Abstract
Aims
We collected the different prescription patterns of diabetes medications in a cohort of patients with heart failure with reduced ejection fraction (HFrEF) and analysed the impact of different prescription patterns on clinical outcomes.
Methods and results
Consecutive diabetic patients with HFrEF from a heart failure referral centre were retrospectively analysed between 2015 and 2016. Exclusion criteria include being lost to follow‐up, not receiving diabetes medications, and having severe renal impairment with a glomerular filtration rate < 30 mL/min/1.73 m2. Prescription of diabetes medications and the respective clinical outcomes were collected between 2016 and 2018. Among 381 patients (mean age, 64.8 ± 12.8 years; 71.9% male; mean left ventricular ejection fraction, 27.6 ± 7.0%; mean body mass index, 26.1 ± 4.7 kg/m2), the prescription rates of sodium‐glucose co‐transporter 2 inhibitor (SGLT2i) increased from 10.3% in 2016 to 17.6% in 2017 and 26.5% in 2018 (P < 0.001); the prescription rates of metformin, sulfonylurea, insulin, and dipeptidyl peptidase‐4 inhibitors did not change significantly over time. The prescription rates of metformin and SGLT2i were significantly higher in patients managed by cardiologists than non‐cardiologists (in 2018, 71.1% vs. 44.2% for metformin, 45.4% vs. 9.9% for SGLT2i, both P < 0.001). During the study period, annualized event rates of cardiovascular death or first unplanned HF hospitalization were 19.0 per 100 patient‐years. After a multivariate analysis, prescriptions of metformin {odds ratio (OR): 0.49 [95% confidence interval (CI) 0.27–0.51], P < 0.001} and SGLT2i [OR: 0.52 (95% CI 0.28–0.98), P = 0.042] were independently associated with lower annualized event rates of cardiovascular death or unplanned HF hospitalization.
Conclusions
Prescription patterns of diabetes medications in diabetics with HFrEF were diverse among different specialists. Prescriptions of metformin and SGLT2i were associated with favourable clinical outcomes. Our finding indicates the importance of awareness of beneficial effect of different classes of diabetes medications and collaboration between specialists in the management of diabetic HFrEF patients.
Keywords: Heart failure, Sodium‐glucose co‐transporter 2 inhibitor (SGLT2i), Metformin, Diabetes mellitus
1. Introduction
Chronic heart failure (HF) is one of the leading causes of morbidity and mortality worldwide and a major burden on the global health care system. Owing to the rapidly aging population and prolonged survival of patients suffering from HF, the HF population is increasing rapidly worldwide.1, 2, 3
Diabetes mellitus (DM) is one of the most prevalent chronic diseases worldwide. Patients with DM have a 2.5‐fold increased risk of developing HF. Patients with type 2 DM and poor glycaemic control and obesity are at high risk of developing HF.4 Diabetes patients also have increased risk of dying from HF.5 Furthermore, in HF patients who were not treated for DM, higher haemoglobin A1c (HbA1c) was independently associated with higher risk of cardiovascular (CV) events.6, 7
Although guideline‐recommended HF therapies such as renin–angiotensin system (RAS) blockers, beta‐blockers, and mineralocorticoid receptor antagonists (MRAs) work similarly well in patients with and without DM,5 different classes of diabetes medications have different pharmacological effects on patients with HF. In particular, thiazolidinedione (TZD) causes sodium and water retention, increases risk of worsening HF and hospitalization, and is therefore contraindicated in HF patients.8, 9 In addition to TZD, other commonly used diabetes medications, such as sulfonylurea (SU) derivatives and some classes of dipeptidyl peptidase‐4 inhibitor (DPP4i), possibly increase risks of CV events and worsening HF.5, 9, 10 Insulin as well, which acts as a potent sodium‐retaining hormone through reducing urinary sodium excretion via various sodium channels along renal tubules, exacerbates fluid retention and can therefore potentially deteriorate the congestion symptoms in HF patients.11, 12, 13, 14 Currently, metformin is regarded as the better treatment of choice in HF patients with DM.15 Although there was a lack of a large‐scale, randomized controlled trial confirming the beneficial effects of metformin on HF, a systemic review of nine observational studies including 34 000 subjects demonstrated no increase in adverse events among diabetic patients with HF with reduced ejection fraction (HFrEF) treated with metformin.16 The sodium‐glucose co‐transporter 2 inhibitor (SGLT2i) has been demonstrated to reduce HF hospitalization in patients who had or were at risk of developing atherosclerotic CV disease and therefore has potential benefit in patients with both DM and HF.17, 18, 19 A recent published meta‐analysis of randomized, placebo‐controlled, CV outcome trials of SGLT2i demonstrated that it could reduce risk of HF hospitalization by 31%.20 Although only few patients with baseline cardiac failure of reduced left ventricular ejection fraction (LVEF) were enrolled in randomized trials of SGLT2i, the effect of SGLT2i to reduce HF hospitalization seems to be comparable and potentially superior in patients with HF than in those without HF.17, 21
The guideline from the American Diabetes Association standard of medical care in diabetes recommends that in type 2 DM patients with CV disease, diabetic therapy should begin with lifestyle management and metformin. If HF predominates in these patients, SGLT2i with evidence of reducing HF hospitalization should be considered as the treatment strategy.22 In patients with HFrEF, physician's adherence to treatment guideline was closely associated with clinical outcome and prognosis.23 However, the prescription rates of various diabetes medications and treatment outcomes in real‐world HF patients have not be widely reported. Therefore, the aim of our study was to analyse the physicians' prescription pattern of diabetes medications in HF patients and to evaluate the impact of prescription pattern on clinical outcomes.
2. Methods
2.1. Study design and study population
Our study aimed to evaluate different prescription patterns and effects of diabetes medications in HFrEF patients. Our study complied with the ethical principles of the Declaration of Helsinki and was approved by the institutional ethics committee of Cheng Hsin General Hospital. The definition of HF is consistent with the European Society of Cardiology (ESC) guideline: presentation of typical HF symptoms accompanied by HF signs caused by a structural and/or functional cardiac abnormality.9 The definition of HFrEF patient is patient with New York Heart Association (NYHA) class II, III, or IV HF symptoms and with LVEF < 40%.
Between 2015 and 2016, all patients had been retrospectively and consecutively screened from the HF database in the Cheng Hsin General Hospital, which is a tertiary referral centre for HF management and cardiac transplant in Taiwan. Inclusion criteria for the current study included (i) male or female, with age ≥ 20 years at screening; (ii) patients with chronic HF and NYHA Functional Classification (Fc) II to IV; (iii) documented LVEF < 40% by echocardiography before screening; and (iv) DM with at least one type of oral diabetes medication and/or insulin injection. The exclusion criteria for the current study included (i) patients refused medical advice or were lost to follow‐up; (ii) HF with echocardiographic LVEF ≥ 40%; (iii) HF primarily from right ventricular failure, pericardial disease, or congenital heart disease; (iv) severe renal impairment, defined as a glomerular filtration rate (GFR) < 30 mL/min/1.73 m2 or requiring dialysis; and (v) patients with type 1 DM or gestational DM.
After inclusion and exclusion criteria were applied, a total of 381 eligible diabetic patients with HFrEF were enrolled (Figure 1 ). Baseline characteristics were recorded. The prescription patterns of diabetes medications were collected annually since the beginning of the next year after enrolment until the end of 2018. A total of 319, 340, and 324 patients had adequate data for analyses in 2016, 2017, and 2018, respectively.
Figure 1.
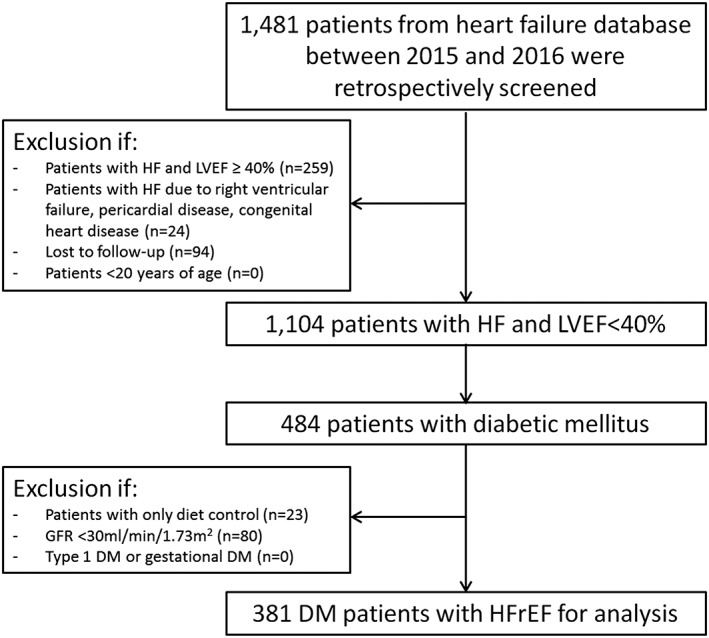
The inclusion and exclusion criteria flow chart. DM, diabetes mellitus; GFR, glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction.
Standard HF treatment of these patients, including RAS blockers, beta‐blockers, MRA, diuretics, and programming of implantable cardiac device, was prescribed and performed by cardiologists. Diabetes medications were prescribed by cardiologists, endocrinologists, or internal medicine physicians. Patients could freely decide which specialists to see for diabetic control. The study protocol was approved by the institutional review board.
2.2. Echocardiography studies
Echocardiographic images were acquired at baseline in every patient. Left ventricular end‐diastolic diameter was measured at end‐diastole, and left ventricular end‐systolic diameter and left atrial anteroposterior dimension were measured at end‐systole on parasternal views. The LVEF was calculated using the biplane Simpson's method on apical four‐chamber and two‐chamber views. Continuous wave Doppler of the tricuspid regurgitation trace is used to measure and estimate pulmonary artery systolic pressure (PASP).
2.3. Prescription of diabetes medications and adherence to guideline
The classes of diabetes medications were classified as follows: metformin, SGLT2i, SU, DPP4i, alpha‐glucosidase inhibitor (AGI), insulin, and TZD. Physicians prescribing diabetes medications were classified into three groups including cardiologists and endocrinologists. The data of prescriptions were collected annually in 2016, 2017, and 2018. Diabetes medications prescribed for >50% of treatment period in the particular year would be regarded as ‘prescribed medications’ for that year.
Prescription patterns of anti‐hyperglycaemic agents were further classified as follows: Pattern A, metformin mono‐therapy, metformin‐based therapy with SGLT2i, and SGLT2i‐based therapy without metformin; Pattern B, metformin‐based therapy with anti‐hyperglycaemic medication other than SGLT2i; and Pattern C, anti‐hyperglycaemic medication without metformin or SGLT2i.
2.4. Clinical outcomes
Death from CV causes or first unplanned hospitalization for HF was defined as the primary outcome of our study. In addition, death from CV causes alone, death from any cause, sudden cardiac death, and total incident of unplanned re‐hospitalization for HF were also collected. Severe hypoglycaemia was defined as having low blood glucose levels that required hospitalization.
2.5. Statistical analysis
Quantitative data were expressed as mean ± standard deviation or as median and inter‐quartile range, and categorical variables were presented as percentages. Descriptive summaries were presented for different groups of patients. The Student t‐test or the Mann–Whitney U‐test was used for comparisons between continuous data, and a χ 2 test was used for comparisons between categorical data. For the clinical outcome analysis, annualized event rates were estimated using the total number of clinical outcomes during the follow‐up period divided by 100 patient‐years at risk. Baseline characteristics, vital signs, HbA1c, renal function, echocardiographic parameters, HF, and diabetes medications were used for a univariate analysis. A multivariate logistic regression analysis with forward selection was performed to assess the predictability of variables on the primary outcome presented as odds ratios and 95% confidence intervals (CIs) using P < 0.1 in univariate analyses for inclusion. A P value of <0.05 was considered to be statistically significant. All tests were two‐sided. All the statistical analyses were performed using the SPSS Statistics 17.0 software (Chicago, IL, USA).
3. Results
3.1. General information and baseline heart failure management
Our study included 381 diabetic patients (age 64.8 ± 12.8 years, 71.9% male). The LVEF of all the patients were <40% at baseline, and the mean LVEF was 27.6 ± 7.0%. The baseline characteristics are shown in Table 1. The prescription rates of RAS blockers, beta‐blockers, and MRAs were 82.9%, 80.6%, and 63.5%, respectively. A total of 35 (9.2%) patients received cardiac resynchronization therapy and/or implantable cardioverter‐defibrillator. The HF medications and cardiac implantable devices were prescribed, programmed, and monitored by cardiologists.
Table 1.
Baseline characteristics of study patients
| Diabetic patients with HFrEF (n = 381) | |
|---|---|
| Age (year) | 64.8 ± 12.8 |
| Male gender, n (%) | 274 (71.9) |
| Body mass index (kg/m2) | 26.1 ± 4.7 |
| Systolic BP (mmHg) | 122.4 ± 18.7 |
| Heart rate (b.p.m.) | 82.7 ± 14.6 |
| NYHA Fc III or IV, n (%) | 86 (22.6) |
| Medical history, n (%) | |
| Non‐ischaemic cardiomyopathy | 166 (43.6) |
| Hypertension | 232 (60.9) |
| Old myocardial infarction | 157 (41.2) |
| Stroke/TIA | 56 (14.7) |
| Atrial fibrillation | 117 (30.7) |
| Previous HF hospitalization | 242 (63.5) |
| Previous valvular surgery | 31 (8.1) |
| Hyperlipidaemia | 224 (58.8) |
| COPD/asthma | 42 (11.0) |
| Chronic kidney disease | 150 (39.4) |
| Heart failure treatment, n (%) | |
| RAS blocker | 316 (82.9) |
| Beta‐blocker | 307 (80.6) |
| MRA | 242 (63.5) |
| CRT/ICD | 35 (9.2) |
| Haemoglobin A1c (%) | 7.7 ± 1.8 |
| GFR (mL/min/1.73 m2) | 67.0 ± 24.2 |
| GFR ≥90 mL/min/1.73 m2, n (%) | 58 (15.2) |
| GFR 60–90 mL/min/1.73 m2, n (%) | 165 (43.3) |
| GFR 30–60 mL/min/1.73 m2, n (%) | 158 (41.5) |
| Echocardiographic parameters | |
| LVEF (%) | 27.6 ± 7.0 |
| LA diameter (mm) | 48.5 ± 6.6 |
| LVEDD (mm) | 55.9 ± 8.2 |
| LVESD (mm) | 45.8 ± 9.8 |
| PASP (mmHg) | 40.5 ± 16.0 |
| Severe mitral regurgitation, n (%) | 94 (24.7) |
| Severe tricuspid regurgitation, n (%) | 60 (15.7) |
BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; GFR, glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter‐defibrillator; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MRA, mineralocorticoid receptor antagonists; NYHA Fc, New York Heart Association Functional Classification; PASP, pulmonary artery systolic pressure; RAS, renin–angiotensin system; TIA, transient ischaemic attack.
3.2. Prescription rates and patterns of different anti‐hyperglycaemic agents
Patients in the current study received 2 ± 1 types of diabetes medications for glycaemic control. The average duration of diabetes was 9.1 ± 4.1 years. Approximately 45% of patients received diabetes medications from cardiologists. Diabetes was managed by either endocrinologists or other specialists in 30% and 25% of patients, respectively. This trend did not differ throughout the study period, as shown in Table 2.
Table 2.
Distribution of physician specialties and prescription patterns of anti‐hyperglycaemic agents in study patients
| Year |
2016 (n = 319) |
2017 (n = 340) |
2018 (n = 324) |
P value |
|---|---|---|---|---|
| Numbers of anti‐hyperglycaemic agents prescribed | 2.1 ± 1.0 | 2.1 ± 1.0 | 2.1 ± 1.0 | 0.712 |
| Physicians who prescribe anti‐hyperglycaemic agents, n (%) | ||||
| Cardiologists | 135 (42.3) | 157 (46.2) | 152 (46.9) | 0.778 |
| Endocrinologists | 102 (32.0) | 99 (29.1) | 97 (29.9) | |
| Others | 82 (25.7) | 84 (24.7) | 75 (23.1) | |
| Prescribed anti‐hyperglycaemic agents, n (%) | ||||
| Metformin | 159 (49.8) | 179 (52.6) | 184 (56.8) | 0.206 |
| SGLT2i | 33 (10.3) | 60 (17.6) | 86 (26.5) | <0.001 |
| DPP4i | 157 (49.2) | 164 (48.2) | 138 (42.6) | 0.189 |
| SU | 156 (48.9) | 158 (46.5) | 142 (43.8) | 0.435 |
| AGI | 71 (22.3) | 66 (19.4) | 62 (19.1) | 0.551 |
| Insulin | 63 (19.7) | 69 (20.3) | 68 (21.0) | 0.926 |
| TZD | 4 (1.3) | 7 (2.1) | 6 (1.9) | 0.715 |
AGI, alpha‐glucosidase inhibitor; DPP4i, dipeptidyl peptidase‐4 inhibitor; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Table 2 also shows the prescription rates of different diabetes medications over time. The prescription rates of SGLT2i increased significantly from 10.3% in 2016 to 17.6% in 2017 and to 26.5% in 2018 (P < 0.001), with an ~8% annual increment. The prescription rates of metformin were also increased from 2016 to 2018, with a 3‐percentage annual increment, but this was not statistically significant (P = 0.206). The prescription rates of DPP4i, SU, AGI, and insulin did not differ significantly throughout the study period. Patients who had ever been treated with insulin therapy tend to have longer duration of diabetes than had those who did not require insulin (11 ± 4.4 vs. 8.5 ± 3.7 years, P < 0.001). The prescription rates of TZD were ~2% annually. None of the study patients received glucagon‐like peptide‐1 receptor agonist treatment.
Figure 2 demonstrates the different prescription patterns among different specialists and different years. Overall, the most commonly prescribed diabetes medication by cardiologists was metformin. The most commonly prescribed diabetes medication by endocrinologists was DPP4i, but endocrinologists also prescribed metformin, SU, AGI, and insulin equally in 40% of their patients. The most commonly prescribed diabetes mediation by other physicians was SU. Compared with different physicians, cardiologists preferred to prescribe metformin and SGLT2i than did other specialists. The prescription rates of metformin by cardiologists were ~60% in 2016, and it further increased to about 70% in 2018. On the contrary, the prescription rates of metformin by non‐cardiologists were ~40%, which did not increase significantly over time. The prescription rates of SGLT2i by cardiologists increased significantly from 18.3% in 2016 to 30.5% in 2017 and to 46.3% in 2018. SGLT2i was the fourth most commonly prescribed diabetes medication by cardiologists in 2016 and 2017, but it became the second most commonly prescribed diabetes medication in 2018. The prescription rates of SGLT2i by endocrinologists also increased from 8.8% in 2016 to 10.1% in 2017 and to 16.5% in 2018. The prescription rate of SGLT2i by other physicians was <4%. Endocrinologists were more likely to prescribe insulin, AGI, and DPP4i than were other specialists. The prescription rates of SU were similar among different specialists. These trends did not change significantly throughout the study period.
Figure 2.
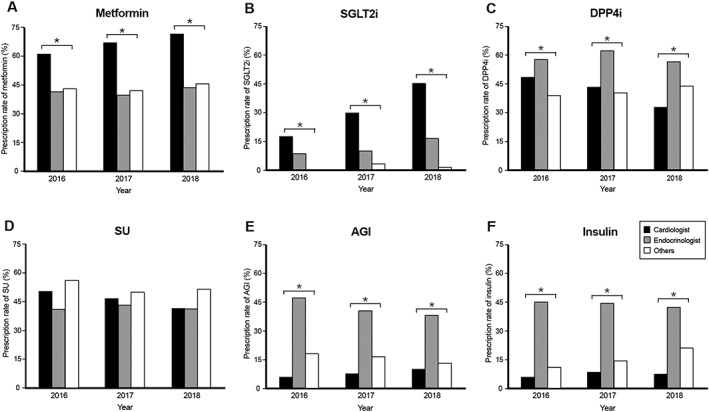
(A–F) The distribution of prescribed diabetes medications over time. Asterisk indicates P value < 0.05. AGI, alpha‐glucosidase inhibitor; DPP4i, dipeptidyl peptidase‐4 inhibitor; SGLT2i, sodium‐glucose co‐transporter 2 inhibitor; SU, sulfonylurea.
Figure 3 demonstrates the prescription patterns of diabetes medications among different specialists across different years. Pattern A increased gradually over the year [62 patients (19.4%), 92 (27.1%), and 114 (35.2%) in 2016, 2017 and 2018, respectively], and the upward trend was mainly driven by the prescription increase by cardiologists. Pattern B decreased from 103 patients (32.3%) in 2016 to 98 (28.8%) in 2017 and 86 (26.5%) in 2018, and Pattern C also decreased from 154 patients (48.3%) in 2016 to 150 (44.1%) in 2017 and 124 (38.3%) in 2018.
Figure 3.
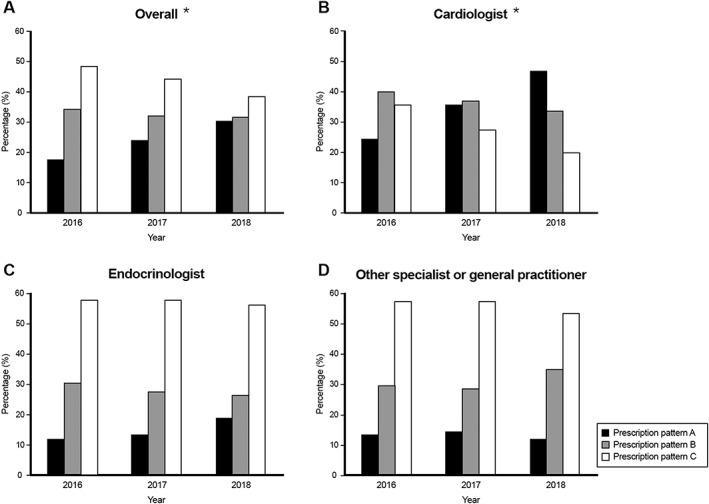
(A–D) The distribution of diabetes medications prescription pattern over time. Asterisk indicates statistical difference among years, P value < 0.05.
3.3. Clinical outcomes and predictors
Table 3 summarizes the clinical outcomes of our study. The incidence rate of death from CV causes or first unplanned hospitalization for HF was 19.0% per patient‐year. The incidence rates of death from any cause, death from CV causes, and sudden cardiac death were 7.3%, 5.4%, and 3.0% per patient‐year, respectively. The incidence rate of total unplanned HF hospitalization was 25.3% per patient‐year.
Table 3.
The cardiovascular clinical outcomes of patients
| (A) | ||||
|---|---|---|---|---|
| Events | Overall | Treated with metformin and/or SGLT2i | Not treated with metformin and/or SGLT2i | P value |
| Incidence rate, n (%/patient‐years) | Incidence rate, n (%/patient‐years) | Incidence rate, n (%/patient‐years) | ||
| CV death and/or 1st HFH | 187 (19.0) | 56 (10.1) | 131 (30.6) | <0.001 |
| All‐cause mortality | 72 (7.3) | 20 (3.6) | 52 (12.1) | <0.001 |
| Cardiovascular death | 53 (5.4) | 16 (2.9) | 37 (8.6) | <0.001 |
| Total HFH | 249 (25.3) | 72 (13.0) | 177 (41.4) | <0.001 |
| Sudden cardiac death | 29 (3.0) | 10 (1.8) | 19 (4.4) | 0.016 |
| Severe hypoglycaemia | 5 (0.5) | 1 (0.2) | 4 (0.9) | 0.095 |
| (B) | ||||
|---|---|---|---|---|
| Events | Pattern A | Pattern B | Pattern C | P value |
| Incidence rate, n (%/patient‐years) | Incidence rate, n (%/patient‐years) | Incidence rate, n (%/patient‐years) | ||
| CV death and/or 1st HFH | 24 (9.0) | 32 (11.1) | 131 (30.6) | <0.001 |
| All‐cause mortality | 6 (2.2) | 14 (4.9) | 52 (12.1) | <0.001 |
| Cardiovascular death | 5 (1.9) | 11 (3.8) | 37 (8.6) | <0.001 |
| Total HFH | 27 (10.1) | 45 (15.7) | 177 (41.4) | <0.001 |
| Sudden cardiac death | 3 (1.1) | 7 (2.4) | 19 (4.4) | 0.035 |
| Severe hypoglycaemia | 0 (0) | 1 (0.3) | 4 (0.9) | 0.084 |
In patients receiving metformin therapy with or without SGLT2i, the incidence of death from CV causes or first unplanned hospitalization for HF was 10.1% per patient‐year, which was significantly lower than that of patients not taking metformin with or without SGLT2i therapy (30.6% per patient‐year, P < 0.001, Table 3 A). Incidence of death from any cause, death from CV cause, sudden cardiac death, and total numbers of unplanned re‐hospitalization for HF were all significantly lower during the treatment period of metformin therapy with or without SGLT2i.
Regarding different diabetes medications prescription patterns, Table 3 B demonstrates that unfavourable clinical outcomes were least likely to occur during the treatment period of Pattern A and most likely to occur during the treatment period of Pattern C.
Figure 4 demonstrates the differences of annual incidence rate of death from CV causes or first unplanned hospitalization for HF, stratified according to prescriber (Figure 3 A) and baseline renal function (Figure 3 B). The incidence of CV death or first unplanned hospitalization for HF was significantly lower among patients receiving metformin and/or SGLT2i than among those who did not receive these medications, regardless of the prescriber. Similar statistical differences were also observed in the patients with GFR of 30–89 mL/min/1.73 m2. However, the event rates were low and did not differ significantly in patients with GFR ≥ 90 mL/min/1.73 m2.
Figure 4.
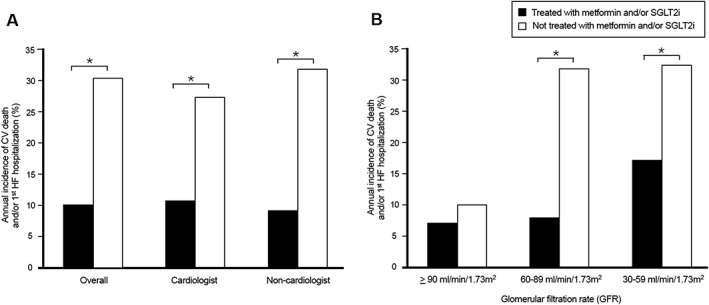
Annual incidence of death from cardiovascular causes or first unplanned hospitalization for heart failure in diabetic HFrEF patients, stratified according to diabetes medication prescriber (A) and baseline renal function (B). Asterisk indicates P value < 0.05. CV, cardiovascular; HFrEF, heart failure with reduced ejection fraction; SGLT2i, sodium‐glucose co‐transporter 2 inhibitor.
After adjustment for baseline characteristics, echocardiographic parameters, HF medications, diabetes medications, and physicians, the multivariate logistic regression analysis showed that the incidence of CV death or first unplanned hospitalization for HF was associated with history of chronic obstructive pulmonary disease, stroke or transient ischaemic attack, severe HF symptoms, worse LVEF, and higher baseline PASP. On the contrary, the prescriptions of RAS blocker, beta‐blocker, metformin, and SGLT2i were all independently associated with favourable outcomes (Table 4, Model 1). In Model 2, diabetes medication prescription pattern was again demonstrated as an independent predictor of favourable clinical outcomes.
Table 4.
Multivariate analysis for risk factors associated with cardiovascular death or first unplanned hospitalization for heart failure
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Event (+) | Event (−) | P value | OR (95% CI) | P value | |
| Model 1 including different anti‐hyperglycaemic agents | |||||
| Age (years) | 66.7 ± 12.7 | 64.8 ± 12.2 | 0.036 | — | NS |
| Stroke/TIA | 21.4% | 13.8% | 0.009 | 1.72 (1.09–2.71) | 0.019 |
| Atrial fibrillation | 37.4% | 30.2% | 0.049 | — | NS |
| Previous HF hospitalization | 78.1% | 59.0% | <0.001 | — | NS |
| COPD/asthma | 19.3% | 8.3% | <0.001 | 2.16 (1.30–3.59) | 0.003 |
| NYHA Fc III or IV | 46.5% | 13.6% | <0.001 | 3.93 (2.67–5.79) | <0.001 |
| Systolic BP (mmHg) | 119.0 ± 19.4 | 123.9 ± 18.2 | <0.001 | — | NS |
| LVEF (%) | 26.2 ± 7.2 | 28.3 ± 6.9 | <0.001 | 0.96 (0.94–0.99) | 0.007 |
| LA diameter (mm) | 50.4 ± 5.9 | 47.9 ± 6.6 | <0.001 | — | NS |
| PASP (mmHg) | 45.6 ± 17.0 | 38.1 ± 15.3 | <0.001 | 1.02 (1.01–1.03) | 0.001 |
| GFR (mL/min/1.73 m2) | 57.6 ± 17.7 | 70.0 ± 25.2 | <0.001 | — | NS |
| Heart failure medication | |||||
| Prescription of RAS blocker | 74.3% | 87.1% | <0.001 | 0.49 (0.31–0.76) | 0.002 |
| Prescription of beta‐blocker | 71.7% | 83.2% | <0.001 | 0.65 (0.43–0.99) | 0.049 |
| Diabetes mellitus medication | |||||
| Prescription of metformin | 26.7% | 59.3% | <0.001 | 0.40 (0.27–0.59) | <0.001 |
| Prescription of SGLT2i | 8.0% | 20.6% | <0.001 | 0.52 (0.28–0.98) | 0.042 |
| Prescription of DPP4i | 55.1% | 44.7% | 0.011 | — | NS |
| Prescription of insulin | 28.9% | 18.3% | 0.001 | — | NS |
| Prescribed by cardiologist | 36.4% | 47.2% | 0.007 | — | NS |
| Model 2 including different prescription patterns | |||||
| Age (years) | 66.7 ± 12.7 | 64.8 ± 12.2 | 0.036 | — | NS |
| Stroke/TIA | 21.4% | 13.8% | 0.009 | 1.74 (1.11–2.74) | 0.017 |
| Atrial fibrillation | 37.4% | 30.2% | 0.049 | — | NS |
| Previous HF hospitalization | 78.1% | 59.0% | <0.001 | — | NS |
| COPD/asthma | 19.3% | 8.3% | <0.001 | 2.36 (1.41–3.96) | 0.002 |
| NYHA Fc III or IV | 46.5% | 13.6% | <0.001 | 4.24 (2.88–6.25) | <0.001 |
| LVEF (%) | 26.2 ± 7.2 | 28.3 ± 6.9 | <0.001 | 0.96 (0.94–0.99) | 0.006 |
| LA diameter (mm) | 50.4 ± 5.9 | 47.9 ± 6.6 | <0.001 | — | NS |
| PASP (mmHg) | 45.6 ± 17.0 | 38.1 ± 15.3 | <0.001 | 1.02 (1.01–1.03) | 0.001 |
| GFR (mL/min/1.73 m2) | 57.6 ± 17.7 | 70.0 ± 25.2 | <0.001 | — | NS |
| Heart failure medication | |||||
| Prescription of RAS blocker | 74.3% | 87.1% | <0.001 | 0.47 (0.30–0.73) | 0.001 |
| Prescription of beta‐blocker | 71.7% | 83.2% | <0.001 | 0.64 (0.42–0.97) | 0.038 |
| Diabetes mellitus prescription pattern | <0.001 | 2.05 (1.57–2.68) | <0.001 | ||
| Pattern A | 12.8% | 30.7% | |||
| Pattern B | 17.1% | 32.0% | |||
| Pattern C | 70.1% | 37.3% | |||
| Diabetes medications prescribed by cardiologist | 36.4% | 47.2% | 0.007 | — | NS |
BP, blood pressure; COPD, chronic obstructive pulmonary disease; DPP4i, dipeptidyl peptidase‐4 inhibitors; GFR, glomerular filtration rate; HF, heart failure; LA, left atrial; LVEF, left ventricular ejection fraction; NYHA Fc, New York Heart Association Functional Classification; PASP, pulmonary artery systolic pressure; RAS, renin–angiotensin system; SGLT2i, sodium‐glucose co‐transporter 2 inhibitor; TIA, transient ischaemic attack.
The incidence of severe hypoglycaemia was 0.5 per 100 patient‐years. Severe hypoglycaemic event did not occur during the treatment period of Pattern A. There was numerically higher incidence of hypoglycaemia during the treatment period of Patterns B and C (0.3 and 0.9 per 100 patient‐years, respectively, P = 0.084).
4. Discussion
4.1. The prescribing trend of diabetes medications
The prescription rates of SGLT2i were significantly increased across the study period, reflecting the impact of growing evidence on HF reduction by SGLT2i. However, SGLT2i was only the fourth most commonly prescribed diabetes medications among the seven classes of drugs in 2018, after metformin (56.8%), DPP4i (43.8%), and SU (42.6%).
Metformin treatment was recommended as the first‐line medication in the current guideline, and therefore, it was naturally the most prescribed anti‐hyperglycaemic drug, although its gastrointestinal side effects were common and might restrict its usage.22 In the DAPA‐HF trial, which evaluated the effect of dapagliflozin in HFrEF patients, the most commonly prescribed baseline diabetes medication was metformin (50.8%).24 The ASIAN‐HF registry enrolled 5276 HFrEF patients from Northeast Asia, South Asia, and Southeast Asia between October 2012 and December 2015, which demonstrated that the most commonly prescribed diabetes medications were metformin (54%).25 The prescription rates of metformin in both studies were similar to those in our study.
The current study showed that SU and DPP4i were respectively the second and third most commonly prescribed medications between 2016 and 2018. In the ASIAN‐HF registry, SU was also the second most commonly prescribed diabetes medication (53%), and the prescription rates of insulin were the lowest in Taiwan and South Korea, whereas the prescription rates of DPP4i were the highest in Taiwan and Japan. The reasons for the heterogeneity of prescription pattern were not addressed, but the relatively high prescription rate of DPP4i was also shown in our study.25
4.2. Role of Dipeptidyl Peptidase‐4 Inhibitors on cardiovascular system
Previously, the SAVOR TIMI‐53 trial demonstrated that the saxagliptin group showed a significantly higher incidence of HF hospitalization than did the control group.26 In the EXAMINE study, patients with recent acute myocardial infarction treated with alogliptin also showed a trend of slight increase in admission to hospital for HF, although not statistically significant [hazard ratio (HR) 1.19, 95% CI 0.89–1.58, P = 0.22].27 On the contrary, the VIVIDD study, which evaluated the effect of vildagliptin on ventricular function in HFrEF diabetic patients with NYHA Fc I to III and LVEF < 40%, demonstrated that although change of LVEF was not significant between vildagliptin and control groups, the left ventricular end‐diastolic volume was significantly increased in the vildagliptin group than in the control group after 52 weeks of follow‐up (P = 0.007).28 As for another commonly prescribed DPP4i, linagliptin, the recently published CARMELINA trial, enrolling subjects with DM, high CV risk, and high renal risk, showed that adding linagliptin to usual care, when compared with placebo, resulted in a non‐inferior risk of a composite CV outcome.29 Despite the slight incoherent results between different classes of DPP4i, the prescription rate of DPP4i remained high not only in our study group but also in the larger‐scaled, aforementioned ASIAN‐HF registry.25 Based on our analysis, DPP4i is associated with a higher rate of CV death or unplanned hospitalization for HF in the univariate analysis, but not after multivariate adjustment.
4.3. Role of insulin on cardiovascular system
The interaction between insulin signalling pathway and HF is complicated. Various studies demonstrated that insulin resistance and hyperinsulinaemia are associated with cardiomyocyte mitochondrial dysfunction, LV remodelling with hypertrophy, and subsequent HF, likely through the regulation of insulin receptor substrate, PI‐3K, Akt kinase, and Foxo‐1.30 Moreover, Defronzo et al. had described the anti‐natriuretic effect of insulin.13 Therefore, insulin could worsen fluid retention, and this raises the concern of exacerbation of HF symptoms.
Some observational studies had shown worse prognosis in patients treated with insulin.31, 32 However, in the ORIGIN trial, among patients with CV risk with diabetes or pre‐diabetes, insulin glargine was not related to the increase in composite primary outcome of non‐fatal myocardial infarction, non‐fatal stroke, or death from CV causes as well as revascularization or hospitalization for HF.33
In our study cohort, the insulin‐treated group had a higher event rate only after the univariate analysis, but the difference became insignificant after the multivariate analysis.
4.4. Benefit of sodium‐glucose co‐transporter 2 inhibitor and metformin in heart failure patients
The 2016 ESC Guideline of HF indicated that empagliflozin should be considered in diabetic patients to prevent or delay the onset of HF.9 According to the recently published 2019 ESC expert consensus, the ability of all SGLT2i's to prevent the hospitalizations for HF in patients with DM is regarded as a class effect.34 However, this consensus also emphasized that no specific recommendations for the use of SGLT2i in patients with established HF can be concluded owing to a lack of definite evidence. The upcoming randomized controlled trials such as DAPA‐HF24 and EMPEROR35 could help to address this question.
The limitations of previous SGLT2i CV outcome trials were that the diagnosis, phenotype, and severity of HF had not been well characterized in general. In our current study exclusively enrolling DM patients with HFrEF, we found that patients who received metformin‐based therapy alone or with SGLT2i had significantly lower risk of all‐cause mortality (3.6 vs. 12.1 per 100 patient‐years, P < 0.001), death from CV causes (2.9 vs. 8.6 per 100 patient‐years, P < 0.001), sudden cardiac death (1.8 vs. 4.4 per 100 patient‐years, P = 0.016), and re‐hospitalizations for HF (13.0 vs. 41.4 per 100 patient‐years, P < 0.001) than had those without the therapy. The result basically echoes our current concept that SGLT2i is one of the most beneficial medications for decreasing HF and death. In the subgroup analysis of DECLARE‐TIMI 58 trial, dapagliflozin reduced the risk of CV death or HF hospitalizations, CV death alone, HF hospitalizations alone, and all‐cause mortality in patients with HFrEF. The benefit of dapagliflozin in patients with HFrEF occurred soon after drug initiation; in contrast, the benefit in HF patients with preserved LVEF was observed only after 1 year of administration of dapagliflozin.21 This result echoed our study finding that early intervention with SGLT2i could allow early improvement of the clinical outcomes within a year.
The role of metformin on the CV system is less well established. Metformin acts through decreasing hepatic glucose production. It also functions as insulin sensitizer to enhance peripheral glucose uptake and utilization. To date, there has not been any large, randomized, controlled study focusing on the safety or efficacy of metformin in those with HF. However, Eurich et al. have conducted a systemic review of nine observational studies, including 34 000 patients with both DM and HF, to evaluate the association of metformin use on all‐cause mortality and all‐cause hospitalization. According to their analysis, metformin was associated with a small reduction in all‐cause hospitalizations. Besides, metformin was not associated with increased risk of lactic acidosis. Therefore, the author concluded that metformin is at least as safe as other glucose‐lowering agents in the population.16 Chia et al. demonstrated that only metformin therapy was associated with reduced risk of the 1 year all‐cause mortality or HF hospitalization in the ASIAN‐HF registry after propensity score adjustment and that no significant risk reduction was found with other diabetes medications.25
In line with the aforementioned studies, our study also demonstrated a reduced risk of CV death and all‐cause mortality, and hospitalization for HF in patients treated with metformin and SGLT2i. The DPP4i, along with ‘insulin provision therapies’, including insulin itself and SU, was negatively associated with CV events.
It is worth noting that the benefits of metformin‐based therapy alone or with SGLT2i in current study were noted in patients with GFR of 30 to 90 mL/min/1.73 m2 but not in patients with GFR ≥ 90 mL/min/1.73 m2. Baseline risks among these patients with preserved renal function were relatively low, and hence, it was difficult to demonstrate the benefit of drug effects with a smaller sample size. Interestingly, GFR of 30 to 90 mL/min/1.73 m2 was also the inclusion criterion of the CREDENCE trial, which enrolled patients at high risk of CV disease and demonstrated that canagliflozin could reduce HF hospitalization by 39% and CV death by 22%.36 This finding further emphasizes the importance of optimal diabetes treatment for high‐risk HFrEF patients with mild‐to‐moderate chronic kidney disease.
4.5. Study limitations
There were several limitations in the present study. First, in this observational, retrospective study, despite covariate adjustment, other unmeasured confounding factors might affect clinical outcomes. For example, N‐terminal pro‐BNP level is a useful tool to predict prognosis, but it was not extensively studied in our patients. Second, the reasons for not initiating metformin or SGLT2i treatment were not obtained; therefore, we could not provide a detailed explanation on not prescribing these medications. However, some patients could have discontinued these medications because of acute illness and worsening renal function, which could possibly correlate with subsequent CV events. Third, we did not measure the baseline insulin level and C‐peptide level, and we did not regularly examine the change of echocardiography after the adjustment of diabetes medications.
5. Conclusions
The prescription patterns of diabetes medications in diabetics patients with HFrEF were diverse among different specialists. The treatment with metformin and SGLT2i was associated with better clinical outcomes. Establishing educational programme for physicians to increase awareness of the evidence‐based diabetes medications in treating HFrEF patients is important to improve clinical outcome.
Conflict of interest
None declared.
Funding
This work was supported by Cheng Hsin General Hospital [CHGH109‐(IP)1‐01]
Chang, H.‐Y. , Su, Y.‐W. , Feng, A.‐N. , Fong, M.‐C. , Huang, K.‐C. , Chong, E. , Chen, K.‐C. , and Yin, W.‐H. (2020) Prescription patterns of diabetes medications influencing clinical outcomes of heart failure patients with reduced ejection fraction. ESC Heart Failure, 7: 604–615. 10.1002/ehf2.12617.
References
- 1. Bonneux L, Barendregt JJ, Meeter K, Bonsel GJ, van der Maas PJ. Estimating clinical morbidity due to ischemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health 1994; 84: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, Suzuki K, Tanabe N, Kodama M, Aizawa Y. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J 2008; 72: 489–491. [DOI] [PubMed] [Google Scholar]
- 4. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004; 27: 1879–1884. [DOI] [PubMed] [Google Scholar]
- 5. Gilbert RE, Krum H. Heart failure in diabetes: effects of anti‐hyperglycaemic drug therapy. Lancet 2015; 385: 2107–2117. [DOI] [PubMed] [Google Scholar]
- 6. Gerstein HC, Swedberg K, Carlsson J, McMurray JJV, Michelson EL, Olofsson B, Pfeffer MA, Yusuf S, CHARM Program Investigators . The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med 2008; 168: 1699–1704. [DOI] [PubMed] [Google Scholar]
- 7. Goode KM, John J, Rigby AS, Kilpatrick ES, Atkin SL, Bragadeesh T, Clark AL, Cleland JG. Elevated glycated haemoglobin is a strong predictor of mortality in patients with left ventricular systolic dysfunction who are not receiving treatment for diabetes mellitus. Heart 2009; 95: 917–923. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta‐analysis and meta‐regression analysis of placebo‐controlled randomized clinical trials. Am J Cardiovasc Drugs 2011; 11: 115–128. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 10. Savarese G, Perrone‐Filardi P, D'Amore C, Vitale C, Trimarco B, Pani L, Rosano GM. Cardiovascular effects of dipeptidyl peptidase‐4 inhibitors in diabetic patients: a meta‐analysis. Int J Cardiol 2015; 181: 239–244. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien MJ, Karam SL, Wallia A, Kang RH, Cooper AJ, Lancki N, Moran MR, Liss DT, Prospect TA, Ackermann RT. Association of second‐line antidiabetic medications with cardiovascular events among insured adults with type 2 diabetes. JAMA Netw Open 2018; 1: e186125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bubien JK. Epithelial Na+ channel (ENaC), hormones, and hypertension. J Biol Chem 2010; 285: 23527–23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Defronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975; 55: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int J Hypertens 2011; 2011: 391762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacDonald MR, Eurich DT, Majumdar SR, Lewsey JD, Bhagra S, Jhund PS, Petrie MC, McMurray JJ, Petrie JR, McAlister FA. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case‐control study from the U.K. General Practice Research Database. Diabetes Care 2010; 33: 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013; 6: 395–402. [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 18. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 19. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 20. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 21. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018; 41: S73–S85. [DOI] [PubMed] [Google Scholar]
- 23. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS, QUALIFY Investigators . Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017; 19: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; Committees DAPA‐HF and Investigators. The Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure (DAPA‐HF) trial: baseline characteristics. Eur J Heart Fail 2019; 21:1402‐1411. [DOI] [PubMed] [Google Scholar]
- 25. Chia YMF, Teng TK, Tay WT, Anand I, MacDonald MR, Yap J, Chandramouli C, Richards AM, Tromp J, Ouwerkerk W, Ling LH, Lam CSP, Investigators ASIAN‐HF. Prescription patterns of anti‐diabetic medications and clinical outcomes in Asian patients with heart failure and diabetes mellitus. Eur J Heart Fail 2019; 21: 685–688. [DOI] [PubMed] [Google Scholar]
- 26. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR‐TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 27. Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB, EXAMINE Investigators . Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double‐blind trial. Lancet 2015; 385: 2067–2076. [DOI] [PubMed] [Google Scholar]
- 28. McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W, Lewsey JD, Krum H, VIVIDD Trial Committees and Investigators . Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure. JACC Heart Fail 2018; 6: 8–17. [DOI] [PubMed] [Google Scholar]
- 29. Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK, CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA Randomized Clinical Trial. JAMA 2019; 321: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riehle C, Abel ED. Insulin Signaling and Heart Failure. Circ Res 2016; 118: 1151–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murcia AM, Hennekens CH, Lamas GA, Jiménez‐Navarro M, Rouleau JL, Flaker GC, Goldman S, Skali H, Braunwald E, Pfeffer MA. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med 2004; 164: 2273–2279. [DOI] [PubMed] [Google Scholar]
- 32. Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006; 27: 65–75. [DOI] [PubMed] [Google Scholar]
- 33. Trial Investigators ORIGIN, Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 34. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 35. Butler J, Hamo CE, Filippatos G, Pocock SJ, Bernstein RA, Brueckmann M, Cheung AK, George JT, Green JB, Januzzi JL, Kaul S, Lam CSP, Lip GYH, Marx N, McCullough PA, Mehta CR, Ponikowski P, Rosenstock J, Sattar N, Salsali A, Scirica BM, Shah SJ, Tsutsui H, Verma S, Wanner C, Woerle HJ, Zannad F, Anker SD, Trials Program EMPEROR. The potential role and rationale for treatment of heart failure with sodium‐glucose co‐transporter 2 inhibitors. Eur J Heart Fail 2017; 19: 1390–1400. [DOI] [PubMed] [Google Scholar]
- 36. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Trial Investigators CREDENCE. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]


