitory system. Signal transduction of the gonadotropin inhibitory hormone receptor was not regulated by the stimulatory G protein, but by the inhibitory G protein (4). Precocious puberty has not been reported in cases of nearby frameshift mutations, such as c.565_568delGACT (5). It is unlikely that such truncated GNAS proteins preserving approximately 190 amino acids in the amino terminus are constitutively active even in a tissue-specific manner. This case showed that central precocious puberty could occur in patients with PHP Ia, who show less resistance to gonadotropin when gonadotropin secretion is accelerated early. However, the specific cause for this occurrence in these patients is still unclear.
In this case, central precocious puberty secondary to peripheral precocious puberty is highly improbable for the following two reasons. First, the testosterone and gonadotropin levels were suppressed following leuprorelin treatment. Second, it is unlikely that the function of the truncated GNAS protein in our patient would depend on temperature. Peripheral precocious puberty was only reported in a case of PHP Ia with the mutant GNAS protein, p.Ala366Ser, that showed both gain and loss of functions in a temperature-dependent manner (3). Thus, patients with PHP Ia can develop not only peripheral precocious puberty with a mutation-specific cause but also central precocious puberty with other causes.
In summary, we report a boy with central precocious puberty who had PHP Ia due to a novel GNAS mutation.
Conflict of Interests
Tomonobu Hasegawa has the following financial relationships to disclose: Research funding from Novo Nordisk Pharma Ltd. and JCR Pharmaceuticals Co., Ltd.
Acknowledgements
We thank the patient’s family for participating in this study. We also thank Dr. Masayo Kagami (National Center for Child Health and Development) for technical assistance and Dr. Gen Nishimura for helpful discussions.
References
- 1.Lemos MC, Thakker RV. GNAS mutations in pseudohypoparathyroidism type 1a and related disorders. Hum Mutat 2015;36: 11–9. doi: 10.1002/humu.22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Sanctis C, Lala R, Matarazzo P, Andreo M, de Sanctis L. Pubertal development in patients with McCune-Albright syndrome or pseudohypoparathyroidism. J Pediatr Endocrinol Metab 2003;16(Suppl 2): 293–6. [PubMed] [Google Scholar]
- 3.Iiri T, Herzmark P, Nakamoto JM, van Dop C, Bourne HR. Rapid GDP release from Gs α in patients with gain and loss of endocrine function. Nature 1994;371: 164–8. doi: 10.1038/371164a0 [DOI] [PubMed] [Google Scholar]
- 4.Son YL, Ubuka T, Tsutsui K. Molecular mechanisms of gonadotropin-inhibitory hormone (GnIH) actions in target cells and regulation of GnIH expression. Front Endocrinol (Lausanne) 2019;10: 110. doi: 10.3389/fendo.2019.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rickard SJ, Wilson LC. Analysis of GNAS1 and overlapping transcripts identifies the parental origin of mutations in patients with sporadic Albright hereditary osteodystrophy and reveals a model system in which to observe the effects of splicing mutations on translated and untranslated messenger RNA. Am J Hum Genet 2003;72: 961–74. doi: 10.1086/374566 [DOI] [PMC free article] [PubMed] [Google Scholar]