Abstract
Aims
Soluble tumour necrosis factor‐α receptor 1 (sTNF‐αR1) and interleukin‐2 receptor α (sIL‐2Rα) predict incident heart failure (HF) in the elderly population. However, the association of these biomarkers with HF in a multi‐ethnic asymptomatic population is unclear. We aimed to investigate the association of sTNF‐αR1 and sIL‐2Rα with incident HF in a multi‐ethnic population of middle age and older participants.
Methods and results
The multi‐ethnic study of atherosclerosis is a prospective population‐based study of 6814 participants aged 45–84 years who were free of clinical cardiovascular disease at enrolment. We included 2869 participants with available sTNF‐αR1 or sIL‐2Rα level measurement at baseline multi‐ethnic study of atherosclerosis exam (2000–2002). We used Cox proportional‐hazards model to investigate the association between sTNF‐αR1 and sIL‐2Rα with incident HF after adjusting for traditional cardiovascular risk factors and coronary artery calcium score measured by cardiac computed tomography. Among the included participants, the mean (standard deviation) age was 61.6 (10.2) years and 46.7% were men. The median (interquartile range) sTNF‐αR1 and sIL‐2Rα were 1293 (1107–1547) and 901 (727–1154) pg/mL. During a median follow‐up of 14.2 (interquartile range: 11.7–14.8) years, 130 participants developed HF. In multivariable analysis, the hazard ratio (95% confidence interval, P value) of incident HF for each standard deviation increment of log‐transformed sTNF‐αR1 and sIL‐2Rα was 1.43 (1.21–1.7, P ≤ 0.001) and 1.26 (1.04–1.53, P = 0.02), respectively. Excluding participants with interim coronary heart disease, we found a statistically significant association between sTNF‐αR1 and HF with hazard ratio of 1.39 (95% confidence interval: 1.11 to 1.74, P = 0.005) and sIL‐2Rα and HF showing a hazard ratio of 1.39 (95% confidence interval: 1.09 to 1.76, P = 0.007).
Conclusions
sTNF‐αR1 and sIL‐2Rα are associated with a higher risk of incident HF in a multi‐ethnic cohort without a previous history of cardiovascular disease.
Keywords: Inflammation, Heart failure, Tumour necrosis factor‐α, Interleukin‐2, Soluble cytokine receptors
1. Introduction
Physiologic activation of the immune system has a critical role in tissue repair after myocardial injury.1 However, chronic or excessive immune activation leads to pathological changes in cardiac function and structure.2, 3 Elevated circulating levels of pro‐inflammatory cytokines including tumour necrosis factor‐α (TNF‐α) and their soluble receptors are associated with incident heart failure (HF),4 the severity of HF,5 and adverse outcomes.6, 7 Soluble cytokine receptors have a longer half‐life than respective cytokines and consequently are more reliable indicators of present and past inflammatory responses.8 Nevertheless, only one epidemiologic study reported the association of soluble TNF‐α receptors (sTNF‐αRs) with incident HF in an older population free of HF and coronary heart disease (CHD).4
In addition to innate immunity, adaptive immunity is implicated in the pathogenesis of HF.9 One previous study showed that soluble interleukin‐2 receptor α (sIL‐2Rα), a marker of T cell activation, was associated with HF risk among population aged above 65 years.10 However, the association of sIL‐2Rα level and development of HF in the younger population are yet to be studied. Therefore, we assessed the association of sTNF‐αR1 and sIL‐2Rα with incident HF independent of subclinical and clinical coronary artery disease (CAD) in a multi‐ethnic population of individuals without prior cardiovascular disease, consisting of middle age and older age participants.
2. Materials and methods
The design of the multi‐ethnic study of atherosclerosis (MESA) has been described in previous publications.11 Briefly, MESA is a prospective observational cohort study consisting of 6814 men and women aged 45–84 years who were recruited from six US field centres (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and St Paul, MN). Participants identified their ethnicity as White, African American, Chinese American, and Hispanics and were free of clinical cardiovascular disease at enrolment (Exam 1: between July 2000 and August 2002). All study protocols were reviewed and approved by institutional review boards of each field centre, and all participants signed written consent. In this study, we included participants with available sTNF‐αR1 or sIL‐2Rα measurement at the baseline exam (Exam 1) and with follow‐up data for HF. This subgroup of MESA participants was selected equally and randomly from four race/ethnic groups (n = 720 from each race/ethnic group) for another MESA ancillary study (candidate gene).12
Standard questionnaires were used to gather demographic information, medical history, medication use, highest educational level, and smoking status (current, former, or never smoker). Resting blood pressure was measured three times in a seated position, and the average of the last two was used for data analysis. Fasting blood samples were collected, and blood glucose, total, and high‐density lipoprotein (HDL) cholesterol were measured. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL or the use of any hypoglycaemic medications. sTNF‐αR1 and sIL‐2Rα were measured by ultrasensitive ELISA (R&D Systems, Minneapolis, MN, USA) with coefficients of variation of 5%13 and 4.6–7.2%14, respectively. Agatston's method was used to calculate the coronary artery calcium (CAC) score. The details of the acquisition and interpretation of cardiac computed tomography images have been reported previously.15, 16
Every 9–12 months, a telephone interviewer called each participant (or family member) to ask about any interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Two independent physicians reviewed all collected records for endpoint classification and assignment of incidence dates. CHD was defined as a combination of myocardial infarction, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), and CHD death. The diagnosis of HF was made only in participants with symptoms of HF such as peripheral oedema and shortness of breath. HF was considered as definite if one or more of the following criteria were present: (i) pulmonary oedema/congestion in chest X‐ray, (ii) dysfunctional or dilated left ventricle (LV) detected by a cardiac imaging method, or (iii) evidence of LV diastolic dysfunction. HF was classified as probable if the diagnosis was made by a physician and the participant was receiving medical treatment for HF. The combination of probable and definite HF was used as the endpoint of our study.
2.1. Statistical analysis
Baseline characteristics of participants with and without HF were presented as mean ± standard deviation (SD), median [interquartile range (IQR)], or frequency (%). Student's t‐test/Wilcoxon rank‐sum test and chi‐square test were used to compare the means/medians (for continuous variables) and frequency (for categorical variables) between participants with and without HF. Natural logarithm of CAC score [In (CAC +1)], sTNF‐αR1, and sIL‐2Rα were used as continuous variables. Cox proportional‐hazards regression was deployed to analyse the association between biomarkers and time to incident HF. The following four models were created for each biomarker separately: model 1: unadjusted; model 2: adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, total cholesterol, HDL cholesterol, use of lipid‐lowering medication, diabetes, cigarette smoking status, and highest education level; model 3: model 2 adjusted for baseline CACs; and model 4: model 2 after excluding participants with interim CHD. Restricted cubic spline curves with three knots showed a linear relationship between each biomarker and hazard ratio (HR) for incident HF. The HR (with a 95% confidence interval, CI) for incident HF was calculated per 1 SD increase in each log‐transformed biomarker level (pg/mL). Stata statistical software (version 14.1, College Station, Texas) was used to compute all statistical results. P values less than 0.05 were considered statistically significant.
3. Results
A total of 2869 participants who met our inclusion criteria were included in the study. The mean (SD) age was 61.6 (10.2) years, and 46.7% were men. Of those, 25.4% were Caucasian, 25% were Chinese, 24.9% were Hispanic, and 24.7% were African American. sTNF‐αR1 and sIL‐2Rα were measured in 2859 and 2849 of study participants, respectively. sTNF‐αR1 was too high to measure among three participants (>5544 pg/mL), and sIL‐2Rα was too low to measure among three participants (<78.1 pg/mL), which were recoded to the closest value. The median (IQR) biomarker levels were 1293 pg/mL (1107–1547 pg/mL) for sTNF‐αR1 and 901 pg/mL (727–1154 pg/mL) for sIL‐2Rα (Figures 1 and 2 ).
Figure 1.
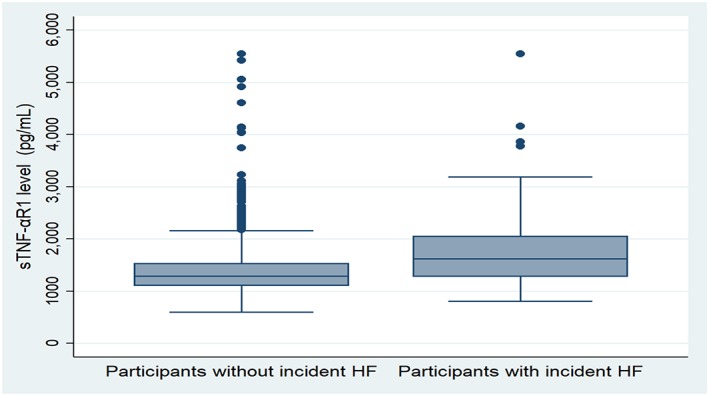
Boxplot showing the distribution of soluble tumour necrosis factor‐α receptor 1 (sTNF‐αR1). HF, heart failure.
Figure 2.
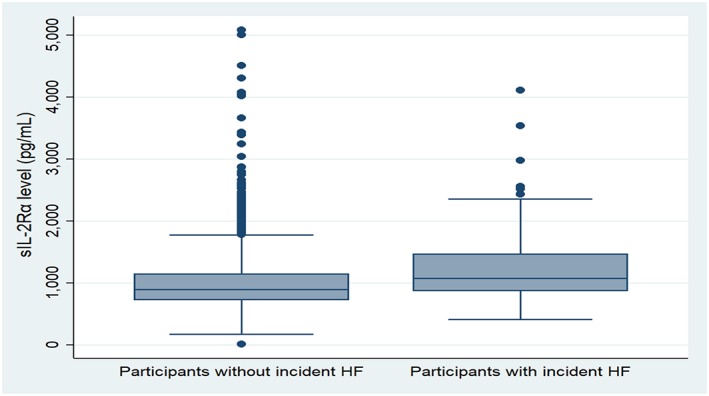
Boxplot showing the distribution of soluble interleukin‐2 receptor α (sIL‐2Rα). HF, heart failure.
Over 14.2 median (IQR: 11.7–14.8) years of follow‐up, 130 participants developed incident HF. Of those, 50 also developed interim CHD. The baseline characteristics of participants with and without HF are provided in Table 1. Participants who developed HF were more likely to be older men with a higher prevalence of the traditional cardiovascular risk factors compared with participants without HF. The median CAC score, sTNF‐αR1 (Figure 1 A), and sIL‐2Rα (Figure 1 B) were all higher in a participant with HF compared with participants without HF. Total baseline cholesterol was lower in participants with HF, and the use of lipid‐lowering medication was higher among participants with HF.
Table 1.
Baseline characteristics of participants with and without incident heart failure
| Heart failure | Yes | No | P value |
|---|---|---|---|
| Participants | 130 (4.5) | 2739 (95.5) | |
| Male | 84 (64.6) | 1255 (45.8) | <0.001 |
| Age (years) | 68.5 ± 8.6 | 61.2 ± 10.2 | <0.001 |
| Race | 0.09 | ||
| Caucasian | 33 (25.4) | 697 (25.5) | |
| African American | 37 (28.5) | 672 (24.5) | |
| Hispanic | 39 (30) | 675 (24.6) | |
| Chinese | 21 (16.2) | 695 (25.4) | |
| Education ≤12 years | 64 (49.6) | 1074 (39.3) | 0.02 |
| BMI (kg/m2) | 29.7 ± 6 | 27.8 ± 5.4 | <0.001 |
| Systolic blood pressure (mmHg) | 137 ± 22 | 125.5 ± 21.2 | <0.001 |
| Diastolic blood pressure (mmHg) | 73.3 ± 10.5 | 71.8 ± 10.1 | 0.11 |
| Blood pressure medication | 77(59.2) | 945(34.5) | <0.001 |
| Total cholesterol (mg/dL) | 185.9 ± 34.4 | 194.9 ± 35.4 | 0.005 |
| HDL cholesterol (mg/dL) | 47.1 ± 13.4 | 50.7 ± 14.4 | 0.005 |
| Lipid‐lowering medication | 31(24) | 414(15.2) | 0.007 |
| Smoking status | 0.08 | ||
| Never smoker | 58 (45) | 1495 (54.7) | |
| Former smoker | 52 (40.3) | 865 (31.7) | |
| Current smoker | 19 (14.7) | 373 (13.7) | |
| Diabetes mellitus | 48 (36.9) | 326 (11.9) | <0.001 |
| Coronary artery calcium score | 132 (0–471) | 0 (0–62) | <0.001 |
| sTNF‐αR1(pg/mL) | 1626 (1280–2055) | 1287 (1104–1531) | <0.001 |
| sIL‐2R (pg/mL) | 1076 (871–1466) | 896 (723–1145) | <0.001 |
BMI, body mass index; HDL, high‐density lipoprotein; sTNF‐αR1, soluble tumour necrosis α receptor; sIL‐2R, soluble interleukin‐2 receptor.
Figures are numbers (%), mean ± standard deviation, and median (interquartile range).
Table 2 shows the association between each biomarker and incident HF in unadjusted and adjusted models. sTNF‐αR1 and sIL‐2Rα levels were associated with incident HF independent of traditional cardiovascular risk factors and CACs. In a multivariable analysis for every 1 SD increase in baseline log‐transformed sTNF‐αR1 and log‐transformed sIL‐2Rα levels, the HRs of HF were 1.43 (95% CI: 1.21–1.7) and 1.26 (95% CI: 1.04–1.53), respectively. Excluding participants with interim CHD, there was a statistically significant association between sTNF‐αR1 and HF (HR: 1.39, 95% CI: 1.11–1.74) and sIL‐2Rα and HF (HR: 1.39, 95% CI: 1.09–1.76). There was no statistically significant interaction between gender (P = 0.64) or race/ethnicity (Caucasian‐Ref; African American: P = 0.74; Hispanic: P = 0.17; and Chinese: P = 0.76) with each level of log‐transformed sTNF‐αR1 and between gender (P = 0.70) or race/ethnicity (Caucasian‐Ref; African American: P = 0.12; Hispanic: P = 0.17; and Chinese: P = 0.67) with each level of log‐transformed sIL‐2Rα.
Table 2.
Showing the association between biomarker levels and incident heart failure
| Biomarkers | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| sTNF‐αR1 | 2.1 (1.84–2.39) | <0.001 | 1.44 (1.22–1.70) | <0.001 | 1.43 (1.21–1.70) | <0.001 | 1.39 (1.11–1.74) | 0.005 |
| sIL‐2Rα | 1.72 (1.48–2.01) | <0.001 | 1.30 (1.08–1.57) | 0.006 | 1.26 (1.04–1.53) | 0.02 | 1.39 (1.09–1.76) | 0.007 |
CI, confidence interval; sTNF‐αR1, soluble tumour necrosis α receptor; sIL‐2Rα, soluble interleukin‐2 receptor.
Model 1: unadjusted. Model 2: adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, total cholesterol, high‐density lipoprotein (HDL) cholesterol, use of lipid‐lowering medication, diabetes, cigarette smoking status, and highest education level. These two models were created for each biomarker separately. Model 3: model 2 adjusted for baseline coronary artery calcium score. Model 4: model 2 after excluding participants with interim coronary heart disease. Hazard ratios (HR) per 1 standard deviation increment of log‐transformed biomarker levels.
4. Discussion
In this study, we investigate the association of two key inflammatory biomarkers, sTNF‐αR1 (innate immunity) and sIL‐2Rα (adaptive immunity), with the development of symptomatic HF in a multi‐ethnic population. Our results demonstrate that sTNF‐αR1 and sIL‐2Rα levels are associated with future HF independent of traditional cardiovascular risk factors, subclinical coronary atherosclerosis, and clinical CHD.
Inflammation plays a significant role in the pathophysiology of HF.17 Levine et al. reported the presence of high TNF‐α levels in advanced HF.18 Since then, several studies have shown that elevated serum levels of pro‐inflammatory cytokines including TNF‐α and its soluble receptors are associated with severity5 and prognosis of HF.8 However, only a few population‐based studies investigated the independent association between TNF‐α or sTNF‐αRs levels with incident HF.4, 19, 20 Vasan et al.19 in a study of 732 Framingham study participants (mean age 78 years) demonstrated the association between increased spontaneous production of TNF‐α by peripheral blood mononuclear cell and future HF over a mean follow‐up of 5.2 years. Since the study was performed in elderly Whites, the results may not be extrapolated to the general population, including younger individuals of different ethnicities and no history of heart disease. Investigators from the Health, Aging, and Body Composition (Health ABC) study showed the association between baseline TNF‐α levels with incident HF among elderly African Americans and White individuals.20 In another study from Health ABC study, Marti et al.4 also reported a significant association between TNF‐α and sTNF‐αR1 levels with incident HF. These associations were independent of the ankle‐arm index (a measure of subclinical vascular disease) and incident coronary events. Similarly, we found that higher sTNF‐αR1 was associated with incident HF independent of subclinical CAD and clinical CHD. Studies on transgenic mice and other experimental studies have shown that the pathological level of TNF‐α has a deleterious effect on cardiac function through different mechanisms including negative ionotropic effect,21 β‐adrenergic receptor uncoupling,4 increasing nitric oxide production,22 and changing intracellular calcium haemostasis.23 Additionally, sustained or excessive expression of TNF‐α changes the cardiac structure by promoting cardiomyocytes apoptosis,24 myocardial hypertrophy,25 remodelling,26 and fibrosis.27
In contrast to sTNF‐αR1, previous studies have reported conflicting results regarding the association of sIL‐2R and HF.5, 10, 28 In a cross‐sectional study, Testa et al.5 showed that IL‐2 and sIL‐2R were not elevated in patients with HF secondary to CAD or hypertension. Additionally, another cross‐sectional study of 499 participants of the Health ABC study failed to show any significant association between sIL‐2R and clinical cardiovascular disease.28 On the other hand, in a more recent study, Durda et al.10 demonstrated that sIL‐2Rα was an independent predictor of incident HF but again in an older and primarily White population. Our results extend previous findings to a younger and diverse population. Our knowledge about the central role of IL‐2 in the inflammatory response has evolved over time.29 IL‐2 induces inflammation by promoting the activation and proliferation of effector T cells. On the other hand, IL‐2 also increases the function and survival of regulatory T cells.30 Regulatory T cells control the extent of the immune response31 and can consequently reduce myocardial remodelling and fibrosis.30 A dose‐dependent response has been proposed to explain the dual role of IL‐2 in the immune system activation.29
Our study has limitations. Firstly, there is a possibility of poor external validity as we included only MESA participants who were free of cardiovascular disease at enrolment, and thus, our results can only be directly translated to individuals without prior cardiovascular diseases. Secondly, we cannot exclude the possibility of subclinical LV dysfunction at the time of enrolment in MESA participants. However, for the entire MESA cohort at baseline, left ventricular ejection fraction measured by magnetic resonance imaging was normal. Thirdly, because ejection fraction was not available for all study participants at the time of HF diagnosis, we could not differentiate HF with reduced ejection fraction from HF with preserved ejection fraction. Lastly, sTNF‐αR1 and sIL‐2Rα were measured only one time, and therefore, we could not account for possible intra‐individual variability of these biomarkers.
5. Conclusions
sTNF‐αR1 and sIL‐2Rα are associated with the development of symptomatic HF independent of traditional cardiovascular risk factors and of CAC score in a multi‐ethnic cohort including middle age and older individuals. These associations remained statistically significant after excluding participants who died from cardiac causes and those with non‐fatal myocardial infarction.
Conflict of interest
Dr Joao A. C. Lima discloses grant support from Canon (formerly Toshiba) Medical Systems. The following individuals declare no disclosures or conflict of interest: Hooman Bakhshi, Vinithra Varadarajan, Bharath Ambale‐Venkatesh, Zahra Meyghani, Mohammad R. Ostovaneh, Mary Cushman, Peter Durda, Colin O. Wu, Russell P.Tracy, Mary Cushman, and David A. Bluemke.
Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005,N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences(NCATS).
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Bakhshi, H. , Varadarajan, V. , Ambale‐Venkatesh, B. , Meyghani, Z. , Ostovaneh, M. R. , Durda, P. , Wu, C. O. , Tracy, R. P. , Cushman, M. , Bluemke, D. A. , and Lima, J. A. C. (2020) Association of soluble interleukin‐2 receptor α and tumour necrosis factor receptor 1 with heart failure: The Multi‐Ethnic Study of Atherosclerosis. ESC Heart Failure, 7: 639–644. 10.1002/ehf2.12623.
References
- 1. Hartupee J, Mann DL. Positioning of inflammatory biomarkers in the heart failure landscape. J Cardiovasc Transl Res 2013; 6: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topkara VK, Evans S, Zhang W, Epelman S, Staloch L, Barger PM, Mann DL. Therapeutic targeting of innate immunity in the failing heart. J Mol Cell Cardiol 2011; 51: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 2015; 116: 1254–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marti CN, Khan H, Mann DL, Georgiopoulou VV, Bibbins‐Domingo K, Harris T, Koster A, Newman A, Kritchevsky SB, Kalogeropoulos AP, Butler J. Soluble tumor necrosis factor receptors and heart failure risk in older adults: Health, Aging, and Body Composition (Health ABC) Study. Circ Heart Fail 2014; 7: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, LeJemtel TH. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol 1996; 28: 964–971. [DOI] [PubMed] [Google Scholar]
- 6. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001; 103: 2055–2059. [DOI] [PubMed] [Google Scholar]
- 7. Herrmann R, Sandek A, von Haehling S, Doehner W, Schmidt HB, Anker SD, Rauchhaus M. Risk stratification in patients with chronic heart failure based on metabolic‐immunological, functional and haemodynamic parameters. Int J Cardiol 2012; 156: 62–68. [DOI] [PubMed] [Google Scholar]
- 8. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000; 102: 3060–3067. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez‐Trujillo L, Vazquez‐Garza E, Castillo EC, Garcia‐Rivas G, Torre‐Amione G. Role of adaptive immunity in the development and progression of heart failure: new evidence. Arch Med Res 2017; 48: 1–11. [DOI] [PubMed] [Google Scholar]
- 10. Durda P, Sabourin J, Lange EM, Nalls MA, Mychaleckyj JC, Jenny NS, Li J, Walston J, Harris TB, Psaty BM, Valdar W, Liu Y, Cushman M, Reiner AP, Tracy RP, Lange LA. Plasma levels of soluble interleukin‐2 receptor alpha: associations with clinical cardiovascular events and genome‐wide association scan. Arterioscler Thromb Vasc Biol 2015; 35: 2246–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 12. Weiner SD, Ahmed HN, Jin Z, Cushman M, Herrington DM, Nelson JC, Di Tullio MR, Homma S. Systemic inflammation and brachial artery endothelial function in the multi‐ethnic study of atherosclerosis (MESA). Heart 2014; 100: 862–866. [DOI] [PubMed] [Google Scholar]
- 13. Kawut SM, Barr RG, Johnson WC, Chahal H, Tandri H, Jain A, Bristow MR, Kizer JR, Bagiella E, Lima JA, Bluemke DA. Matrix metalloproteinase‐9 and plasminogen activator inhibitor‐1 are associated with right ventricular structure and function: the MESA‐RV study. Biomarkers 2010; 15: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al Rifai M, DeFilippis AP, McEvoy JW, Hall ME, Acien AN, Jones MR, Keith R, Magid HS, Rodriguez CJ, Barr GR, Benjamin EJ, Robertson RM, Bhatnagar A, Blaha MJ. The relationship between smoking intensity and subclinical cardiovascular injury: the multi‐ethnic study of atherosclerosis (MESA). Atherosclerosis 2017; 258: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of multi‐ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology 2005; 234: 35–43. [DOI] [PubMed] [Google Scholar]
- 16. Bakhshi H, Ambale‐Venkatesh B, Yang X, Ostovaneh MR, Wu CO, Budoff M, Bahrami H, Wong ND, Bluemke DA, Lima JAC. Progression of coronary artery calcium and incident heart failure: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc 2017; 6: 005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev 2010; 15: 331–341. [DOI] [PubMed] [Google Scholar]
- 18. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323: 236–241. [DOI] [PubMed] [Google Scholar]
- 19. Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 2003; 107: 1486–1491. [DOI] [PubMed] [Google Scholar]
- 20. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010; 55: 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller‐Werdan U, Engelmann H, Werdan K. Cardiodepression by tumor necrosis factor‐alpha. Eur Cytokine Netw 1998; 9: 689–691. [PubMed] [Google Scholar]
- 22. Funakoshi H, Kubota T, Machida Y, Kawamura N, Feldman AM, Tsutsui H, Shimokawa H, Takeshita A. Involvement of inducible nitric oxide synthase in cardiac dysfunction with tumor necrosis factor‐alpha. Am J Physiol Heart Circ Physiol 2002; 282: H2159–H2166. [DOI] [PubMed] [Google Scholar]
- 23. Berthonneche C, Sulpice T, Boucher F, Gouraud L, de Leiris J, O'Connor SE, Herbert JM, Janiak P. New insights into the pathological role of TNF‐alpha in early cardiac dysfunction and subsequent heart failure after infarction in rats. Am J Physiol Heart Circ Physiol 2004; 287: H340–H350. [DOI] [PubMed] [Google Scholar]
- 24. Chen ZW, Qian JY, Ma JY, Chang SF, Yun H, Jin H, Sun AJ, Zou YZ, Ge JB. TNF‐alpha‐induced cardiomyocyte apoptosis contributes to cardiac dysfunction after coronary microembolization in mini‐pigs. J Cell Mol Med 2014; 18: 1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor‐alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 2007; 115: 1398–1407. [DOI] [PubMed] [Google Scholar]
- 26. Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor‐alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res 2002; 53: 822–830. [DOI] [PubMed] [Google Scholar]
- 27. Wang JH, Su F, Wang S, Lu XC, Zhang SH, Chen D, Chen NN, Zhong JQ. CXCR6 deficiency attenuates pressure overload‐induced monocytes migration and cardiac fibrosis through downregulating TNF‐alpha‐dependent MMP9 pathway. Int J Clin Exp Pathol 2014; 7: 6514–6523. [PMC free article] [PubMed] [Google Scholar]
- 28. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (the Health, Aging and Body Composition [Health ABC] study). Am J Cardiol 2003; 92: 522–528. [DOI] [PubMed] [Google Scholar]
- 29. Abbas AK, Trotta E, Simeonov RS, Marson A, Bluestone JA. Revisiting IL‐2: biology and therapeutic prospects. Sci Immunol 2018; 3: eaat1482. [DOI] [PubMed] [Google Scholar]
- 30. Zeng Z, Yu K, Chen L, Li W, Xiao H, Huang Z. Interleukin‐2/anti‐interleukin‐2 immune complex attenuates cardiac remodeling after myocardial infarction through expansion of regulatory T cells. J Immunol Res 2016;8493767: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsumoto K, Ogawa M, Suzuki J, Hirata Y, Nagai R, Isobe M. Regulatory T lymphocytes attenuate myocardial infarction‐induced ventricular remodeling in mice. Int Heart J 2011; 52: 382–387. [DOI] [PubMed] [Google Scholar]


