Abstract.
In females, endogenous estrogen secretion increases gradually before pubertal development. The benefits of low-dose estrogen therapy in patients with Turner syndrome were originally discussed by Ross et al. and Quigley et al. These seminal studies used ethinyl estradiol (EE2), starting at a dose of 25 ng/kg/d. We hypothesized that the initial dosage of estrogen could be titrated to more closely mimic physiological increments of endogenous estrogen. Therefore, our recent study initiated EE2 treatment at a dosage of 1–2 ng/kg/d, an ultra-low-dose estrogen therapy in pediatric patients with Turner syndrome. The ultra-low-dose estrogen therapy in this syndrome produced a good final height outcome but achieved suboptimal bone mineral density (BMD). In the present review, we have explained our findings to clarify the merits and demerits of this new therapy and to promote further discussion and research. This type of ultra-low-dose estrogen therapy, initiated at an early age, could be ideal for estrogen replacement in female patients with hypogonadism, such as Turner syndrome.
Keywords: ultra-low-dose, estrogen therapy, Turner syndrome, hypogonadism
Introduction
In female patients with hypogonadism, puberty induction is initiated with estrogen. Based on the assumption that endogenous estrogen secretion increases gradually with pubertal development in females, the benefits of low-dose estrogen therapy for patients with Turner syndrome (TS) were originally discussed by Ross et al. (2011) (1) and Quigley et al. (2014) (2). These seminal studies used ethinyl estradiol (EE2) at a starting dose of 25 ng/kg/day. In our recently published study, we hypothesized that the initial dosage of estrogen could be titrated to mimic physiological increments of endogenous estrogen more closely and initiated EE2 treatment at a dosage of 1–2 ng/kg/d (5) in pediatric patients with TS. In the present mini-review, we addressed the following topics to clarify the merits of this new therapy, ultra-low-dose estrogen therapy in this manuscript, and to promote further discussion.
1) What is the background of this new therapy?
2) What have we observed in our study of this therapy?
(1) The main results of our recent study
(2) The strengths of our study
(3) The weaknesses of our study
3) How can the findings be applied in clinical practice?
4) What are the future directions in research on this therapy?
1) What is the Background of This New Therapy?
The definition of low-dose and ultra-low-dose estrogen therapy used is that of Ross et al. (1) and Quigley et al. (2) and our own (3). Low-dose therapy starts at 25 ng/kg/d whereas ultra-low-dose therapy starts at 1–2 ng/kg/d. In comparison, the adult EE2 dosage for complete hypogonadism is 400 ng/kg.
Ross et al. and Quigley et al. attempted to mimic the physiological increase in estrogen by initiating treatment at a low dose, and then gradually increasing the dosage. Their concept is physiologically sound, and in our study, we mainly followed their protocol while lowering the dosage even further. Although low-dose estrogen therapy, hereafter referred to as “classical therapy”, sometimes reportedly causes breast development in pediatric patients with TS at the initial dose (1, 2) of 25 ng/kg/d of EE2, the ultra-low-dose regimen of 1–2 ng/kg/d of EE2 used in our study did not demonstrate similar results (3). Therefore, the dosages used in classical and our ultra-low-dose therapy may simulate the estrogen levels responsible for the early-pubertal effects of the hormone, including breast development on one hand and pre-pubertal effects, including bone maturation and growth acceleration, on the other, respectively.
In our study, the regimen used closely approximates estrogen activity during the period preceding breast development. First, longitudinal height curves clearly reflect a gradual growth acceleration during this period, often referred to clinically as a “take-off”, although this take-off is less evident in the cross-sectional height curves more often used worldwide, including in Japan (4). Second, in females, the maturation of the pharynx, carpal bones, ulna, and radius is faster before puberty than in males. This difference in the speed of maturation could be explained by a low blood concentration of estrogen presumably observed in females during this period, although the assays used in our clinical practice failed to measure these low levels of estradiol.
2) What Have We Observed in Our Study of This Therapy? (3)
(1) The main results of our recent study
We described the final height and bone mineral density (BMD) in a group of patients with TS (group E) who had received ultra-low dose (1–2 ng/kg/d) EE2 treatment, comparing these parameters with those in the two other historical groups (groups L and S). Thus, the design was prospective in group E and retrospective in groups L and S. This study had been approved by the ethical committee of our hospital.
Group E comprised 17 female patients who had received GH 0.35 mg/kg/wk (six or seven d/wk) from the average age of 7.4 yr. Ultra-low-dose EE2 treatment was started at the age of 9.8–13.7 yr. Group L comprised 30 female patients who received the same GH treatment as group E. Conjugated estrogen 0.3125 mg/wk (once a wk) ~0.3125 mg/d was initiated at the age of 12.2–18.7 yr. Group S comprised 21 patients who had experienced breast development and menarche spontaneously.
The final height (height velocity < 2 cm/yr) in group E (N = 17) has been demonstrated as 152.4 ± 3.4 cm, with an SD +2.02 ± 0.62 SD for TS. The final height in group L (N = 30) was reported as 148.5 ± 3.0 cm, with an SD of +1.30 ± 0.55 for TS. The height (cm) differed significantly between groups E and L (E > L; p = 0.0002).
The volume-adjusted BMD (vBMD) results for groups E, L, and S are shown in Table 1. No significant difference in the vBMD was observed between groups E and L whereas the vBMD in group S differed significantly from that of groups E and L (p = 0.0056 and p = 0.0019, respectively). Thus, in our report, we concluded that ultra-low-dose estrogen therapy in group E produced a good final height outcome but achieved suboptimal BMD.
Table 1. Volumetric bone mineral density at final height.
(2) What are the strengths of our study?
Our study has several strengths. First, we chose TS as a model. TS is a well-defined entity despite manifesting wide clinical variations in the severity of hypogonadism, from complete absence to the presence of ovarian function. Second, we observed the final height and peak bone mass in our patients. It was ideal in a way that long-term, rather than short-term, prognosis was evaluated in the study. Third and most importantly, we attempted to simulate physiological estrogen levels more accurately than previous studies by calibrating the EE2 dosage as early and accurately as possible. For example, the period of initial EE2 administration (1, 2, 4–5 ng/kg/d), which did not induce any breast development physically as explained above, was 1.66 ± 0.20 yr (range: 1.21–1.89; N = 14), except in three patients in whom the duration was 0.50–0.60 yr.
In terms of final height, our results were comparable to the best outcomes reported to date (3, 5). Among the patients who had received ultra-low-dose estrogen treatment in our study (3), the patient with the best outcomes demonstrated excellent growth and BMD. Particularly, in this patient, GH was started at the age of 2 ys and 2 mo, when her height was –2.0 SD for Japanese females. The ultra-low-dose estrogen treatment was started at 9 yr and 10 mo when serum FSH was elevated to 51 mIU/mL. A progesterone derivative was added at 13 yr and 9 mo to successfully induce menstruation. Furthermore, the patient achieved a final height of 159.2 cm or 0.1 SD for Japanese females, which was above the 157 cm target height of the parents. Furthermore, the peak bone mass at the age of 26 yr was 0.1 SD for healthy Japanese adults. Other studies in which GH therapy was initiated early, such as at age of 6 yr or younger (reviewed in 5), have reported similar favorable outcomes.
(3) What are the weaknesses of our study?
Our study had several limitations. First, our study was, at least partially, retrospective. Although we planned the protocol for group E, group L was a historical group with no clearly defined treatment. Second, the mean age (11.6 yr) at the initiation of EE2 treatment in group E was greater than 10 yr, the mean age at which breast development begins among healthy Japanese females (6). We believe that ultra-low-dose EE2 treatment should be initiated at the age of 8–9 yr or before the start of breast development if patients demonstrate a complete lack of endogenous estrogen. In our study, the fact that breast development did not occur at 1–5 ng/kg/d EE2 suggests that this dosage may match endogenous estrogen levels before breast development. Third, we administered EE2 similarly to the method employed by Ross et al. (1) and Quigley et al. (2) by using the same preparations. Most recent articles claim that transdermal delivery is preferable (5). Although induction of pubertal development by using transdermal estradiol has been attempted in a Swedish group, the initial dose, which was roughly one-sixteenth the adult dose (7), is not titrated as a low dose as in our investigation (one-80th to one-400th).
Finally, we were unable to appropriately estimate endogenous and exogenous estrogen. First, our estradiol assay sensitivity was 5 pg/mL, and endogenous estrogen during the early pubertal period cannot be detected. In the future, when an advanced technology such as liquid chromatography-tandem-mass spectrometry technique is more easily accessible, estradiol can be measured before and after initiating replacement therapy (8). Second, our estradiol assay failed to cross-react with EE2. We were unable to quantify the estrogen treatment effects due to the lack of suitable means, the only available method at present being bone age assessment, which can be readily performed but is not totally objective.
3) How Can the Findings be Applied in Clinical Practice?
There are a few considerations relevant to introducing this therapy into clinical practice. The first is the timing of ultra-low-dose estrogen therapy initiation before breast development in a given ethnic population. In Japan, breast development begins at around 10 yr of age as explained above; Japanese subjects have a relatively faster maturation rate than Caucasians. As growth acceleration precedes breast development by more than one year, ultra-low-dose estrogen therapy should ideally be initiated, for example, at the age of 8 or 9 yr in patients with complete ovarian failure. Table 2 presents a tentative oral EE2 regimen at 8, 9, 10, 11, and 12 yr in Japanese TS patients with a completely null gonadal function. If patients do possess some ability to secrete estrogen and their FSH levels are not above 10 mIU/mL, we recommend that the dosage in 8–9-yr-old patients not be increased as shown in Table 2, but that the initial dose (1 ng/kg/d) be maintained until 10 yr of age when the ovarian prognosis can be better assessed by measuring FSH (9).
Table 2. Tentative plans for ultra-low-dose ethinyl estradiol (EE2) regimen in Turner syndrome patients with no endogenous estrogen secretion capacity.
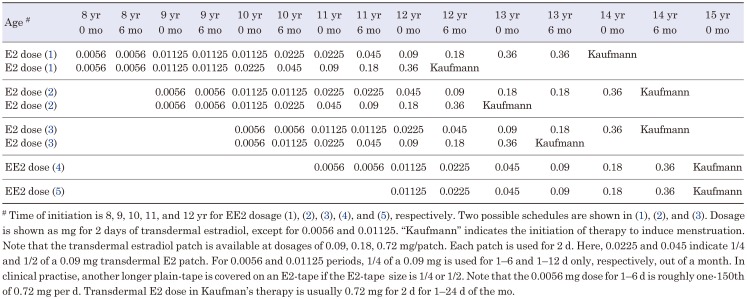
Another consideration is the use of different delivery systems, such as transdermal methods via patch or cream. As mentioned previously, the most recent expert opinion paper published in 2017 recommends transdermal estrogen administration (5). Table 3 presents our tentative plans for transdermal estrogen therapy using commercially available Estrana tapes (Hisamitsu Inc, Japan) in Japanese TS patients with a complete absence of gonadal function. A dose of Kaufman therapy in these tapes are empirically 0.72 mg in most patients with TS. Notably, the patch used in Table 3 differs from the Swedish one used by Donaldson et al. (7). However, considering scientific neutrality towards the mode of delivery, we must acknowledge that further research is crucial to compare oral and transdermal therapy. Reportedly, estradiol transdermal delivery induced higher liver enzymes and HbA1c concentrations than oral estrogen (10).
Table 3. Tentative plans of transdermal E2 dose in ultra-low-dose therapy in Turner syndrome patients with no endogenous estrogen secretion capacity.
Finally, the rate of estrogen increments should be decided after the patients are fully informed of the merits and potential demerits. The timings of estrogen increments suggested in Tables 2 and 3 are some examples. Notably, Tables 2 and 3 represent the plans for patients without endogenous estrogen production as described above. In addition to endogenous estrogen production, the rate should be individualized depending on 1) psychosocial development, 2) height prognosis, and 3) bone density.
4) What Are the Future Directions of Research in This Therapy?
We suggest the following three directions for future research based on our findings. First, the final height data obtained in our study are among the best published thus far, necessitating further confirmation. Fully designed, prospective studies enrolling a larger number of participants could be performed to meet this purpose.
Second, further research is crucial to improve patient prognosis in terms of BMD. In our study, the BMD values obtained were suboptimal. We have observed (Itonaga et al., unpublished data) that the BMD data are lower in pediatric and young adult patients with TS than in healthy Japanese females of comparable age. Furthermore, one of the prognostic factors of peak bone mass may be the early initiation of estrogen treatment. To date, the only prepubertal data for TS available were published by our group (11), indicating that BMD, expressed in mg/cm3, starts to decrease at the age of 7–10 yr. Therefore, future prospective studies of therapeutic approaches are needed to generate better BMD outcomes. We believe that initiating estrogen earlier in patients with TS as suggested in Tables 2 and 3, is desirable.
Finally, further research should include clinical endpoints other than final height and peak bone mass. The early initiation of estrogen in TS patients may have an impact on other functions, such as lipid metabolism and psychological development, during the pubertal period (5) and lead to better outcomes in adulthood.
5) Conclusions
In conclusion, the ultra-low-dose estrogen therapy used in our study produced encouraging outcomes in female pediatric patients with TS and will hopefully encourage future research. TS is a prototypic disorder, suitable for research. Furthermore, we believe that the results from TS investigations may be applicable to puberty induction in patients with other types of congenital or early-onset primary hypogonadism.
Acknowledgements
We are indebted to Mr. James Robert Valera for his assistance in editing this manuscript.
References
- 1.Ross JL, Quigley CA, Cao D, Feuillan P, Kowal K, Chipman JJ, et al. Growth hormone plus childhood low-dose estrogen in Turner’s syndrome. N Engl J Med 2011;364: 1230–42. doi: 10.1056/NEJMoa1005669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley CA, Wan X, Garg S, Kowal K, Cutler GB, Jr, Ross JL. Effects of low-dose estrogen replacement during childhood on pubertal development and gonadotropin concentrations in patients with Turner syndrome: results of a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 2014;99: E1754–64. doi: 10.1210/jc.2013-4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa Y, Ariyasu D, Izawa M, Igaki-Miyamoto J, Fukuma M, Hatano M, et al. Gradually increasing ethinyl estradiol for Turner syndrome may produce good final height but not ideal BMD. Endocr J 2017;64: 221–7. doi: 10.1507/endocrj.EJ16-0170 [DOI] [PubMed] [Google Scholar]
- 4.Suwa S, Tachibana K. Standard growth charts for height and weight of Japanese children from birth to 17 years based on a cross-sectional survey of national data. Clin Pedtric Endocrinol 1993;2: 87–97. doi: 10.1297/cpe.2.87 [DOI] [Google Scholar]
- 5.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 2017;177: G1–70. doi: 10.1530/EJE-17-0430 [DOI] [PubMed] [Google Scholar]
- 6.Matsuo N.Skeletal and sexual maturation in Japanese children. Clin Pediatr Endocrinol 1993;2 (Suppl): 1–4. [Google Scholar]
- 7.Donaldson M, Kriström B, Ankarberg-Lindgren C, Verlinde S, van Alfen-van der Velden J, Gawlik A, et al. on behalf of the European Society for Paediatric Endocrinology Turner Syndrome Working Group. Optimal pubertal induction in girls with Turner syndrome using either oral or transdermal estradiol: A proposed modern strategy. Horm Res Paediatr 2019;91: 153–63. doi: 10.1159/000500050 [DOI] [PubMed] [Google Scholar]
- 8.Mauras N, Torres-Santiago L, Santen R, Mericq V, Ross J, Colon-Otero G, et al. Impact of route of administration on genotoxic oestrogens concentrations using oral vs transdermal oestradiol in girls with Turner syndrome. Clin Endocrinol (Oxf) 2019;90: 155–61. doi: 10.1111/cen.13869 [DOI] [PubMed] [Google Scholar]
- 9.Aso K, Koto S, Higuchi A, Ariyasu D, Izawa M, Miyamoto Igaki J, et al. Serum FSH level below 10 mIU/mL at twelve years old is an index of spontaneous and cyclical menstruation in Turner syndrome. Endocr J 2010;57: 909–13. doi: 10.1507/endocrj.K10E-092 [DOI] [PubMed] [Google Scholar]
- 10.Cameron-Pimblett A, Davies MC, Burt E, Talaulikar VS, La Rosa C, King TFJ, et al. Effects of estrogen therapies on outcomes in Turner syndrome: Assessment of induction of puberty and adult estrogen use. J Clin Endocrinol Metab 2019;104: 2820–6. doi: 10.1210/jc.2018-02137 [DOI] [PubMed] [Google Scholar]
- 11.Nanao K, Tsuchiya Y, Kotoh S, Hasegawa Y. Low vertebral cancellous bone density in peripubertal girls with Turner’s syndrome and boys with hypogonadism. J Pediatr Endocrinol Metab 2002;15: 1537–42. doi: 10.1515/JPEM.2002.15.9.1537 [DOI] [PubMed] [Google Scholar]