Abstract
Aims
RBP4 is an adipokine with adverse effects on cardiovascular system. Increased circulating retinol‐binding protein 4 (RBP4) has been linked to chronic heart failure (CHF). However, whether elevated RBP4 is correlated with a poor prognosis in elderly patients with CHF remains unclear. The aim of this study was to evaluate the prognostic value of serum RBP4 in elderly patients with CHF.
Methods and results
We enrolled 934 consecutive elderly patients (aged 60 years and older) with CHF and 138 age‐matched and sex‐matched control subjects in a prospective cohort study and explored the association of serum RBP4 levels with the clinical outcomes using multivariate Cox regression analyses. Serum RBP4 levels were elevated in CHF patients when compared with controls (46.66 ± 12.38 μg/mL vs. 40.71 ± 7.2 μg/mL, P < 0.001). Patients with the highest RBP4 concentrations had higher N terminal pro brain natriuretic peptide (NT‐proBNP) levels but lower left ventricular eject fraction (LVEF) and estimated glomerular filtration rate (P < 0.001). Serum RBP4 levels were increased as the New York Heart Association functional class increased and LVEF decreased (P < 0.001) and were negatively correlated with LVEF (r = −0.154, P < 0.001) but positively correlated with NT‐proBNP levels (r = 0.074, P = 0.023). Multivariate Cox regression analysis suggested that log RBP4 was an independent predictor for major adverse cardiac event(s) [hazard ratio (HR) = 2.61, 95% confidence interval (CI) = 1.19–5.70], together with age, male, LVEF, log NT‐proBNP, and estimated glomerular filtration rate. Moreover, log RBP4 was also an independent predictor for cardiovascular mortality (HR = 2.24, 95% CI = 1.35–5.39) and CHF rehospitalization (HR = 2.54, 95% CI = 1.09–5.60) even after adjustment for the established adverse prognostic factors for CHF. The Kaplan–Meier survival curves showed that high concentration of RBP4 was a prognostic indicator of major adverse cardiac event(s) in patients with CHF.
Conclusions
Our findings demonstrate for the first time that elevated serum RBP4 is correlated with worse outcome in elderly patients with CHF.
Keywords: Chronic heart failure, Elderly, Etinol‐binding protein 4, Major adverse cardiac events
1. Introduction
Chronic heart failure (CHF) is a major cause of hospitalization in the elderly, leading to a high risk of mortality, disability, and rehospitalization.1 Due to poor prognosis and high costs of therapy, adequate risk assessments and optimized medical treatment are essential in elderly patients with CHF.2
Retinol‐binding protein 4 (RBP4) is an approximately 21‐kDa secreted protein that mediates the transport of vitamin A (retinol) in circulation.3 RBP4 has been well known as an important adipokine that contributes to insulin resistance and obesity.4, 5 Recent clinical studies have also linked higher circulating RBP4 to various cardiovascular diseases.6 Serum RBP4 levels were found to be increased in patients with advanced heart failure and correlated with insulin resistance.7 Another clinical study showed that plasma RBP4 was increased in patients with inflammatory cardiomyopathy.8 Our previous study demonstrated that RBP4 could promote cardiac hypertrophy via inducing proinflammatory responses in cardiomyocytes through Toll‐like receptor 4/myeloid differentiation primary response gene 88 pathway.9 However, whether elevated RBP4 is correlated with a poor prognosis in patients with CHF remains unclear. Therefore, we carried out a prospective cohort study to evaluate the prognostic value of serum RBP4 in elderly patients with CHF.
2. Methods
2.1. Study population
A total of 934 consecutive elderly patients (aged 60 years and older) with CHF admitted to the affiliated hospitals of Nanjing Medical University were recruited between 1 October 2010 and 31 July 2013. The control subjects were selected during the same period in the same hospital from the health examination centre. This study was performed in accordance with the principles outlined in the Declaration of Helsinki 10 and approved by the Ethics Committee of Nanjing Medical University. The diagnosis of CHF was on the basis of typical symptoms and signs and evidence of left ventricular diastolic and/or systolic dysfunction, according to the American College of Cardiology/American Heart Association guidelines.11 All patients had a history of CHF for at least 6 months and were in stable condition on medication for at least 1 month before blood sampling. All subjects included in this study had no history of significant concomitant diseases, including severe hepatic or renal diseases, bleeding disorders, previous thoracic irradiation therapy, autoimmune disease, and malignant diseases. All patients received standard medical treatment, and written informed consent was obtained from each participant.
2.2. Serum RBP4 measurements
Serum RBP4 levels were assayed in duplicate by using a sandwich enzyme‐linked immunosorbent assay kit (R&D, Minneapolis, MN, USA) according to the manufacturer's protocol. The intra‐assay and inter‐assay coefficients of variance were 2.32% and 2.95%, respectively. The analytic sensitivity of the assays was 0.021 ng/mL.
2.3. Clinical outcomes
The primary clinical outcome was major adverse cardiac event(s) (MACE), which was defined as cardiovascular death and rehospitalization due to worsening CHF. The secondary endpoints were the individual components of the primary outcome, including cardiovascular mortality and CHF rehospitalization. Endpoints were obtained by reviewing the hospital database and by contacting each patient individually.
2.4. Statistical analysis
Normality of distribution was assessed using the Kolmogorov–Smirnov test. RBP4 were normally distributed parameters and presented as the mean ± standard deviation. Skewed data were expressed as median and quartile ranges, and comparisons were analysed by the Mann–Whitney U test. Pearson χ2 test was used to compare qualitative variables represented as frequencies. The correlations between serum RBP4 levels and cardiac function variables were calculated using Spearman correlation coefficient. The association between baseline variables and MACE was evaluated using univariable and multivariable Cox proportional hazards analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The factors entered into the Cox regression model were age, sex, body mass index (BMI), ischaemic aetiology, hypertension, diabetes, smoking, New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), N terminal pro brain natriuretic peptide (NT‐proBNP), estimated glomerular filtration rate (eGFR), medical treatments, and RBP4. Kaplan–Meier analysis was conducted to compare the differences of survival rates between patients with high and low levels of RBP4 using the log‐rank test. All tests were two sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using PASW 18.0 (IBM SPSS, Inc., Chicago, USA).
3. Results
3.1. Baseline characteristics
The baseline characteristics of the participants are shown in Table 1. There were no statistically significant differences between CHF patients and control subjects with respect to age, proportion of male, BMI, rates of smoking, hypertension and diabetes, high‐density lipoprotein cholesterol, and low‐density lipoprotein cholesterol. However, CHF patients had lower levels of eGFR and higher levels of total cholesterol and triglyceride (P < 0.001). In addition, serum RBP4 levels were increased in CHF patients when compared with the control subjects (46.66 ± 12.38 μg/mL vs. 40.71 ± 7.2 μg/mL, P < 0.001). We further divided the elderly patients with CHF into four subgroups according to the quartile values of serum RBP4 (Table 2). We found that patients with the highest RBP4 concentrations had higher NT‐proBNP levels but lower LVEF and eGFR (P < 0.001), as well as lower rates of medical treatments, including diuretics, spironolactone, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and beta‐blockers (P < 0.05). The median length of follow‐up was 736 days (range 102 to 1591 days). During the follow‐up period, 207 patients died, and 360 patients were readmitted due to CHF. No patient was lost to follow‐up during the study.
Table 1.
Baseline characteristics of the control subjects and elderly patients with CHF
| Variables | Control (n = 138) | CHF (n = 934) | P value |
|---|---|---|---|
| Age (years) | 69 (64–76) | 68 (66–74) | 0.257 |
| Male, n (%) | 96 (69.6) | 611 (65.4) | 0.337 |
| BMI (kg/m2) | 24.51 (22.66–27.00) | 24.68 (22.49–26.67) | 0.886 |
| Smoking, n (%) | 54 (39.1) | 411 (44.0) | 0.281 |
| Hypertension, n (%) | 60 (43.5) | 392 (42.0) | 0.782 |
| Diabetes, n (%) | 50 (36.2) | 402 (43.0) | 0.131 |
| eGFR (mL/min/1.73 m2) | 104 (96–117) | 75 (64–82) | <0.001 |
| TC (mmol/L) | 4.01 (3.39–5.04) | 4.59 (3.90–5.20) | <0.001 |
| TG (mmol/L) | 1.25 (0.93–1.76) | 1.50 (1.07–2.04) | <0.001 |
| HDL‐C (mmol/L) | 1.04 (0.88–1.18) | 1.07 (0.91–1.24) | 0.057 |
| LDL‐C (mmol/L) | 2.63 (2.15–3.40) | 2.68 (2.26–3.28) | 0.595 |
| RBP4 (μg/mL) | 40.71 ± 7.28 | 46.66 ± 12.38 | <0.001 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; RBP4, retinol‐binding protein 4; TC, total cholesterol; TG, triglyceride.
RBP4 is presented as mean ± standard deviation. Other data are presented as median with interquartile range or number with percentage in parentheses.
Table 2.
Baseline characteristics of the patients according to quartile levels of serum RBP4
| Variables | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P value |
|---|---|---|---|---|---|
| Age (years) | 67 (66–73) | 68 (67–74) | 68 (66–76) | 69 (67–75) | 0.351 |
| Male, n (%) | 158 (67.8) | 150 (64.1) | 154 (66.1) | 149 (63.7) | 0.770 |
| BMI (kg/m2) | 24.61 (22.86–26.64) | 24.80 (22.47–26.71) | 24.67 (22.52–26.83) | 24.67 (22.19–26.54) | 0.742 |
| Smoking, n (%) | 106 (45.5) | 97 (41.5) | 107 (45.9) | 101 (43.2) | 0.743 |
| Hypertension, n (%) | 85 (36.5) | 106 (45.3) | 101 (43.3) | 100 (42.7) | 0.242 |
| Diabetes, n (%) | 95 (40.8) | 88 (37.6) | 103 (44.2) | 116 (49.6) | 0.057 |
| Ischaemic aetiology, n (%) | 103 (44.2) | 99 (42.3) | 105 (45.1) | 114 (48.7) | 0.562 |
| NYHA class | |||||
| I | 50 (21.5) | 49 (20.9) | 48 (20.6) | 37 (15.8) | 0.386 |
| II | 73 (31.3) | 69 (29.5) | 79 (33.9) | 65 (27.8) | 0.518 |
| III | 74 (31.8) | 75 (32.1) | 74 (31.8) | 87 (37.2) | 0.523 |
| IV | 38 (16.3) | 39 (16.7) | 31 (13.3) | 46 (19.7) | 0.330 |
| LVEF (%) | 44 (37–52) | 40 (34–53) | 40 (34–51) | 38 (34–46) | <0.001 |
| NT‐proBNP (pg/mL) | 1349.17 (935.12–1608.91) | 1507.83 (1265.88–1932.40) | 1682.12 (1336.70–3297.59) | 2634.96 (1571.93–4949.26) | <0.001 |
| eGFR (mL/min/1.73 m2) | 78 (66–88) | 73 (66–89) | 72 (56–78) | 68 (54–79) | <0.001 |
| TC (mmol/L) | 4.61 (3.97–5.11) | 4.59 (3.94–5.08) | 4.57 (3.89–5.15) | 4.60 (3.97–5.32) | 0.767 |
| TG (mmol/L) | 1.51 (1.13–2.02) | 1.50 (1.04–1.97) | 1.46 (1.06–2.23) | 1.52 (1.06–2.19) | 0.616 |
| HDL‐C (mmol/L) | 1.07 (0.92–1.20) | 1.08 (0.90–1.23) | 1.07 (0.92–1.27) | 1.07 (0.92–1.25) | 0.914 |
| LDL‐C (mmol/L) | 2.73 (2.31–3.32) | 2.63 (2.18–3.15) | 2.67 (2.29–3.28) | 2.71 (2.26–3.30) | 0.442 |
| Medical treatment | |||||
| Diuretics, n (%) | 227 (97.4) | 229 (97.9) | 222 (95.3) | 218 (93.2) | 0.038 |
| Spironolactone, n (%) | 182 (78.1) | 192 (82.1) | 197 (84.5) | 141 (60.3) | <0.001 |
| ACEI/ARB, n (%) | 211 (90.6) | 206 (88.0) | 206 (88.4) | 189 (80.8) | 0.011 |
| Beta‐blocker, n (%) | 128 (54.9) | 145 (62.0) | 134 (57.5) | 90 (38.5) | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor antagonist; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular eject fraction; NT‐proBNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; RBP4, retinol‐binding protein 4; TC, total cholesterol; TG, triglyceride.
Data are presented as median with interquartile range or number with percentage in parentheses.
3.2. Correlation between RBP4 and cardiac function
Serum RBP4 levels were increased as the NYHA class increased (Figure 1 A). Serum RBP4 levels were 43.16 ± 8.44, 43.96 ± 9.97, 48.73 ± 13.39, and 51.60 ± 15.51 μg/mL in patients with NYHA Classes I–IV, respectively (P < 0.001). Serum RBP4 levels were also increased as LVEF decreased, with 43.70 ± 9.15 μg/mL in HF with preserved ejection fraction (LVEF ≥50%), 45.32 ± 11.19 μg/mL in HF with mid‐range ejection fraction (LVEF 40–49%), and 48.73 ± 13.39 μg/mL in HF with reduced ejection fraction (LVEF <40%) (Figure 1 B). Moreover, serum RBP4 levels were negatively correlated with LVEF (r = −0.154, P < 0.001, Figure 1 C) and positively correlated with NT‐proBNP levels (r = 0.074, P = 0.023, Figure 1D).
Figure 1.
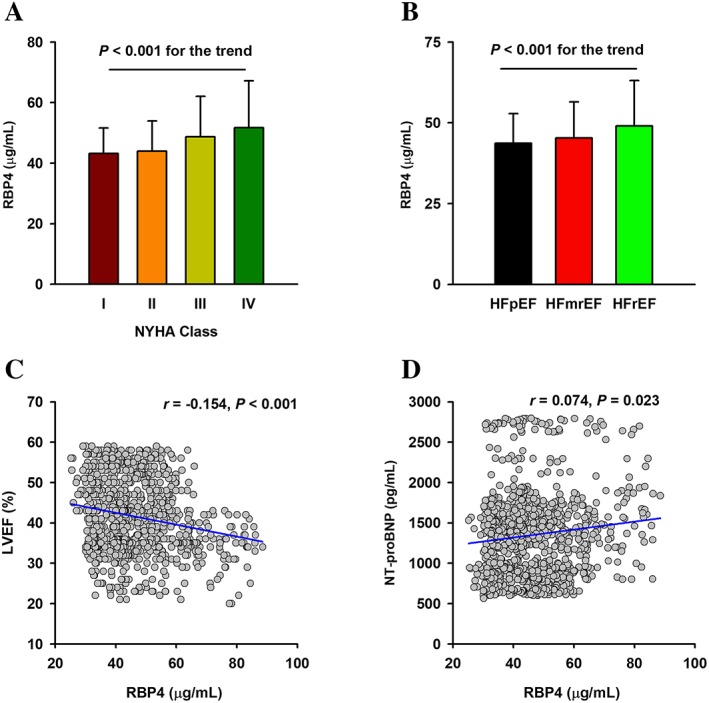
Association of RBP4 with the severity of cardiac dysfunction in elderly patients with CHF. (A) Changes of serum RBP4 in patients with different NYHA functional class. (B) Changes of serum RBP4 in patients with different LVEF subgroups. (C) Correlation of serum RBP4 levels with LVEF. (D) Correlation of serum RBP4 levels with NT‐proBNP. CHF, chronic heart failure; LVEF, left ventricular eject fraction; NYHA, New York Heart Association; NT‐proBNP, N terminal pro brain natriuretic peptide; RBP4, retinol‐binding protein 4; HFpEF, HF with preserved ejection fraction; HFmrEF, HF with mid‐range ejection fraction; HFrEF, HF with reduced ejection fraction.
3.3. Primary endpoint
Univariate Cox regression analyses showed that age, ischaemic aetiology, NYHA class, LVEF, log NT‐proBNP, eGFR, and log RBP4 were predictors for the primary endpoint of MACE (Table 3). In multivariable Cox regression analyses, log RBP4 was still associated with 1.6 times higher risk of MACE (HR = 2.61, 95% CI = 1.19–5.70), besides age (HR = 1.02, 95% CI = 1.01–1.04), male (HR = 1.24, 95% CI = 1.04–1.49), LVEF (HR = 1.06, 95% CI = 1.03–1.09), log NT‐proBNP (HR = 2.63, 95% CI = 1.47–4.69), and eGFR (HR = 1.01, 95% CI = 1.00–1.01) (Table 3). Similar results were obtained by using serum RBP4 as a ranked variable (4th quartile vs. 1st quartile: adjusted OR = 1.39, 95% CI = 1.25–1.55, P < 0.01) (Table 4).
Table 3.
Cox regression analyses for MACE
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 1.06 (1.05–1.07) | <0.001 | 1.02 (1.01–1.04) | 0.001 |
| Male | 1.10 (0.93–1.31) | 0.277 | 1.24 (1.04–1.49) | 0.017 |
| BMI | 1.00 (0.98–1.03) | 0.918 | 1.00 (0.98–1.03) | 0.989 |
| Ischaemic aetiology | 1.50 (1.27–1.77) | <0.001 | 0.96 (0.80–1.15) | 0.634 |
| Hypertension | 0.98 (0.83–1.16) | 0.801 | 1.04 (0.88–1.24) | 0.625 |
| Diabetes | 0.96 (0.81–1.13) | 0.585 | 0.88 (0.74–1.04) | 0.129 |
| Smoking | 1.09 (0.92–1.28) | 0.329 | 1.02 (0.86–1.21) | 0.790 |
| NYHA class | 1.86 (1.70–2.04) | <0.001 | 1.06 (0.91–1.22) | 0.454 |
| LVEF | 1.08 (1.05–1.11) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Log NT‐proBNP | 3.22 (1.83–7.54) | <0.001 | 2.63 (1.47–4.69) | 0.001 |
| eGFR | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.00–1.01) | 0.042 |
| Log RBP4 | 3.26 (1.92–7.27) | <0.001 | 2.61 (1.19–5.70) | 0.016 |
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular eject fraction; MACE, major adverse cardiac event(s); NT‐proBNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; RBP4, retinol‐binding protein 4.
Table 4.
Associations of serum RBP4 with clinical outcomes in CHF patients
| Model | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile |
|---|---|---|---|---|
| MACEs | ||||
| Crude model | 1 | 1.45 (1.10–1.90)* | 1.47 (1.29–1.68)* | 1.53 (1.40–1.66)* |
| Adjusted model | 1 | 1.14 (0.85–1.55) | 1.17 (0.99–1.38) | 1.39 (1.25–1.55)* |
| Cardiovascular mortality | ||||
| Crude model | 1 | 1.64 (1.41–1.91)* | 1.73 (1.36–2.19)* | 2.23 (1.38–3.60)* |
| Adjusted model | 1 | 1.19 (0.89–1.62) | 1.22 (0.91–1.63) | 1.33 (1.09–1.61)* |
| Rehospitalization | ||||
| Crude model | 1 | 1.25 (0.88–1.76) | 1.35 (1.15–1.60)* | 1.48 (1.34–1.64)* |
| Adjusted model | 1 | 1.01 (0.69–1.47) | 1.15 (0.95–1.41) | 1.39 (1.22–1.59)* |
CI, confidence interval; HR, hazard ratio; MACE, major adverse cardiac event(s); RBP4, retinol‐binding protein 4.
The adjusted model included age, sex, BMI, ischaemic aetiology, hypertension, diabetes, smoking, NYHA functional class, LVEF, NT‐proBNP, eGFR, and medical treatments.
We further divided the CHF patients into diabetes and non‐diabetes subgroups. Serum RBP4 levels were higher in patients with diabetes than those without diabetes (45.62 ± 11.70 μg/mL vs. 48.03 ± 13.13 μg/mL, P = 0.003). Multivariable Cox regression analyses indicated that LVEF (HR = 1.04, 95% CI = 1.02–1.08), log NT‐proBNP (HR = 3.40, 95% CI = 1.38–8.36), and log RBP4 (HR = 2.97, 95% CI = 1.09–5.71) were independent predictors for MACE in patients with diabetes, while age (HR = 1.02, 95% CI = 1.01–1.03), male (HR = 1.26, 95% CI = 1.04–1.51), LVEF (HR = 1.06, 95% CI = 1.02–1.09), log NT‐proBNP (HR = 2.69, 95% CI = 1.47–4.90), eGFR (HR = 1.01, 95% CI = 1.00–1.02), and log RBP4 (HR = 2.96, 95% CI = 1.31–6.67) were significant predictors for MACE in patients without diabetes (Supporting Information, Table S 1). In addition, there was also no significant difference of serum RBP4 between men and women (47.21 ± 12.77 μg/mL vs. 46.37 ± 12.18 μg/mL, P = 0.325). Multivariable Cox regression analyses indicated that LVEF, log NT‐proBNP, and log RBP4 were independent predictors for MACE both in male and female patients (Supporting Information, Table S 2).
3.4. Secondary endpoints
Multivariable Cox regression analysis showed that log RBP4 was an independent predictor for cardiovascular mortality (HR = 2.24, 95% CI = 1.35–5.39) after adjustment for age, sex, BMI, ischaemic aetiology, hypertension, smoking, NYHA functional class, LVEF, log NT‐proBNP, and eGFR. Moreover, our data indicated that log RBP4 was also an independent predictor for CHF rehospitalization (HR = 2.54, 95% CI = 1.09–5.60) after adjustment for the aforementioned risk factors (Table 5). Similar results were obtained by using serum RBP4 as a ranked variable (4th quartile vs. 1st quartile: adjusted OR = 1.33, 95% CI = 1.09–1.61, P < 0.01 for cardiovascular mortality; adjusted OR = 1.39, 95% CI = 1.22–1.59, P < 0.01 for rehospitalization) (Table 4).
Table 5.
Cox regression analyses for cardiovascular mortality and rehospitalization
| Cardiovascular mortality | Rehospitalization | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 1.04 (1.02–1.07) | <0.001 | 1.03 (1.01–1.05) | 0.002 |
| Male | 1.43 (1.05–1.94) | 0.024 | 1.38 (1.08–1.76) | 0.011 |
| BMI | 0.96 (0.92–1.01) | 0.074 | 1.00 (0.96–1.04) | 0.868 |
| Ischaemic aetiology | 2.86 (1.99–4.09) | <0.001 | 0.95 (0.74–1.21) | 0.634 |
| Hypertension | 0.98 (0.73–1.30) | 0.872 | 1.04 (0.83–1.31) | 0.731 |
| Smoking | 1.19 (0.90–1.58) | 0.227 | 0.99 (0.78–1.25) | 0.920 |
| NYHA class | 1.47 (1.15–1.89) | 0.003 | 0.97 (0.79–1.18) | 0.454 |
| LVEF | 0.98 (0.95–1.01) | 0.109 | 1.07 (1.05–1.09) | <0.001 |
| Log NT‐proBNP | 3.17 (1.21–8.31) | 0.019 | 2.63 (1.21–5.74) | 0.015 |
| eGFR | 1.01 (0.99–1.02) | 0.324 | 1.01 (1.00–1.02) | 0.046 |
| Log RBP4 | 2.24 (1.35–5.39) | 0.021 | 2.54 (1.09–5.60) | 0.035 |
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular eject fraction; MACE, major adverse cardiac event(s); NT‐proBNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; RBP4, retinol‐binding protein 4.
3.5. Kaplan–Meier survival analysis
ROC curve analysis showed that the optimal cut‐off value of RBP4 for the prediction of MACEs was 43.28 μg/mL, with a sensitivity of 65.8% and a specificity of 70.3% (area under the curve = 0.74, 95% CI = 0.71–0.77, P < 0.001). While for NT‐proBNP, the optimal cut‐off value was 1604.42 pg/mL, with a sensitivity of 67.7% and a specificity of 77.7% (area under the curve = 0.77, 95% CI = 0.74–0.80, P < 0.001). We further separated CHF patients into four groups according to the levels of NT‐proBNP (1604.42 pg/mL) and RBP4 (43.28 μg/mL). Kaplan–Meier survival analysis showed that high RBP4 was a potential valuable predictive factor of MACE both in patients with low and high NT‐proBNP levels (Figure 2 ). Patients with higher levels of RBP4 had a significantly higher incidence of MACE compared with those with lower levels of RBP4.
Figure 2.
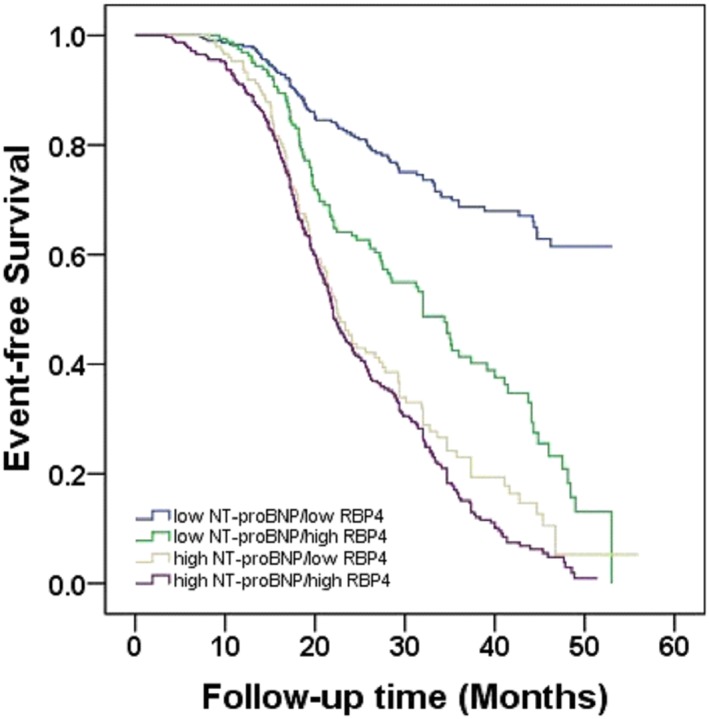
Kaplan–Meier survival analysis. The event‐free survival for MACE in elderly patients with CHF, stratified according to the cut‐off values of RBP4 and NT‐proBNP. CHF, chronic heart failure; MACE, major adverse cardiac event(s); NT‐proBNP, N terminal pro brain natriuretic peptide; RBP4, retinol‐binding protein 4.
4. Discussion
To our knowledge, this is the first prospective cohort study that investigated the association of RBP4 with the prognosis of heart failure. The main finding of our study is that increased serum RBP4, a well‐known adipokine with adverse effects on cardiovascular system, was correlated with the severity of cardiac dysfunction in elderly patients with CHF. Furthermore, elevated serum RBP4 levels were associated with worse outcome in elderly patients with CHF. Serum RBP4 appears to be a valuable additional prognostic marker for the risk stratification of elderly patients with CHF.
RBP4 is mainly secreted by liver and adipose tissue, and elevated circulating RBP4 levels have been linked to diabetes, obesity, and metabolic disorders.12 In the present study, we also found that serum RBP4 levels were increased in patients with diabetes. Recently, growing evidence has suggested that RBP4 is actively involved in cardiovascular diseases, especially in the elderly population.6 Plasma RBP4 concentrations were increased in older patients with hypertension and correlated with the number of antihypertensive drugs.13 Another study of individuals aged over 70 years old showed that circulating RBP4 concentrations were associated with intima‐media and plaque echogenicity in carotid arteries.14 Our previous study demonstrated that serum RBP4 was increased in patients with subclinical hypothyroidism and was associated with the presence and severity of coronary artery disease in patients with subclinical hypothyroidism.15 However, to date, data on the role of RBP4 in patients with CHF are scarce. We here showed that in the elderly patients with CHF, serum RBP4 levels were increased as the cardiac function decreased, which was quantified by the NYHA class and LVEF. Moreover, serum RBP4 levels were negatively correlated with LVEF and positively correlated with NT‐proBNP in patients with CHF. The results are consistent with a previous study showing that serum RBP4 levels were increased in patients with advanced heart failure and remarkably decreased in response to mechanical unloading and hemodynamic correction after the implantation of left ventricular assist device.7 However, another study of elderly subjects found no significant differences of plasma RBP4 levels between subjects with low levels (<125 ng/mL) and high levels (≥125 ng/mL) of NT‐proBNP, as well as subjects with and without a history of hospitalization for heart failure.16 Moreover, no relationship was observed between the levels of RBP4 and NT‐proBNP in their study.16 The discrepancies might partly attribute to the heterogeneity in study design. Our study population was restricted to elderly patients who were hospitalized for heart failure, while the study of Majerczyk et al.16 was a community‐based study composed of both CHF patients and subjects with normal cardiac function. Another potential confounder might be renal function since RBP4 is mainly excreted by the kidney.17, 18 Indeed, eGFR was lower in patients with high concentration of RBP4 in our study, indicating increased circulating RBP4 may serve as a biomarker for renal dysfunction in patients with CHF.
A series of adipokines have been reported as the predictors for unfavourable outcomes in patients with CHF.19 In the present cohort study, multivariate Cox regression analysis indicated that high level of RBP4 was an independent predictor for poor outcomes in the elderly patients with CHF even after adjustment for the well‐known adverse prognostic factors for CHF. Similar results were found in the study of patients with ischaemic stroke, showing that elevated serum levels of RBP4 were associated with the severity and poor prognosis of acute ischaemic stroke.20 However, another two studies of patients admitted in intensive care unit found that low serum RBP4 levels were related to increased mortality in patients with acute exacerbations of chronic obstructive pulmonary disease21 and underlying liver disease22, respectively. The decreased RBP4 levels might attribute to the malnutritional status in these critically ill patients, because circulating RBP4 concentration depends on vitamin A status.23 It was reported that severe calorie restriction could reduce the circulating and adipose tissue messenger ribonucleic acid expression of RBP4.24 Although sex hormones might affect the expression of RBP4 and its relationship with cardiovascular disease,25 no significant difference of serum RBP4 levels was observed between male and female patients in our study. Moreover, our subgroup analysis found that log RBP4, age, LVEF, and log NT‐proBNP were predictors of MACE both in male and female patients with CHF. Nevertheless, addition of RBP4 to the traditional risk factors may lead to an improvement in risk stratification for patients with CHF.
Previous experimental studies have revealed the pro‐hypertrophic function of RBP4 in heart. Kraus et al.26 found that angiotensin‐II induced cardiac hypertrophy was reduced in RBP4 knockout mice. Moreover, recombinant RBP4 stimulation doubled the angiotensin‐II induced hypertrophic response in cardiomyocytes.26 Consistently, our previous study showed that RBP4 enhanced protein synthesis, increased the cell size, and elevated expression of hypertrophic markers in cardiomyocytes.9 Interestingly, RBP4 also directly impairs glucose transporter‐4 expression and insulin‐stimulated glucose uptake in cardiomyocytes. There have been mounting evidence showing that insulin resistance and cardiac hypertrophy are not only associated but also they actually form a vicious cycle that leads to high mortalities in patients with CHF and diabetes.27 Therefore, we speculated that RBP4 may be a key mediator that promotes the vicious cycle of insulin resistance and cardiac hypertrophy, which in turn causes the poor outcomes in patients with CHF. The mechanism underlying the effects of RBP4 in cardiac hypertrophy was related to RBP4‐induced inflammation mediated by the activation of Toll‐like receptor 4/myeloid differentiation primary response gene 88 pathway.9 However, additional in‐depth investigation is required to elucidate the precise mechanism governing the pathological effect of RBP4 on the progression of CHF.
4.1. Study limitations
Firstly, as the present study was performed only in Chinese Han population, our findings need to be confirmed in other regions and ethnicities. Secondly, the patients in our study were all CHF patients but not acute heart failure patients. The prognostic role of RBP4 in patients with acute heart failure needs to be further delineated. Thirdly, we only measured baseline RBP4 levels and did not dynamically monitor the concentration of RBP4 during the follow‐up period. Fourthly, further studies on animals and patients are needed to elucidate the exact role of RBP4 in the treatment of CHF. Finally, the role of residual confounding could not be entirely ruled out in observational studies.
5. Conclusions
In summary, our findings demonstrate for the first time that elevated serum RBP4 is correlated with worse outcome in elderly patients with CHF. Addition of RBP4 to the traditional risk factors may lead to an improvement in risk stratification for the elderly patients with CHF. However, long‐term prospective cohort studies are still needed to confirm the prognostic value of RBP4 in heart failure.
Conflict of interest
None declared.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81470501 and 81770440 to Xiang Lu and no. 81700331 to Wei Gao), a grant from the Natural Science Foundation of Jiangsu Province (no. BK20171051 to Wei Gao), the Jiangsu Province Health Development Project with Science and Education (no. QNRC201685 to Wei Gao), a grant from the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (no. 17KJB320003 to Wei Gao), a grant from the Six One Project of Jiangsu Province (no. LGY2018100 to Wei Gao), and a grant from the Six Talent Peaks Project of Jiangsu Province (no. WSN‐175 to Wei Gao).
Supporting information
Table S1. Cox regression analyses for MACE in patients with and without diabetes.
Table S2. Cox regression analyses for MACE in male and female patients.
Li, X.‐Z. , Zhang, K.‐Z. , Yan, J.‐J. , Wang, L. , Wang, Y. , Shen, X.‐Y. , Sun, H.‐X. , Liu, L. , Zhao, C. , He, H.‐W. , Wang, L.‐S. , Gao, W. , and Lu, X. (2020) Serum retinol‐binding protein 4 as a predictor of cardiovascular events in elderly patients with chronic heart failure. ESC Heart Failure, 7: 542–550. 10.1002/ehf2.12591.
Contributor Information
Wei Gao, Email: gaowei84@njmu.edu.cn.
Xiang Lu, Email: luxiang66@njmu.edu.cn.
References
- 1. Dharmarajan K, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin 2017; 13: 417–426. [DOI] [PubMed] [Google Scholar]
- 2. Alghamdi F, Chan M. Management of heart failure in the elderly. Curr Opin Cardiol 2017; 32: 217–223. [DOI] [PubMed] [Google Scholar]
- 3. Blaner WS. Retinol‐binding protein: the serum transport protein for vitamin A. Endocr Rev 1989; 10: 308–316. [DOI] [PubMed] [Google Scholar]
- 4. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005; 436: 356–362. [DOI] [PubMed] [Google Scholar]
- 5. Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol‐binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006; 354: 2552–2563. [DOI] [PubMed] [Google Scholar]
- 6. Zabetian‐Targhi F, Mahmoudi MJ, Rezaei N, Mahmoudi M. Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv Nutr 2015; 6: 748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chavarria N, Kato TS, Khan R, Chokshi A, Collado E, Akashi H, Takayama H, Naka Y, Farr M, Mancini D, Schulze PC. Increased levels of retinol binding protein 4 in patients with advanced heart failure correct after hemodynamic improvement through ventricular assist device placement. Circ J 2012; 76: 2148–2152. [DOI] [PubMed] [Google Scholar]
- 8. Bobbert P, Weithauser A, Andres J, Bobbert T, Kuhl U, Schultheiss HP, Rauch U, Skurk C. Increased plasma retinol binding protein 4 levels in patients with inflammatory cardiomyopathy. Eur J Heart Fail 2009; 11: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 9. Gao W, Wang H, Zhang L, Cao Y, Bao JZ, Liu ZX, Wang LS, Yang Q, Lu X. Retinol‐binding protein 4 induces cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway. Endocrinology 2016; 157: 2282–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rickham PP. Human experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 12. Blaner WS. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther 2019; 197: 153–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Majerczyk M, Choreza P, Bozentowicz‐Wikarek M, Brzozowska A, Arabzada H, Owczarek A, Mossakowska M, Grodzicki T, Zdrojewski T, Wiecek A, Olszanecka‐Glinianowicz M, Chudek J. Increased plasma RBP4 concentration in older hypertensives is related to the decreased kidney function and the number of antihypertensive drugs‐results from the PolSenior substudy. J Am Soc Hypertens 2017; 11: 71–80. [DOI] [PubMed] [Google Scholar]
- 14. Ingelsson E, Lind L. Circulating retinol‐binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care 2009; 32: 733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun HX, Ji HH, Chen XL, Wang L, Wang Y, Shen XY, Lu X, Gao W, Wang LS. Serum retinol‐binding protein 4 is associated with the presence and severity of coronary artery disease in patients with subclinical hypothyroidism. Aging 2019; 11: 4510–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majerczyk M, Choreza P, Mizia‐Stec K, Bozentowicz‐Wikarek M, Brzozowska A, Arabzada H, Owczarek AJ, Szybalska A, Grodzicki T, Wiecek A, Olszanecka‐Glinianowicz M, Chudek J. Plasma level of retinol‐binding protein 4, N terminal proBNP and renal function in older patients hospitalized for heart failure. Cardiorenal med 2018; 8: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Domingos MAM, Queiroz M, Lotufo PA, Bensenor IJ, Titan SMO. Serum RBP4 and CKD: association with insulin resistance and lipids. J Diabetes Complications 2017; 31: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 18. Norden AG, Lapsley M, Unwin RJ. Urine retinol‐binding protein 4: a functional biomarker of the proximal renal tubule. Adv Clin Chem 2014; 63: 85–122. [DOI] [PubMed] [Google Scholar]
- 19. Shibata R, Ouchi N, Ohashi K, Murohara T. The role of adipokines in cardiovascular disease. J Cardiol 2017; 70: 329–334. [DOI] [PubMed] [Google Scholar]
- 20. Zhu YY, Zhang JL, Liu L, Han Y, Ge X, Zhao S. Evaluation of serum retinol‐binding protein‐4 levels as a biomarker of poor short‐term prognosis in ischemic stroke. Biosci Rep 2018; 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin Q, Chen Y, Lou Y, He X. Low serum retinol‐binding protein‐4 levels in acute exacerbations of chronic obstructive pulmonary disease at intensive care unit admission is a predictor of mortality in elderly patients. J Inflamm (Lond) 2013; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen WT, Lee MS, Chang CL, Chiu CT, Chang ML. Retinol‐binding protein‐4 expression marks the short‐term mortality of critically ill patients with underlying liver disease: lipid, but not glucose, matters. Sci Rep 2017; 7: 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol‐binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972; 247: 2542–2550. [PubMed] [Google Scholar]
- 24. Vitkova M, Klimcakova E, Kovacikova M, Valle C, Moro C, Polak J, Hanacek J, Capel F, Viguerie N, Richterova B, Bajzova M, Hejnova J, Stich V, Langin D. Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol‐binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet‐induced changes in insulin sensitivity. J Clin Endocrinol Metab 2007; 92: 2330–2335. [DOI] [PubMed] [Google Scholar]
- 25. Wang H, Zhou P, Zou D, Liu Y, Lu X, Liu Z. The role of retinol‐binding protein 4 and its relationship with sex hormones in coronary artery disease. Biochem Biophys Res Commun 2018; 506: 204–210. [DOI] [PubMed] [Google Scholar]
- 26. Kraus BJ, Sartoretto JL, Polak P, Hosooka T, Shiroto T, Eskurza I, Lee SA, Jiang H, Michel T, Kahn BB. Novel role for retinol‐binding protein 4 in the regulation of blood pressure. FASEB J: Offic Pub Fed Am Soc Exp Biol 2015; 29: 3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin 2012; 8: 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox regression analyses for MACE in patients with and without diabetes.
Table S2. Cox regression analyses for MACE in male and female patients.


