Abstract
Aims
Echocardiographic response after cardiac resynchronization therapy (CRT) is often lesser in ischaemic cardiomyopathy (ICM) than non‐ischaemic dilated cardiomyopathy (NIDCM) patients. We assessed the association of heart failure aetiology on the amount of reverse remodelling and outcome of CRT.
Methods and results
Nine hundred twenty‐eight CRT patients were retrospectively included. Reverse remodelling and endpoint occurrence (all‐cause mortality, heart transplantation, or left ventricular assist device implantation) was assessed. Two response definitions [≥15% reduction left ventricular end systolic volume (LVESV) and ≥5% improvement left ventricular ejection fraction] and the most accurate cut‐off for the amount of reverse remodelling that predicted endpoint freedom were assessed.
Mean follow‐up was 3.8 ± 2.4 years. ICM was present in 47%. ICM patients who were older (69 ± 7 vs. 63 ± 11), more often men (83% vs. 58%), exhibited less LVESV reduction (13 ± 31% vs. 23 ± 32%) and less left ventricular ejection fraction improvement (5 ± 11% vs. 10 ± 12%) than NIDCM patients (all P < 0.001). Nevertheless, every 1% LVESV reduction was associated with a relative reduction in endpoint occurrence: NIDCM 1.3%, ICM 0.9%, and absolute risk reduction was similar (0.4%). The most accurate cut‐off of LVESV reduction that predicted endpoint freedom was 17.1% in NIDCM and 13.2% in ICM.
Conclusions
ICM patients achieve less reverse remodelling than NIDCM, but the prognostic gain in terms of survival time is the same for every single percentage of reverse remodelling that does occur. The assessment and expected magnitude of reverse remodelling should take this effect of heart failure aetiology into account.
Keywords: Cardiac resynchronization therapy, Echocardiography, Clinical response, Aetiology
Introduction
Cardiac resynchronization therapy (CRT) is an effective therapy for symptomatic heart failure patients with impaired left ventricular (LV) function and electrical dyssynchrony, despite optimal medical therapy.1, 2 Landmark trials have shown improvement in symptoms and cardiac function and reduction in morbidity and mortality.3, 4, 5, 6, 7
The effectiveness of CRT is related to its ability to reverse the adverse LV remodelling that characterizes these patients.8, 9, 10 Electrical resynchronization enables optimized filling time, the reduction of intraventricular dyssynchrony, and optimization of right–left ventricular interaction, and more pronounced reverse remodelling favourably influences prognosis.11, 12 Reduction in LV end systolic volume (LVESV reduction of ≥15%) or an increase in LV ejection fraction (LVEF increase of ≥5%) are two of the most common echocardiographic markers of reverse remodelling, and they provide important prognostic information on therapy outcome.10, 13
Echocardiographic response can occur in both patients with non‐ischaemic dilated cardiomyopathy (NIDCM) as well as those with ischaemic cardiomyopathy (ICM) but often to a lesser extent in the latter.14, 15, 16, 17, 18 The question is whether viewing response as a binary entity does justice to the reverse remodelling and the associated prognostic outcome effect that still occurs in ICM patients, albeit to a lesser degree.14, 15, 16, 17, 18
We set out to study the association between heart failure aetiology and the amount of reverse remodelling and long‐term clinical outcome of CRT in a real‐world CRT cohort.
Methods
Maastricht–Utrecht–Groningen cohort
The Maastricht–Utrecht–Groningen cohort consists of 1946 patients who received a CRT in one of three university hospitals in the Netherlands between January 2001 and January 2015 (Maastricht University Medical Center, January 2010–January 2015; University Medical Center Utrecht, January 2005–January 2015; and University Medical Center Groningen, January 2001–January 2015).19 There were no formal inclusion criteria. CRT indication, device implantation, and lead positioning were according to prevailing European Society of Cardiology (ESC) guidelines at the time of implantation and local hospital protocols. All commercially available devices and leads could be used. For the current analysis, we included patients with a de novo CRT implantation. Patients were excluded if they had right ventricular pacing from a pacemaker or implantable cardioverter defibrillator at baseline (N = 340, 17%), a QRS duration less than 120 ms (N = 119, 6%), or no paired echocardiographic data at baseline and follow‐up (N = 559, 29%). The final study cohort consisted of 928 patients. See Figure 1 for a flowchart.
Figure 1.
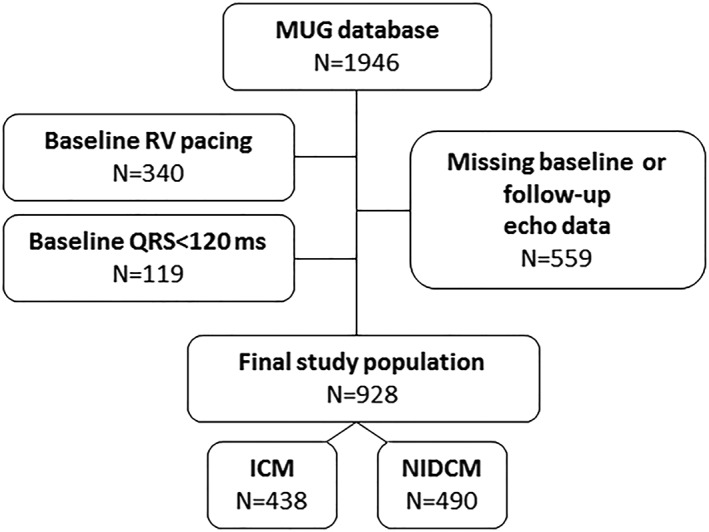
Flowchart of the Maastricht–Utrecht–Groningen database19 depicting the final study cohort with available echocardiographic and outcome data. ACM, all‐cause mortality; FU, follow‐up; HTx, heart transplantation; ICM, ischaemic cardiomyopathy; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; MUG, Maastricht–Utrecht–Groningen cohort; NIDCM, non‐ischaemic dilated cardiomyopathy; RV, right ventricular.
Data selection
The Dutch Central Committee on Human‐Related Research (Centrale Commissie Mensgebonden Onderzoek) allows the use of anonymous data without prior approval of an institutional research board provided that the data are acquired from routine patient care. Demographic characteristics, comorbidities, heart failure aetiology (deemed ischaemic when there was clear evidence of myocardial infarction or coronary artery bypass graft in the medical history), medical therapy, baseline electrocardiography, and echocardiography were collected from local electronic medical record. Device data were retrieved from device specific databases at each centre; optimization of device counters was up to the discretion of the patient's treating physician. Fluoroscopic images or chest X‐rays were used to determine LV lead position. Data were handled anonymously.
Electrocardiography
Recorded baseline 12‐lead electrocardiograms (ECGs) were stored digitally in the MUSE Cardiology Information system (GE Medical System) at the three hospitals. QRS duration and baseline ECG parameters were evaluated using automated ECG readings. Left bundle branch block (LBBB) morphology was defined according to ESC guideline criteria, namely, QRS duration ≥120 ms; QS or rS in lead V1; broad (frequently notched or slurred) R waves in leads I, aVL, V5, or V6; and absent Q waves in leads V5 and V6.2
Echocardiography
Transthoracic echocardiography was performed at baseline and during follow‐up (median = 6.6 ± 2.7 months after implantation) as part of routine clinical care by experienced cardiac sonographers at the echocardiographic core lab of the three respective centres. The Simpson's modified biplane method, using apical two‐chamber and four‐chamber views, was used to measure LVESV and LVEF at baseline and follow‐up. Response to CRT was quantified as the change in LVESV and LVEF (Δ in percentage) during follow‐up. Next, to response as a continuous variable, two definitions of response were studied: reduction in LVESV ≥ 15% and LVEF improvement of ≥5%.
Endpoint
Follow‐up and survival status until 1 January 2016 were obtained from electronic hospital records linked to municipal registries. Mean follow‐up time was 3.8 ± 2.4 years. Endpoint occurrence was defined as a composite of all‐cause mortality, heart transplantation, or LV assist device implantation. All patients had available follow‐up data.
Statistical analyses
Data are presented as mean ± standard deviation or median (interquartile range) for continuous variables. Normality was checked using the Shapiro–Wilk statistic. Categorical data were expressed as numbers and percentages. Differences between heart failure aetiologies were evaluated using the Student's t‐test, Mann–Whitney U‐test, χ 2 test, and Fisher's exact test, depending on normality and type of data. To study effect modification, the amount of reverse remodelling was determined in several predefined subgroups, including sex, LBBB presence, and baseline QRS duration < 150 or ≥150 ms. Adjusted hazard ratios (HRs) for outcome in the ICM and NIDCM patients were calculated by Cox regression analysis after correcting for age, sex, and amount of LVESV remodelling. A multivariable Cox regression model was made from significant univariate parameters (P < 0.1) in the total population and ICM and NIDCM groups separately. First line interactions were tested. Tested univariate variables were based on baseline variables that differed between the ICM and NIDCM group. Optimal relationship between change in LVESV (continuous variable) and LVEF (continuous variable) and absence of the endpoint was investigated using receiver operating characteristics (ROCs). Optimal cut‐off point was identified by the Youden index point (sensitivity + specificity − 1). All tests of significance were two‐sided, with P values of <0.05 assumed to indicate significance. All analyses were generated using SPSS version 23.0 for Windows (IBM Corp, Chicago, IL, USA).
Results
Baseline characteristics
Baseline characteristics are listed in Table 1. Mean age was 66 ± 11 years, 70% were men. Most patients were in New York Heart Association (NYHA) class III (55%). Ischaemic aetiology was present in 47% of patients. ICM patients were significantly older (69 ± 7 vs. 63 ± 11; P < 0.001), more often men (83% vs. 58%; P < 0.001), and suffered more from diabetes mellitus (27% vs. 18%; P < 0.001), than NIDCM patients. Patients with ICM less often had LBBB (75% vs. 86%; P < 0.001) and larger baseline LV end‐diastolic and end‐systolic volumes (both P < 0.001). ICM patients had higher N terminal pro brain natriuretic peptide (NT‐proBNP) levels [1490 (750–3034) pg/mL vs. 1107 (394–2770) pg/mL; P = 0.002] and worse renal function [estimated glomerular filtration rate 60 (44–79) mL/min/1.73 m2 vs 71 (51–96) mL/min/1.73 m2; P < 0.001]. There were no differences in body mass index, NYHA class, and presence of hypertension or atrial fibrillation.
Table 1.
Baseline characteristics
| Total cohort (N = 928) | ICM (N = 438) | NIDCM (N = 490) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Men, % (n) | 647 (70) | 362 (83) | 285 (58) | <0.001 |
| Age, years | 66 ± 11 | 69 ± 7 | 63 ± 11 | <0.001 |
| BMI, kg/m2 | 26.7 ± 4.6 | 26.8 ± 4.2 | 26.7 ± 5.0 | 0.79 |
| Weight, kg | 81 ± 16 | 82 ± 14 | 80 ± 18 | 0.19 |
| Height, cm | 174 ± 9 | 175 ± 8 | 173 ± 10 | 0.01 |
| Medical history | ||||
| Hypertension, % (n) | 397 (43) | 193 (44) | 204 (42) | 0.51 |
| Diabetes mellitus, % (n) | 207 (22) | 120 (27) | 84 (18) | 0.001 |
| (History of) AF, % (n) | 130 (14) | 60 (14) | 70 (14) | 0.85 |
| Clinical profile | ||||
| NYHA class, % (n) | 0.19 | |||
| I | 19 (2) | 4 (1) | 15 (3) | |
| II | 345 (37) | 165 (37) | 180 (37) | |
| III | 515 (55) | 246 (56) | 265 (54) | |
| IV | 39 (4) | 20 (5) | 19 (4) | |
| Missing | 14 (2) | 3 (1) | 11 (2) | |
| ECG | ||||
| Heart rate, bpm | 73 ± 15 | 71 ± 15 | 74 ± 16 | 0.02 |
| PQ duration, ms | 189 ± 38 | 195 ± 38 | 184 ± 38 | <0.001 |
| QRS duration, ms | 161 ± 20 | 160 ± 19 | 162 ± 21 | 0.11 |
| QT duration, ms | 485 ± 41 | 481 ± 41 | 489 ± 40 | 0.002 |
| LBBB, % (n) | 746 (80) | 327 (75) | 419 (86) | <0.001 |
| Echocardiography | ||||
| LVEF, % | 24 ± 9 | 24 ± 8 | 25 ± 9 | 0.02 |
| LVEDV, mL | 220 ± 89 | 231 ± 83 | 211 ± 93 | 0.001 |
| LVESV, mL | 169 ± 78 | 178 ± 74 | 161 ± 80 | 0.001 |
| Mitral regurgitation, % (n) | 0.03 | |||
| Mild | 271 (33) | 118 (41) | 153 (31) | |
| Mild‐moderate | 174 (21) | 98 (22) | 76 (16) | |
| Moderate‐severe/severe | 130 (16) | 66 (15) | 64 (13) | |
| Implantation | ||||
| Device type | 0.11 | |||
| CRT‐P, % (n) | 60 (7) | 22 (5) | 38 (8) | |
| CRT‐D, % (n) | 868 (93) | 416 (95) | 452 (92) | |
| Lead position | 0.96 | |||
| Anterior | 7 (1) | 4 (1) | 3 (1) | |
| Anterolateral | 94 (10) | 46 (11) | 48 (10) | |
| Lateral | 320 (34) | 146 (33) | 174 (35) | |
| Posterolateral | 405 (44) | 188 (43) | 217 (44) | |
| Posterior | 76 (8) | 36 (8) | 40 (8) | |
| Missing | 26 (3) | 18 (4) | 8 (2) | |
| Medication use | ||||
| β‐blocker, % (n) | 794 (86) | 377 (86) | 417 (85) | 0.71 |
| ACEi or ARB, % (n) | 850 (92) | 396 (90) | 454 (93) | 0.24 |
| MRA, % (n) | 254 (27) | 107 (24) | 147 (30) | 0.80 |
| Diuretics, % (n) | 741 (80) | 358 (82) | 383 (78) | 0.19 |
| Statin, % (n) | 531 (57) | 353 (81) | 178 (36) | <0.001 |
| Digoxin, % (n) | 134 (14) | 57 (13) | 77 (16) | 0.26 |
| Antiarrhythmic drugs, % (n) | 93 (10) | 49 (11) | 44 (9) | 0.28 |
| Laboratory values | ||||
| NT‐proBNP (pg/mL) | 1301 [541–2856] | 1490 [750–3034] | 1107 [394–2770] | 0.002 |
| Hb (mmol/L) | 8.5 [7.7–9.1] | 8.5 [7.7–9.1] | 8.5 [7.8–9.0] | 0.99 |
| Creatinine (umol/L) | 102 [83–129] | 111[91–137] | 92 [79–119] | <0.001 |
| eGFR (mL/min/1.73m2) | 65 [48–90] | 60 [44–79] | 71 [51–96] | <0.001 |
ACEi, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CRT‐P, cardiac resynchronization therapy pacemaker; CRT‐D, cardiac resynchronization therapy defibrillator; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; ICM, ischaemic cardiomyopathy; LBBB, left bundle branch block; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; MRA, mineralocorticoid receptor antagonist; NIDCM, non‐ischaemic dilated cardiomyopathy; NT‐proBNP, N terminal brain natriuretic peptide; NYHA, New York Heart Association.
Reverse remodelling
Patients with ICM exhibited less reduction in LVESV (13 ± 31% vs. 23 ± 32%; P < 0.001) and less increase in LVEF (5 ± 11% vs. 10 ± 12%; P < 0.001) (see Supporting Information for dispersion graph). Fifty‐six percent of all patients were classified as LVESV responders (47% ICM vs. 63% NIDCM; P < 0.001) and 57% as LVEF responders (47% ICM vs. 66% NIDCM; P < 0.001). For several subgroups, including sex, LBBB presence, and baseline QRS duration, ICM patients achieved significantly less reverse remodelling (Figure 2 ).
Figure 2.
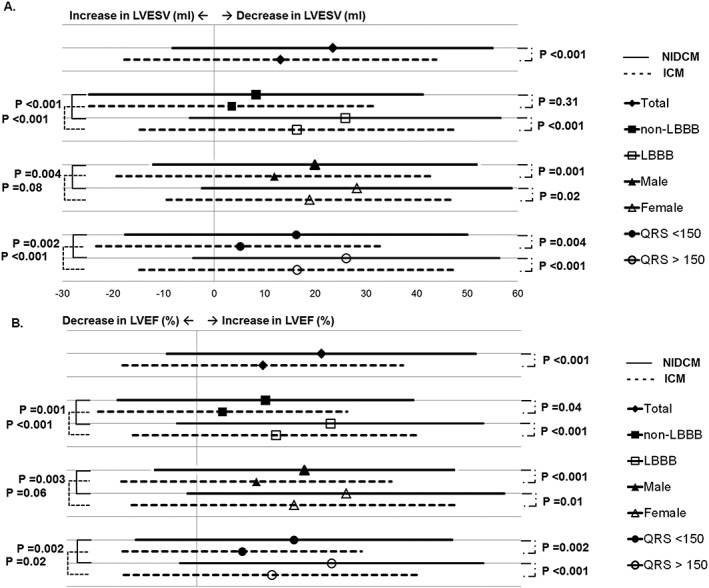
Amount of reverse remodelling. Amount of reduction in left ventricular end systolic volume during follow‐up (%) (A) and improvement in left ventricular ejection fraction (%) (B) for ischaemic cardiomyopathy and non‐ischaemic dilated cardiomyopathy patients in the total population and different predefined sub‐groups (sex, left bundle branch block presence, and QRS duration). ICM, ischaemic cardiomyopathy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; NIDCM, non‐ischaemic dilated cardiomyopathy.
Long‐term clinical outcome
Endpoint free survival in ischaemic cardiomyopathy and non‐ischaemic dilated cardiomyopathy patients
Patients with ICM experienced more events [167 (38%) vs. 131 (27%); P < 0.001]. After adjustment for age and sex, ICM remained associated with a worse outcome [HR 1.24, 95% confidence interval (CI) 1.02–1.50, and P = 0.04]. After adding the amount of reverse remodelling, it was no longer significant (HR 1.05, 95% CI 0.82–1.34, and P = 0.70). There was no significant interaction between Δ reverse remodelling and heart failure aetiology on outcome (P = 0.176); interaction was significant between age and Δ reverse remodelling (P = 0.008)
Clinical outcome in non‐responders vs. responders
Overall, CRT non‐responders had a worse clinical outcome. This was observed for both the LVESV definition of response, as well as for the LVEF definition of response (see Figure 3 for unadjusted and adjusted HRs) In NIDCM patients, this was also observed. NIDCM non‐responders had a worse outcome according to both the LVESV and LVEF definition of response. In ICM patients, only the LVESV definition was associated with clinical outcome, but LVEF increase < 5% or ≥5% was not associated with outcome.
Figure 3.
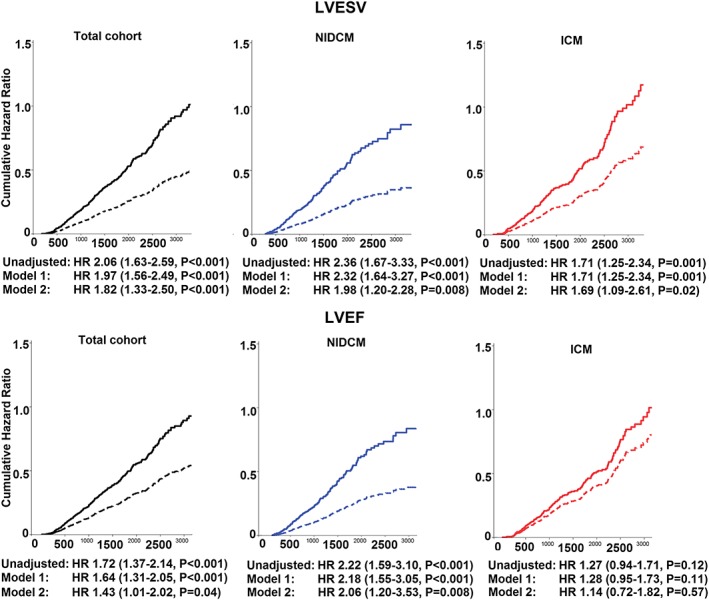
Left ventricular end systolic volume (LVESV) and left ventricular ejection fraction (LVEF) response in the total population, non‐ischaemic dilated cardiomyopathy, and ischaemic cardiomyopathy patients. Upper row, long‐term outcome according to LVESV response; and lower row, long‐term outcome according to LVEF response. Bold line = responders and dashed line = non‐responders. Model 1, age and sex; and model 2, age, sex, device type, estimated glomerular filtration rate, (history of) atrial fibrillation, LVESV at baseline, LVEF at baseline, N terminal pro brain natriuretic peptide, β‐blocker use, angiotensin converting enzyme inhibitors/angiotensin‐receptor blockers use, and diuretic use. HR, hazard ratio; ICM, ischaemic cardiomyopathy; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; NIDCM, non‐ischaemic dilated cardiomyopathy.
Amount of reverse remodelling
For NIDCM patients, every 1% reduction in LVESV was associated with a 1.3% relative reduction in the risk of all‐cause mortality, heart transplantation, or left ventricular assist device. For ICM patients the relative risk reduction was 0.9% per every 1% reduction in LVESV. Absolute risk reduction per 1% LVESV reverse remodelling was similar (0.4% in both ICM and NIDCM patients). EF improvement was not associated with endpoint free survival after multivariable adjustment in both groups.
Multivariable associated parameters to endpoint occurrence
For the entire population, LVESV reduction, NT‐proBNP, male sex, and diuretic use were associated with endpoint occurrence (Table 2, see Supporting Information for univariate variables). For NIDCM patients, associated variables were LVESV reduction, NT‐proBNP, and LBBB; and for ICM patients, LVESV reduction, NT‐proBNP, and diuretic use.
Table 2.
Multivariate Cox regression model
| Total population (N = 928) | NIDCM (N = 490) | ICM (N = 438) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P value | Variables | HR (95% CI) | P value | Variables | HR (95% CI) | P value |
| Δ LVESV, % | 0.989 (0.985–0.993) | <0.001 | Δ LVESV, % | 0.987 (0.981–0.993) | <0.001 | Δ LVESV, % | 0.991 (0.985–0.996) | 0.001 |
| NT‐proBNP, per 1000 pg/mL | 1.07 (1.05–1.08) | <0.001 | NT‐proBNP, per 1000 pg/mL | 1.09 (1.05–1.13) | <0.001 | NT‐proBNP, per 1000 pg/mL | 1.06 (1.04–1.08) | <0.001 |
| Male sex | 1.44 (1.02–2.05) | 0.04 | LBBBa | 0.50 (0.29–0.88) | 0.02 | Diuretic use | 2.66 (1.30–5.47) | 0.01 |
| Diuretic use | 2.42 (1.44–4.04) | 0.001 | ||||||
CI, confidence interval; HR, hazard ratio; ICM, ischaemic cardiomyopathy; LBBB, left bundle branch block; LVESV, left ventricular end systolic volume; NIDCM, non‐ischaemic dilated cardiomyopathy; NT‐proBNP, N terminal pro brain natriuretic peptide.
LBBB defined according to ESC guidelines.
Receiver operating characteristics curves and Youden index
To further evaluate the relationship between reverse remodelling and outcome ROC, curve analysis and Youden index determination was performed to determine what amount of LV reverse remodelling and LVEF improvement optimally predicted endpoint freedom (see Supporting Information for ROC curves). A cut‐off value of 16.6% reduction in LVESV yielded a sensitivity of 61% with a specificity of 62% in the total cohort [area under the curve (AUC) 0.63, 95% CI 0.59–0.67, and P < 0.001]. For NIDCM patients, optimal cut‐off was 17.1% reduction in LVESV (sensitivity 67%, specificity 62%; AUC 0.65, 95% CI 0.59–0.71, and P < 0.001); and for ICM patients, optimal cut‐off was 13.2% reduction in LVESV (sensitivity 56%, specificity 59%; AUC 0.59, 95% CI 0.54–0.65, and P = 0.001). An optimal cut‐off of LVEF improvement to predict endpoint free survival could not be found for ICM patients; for NIDCM patients, the optimal LVEF improvement cut‐off value was 4% (sensitivity 75%, specificity 48%; AUC 0.63, 95% CI 0.58–0.69, and P < 0.001).
Discussion
In this large retrospective real‐world CRT cohort, we assessed the association of heart failure aetiology on the magnitude of reverse remodelling and long‐term clinical outcome after CRT. We found that ICM patients, despite achieving lesser reverse ventricular remodelling, have a similar prognostic gain, in terms of survival time, compared with NIDCM patients for every single percentage of achieved reverse remodelling. Furthermore, the most accurate LVESV reverse remodelling ROC curve cut‐off to predict endpoint freedom was lower in ICM patients. LVEF improvement was not suited to predict endpoint freedom in ICM patients. Therefore, assessment of response and expected magnitude of reverse remodelling should be tailored according to underlying heart failure aetiology.
ICM patients have a diminished capacity of reverse remodelling.15, 16, 17, 18 This is often attributed to their higher baseline risk with more comorbidities, older age, and more often the presence of myocardial scarring not amenable by CRT.20 Consequently, ICM patients have a lower chance of echocardiographic response according to frequently used definitions.1 ICM patients also have a higher event rate and worse outcome during follow‐up.14, 15, 16 This too seems to be driven by their advanced age, increased baseline risk, and myocardial substrate, which seems intrinsically associated with a worse outcome.21 But it seems wrong to think that because ICM is associated with less reverse remodelling, and less reverse remodelling with a poor (er) outcome, the magnitude of survival time after CRT with respect to the magnitude of LV reverse remodelling is less in patients with ICM.
Every 1% of reduction in LVESV volume was associated with a 0.9% relative risk reduction in endpoint occurrence in ICM patients. Relative risk reduction was 1.3% for NIDCM patients. Absolute risk reduction was similar, 0.4% in both groups. A sub‐analysis from the Cardiac Resynchronization‐Heart Failure study described similar improvements in all‐cause mortality occurrence, NYHA class, and hospitalization rates in CRT patients with or without ischaemic heart disease.17 Notably, a CRT response cut‐off of ≥5% LVEF improvement was not associated with a better outcome in the ICM patients; no optimal cut‐off point of LVEF change could be found by ROC analysis, and LVEF was not an independent predictor of outcome in both groups.22
In current practice, a disconnect exists between CRT response definitions from large clinical trials, ‘real world' expectations, and achievements in daily practice.23 The effect range among patients receiving CRT is large, spanning from complete normalization of ventricular volume and LVEF to a lack of reverse remodelling. The desire to measure treatment effect using current strict binary definitions results in a large portion of ICM being classified as ‘non‐responder'.1 Furthermore, the natural course of remodelling might differ in various populations, which influences the interpretation of response. There might be patients in whom ‘non‐progression' is already a success for CRT and might improve clinical outcomes.24 In the end, the ultimate goal of CRT should be to meet the patients (and ‘physicians') individually tailored expectations for symptomatic improvement, amount of reverse remodelling, and gain in cardiac function and survival time. The Markers and Response to CRT study prospectively studied markers for response in patients with a guideline indication for CRT.25 A risk score, CAVIAR (CRT–Age–Vectorcardiograhic QRSAREA–Interventricular mechanical delay–Apical Rocking), that functions as a continuous response scale was constructed. Scores such as CAVIAR may help to personalize the notion of response for the individual patient and allow for expectations after implantation to be adjusted accordingly.25 They can be used to identify candidates for CRT, predict the amount of ventricular reverse remodelling that can be achieved, as well as validate the achieved amount of reverse remodelling. Taking into account mechanisms of disease in the individual patient (underlying electrophysiological, contractile, circulatory, and risk factors substrate) will place the patient‐specific extent of reverse remodelling than can be achieved with CRT in context. In the end, a lesser degree of reverse remodelling obtained in a patient with ICM, but meeting its individually predicted maximum amount could be perceived as successful response in an otherwise progressive and debilitating disease.
Strength and limitations
The Maastricht–Utrecht–Groningen cohort is a large group of real‐world CRT recipients with excellent outcome follow‐up. Nonetheless, the current study is inherently limited because of its retrospective design with a long inclusion time. Furthermore, we classified patients into an ischaemic or non‐ischaemic dilated aetiology based on recorded and verifiable history of myocardial infarction and coronary artery bypass graft without knowledge of the actual extent of myocardial scar/fibrosis. Furthermore, LVESV and LVEF assessments were performed after approximately 6 months, and reverse remodelling or increased contractility that may still occur after that period will be missed. Despite following ESC guidelines recommendations, timing and manner of (laboratory) data collection and follow‐up were not uniform across centres, and we cannot exclude that this had an effect on presented results. Also, knowledge and expertise of the individual centres will have increased during the inclusion period. Additionally, there is an inherent baseline risk difference in both groups that will affect the outcome and observed reverse remodelling. We adjusted for this to the best of our ability, but a degree of bias will always remain. The current study should be interpreted as hypothesis generating because the (pathophysiological) processes that underlie the different (outcome) response in ICM and NIDCM patients remain elusive. Future studies might focus on risk reduction effect while taking into account baseline risk and expected amount of reverse remodelling in the individual patient.
Conclusions
ICM patients achieve less reverse remodelling than NIDCM, but the prognostic gain in terms of survival time is the same for every single percentage of reverse remodelling that does occur. The assessment and expected magnitude of reverse remodelling should take this effect of heart failure aetiology into account.
Conflict of interest
AHM reports lecture fees from Medtronic and LivaNova. The other authors have nothing to disclose.
Funding
No specific sources of funding.
Supporting information
Figure S1. Amount of LVESV reverse remodeling.
Figure S2. ROC curves.
Table S1. Univariate outcome parameters.
Table S2. Baseline characteristics according to availability echocardiography data.
Kloosterman, M. , van Stipdonk, A. M. W. , ter Horst, I. , Rienstra, M. , Van Gelder, I. C. , Vos, M. A. , Prinzen, F. W. , Meine, M. , Vernooy, K. , and Maass, A. H. (2020) Association between heart failure aetiology and magnitude of echocardiographic remodelling and outcome of cardiac resynchronization therapy. ESC Heart Failure, 7: 645–653. 10.1002/ehf2.12624.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA) , Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15: 1070–1118. [DOI] [PubMed] [Google Scholar]
- 3. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, MIRACLE Study Group . Multicenter InSync randomized clinical evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 4. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) Investigators. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140–2150. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, MADIT‐CRT Trial Investigators . Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009; 361: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 7. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL, Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators . Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med 2010; 363: 2385–2395. [DOI] [PubMed] [Google Scholar]
- 8. Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ, MADIT‐CRT Investigators . Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation 2010; 122: 985–992. [DOI] [PubMed] [Google Scholar]
- 9. Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006; 113: 266–272. [DOI] [PubMed] [Google Scholar]
- 10. Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long‐term survival after cardiac resynchronization therapy. Circulation 2005; 112: 1580–1586. [DOI] [PubMed] [Google Scholar]
- 11. Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C, Schondube F, Wolfhard U, Bocker D, Krahnefeld O, Kirkels H. Pacing Therapies in Congestive Heart Failure (PATH‐CHF) Study Group. Long‐term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002; 39: 2026–2033. [DOI] [PubMed] [Google Scholar]
- 12. Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344: 873–880. [DOI] [PubMed] [Google Scholar]
- 13. Foley PW, Chalil S, Khadjooi K, Irwin N, Smith RE, Leyva F. Left ventricular reverse remodelling, long‐term clinical outcome, and mode of death after cardiac resynchronization therapy. Eur J Heart Fail 2011; 13: 43–51. [DOI] [PubMed] [Google Scholar]
- 14. Marsan NA, Bleeker GB, van Bommel RJ, Ypenburg C, Delgado V, Borleffs CJ, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Comparison of time course of response to cardiac resynchronization therapy in patients with ischemic versus nonischemic cardiomyopathy. Am J Cardiol 2009; 103: 690–694. [DOI] [PubMed] [Google Scholar]
- 15. McLeod CJ, Shen WK, Rea RF, Friedman PA, Hayes DL, Wokhlu A, Webster TL, Wiste HJ, Hodge DO, Bradley DJ, Hammill SC, Packer DL, Cha YM. Differential outcome of cardiac resynchronization therapy in ischemic cardiomyopathy and idiopathic dilated cardiomyopathy. Heart Rhythm 2011; 8: 377–382. [DOI] [PubMed] [Google Scholar]
- 16. Martens P, Nijst P, Verbrugge FH, Dupont M, Tang WHW, Mullens W. Profound differences in prognostic impact of left ventricular reverse remodeling after cardiac resynchronization therapy relate to heart failure etiology. Heart Rhythm 2018; 15: 130–136. [DOI] [PubMed] [Google Scholar]
- 17. Wikstrom G, Blomstrom‐Lundqvist C, Andren B, Lonnerholm S, Blomstrom P, Freemantle N, Remp T, Cleland JG, CARE‐HF Study Investigators . The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE‐HF trial. Eur Heart J 2009; 30: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barsheshet A, Goldenberg I, Moss AJ, Eldar M, Huang DT, McNitt S, Klein HU, Hall WJ, Brown MW, Goldberger JJ, Goldstein RE, Schuger C, Zareba W, Daubert JP. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT‐CRT. Eur Heart J 2011; 32: 1622–1630. [DOI] [PubMed] [Google Scholar]
- 19. van Stipdonk AMW, Ter Horst I, Kloosterman M, Engels EB, Rienstra M, Crijns HJGM, Vos MA, van Gelder IC, Prinzen FW, Meine M, Maass AH, Vernooy K. QRS area is a strong determinant of outcome in cardiac resynchronization therapy. Circ Arrhythm Electrophysiol 2018; 11: e006497. [DOI] [PubMed] [Google Scholar]
- 20. Rizzello V, Poldermans D, Boersma E, Biagini E, Schinkel AF, Krenning B, Elhendy A, Vourvouri EC, Sozzi FB, Maat A, Crea F, Roelandt JR, Bax JJ. Opposite patterns of left ventricular remodeling after coronary revascularization in patients with ischemic cardiomyopathy: role of myocardial viability. Circulation 2004; 110: 2383–2388. [DOI] [PubMed] [Google Scholar]
- 21. Wikstrom G, Blomstrom‐Lundqvist C, Andren B, Lonnerholm S, Blomstrom P, Freemantle N, Remp T, Cleland JG, CARE‐HF Study Investigators . The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE‐HF trial. Eur Heart J 2009; 30: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bax JJ, Schinkel AF, Boersma E, Elhendy A, Rizzello V, Maat A, Roelandt JR, van der Wall EE, Poldermans D. Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long‐term prognosis. Circulation 2004; 110: II18–II22. [DOI] [PubMed] [Google Scholar]
- 23. Linde C, Ellenbogen K, McAlister FA. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm 2012; 9: S3–S13. [DOI] [PubMed] [Google Scholar]
- 24. Steffel J, Ruschitzka F. Superresponse to cardiac resynchronization therapy. Circulation 2014; 130: 87–90. [DOI] [PubMed] [Google Scholar]
- 25. Maass AH, Vernooy K, Wijers SC, van 't Sant J, Cramer MJ, Meine M, Allaart CP, De Lange FJ, Prinzen FW, Gerritse B, Erdtsieck E, Scheerder COS, Hill MRS, Scholten M, Kloosterman M, Ter Horst IAH, Voors AA, Vos MA, Rienstra M, Van Gelder IC. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. Europace 2018; 20: e1–e10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Amount of LVESV reverse remodeling.
Figure S2. ROC curves.
Table S1. Univariate outcome parameters.
Table S2. Baseline characteristics according to availability echocardiography data.


