Abstract
Currently, the assessment of left ventricular ejection fraction (LVEF) is the cornerstone of the classification of patients with heart failure (HF). The mid‐range LVEF (HFmrEF) category was identified in an attempt to uncover specific characteristics of these patients. So far, the analysis of trials, registries, and observational studies have demonstrated that patients with mid‐range LVEF belong to a patient cohort with generally intermediate clinical profile as compared with other groups but with a remarkable variety of intrinsic phenotypes. This is due to the limitations of LVEF as the sole criterion to categorize patients with HF and characterize their prognosis, above all when it is >40%. To better define the HFmrEF phenotype, it is reasonable to consider other parameters, such as LVEF changes over time, HF aetiology, co‐morbidities, and other imaging parameters. A multiparametric evaluation may contextualize a patient with HFmrEF in a more defined phenotype with a specific prognosis.
Keywords: Heart failure with mid‐range ejection fraction, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, Classification
1. Highlights
New guidelines recommend distinguishing HFmrEF from HFpEF and HFrEF.
Current categorization of patients with heart failure is primarily based on a measure of left ventricular ejection fraction, which has important limitations.
HFmrEF is in general an intermediate clinical profile between HFpEF and HFrEF but is characterized by a number of distinct phenotypes.
2. Introduction
The 2016 European Society of Cardiology (ESC) guidelines have introduced a new classification of heart failure (HF) based on the left ventricular ejection fraction (LVEF) in combination with signs and symptoms of HF, elevated levels of natriuretic peptides, and signs of structural heart disease or diastolic dysfunction. The new guidelines classify patients with HF in three categories: heart failure with preserved ejection fraction (HFpEF), defined by an LVEF ≥50%, heart failure with reduced ejection fraction (HFrEF) if the LVEF is <40%, and heart failure with mid‐range ejection fraction (HFmrEF) if the LVEF is 40–49%.1 The 2013 ACC/AHA Heart Failure Guidelines recognized HFpEF and HFrEF categories and identified the group with mid‐range LVEF as borderline HFpEF (in the range of 40–49%) or as HFrEF‐improved for patients with prior reduced LVEF.2 Recently, Australian and New Zealand guidelines have not recommended the recognition of the HFmrEF category because of the lack of a clearly defined syndrome for this group of patients, without specific recommendations in clinical management. They classify the patients as HFrEF and HFpEF with 50% as cut‐off value between the two categories.3 Thus, there is currently an ongoing debate about the ‘grey area’ of HF with mid‐range LVEF.
3. Left ventricular ejection fraction based classification: Opportunities and limitations
The new classification by ESC was made in a recognition of the lack of knowledge about the patient cohort with LVEF 40–49%, given their exclusion from most of the trials and lack of evidence‐based treatment. Trials, prospective, and retrospective observational studies, which included HFmrEF patients and their outcomes, are summarized respectively in Tables 1, 2, and 3. As acknowledged in the guidelines, the move for this new classification should be seen as an attempt to stimulate research and resolve pending critical questions, rather than an admittance to true phenotypical differences between HFmrEF and other groups. The aim of this review is to identify a clearer profile of the HFmrEF population and to discuss the effective role of the LVEF in the diagnostic and prognostic work up of patients with HF.
Table 1.
Overview of main clinical trials investigating HF patients with mid‐range LVEF
| Study | Year of pubblication | Enrolment/follow up | Geography | Inclusion criteria | Patient number | Prevalence of HFmrEF | Outcomes for HFmrEF |
|---|---|---|---|---|---|---|---|
| Rickenbacher P. et al.4 | 2017 | 2004–2005; median follow up 794 days | Europe | Symptomatic patients, CHF hospitalization within the last year, and elevated NT‐BNP | 824 | 17% |
Mortality 39.7% (no significant differences) All‐cause hospitalization free survival: P = 0.08 Survival: P = 0.92 HF hospitalization free survival: P = 0.29 |
| Toma M. et al.5 | 2014 | May 2007–August 2010 | America, Europe, Asia, and New Zealand | Inclusion criteria of ASCEND‐HF trial with LVEF recorded | 5687 | 11.9% |
180 day mortality HFrEF vs. HFpEF: HR 0.96 (0.75–1.24); P = 0.77 180 day mortality HFmrEF vs. HFpEF: HR 0.91 (0.66–1.3); P = 0.58 Outcomes (HFrEF, HFmrEF, HFpEF; P value) Length of stay (days): 6 (4–10), 7 (4–10), 7 (5–11); 0.007 30 day all‐cause rehospitalization: 11.7, 13.6, 18.1; <0.001 |
| Solomon S.D. et al.6 | 2005 | March 1999–March 2001; follow up: 38 months | Europe, USA, Canada, South Africa, and Australia | Patients enrolled in CHARM programme | 7599 | 17% (LVEF 42–52%) |
In patients with LVEF 42–52%: All‐cause mortality: 5.2% CV death: 4% Non‐CV death: 1.2% Fatal or non‐fatal MI (1st episode): 1.7% CHF hospitalization: 5.7% Fatal or non‐fatal stroke: 1.3% CV death or CHF hospitalization: 7.9% |
| Solomon S.D. et al.7 | 2016 | August 2006–January 2012; follow up through June 2013 | Americas (USA, Canada, Brazil, Argentina) and Europe (Russia and Georgia) | Patients with HF and LVEF ≥ 45% enrolled in TOPCAT | 3444 |
CV death, aborted cardiac arrest, or hospitalization HF (per 100 patient‐years): EF < 50%: HR 7.2 (6.0–8.7), 50% ≤ EF < 55%: HR 6.0 (5.0–7.0), 55% ≤ EF < 60%: HR 5.5 (4.7–6.4), EF ≥ 60% HR 6.7 (5.9–7.5); P = 0.02 HF hospitalization (per 100 patient‐years): EF < 50%: HR 3.8 (2.9–5.0), 50% ≤ EF < 55%: HR 4.1 (3.3–5.0), 55% ≤ EF < 60%: HR 3.7 (3.0, 4.5), EF ≥ 60% HR 4.9 (4.2–5.6) ; P = 0.79 CV death (per 100 patient‐years): EF < 50%: HR 4.1 (3.2–5.2), 50% ≤ EF < 55%: HR 2.8 (2.2–3.6), 55% ≤ EF < 60%: HR 2.7 (2.2–3.3), EF ≥ 60% HR 2.7 (2.2–3.2); P = 0.002 Death (per 100 patient‐years): EF < 50%: HR 5.6 (4.5–6.8), 50% ≤ EF < 55%: HR 4.0 (3.3–4.8), 55% ≤ EF < 60%: HR 4.3 (3.6–5.0), EF ≥ 60% HR 4.3 (3.7, 4.9); P = 0.004 |
|
| Lund L.H. et al.8 | 2018 | March 1999–March 2001; follow up: 2.9 years | Europe, USA, Canada, South Africa, and Australia | Patients enrolled in the CHARM programme | 7598 | 17% |
Outcomes (HFmrEF vs. HFpEF; HFrEF vs. HFpEF) regardless of any treatment CV death + HF hospitalization: HR 1.00 (0.85–1.17) P = 0.98; HR 1.58 (1.40–1.79) P < 0.001 HF hospitalization: HR 0.94 (0.78–1.13) P = 0.55; HR 1.42 (1.23–1.64) P < 0.001 Recurrent HF hospitalization: HR 1.21 (0.98–1.49) P = 0.07; HR 1.96 (1.65–2.23) P < 0.001 CV death: HR 1.21 (0.98–1.51) P = 0.08; HR 2.20 (1.85–2.61) P < 0.001 All‐cause hospitalization: HR 0.89 (0.81–0.98) P = 0.02; HR 0.99 (0.91–1.08) P = 0.85 All‐cause death: HR 0.98 (0.82–1.19) P = 0.88; HR 1.73 (1.49–2.00) P < 0.001 |
CHF, chronic heart failure; CV, cardiovascular; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LVEF, left ventricular ejection fraction; OR, odds ratio.
Table 2.
Overview of main prospective observational studies investigating HF patients with mid‐range LVEF
| Study | Year of pubblication | Enrolment/follow up | Geography | Inclusion criteria | Patient number | Prevalence of HFmrEF | Outcomes for HFmrEF |
|---|---|---|---|---|---|---|---|
| Chioncel O. et al.9 | 2017 | April 2011–January 2015 | Europe | Enrolment in ESC‐HF‐LT Registry | 9134 (outpatients) + 6926 (hospitalized) | 24.20% | Outcomes (HFmrEF, HFrEF, HFpEF) |
| Mortality at 1 year: 7.6%, 8.8%, 6.3% (P = 0.005) | |||||||
| All‐cause hospitalization: 22%, 31.9%, 23.5% (P < 0.001) | |||||||
| HF hospitalization: 8.7%, 14.6%, 9.7% (P < 0.001) | |||||||
| All‐cause deaths or HF hospitalization: 15.0%, 21.2%, 14.6% (P < 0.001) | |||||||
| Koh A.S. et al.10 | 2017 | 2000‐2013 | Sweden | Clinician‐judged HF | 42 061 | 21% | Outcomes (HFmrEF vs. HFpEF, HFrEF vs. HFpEF) |
| 30 day mortality overall cohort: HR 1.06 (0.86–1.30) P = 0.573,HR 1.35 (1.14–1.60) P < 0.001 | |||||||
| 30 day mortality with CAD: HR 1.01 (0.75–1.36) P = 0.945, HR 1.47 (1.16–1.87) P = 0.002 | |||||||
| 30 day mortality without CAD: HR 1.14 (0.86–1.87) P = 0.356, HR 1.21 (0.94–1.55) P = 0.131 | |||||||
| 1 year mortality overall cohort: HR 1.08 (1.00–1.18) P = 0.052, HR 1.26 (1.17–1.35) P < 0.001 | |||||||
| 1 year mortality with CAD: HR 1.14 (1.02–1.28) P = 0.026, HR 1.39 (1.26–1.53) P < 0.001 | |||||||
| 1 year mortality without CAD: HR 1.05 (0.94–1.18) P = 0.395, HR 1.12 (1.01–1.24) P = 0.034 | |||||||
| 3 year mortality overall cohort: HR 1.06 (1.00–1.12) P = 0.066, HR 1.20 (1.14–1.26) P < 0.001 | |||||||
| 3 year mortality with CAD: HR 1.11 (1.02–1.21) P = 0.011, HR 1.34 (1.25–1.44) p < 0.001 | |||||||
| 3 year mortality without CAD: HR 1.02 (0.94–1.12) P = 0.592, HR 1.05 (0.97–1.13) P = 0.225 | |||||||
| Rastogi et al.11 | 2017 | March 2010–August 2013 | USA | Inpatients and outpatients with HF | 168 | 16% | Death |
| HFmrEF improved vs. HFrEF: HR 0.30 (0.11–0.76) P = 0.23 | |||||||
| HFmrEF deteriorated vs. HFpEF: HR 1.11 (0.15–7.96) | |||||||
| Cardiac hospitalization | |||||||
| HFmrEF improved vs. HFrEF: HR 0.21 (0.10–0.45) P = 0.016 | |||||||
| HFmrEF deteriorated vs. HFpEF: HR 1.08 (0.34–3.37) | |||||||
| Death/transplant/any hospitalization | |||||||
| HFmrEF improved vs. HFrEF: HR 0.40 (0.25–0.64) P = 0.011 | |||||||
| HFmrEF deteriorated vs. HFpEF: HR 1.64 (0.62–4.35) | |||||||
| Cheng R.K. et al.12 | 2014 | 1 January 2005–30 December 2011; follow up: end of 2012 | USA | Age ≥ 65 years hospitalized with a diagnosis of HF | 40 239 | 14% | Outcomes (HFmrEF vs. HFpEF; HFrEF vs. HFpEF) |
| Mortality: HR 0.967 (0.917–1.020) P = 0.223, HR 1.040 (0.998–1.084) P = 0.065 | |||||||
| All‐cause readmission: HR 1.032 (0.991–1.074) P = 0.126; HR 0.961 (0.930–0.993) P = 0.016 | |||||||
| CV readmission: HR 1.148 (1.092–1.208) P < 0.001; HR 1.179 (1.132–1.228) p < 0.001 | |||||||
| HF readmission: HR 1.215 (1.142–1.291) | |||||||
| P < 0.001 HR 1.348 (1.284–1.416) b.001 | |||||||
| Composite readmission/mortality: HR 1.022 (0.985–1.061) P = 0.247; HR 0.988 (0.958–1.018) 0.420 | |||||||
| He K.L. et al.13 | 2009 | September 2005–February 2008 | China | Inpatients or outpatients seen at the People's Liberation Army General Hospital with and without HF | 564 | 14% (LVEF 40–55%) |
In patients with LVEF 40–55%: LVEDD 55 ± 7, FS 24 ± 4 (P < 0.005 vs. HFrEF and HFpEF) LVESD 42 ± 6, IVSd12 ± 2, PWTd 11 ± 2 (P < 0.005 vs. HFrEF) LVEDV 148 ± 38, LVEDVI 82 ± 20, LVESV 81 ± 24, LVESVI 45 ± 13, SVI 38 ± 8, LVEDV/mass ratio 0.57 ± 0.14 (P < 0.005 vs. HFrEF and HFpEF) SV 67 ± 16 (P < 0.005 vs. HFrEF) LVM 264 ± 74, LVM/BSA 145 ± 36 (P < 0.005 vs. HFpEF) E 75 ± 28, A 82 ± 22, E/A 1.07 ± 0.7, DT 217 ± 65, E′ 7 ± 2 (P < 0.005 vs. HFrEF) S′ 8 ± 2 (P < 0.005 vs. HFrEF and HFpEF) |
| Sweitzer et al.14 | 2008 | 1 January 2004 | America | Patients hospitalized for acute decompensated HF and enrolled in ADHERE database | 74 863 | 23% (LVEF 40–55%) | Outcomes in HF with LVEF 40–55%: |
| In‐hospital mortality: 3.2% | |||||||
| ICU/CCU admission: 19.0% (significantly different from LVEF >55% at P = 0.017 level) | |||||||
| ICU/CCU length of stay (days): 2.6 | |||||||
| Total hospital length of stay (days): 4.7 | |||||||
| Increase in creatinine 0.5 mg/dL during hospitalization: 14.9% | |||||||
| Nadruz et al.15 | 2016 | July 2007–June 2013; median follow up: 4.4 years (through 31 December 2014) | USA | Patients with HF and CPET | 944 | 29% (LVEF 40–55%) |
Outcomes (improved‐LVEF 40–55% vs. HFrEF; stable LVEF 40–55% vs. HFrEF) Deatha: HR 0.32 (0.17–0.61) P = 0.001; HR 0.74 (0.45–1.21) P = 0.23 Deathb: HR 0.42 (0.21–0.82) P = 0.011; HR 0.87 (0.51–1.46) P = 0.59 Left ventricular assistant device implantation, heart transplantation, or all‐cause mortalitya: HR 0.19 (0.10–0.36) P < 0.001; HR 0.25 (0.13–0.47) P < 0.001 Left ventricular assistant device implantation, heart transplantation, or all‐cause mortalityb: HR 0.60 (0.38–0.95) P = 0.029; HR 0.79 (0.49–1.28) P = 0.34 |
| Löfman I. et al.16 | 2017 | 11 May 2000–3 October 2013 | Sweden HF | Clinician‐judged HF | 40 230 | 21% |
In HFmrEF with eGFR ≥ 60: 1 year mortality: 7.8%; 5 year mortality: 32.0%; death/100 patient‐years: 2.23 In HFmrEF with eGFR < 60: 1 year mortality: 22.4%; 5 year mortality: 63.1%; death/100 patients years: 4.79 Association CKD‐mortality: HFpEF: HR 1.32 (1.24–1.42); HFmrEF: HR 1.51 (1.40–1.63); HFrEF: HR 1.49 (1.42–1.56) |
| Kapoor J.R. et al.17 | 2016 | January 2005–September 2013 | USA | HF admission in the Get With The Guidelines‐HF (GWTG‐HF) database | 99 825 | 13% |
In‐hospital death: HFmrEF 2.62%, HFrEF 3.21%, HFpEF 3.02% (P = 0.0020) Length of stay >4 days: HFmrEF 46.61%, HFrEF 45.24%, HFpEF 48.74% (P < 0.0001) |
| Factors associated with length of stay >4 days in HFmrEF: | |||||||
| Pneumonia/respiratory process: OR 1.31(1.18–1.45) P < 0.0001 | |||||||
| Dyspnoea: OR 1.31 (1.18–1.45) P < 0.0001 | |||||||
| Dietary non‐compliance: OR 0.72 (0.58–0.88) P = 0.0015 | |||||||
| Medication non‐compliance: OR 0.86 (0.75–0.99) P = 0.032 | |||||||
| Factors associated with in‐hospital death in HFmrEF: | |||||||
| Worsening renal failure: OR 1.53 (1.02–2.28) P = 0.039 | |||||||
| Pneumonia/respiratory process: OR 1.48 (1.05–2.10) P = 0.025 | |||||||
| Medication non‐compliance: OR 0.52 (0.27–0.98) P = 0.043 | |||||||
| Tsuji et al.18 | 2017 | October 2006–March 2010; follow up: 1–3 years | Japan | Patients enrolled in CHART‐2 Study with previous history of symptomatic HF and echocardiographic available data at the registration | 3480 | 17% |
HFmrEF: intermediate incidences of all‐cause death, CV death, and HF admission (all P values for trend <0.001); non‐significant differences for non‐CV death, acute MI, or stroke 1 year transition HFmrEF → HFpEF: 44% and HFmrEF → HFrEF: 16% 3 year transition HFmrEF → HFpEF: 45% and HFmrEF → HFrEF: 21% 1 year transition HFrEF → HFmrEF: 22% 3 year transition HFrEF → HFmrEF: 21% 1 year transition HFpEF → HFmrEF: 8% 3 year transition HFrEF → HFmrEF: 8% |
|
All‐cause death according to the transitions after registration to 1 year (vs. HFpEF → HFpEF): HFmrEF → HFpEF: HR 0.81 (0.57–1.16) P = 0.259 HFmrEF → HFmrEF: HR 1.07 (0.74–1.54) P = 0.717 HFmrEF → HFrEF: HR 1.60 (1.05–2.44) P = 0.026 HFpEF → HFmrEF: HR 1.32 (0.96–1.81) P = 0.078 HFrEF → HFmrEF: HR 1.17 (0.73–1.89) P = 0.502 | |||||||
| Lupòn et al.19 | 2017 | August 2001–December 2015; mean follow up: 5.6 ± 3.1 years | Europe | All consecutive ambulatory patients referred to HF unit with echocardiography assessment at the baseline and at 1 year of follow up | 1057 |
(HF recovered = baseline LVEF <45% → LVEF ≥45% at 1 year follow up; HFpEF = stable LVEF ≥ 45% throughout the follow up; HFrEF = stable LVEF <45% throughout the follow up) Outcomes (HFpEF vs. HF recovered, HFrEF vs. HF recovered) CV death + HF hospitalization: HR 1.83 (1.27–2.65) P = 0.001, HR 1.74 (1.31–2.32) P < 0.001 All‐cause death: HR 1.77 (1.20–2.61) P = 0.004, HR 1.54 (1.16–2.04) P = 0.003 CV death: HR 2.17 (1.23–3.82) P = 0.007, HR 2.23 (1.44–3.43) P < 0.001 HF death: HR 2.74 (1.34–5.58) P = 0.006, HR 2.94 (1.68–5.14) P < 0.001 Sudden death: HR 1.14 (0.26–5.05) P = 0.87, HR 2.16 (0.83–5.61) P = 0.11 At 1 year: HFrEF → HFmrEF: 22.%, HFrEF → HFpEF: 13.9%, HFrEF → HFrEF: 63.5% Outcomes (HFrEF → HFmrEF vs. HFrEF → HFpEF, HFrEF → HFrEF vs. HFrEF → HFpEF) CV death + HF hospitalization: HR 1.09 (0.69–1.72) P = 0.700, HR 2.19 (1.50–3.22) P < 0.001 All‐cause death: HR 1.40 (0.89–2.20) P = 0.151, HR 2.23 (1.50–3.31) P < 0.001 CV death: HR 1.43 (0.71–2.89) P = 0.310, HR 3.34 (1.83–6.12) P < 0.001 HF death: HR 1.80 (0.72–4.50) P = 0.210, HR 4.93 (2.20–11.05) P < 0.001 Sudden death: HR 2.17 (0.45–10.36) P = 0.330, HR 4.61 (1.12–18.96) P = 0.034 |
BSA, body surface area; CAD, coronary artery disease; CHF, chronic heart failure; CKD, chronic Kidney disease; CPET, cardiopulmonary exercise testing; CV, cardiovascular; CV, cardiovascular; DT, deceleration time; E/A ratio, ratio between mitral early and late wave velocity; E, Mitral early wave velocity; E′, Tissue Doppler E = ‐wave velocity; eGFR, estimated glomerular filtration rate; FS, fractional shortening; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ICU/CCU, intensive care unit/coronary care unit; IVSd, interventricular septal diameter; LVEDD, left ventricular end diastolic diameter; LVEDV, left ventricular end diastolic volume; LVEDVI, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic diameter; LVESV, left ventricular end systolic volume; LVESVI, left ventricular end systolic volume index; LVM, left ventricular mass; MI, myocardial infarction; OR, odds ratio; PWTd, posterior wall thickness diameter; S′, Tissue Doppler S = ‐wave velocity.
Adjusted for age, sex, glomerular filtration rate, coronary artery disease, post‐chemotherapy, diabetes mellitus, race and haemoglobin with death as outcome and for age, sex, glomerular filtration rate, coronary artery disease and post‐chemotherapy with composite endpoint as outcome.
Further adjusted for use of pacemaker, diuretics, statins and angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers with death as outcome and for use of diuretics, aldosterone antagonists, anticoagulation and angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers with composite endpoint as outcome.
Table 3.
Overview of main retrospective observational studies investigating HF patients with mid‐range LVEF
| Study | Year of pubblication | Type of study | Enrolment/follow up | Geography | Inclusion criteria | Patient number | Prevalence of HFmrEF | Outcomes for HFmrEF |
|---|---|---|---|---|---|---|---|---|
| Coles A.H. et al.20 | 2014 | Retrospective review of study population | Years of 1995, 2000, 2002, and 2004 | USA | Patients hospitalized for acute decompensated HF | 3604 | 14% |
Mortality at 1 year after discharge (HFmrEF vs. HFpEF; HFrEF vs. HFpEF) HR 1.37 (1.14–1.65); HR 1.17 (1.07–1.28) Mortality at 2 years after discharge (HFmrEF vs. HFpEF; HFrEF vs. HFpEF) HR 1.36 (1.20–1.55); HR 1.17 (1.09–1.24) Mortality at 5 years after discharge (HFmrEF vs. HFpEF; HFrEF vs. HFpEF) HR 1.04 (0.98–1.10); HR 1.02 (0.99–1.05) |
| Coles A.H. et al.21 | 2015 | Retrospective review of study population | Years of 1995, 2000, 2002, 2004, and 2006 | USA | Patients hospitalized for acute decompensated HF | 4025 | 13% |
Deaths rates at 1 year after discharge: 34% (HFrEF), 30% (HFmrEF), and 29% (HFpEF) (P = 0.03) Factors significantly associated with 1 year mortality after discharge in HFmrEF and according to age: COPD in >75 years: HR 1.63 (1.07–2.47) Serum sodium >135 mEq/L in <75 years: HR 0.22 (0.10–0.51) Systolic blood pressure (150–159 mmHg) in >75 years: HR 0.47 (0.24–0.91) |
COPD, chronic obstructive pulmonary disease; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio.
Left ventricle ejection fraction remains of fundamental importance for the classification of the patients with HF. The main reason for its pivotal role is that it was used as a main inclusion criterion in trials that identified effective drugs and devices for the treatment of the patients with HF. This is, however, true only for the patients with HFrEF, whereas no treatment has been shown to be effective for the patients with HFpEF, yet.22 Thus, an LVEF‐based classification of the HF population has some notable limitations, and this may be true above all when we have to consider patients with a normal LVEF. First of all, in many instances, the classification of patients as HFmrEF is simply the result of the variability in the measure of LVEF in patient cohorts with different degrees of impairment of myocardial function.23 The large degree of ‘mobility’ between the three LVEF categories is due to the heterogeneous aetiology of patients with HFmrEF with several possible LVEF trajectories [e.g. ischaemic aetiology after revascularization, idiopathic dilated cardiomyopathy with different response to drugs or cardiac resynchronization therapy (CRT), myocarditis, HFpEF with progressive decline of LVEF]. Atrial fibrillation (AF) is an additional factor contributing to the mobility across the LVEF categories. AF can worsen pre‐existing LV dysfunction or underlying cardiomyopathy, with a partial or complete improvement of the LVEF after the restoration of sinus rhythm.24, 25, 26, 27 A substantial number of patients may move in and out of HFmrEF group on serial echocardiograms, without any change of underlying pathology (Figure 1 ).11, 28
Figure 1.
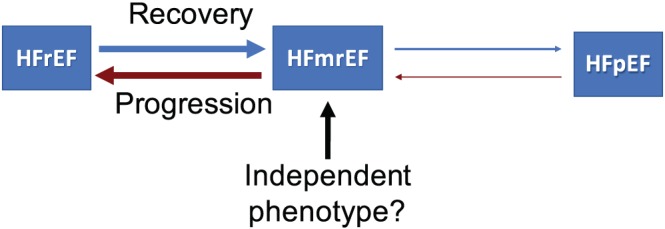
Heart failure phenotypes based on left ventricle ejection fraction. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Although a patient's LVEF underlies a dynamic without a change in underlying pathology, the associated risk can change significantly. Indeed, freedom from death/transplant/cardiac hospitalization in patients with HFmrEF who have recovered from HFrEF is higher than those with HFmrEF who remained stable.11, 29, 30 The same applies to patients with HFmrEF with prior preserved LVEF.15, 29 So, beyond the baseline LVEF assessment, the prognostic implications of longitudinal LVEF changes are becoming increasingly clear, and specific factors have been identified as predictors of increasing or decreasing LVEF.31 Therefore, the attempt to classify patients in LVEF categories does not appropriately account for the spectrum of diverse phenotypes within the LVEF categories.31
Because LVEF is not a static measurement, HFmrEF is certainly a heterogenous condition with variable evolutions rather than a stable phenotype. Only slightly more than one‐third of the HFmrEF population remains in the same category during the long‐term follow up.19, 31 Conversely, the remaining patients reclassify into HFpEF (25–33%) and HFrEF categories (25–37%). LVEF measurements are subject to a wide intra‐variability and inter‐variability and vary between modalities. Further, several variables, such as preload, afterload, contractility, valvular diseases, systolic blood pressure, and heart rate, can temporarily influence the value of LVEF.28 However, evaluation of cardiac function is complex and goes beyond the LVEF and contractility. For example, diastolic dysfunction is more severe in patients with HFmrEF and prior HFpEF than in those with prior HFrEF, indicating its greater value as a determinant of the clinical course of these patients.11 LVEF alone is also not an accurate marker of remodelling. Left ventricular volumes, mass, stroke volume, and their changes in time are more accurate index of maladaptive remodelling and correlate better with the prognosis and response to the therapy.28, 32 Thus, the current use of LVEF for HF classification to some extent impairs our ability to discern true myocardial recovery from myocardial remission in which there are signs of reverse remodelling but without a complete reversal of damage33.
Beyond the LVEF, delayed times of contraction and dyssynchrony are important independent signs of LV dysfunction.28, 34, 35 The assessment of myocardial deformation, such as the strain and strain rate, through the tissue Doppler or global longitudinal strain (GLS)28 or on gated single‐photon emission computed tomography myocardial perfusion imaging could be alternative methods to classify HF patients.34, 35 The GLS is measured on the basis of the LV longitudinal shortening in systole, and it is an index of systolic function.36 It may become abnormal at an earlier stage before a decrease in LVEF.37, 38 GLS has an independent prognostic value in the mid‐range and preserved LVEF group, because of the loss of prognostic value of LVEF in this HF population.39 In particular, GLS is a stronger predictor of all‐cause mortality and outcomes (cardiac death, HF hospitalization, and malignant arrhythmias) than LVEF,40 and the additional predictive value over LVEF for mortality is more pronounced for LVEF >35%.39 GLS allowed a better risk prediction independent of and incremental to the LVEF also in patients with acute myocardial infarction, including those with HFmrEF.41 In addition, peri‐infarct strain is the only independent predictor of malignant cardiac arrhythmias, and GLS could identify patients at risk of ventricular arrhythmias despite LVEF is >35%.39 Even in the setting of acute HF, for a given LVEF, there is a wide distribution of LV dyssynchrony, and LV dyssynchrony appears to have a greater prognostic role than LVEF.42
The current LVEF‐based HF classification does not take into account the underlying HF aetiology and its influence on prognosis.32, 43 Cardiac magnetic resonance (CMR) imaging and late gadolinium enhancement add notable diagnostic and prognostic information in patients with HF, especially in the diagnostic workup of LV hypertrophy. The CMR allows an accurate study of myocardial extracellular volume (ECV) and the detection of fibrosis, interstitial oedema, or deposition of proteins, lipids, or iron. It is a superior imaging method for the identification of the right underlying aetiology of HF.36 The correlation between the ECV and the echocardiographic parameters, such as LVEF, LV volumes, peak ejection, and peak filling rate, is clearer for the HFpEF group but has not been demonstrated for patients with reduced EF.36 Moreover, the ECV expansion is significantly linked with higher rates of mortality and worsening of HF.36 Finally, the epicardial fat detected by CMR is reduced in the HFrEF and increased in the HFmrEF and HFpEF populations compared with healthy controls, suggesting a possible role of fat‐associated inflammation in the mechanisms of HF in the upper value of the LVEF spectrum.44 Given that LVEF is an imaging‐based parameter, it is unable to fully account for the disease trajectory, including predisposition to malignant arrhythmias and the risk of sudden cardiac death.32 Nowadays, it is clear that a better stratification of the arrhythmic risk requires genetic testing, especially in dilated cardiomyopathy. Genetic mutations involved in the cardiomyopathies interfere with the encoding of proteins necessary for the contraction of myocytes or ion channel function in the myocardiac tissue.45 In particular, LMNA and DSP mutations (implicated respectively in the encoding of laminin and desmoplakin) are associated with higher risk of sudden cardiac death.45 These findings suggest that the outcomes correlate more specifically with primary arrhythmia mechanisms than with the degree of left ventricular dysfunction.45 Thus, a complete evaluation of heart chamber sizes, geometry, and function with genetic, clinical, and biomarker data is more appropriate than the measurement of LVEF alone46 (Figure 2 ). Given the limitations of LVEF, the recognition of phenotypes across the LVEF spectrum should consider the disease mechanisms and/or the bio‐profiling of the patients.47 In addition, co‐morbidities, such as diabetes and kidney dysfunction, may have a major role and influence outcomes more than the LVEF itself48.
Figure 2.
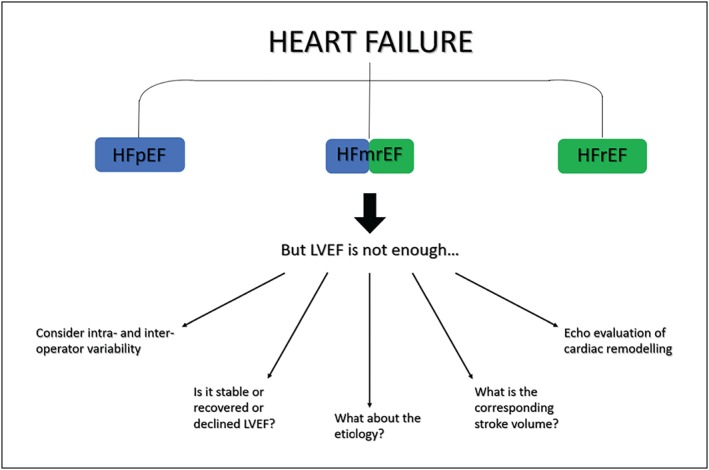
Evaluation of HFmrEF beyond LVEF. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricle ejection fraction.
4. Demographic and clinical characteristics
Real‐world registries and clinical trial data suggest a HFmrEF prevalence of 14–24% among the overall HF population.4, 9, 10, 19, 31 Up until recently, HFmrEF was commonly considered a clinical condition more similar to HFpEF than HFrEF, with higher rates of female patients and more common history of hypertension and atrial fibrillation/flutter.12, 20, 21 With an increased attention to the HFmrEF group, the clinical profile of these patients is becoming clearer. Currently, it seems like the three groups show a continuous relationship of most clinical characteristics. Clinical features, co‐morbidities, medication pattern, and echocardiographic parameters of this population in the different studies are compared in Tables 4 and 5. In Figure 3 , we highlight the intermediate clinical profile of HFmrEF, specifying the aspects of resemblance with the other two HF categories.
Table 4.
Summary of clinical characteristics, medication pattern, and echocardiographic parameters of HFmrEF population included in the main clinical trials considered for our review
| Rickenbacher P. et al. 4 | Toma M. et al. 5 | Solomon S.D. et al. 6, a | Solomon S.D. et al. 7, b | Lund LH et al. 8 | |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 79.0 | 73 | 66.3 ± 10.8 | 66 ± 9 | 65 ± 11 |
| Female gender, % | 46.3 | 41.1 | 32 | 36.5 | |
| BMI, kg/m2, mean ± SD or median (IQR) | 25.5 | 31.5 ± 7.2 | 27.8 (25.0–31.2) | ||
| SBP, mmHg, mean ± SD or median (IQR) | 127 | 130 (117–147) | 135.2 ± 18.9 | 128 ± 14 | 130 (120–145) |
| Heart rate, b.p.m., mean ± SD or median (IQR) | 76 | 78 (68–90) | 71.4 ± 12.4 | 69.98 ± 10.17 | |
| eGFR, mL/min/1.73 m2, mean ± SD or median (IQR) | 49 | 53.8 (38.5–69.9) | 69.6 ± 19.9 | ||
| Creatinine, mg/dL, mean ± SD or median (IQR) | 1.39 | 1.16 ± 0.43 | |||
| Sodium, mmol/L, mean ± SD or median (IQR) | 139 | 140 (137–142) | |||
| NT‐proBNP, median (IQR), ng/L | 3941 (2247–6760) | 3931 (1933‐8269) | |||
| BNP, median (IQR), ng/L | 898 (557–1435) | ||||
| NYHA class III/IV, % | 71.3 | 36.8 | 35.4 | 42.3 | |
| Hypertensive heart disease, % | 27.8 | 12.7 | |||
| Ischaemic aetiology, CAD, % | 56.5 | 69.0 | 72 | 66.9 | |
| Idiopathic dilated CMP, % | 8.3 | 13.1 | |||
| Valve disease and other, % | 7.4 | ||||
| CABG or PCI, % | 32.4 | 29.2 | 36 | 43.6 | |
| CRT, % | |||||
| ICD, % | 2.8 | 3.9 | 1.6 | ||
| Smoker, current, or previous % | 60.2 | 50.7 | 15 | 15.9 | |
| Hypertension, % | 81.9 | 60 | 86.5 | 56.2 | |
| PAOD, % | 18.5 | 19.3 | |||
| Hyperlipidaemia, % | 48.1 | ||||
| Diabetes mellitus, % | 39.8 | 50.2 | 27 | 28.7 | 28.6 |
| COPD, asthma, and lung disease, % | 21.3 | 20.3 | |||
| Prior stroke/TIA and cerebrovascular disease, % | 15.7 | 14.2 | 9.3 | ||
| CKD, % | 63.9 | ||||
| Anaemia, % | 38.0 | ||||
| Depression, % | 13.0 | 11.1 | |||
| Atrial fibrillation, flutter % | 39.6 | 49.9 | 25.6 | ||
| LVEDD, mm, mean ± SD | 52 | ||||
| LVESD, mm, mean ± SD | 42 | ||||
| Beta‐blocker, % | 73.1 | 65.4 | 57 | 78.3 | 57.7 |
| ACE‐I/ARBs, % | 90.7 | 60.7 | 21 | 88.1 | 27.2 |
| Aldosterone receptor blocker, % | 33.3 | 21.2 | 11 | 11.4 | |
| Nitrates, % | 32.4 | 24.5 | |||
| Diuretics % | 89.8 | 97.2 | 64 | 76.2 | 74.4 |
| Statin and lipid‐lowering therapy % | 44.7 | ||||
| Digoxin and digitalis, % | 13.9 | 8.0 | 32 | 35.2 | |
| Calcium channel blocker, % | 26 | 24.1 |
ACE‐I, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; CMP, cardiomiopathy; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IQR, interquantile range; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; PAOD, peripheral arterial occlusive disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD,standard deviation.
population of patients with HF and LVEF 43–52%.
population of patients with HF and LVEF 45–50%.
Table 5.
Summary of clinical characteristics, medication pattern, and echocardiographic parameters of HFmrEF population included in the main prospective observational studies considered for our review
| Chioncel O. et al.9 | Koh A.S. et al.10 | Rastogi et al.11 | Cheng R.K. et al.12 | He K.L. et al.13, c | Sweitzer et al.14, c | Kapoor J.R. et al.17 | Tsuji et al.18 | Lupòn et al.19, a | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years, mean ± SD | 64.2 ± 14.2 | 74 | 56 ± 13.09 | 81 | 66 ± 10 | 73.8 ± 12.9 | 74.44 ± 13.31 | 69.0 ± 12.7 | 63.2 ± 12.4 |
| Female gender, % | 26.4 | 39 | 46 | 50.5 | 21 | 54.3 | 48.85 | 28.2 | |
| BMI, kg/m2, mean ± SD, or median (IQR) | 28.6 ± 5.4 | 27 | 26.5 | 25 ± 4 | 29.9 ± 7.9 | 22.8 ± 5.3 | 27.7 ± 4.9 | ||
| SBP, mmHg, mean ± SD, or median (IQR) | 126.5 ± 21.1 | 131 | 119 ± 18.65 | 142 | 141 ± 31 | 129 ± 22b | 124.7 ± 19.3 | 129.3 ± 22.9 | |
| Heart rate, b.p.m., mean ± SD, or median (IQR) | 73.2 ± 15.9 | 73 | 74 ± 12.88 | 81 | 69 ± 8 | 62 ± 19b | 73.4 ± 14.7 | 70.1 ± 15.5 | |
| eGFR, mL/min/1.73 m2, mean ± SD, or median (IQR) | 62 | 76 ± 29.46 | 71 ± 28 | 58.6 ± 22.1 | 64.2 ± 24.1 | ||||
| Creatinine, mg/dL, mean ± SD, or median (IQR) | 1.22 | 1.0 | 1.3 | 1.33 ± 1.07 | 1.3 (1.0–1.9) | 1.1 ± 0.8 | |||
| Sodium, mmol/L, mean ± SD, or median (IQR) | 140 ± 2.97 | 138 | 138.3 ± 3.3 | ||||||
| NT‐proBNP, median (IQR), ng/L | 2160 (938‐4763) | 2037 ± 3484 | 994 (465–2165) | ||||||
| BNP, median (IQR), ng/L | 790 (421–1487) | 500 ± 627 | 164.5 (83.4–310.7) | ||||||
| NYHA class III/IV, % | 18.4 | 31 | 22 | 11.8 | 18.5 | ||||
| Hypertensive heart disease, % | 9.6 | 14.3 | 9.4 | ||||||
| Ischaemic aetiology, CAD, % | 41.8 | 53 | 20 | 56.7 | 65 | 59.7 | 68.95 | 52.9 | 35.2 |
| Idiopathic dilated CMP, % | 27.6 | 65 | 20.3 | 19.3 | |||||
| Valve disease and other, % | 11.5 | 21 | 20 | 14.4 | 24.8 | 7.2 | 18.9 | ||
| CABG or PCI, % | 35.8 | 17.1 | 43.1 | ||||||
| CRT, % | 8.4 | 0.9 | 1.8 | 1.8 | 4.3 | ||||
| ICD, % | 13.4 | 1.6 | 5.5 | 3.9 | 5.6 | ||||
| Smoker, current, or previous, % | 10.7 | 55 | 8.7 | 12.1 | 14.11 | ||||
| Hypertension, % | 82.4 | 64 | 51 | 77.9 | 77 | 82.18 | 89.8 | ||
| PAOD, % | 10 | 4 | 15.5 | 19.9 | 15.84 | 61.4 | |||
| Hyperlipidaemia, % | 35 | 48.2 | 38.0 | 54.02 | 80.2 | ||||
| Diabetes mellitus, % | 30.5 | 27 | 26 | 41.5 | 48 | 47.7 | 50.16 | 36.1 | 33.0 |
| COPD, asthma, and lung disease, % | 11.6 | 30 | 9 | 29.6 | 31.5 | 36.43 | |||
| Prior stroke/TIA and cerebrovascular disease, % | 8.3 | 8 | 17.1 | 18.0 | 17.10 | 22.1 | |||
| CKD, % | 16.5 | 21.1 | 30.9 | 25.78 | 42.0 | ||||
| Anaemia, % | 35 | 21.3 | 27.02 | 40.3 | |||||
| Depression, % | 7.1 | 10.0 | 12.86 | ||||||
| Atrial fibrillation, flutter % | 22.3 | 58 | 26 | 40.2 | 8 | 32.9 | 45.00 | 43.5 | 21.0 |
| LVEDD, mm, mean ± SD | 58.0 ± 8.4 | 54 ± 7.4 | 55 ± 7 | 55.8 ± 7.9 | |||||
| LVESD, mm, mean ± SD | 41 ± 7.6 | 42 ± 6 | 42.9 ± 6.9 | ||||||
| Beta‐blocker, % | 86 | 88 | 86.5b | 58 | 62.2 | 63.8 | 93.1 | ||
| ACE‐I/ARBs, % | 84 | 86 | 83.5b | 68 | 71.8 | 80.0 | 92.7 | ||
| Aldosterone receptor blocker, % | 24 | 42 | 13.0b | 29.3 | 55.8 | ||||
| Nitrates, % | 17 | 5 | 52.0 | ||||||
| Diuretics, % | 69 | 45 | 63.3 | 89.3 | |||||
| Statin and lipid‐lowering therapy, % | 42 | 39.6 | |||||||
| Digoxin and digitalis, % | 30 | 23 | 37.3 | ||||||
| Calcium channel blocker, % | 3 | 39 | 30.5 | 27.0 |
ACE‐I, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; CMP, cardiomiopathy; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IQR, interquantile range; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; PAOD, peripheral arterial occlusive disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD,standard deviation.
Only for HFmrEF with recovery of LVEF.
After discharge.
Population of patients with HF nad LVEF 40–55%.
Figure 3.
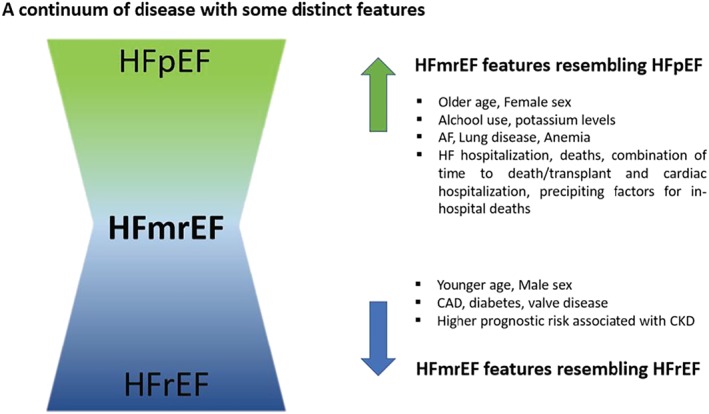
HFmrEF on a continuum of disease. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
5. Biomarkers
Natriuretic peptides (NPs) and other biomarkers can help improve the current HF classification. Recent large randomized controlled trials used specific biomarkers, such as elevated levels of NP, as an enrolment criterion. There is a significant variability in NPs levels across the spectrum of HF.49 Similar to troponin, NPs achieve higher levels in HFrEF, intermediate levels in HFmrEF, and lower concentrations in HFpEF. The association between high N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) levels and increased mortality and hospitalization rates is independent from LVEF. In addition to its absolute values, their changes over time have an important role also in the HFmrEF group.50 The prognostic role of NT‐proBNP is even greater in HFmrEF and HFpEF than in HFrEF, although its absolute levels are lower and more affected by some confounding factors, particularly AF.51 AF has also a major role on NPs trajectories in HFrEF.52
Natriuretic peptides allow a differentiation of HF subtypes in patients with HFmrEF. For example, patients with HFmrEF and recovered LVEF have lower baseline NT‐proBNP levels than patients with a downtrending LVEF.19 Troponin is another commonly used biomarker. Troponins have a prognostic role, regardless of LVEF.53 Generally, the biomarker profile of HFmrEF is intermediate between the two extremes of the LVEF spectrum. In HFmrEF group, both biomarkers of cardiac stretch (NT‐proBNP), typical of HFrEF, and the biomarkers of inflammation, typical of HFpEF, such as endothelin‐1 and galectin‐3, are well represented in the acute and chronic HF setting.54
6. Co‐morbidities
The ESC Heart Failure Long‐Term Registry showed a comparable prevalence of non‐cardiac co‐morbidities in the three LVEF groups,4, 9 except for chronic obstructive pulmonary disease, renal, and hepatic dysfunction, which were more commonly associated with HFrEF.9 Coronary artery disease (CAD) is a leading aetiology of HF in both HFmrEF and HFrEF patients, as compared with HFpEF.4, 10, 13 HFmrEF patients are less often diabetic and anaemic, with less frequent atrial fibrillation, lung disease, or chronic kidney disease (CKD) when compared with HFpEF. HFmrEF is characterized by a higher prevalence of atrial fibrillation, respiratory disease, and aortic stenosis than HFrEF.10, 13, 14 In HFmrEF, the prevalence of CKD is around 50%.16 CKD has a prognostic role in all the three LVEF categories, but the correlation with a mortality seems stronger for the mid‐range and reduced LVEF groups.16 This could be explained by the tight connection between CKD and a more advanced HF stage. In this scenario, both the backward and forward haemodynamic failure and the neurohormonal activation compromise the renal function, and CKD in turn contributes to further cardiac deterioration. Similar to CKD, the prognostic influence of diabetes mellitus on the mortality seems greater in HFmrEF and HFrEF.55 On the contrary, AF has the same prognostic impact across all the EF spectrum, with a similarly increased risk of death, HF hospitalization, and stroke/TIA, regardless of LVEF.56 An analysis of a community‐based prospective observational study has shown a similar impact of co‐morbidities regardless the LVEF values with a linear increase in events rates with an increased number of co‐morbidities and with a major contribution of anaemia, CKD, chronic obstructive pulmonary disease, diabetes mellitus, and peripheral artery disease.57
7. Outcomes
The ESC Long‐Term registry and other studies7, 58 have shown an intermediate rate of mortality at 1 year (7.6%) as compared with reduced and preserved LVEF (8.8% and 6.4%, respectively).9 Most of the data show better outcomes for the patients with HFmrEF, compared with those with HFrEF. Event rates of the patients with HFmrEF are better than in HFrEF and similar to those of the patients with HFpEF, above all after adjustments for patients' characteristics.6, 10, 59 Yet other studies have found similar rates of HF hospitalization and survival between the three LVEF categories.4 In the presence of CAD, HFmrEF group has higher all‐cause death rates at 3 years as compared with HFpEF, probably because of an incomplete myocardial revascularization.10 In the in‐hospital setting, patients with HFmrEF have lower rates of in‐hospital death (2.6%) than preserved and reduced LVEF populations (3.0% and 3.2%, respectively).17 Moreover, precipitating factors for HF decompensation vary by EF group and are independently associated with clinical outcomes.17
8. Medical and non‐medical treatment
Since the introduction of the new LVEF‐based classification of HF, several retrospective analyses have explored differences in medication use and treatment response. In general, the use of medication is comparable between HFmrEF and HFrEF. The use of digoxin, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers is higher in HFrEF, while patients with HFpEF are more likely to receive calcium channel blockers.10, 11, 29 The proportion of patients on mineralocorticoid receptor antagonists has a wide range (23–55%).9, 10, 29 Retrospective analysis of clinical trials and observational studies has explored the effects of drugs and devices in patients with HFmrEF. The results of landmark HF trials stratified for LVEF are reported in Table 6; meanwhile, the summary of the effect of the main HF therapies on outcomes specifically in the HFmrEF population is reported in Table 7. Spironolactone was associated with decreased hospitalization rates in HF patients with LVEF ≥45%, with a stronger benefit for the range of LVEF values from 45% up to 50%.63, 67 HFmrEF patients benefit from spironolactone treatment also in terms of improvement of NYHA class and reduction of BNP and indices of myocardial fibrosis.64 Despite some analyses of observational studies,18, 65 an analysis of CHARM8 showed a reduction of HF hospitalization,66 mortality,10 and of the composite endpoint (cardiovascular death and HF hospitalization)8 with candesartan in the mid‐range LVEF category. Finally, the benefit of beta‐blockers in this population appears still unclear. While the OPTIMIZE‐HF registry65 did not show a benefit from beta‐blockers, other data demonstrated a reduction in mortality in all the EF categories.18 Particularly, beta‐blockers increase the LVEF for the patients with LVEF <50% and reduce the CV death both in HFrEF and in HFmrEF.62 The improvement of the prognosis with beta‐blockers is greater in specific subgroups, such as HFmrEF with CAD.10 Diuretics seem to have negative influence on the prognosis also in patients with HFmrEF.18 In spite of HFrEF, digoxin does not significantly reduce HF hospitalization rates in the HFmrEF population.60 The current evidence for HFmrEF treatment has been summarized in a recent consensus article. It states that candesartan and spironolactone may be considered for ambulatory patients with symptomatic HFmrEF in order to reduce the risk of HF hospitalization and CV death and that beta‐blockers may be considered in ambulatory patients with HFmrEF in sinus rhythm, to reduce all‐cause and cardiovascular death.68
Table 6.
Summarize results of landmark trials stratified for LVEF
| Study | Prevalence (%) | Main outcomes | Outcomes stratified for LVEF | ||||
|---|---|---|---|---|---|---|---|
| HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | ||
| DIG trial60 | 75.42 | 15.34 | 9.23 | Digoxin/placebo HR for HF hospitalization | 0.71 [95% CI 0.65–0.77] | 0.80 [95% CI 0.63–1.03] | 0.85 [95% CI 0.62–1.17] |
| Digoxin/placebo HR for the composite of HF death or HF hospitalization | 0.74 [95% CI 0.68–0.81] | 0.83 [95% CI 0.66–1.05] | 0.88 [95% CI 0.65–1.19] | ||||
| CHARM programme8 | 57 | 17 | 26 | Cardiovascular death or HF hospitalization | 15.9 per 100 p‐y | 8.5 per 100 p‐y | 8.9 per 100 p‐y |
| Candesartan/placebo HF for the composite of cardiovascular death or HF hospitalization | 0.82 [95% CI 0.75–0.91; P < 0.001] | 0.76 [95% CI 0.61–0.96; P = 0.02] | 0.95 [95% CI 0.79–1.14; P = 0.57] | ||||
| Candesartan/placebo incident RR for recurrent HF hospitalization | 0.68 [95% CI 0.58–0.80; P < 0.001] | 0.48 [95% CI 0.33–0.70; P < 0.001] | 0.78 [95% CI 0.59–1.03; P = 0.08] | ||||
| TOPCAT7 | 0 | 15a | 85 | Composite of CV death, aborted cardiac arrest, or HF hospitalization | — | 1.37 [95% CI 1.09–1.72] | Referentb |
| HF hospitalization | — | 1.06 [95% CI 0.79–1.44] | Referentb | ||||
| CV death | — | 1.86 [95% CI 1.35–2.55] | Referent | ||||
| Spironolattone/placebo HR for composite of CV death, aborted cardiac arrest, or HF hospitalization | — | 0.72 [95% CI 0.50–1.05] | 0.97 [95% CI 0.76–1.23; P = 0.046]b | ||||
| Spironolattone/placebo HR for HF hospitalization | — | 0.76 [95% CI 0.46–1.27] | 0.98 [95% CI 0.74–1.30; P = 0.039]b | ||||
| PARAGON‐HF61 | 0 | 52c | 48c | ARNI/valsartan RR for HF hospitalizations and CV death | — | 0.78 [95% CI 0.64–0.95] | 1.00 [95% CI 0.81–1.23] |
| Meta‐analysis on beta‐blockers (Cleland et al.)62 | 94 | 4 | 2 | All‐cause mortality HR for each 5% lower LVEF | 1.16 [95% CI 1.26–1.19; P < 0.0001] | 1.16 [95% CI 1.26–1.19; P < 0.0001] | 1.16 [95% CI 1.26–1.19; P < 0.0001] |
| Beta‐blockers/placebo HR for all‐cause mortality in sinus rhythm | 0.67 [95% CI 0.50–0.90]; P = 0.007d | 0.59 [95% CI 0.34–1.03]; P = 0.066 | 1.79 [95% CI 0.78–4.10]; P = 0.17 | ||||
| Beta‐blockers/placebo HR for CV death in sinus rhythm | 0.72 (0.52–0.99); P = 0.041d | 0.48 [95% CI 0.24–0.97]; P = 0.040 | 1.77 [95% CI 0.61–5.14]; P = 0.29 | ||||
| Beta‐blockers/placebo mean change in LVEF from baseline to follow up in sinus rhythm (SE) | +4.9% (0.9)d | +1.9% (1.1%) | +0.1% (1.2%) | ||||
CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; p‐y, patient‐years; RR, rate ratio; SE, standard error.
LVEF values are ≥45% and <50%.
For LVEF, ≥60%.
In the analysis of pre‐specified groups of PARAGON‐HF trial, the cut‐off value of LVEF is 57% (45–57% and >57%).
These results belong to the subgroups of patients with LVEF 35–39%; for the other subgroups of HFrEF (LVEF <20%, 21–25%, and 26–34%), the HR are superimposable.
Table 7.
Summary of positive (↑) and negative (↓) effect of HF medication on adverse outcomes (mortality and HF hospitalization) in the HFmrEF
| Study | MRA | ACE‐i | ARB | Beta‐blocker | Diuretics | Statin |
|---|---|---|---|---|---|---|
| Pitt et al. TOPCAT63 | ↑ | |||||
| Xiang et al.64 | ↑ | |||||
| Fonarow et al. OPTIMIZE‐HF65 | = | = | ||||
| Tsuji et al.18 | = | = | ↑ | ↓ | = | |
| Yusuf et al. CHARM‐PRESERVED66 | ↑ | |||||
| Koh et al.10 | ↑ | ↑ | ↑ | ↑ | ||
| Lund et al. CHARM programme8 | ↑ |
ACE‐i, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
Recently, the PARAGON‐HF has failed to demonstrate a significant reduction of HF hospitalization and CV death with Sacubutril/Valsartan in HF patients with LVEF ≥45%.61 The inconclusive results from TOPCAT (spironolactone in HFpEF) will hopefully be soon corrected by the two ongoing spironolactone in HFpEF trials (SPIRRIT‐HFpEF and SPIRIT).7 Notably, across PARAGON‐HF and TOPCAT, lower LVEF was associated with improved outcomes. For PARAGON‐HF, the HR of the primary outcomes was 0.76 (95% CI 0.63–0.92) per 10% decrease in LVEF suggesting that in respect to neurohormonal blocker therapy. In the same category of patients, also Vericiguat did not show a change of NT‐proBNP and of left atrial volume 69. Currently, one of the most interesting area of research in the HF concerns the sodium glucose cotransporter 2 inhibitors. Given the reduction of HF hospitalizations and CV death with sodium glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus regardless of history of HF,70, 71 the ongoing EMPEROR‐Preserved, DELIVER, and SOLOIST‐WHF trials have the aim to study the effect of this drug category on the HF outcomes in patients with LVEF ≥45%.
The rates of implanted implantable cardioverter defibrillator (ICD)/CRT‐D in the three LVEF categories have a wide variability.4, 5, 8, 9, 19, 29 Regarding the use of pacemakers, CRT, and/or ICD, HFmrEF resembles more HFpEF.10 Specifically, HFmrEF patients with improved or recovered LVEF have intermediate rates of implanted pacemakers and/or ICD/CRT when compared with HFrEF and HFpEF patients.15, 19
9. Conclusions
The new category of HFmrEF does not simply indicate an intermediate category between HFrEF and HFpEF but comprises a heterogenous population of patients with distinct and heterogeneous prognostic profiles. Thus, HFmrEF represents a new area of investigation and future research. The complexity of this population lies in the heterogeneity of its clinical profile, prognosis, and the underlying pathophysiological substrate. Before the current ESC guidelines, clinical trials and registries often lumped HFpEF and HFmrEF together, potentially introducing a high degree of variability in clinical phenotypes. The complexity of cardiac and extra‐cardiac factors in HF demands a more comprehensive evaluation and classification of patients across the whole spectrum of cardiovascular diagnostic and therapeutic metrics. The current HF classification is based on LVEF measurement alone, but, as demonstrated earlier, there are many limitations to this method. Thus, a more detailed phenotyping may allow to better characterize patients, provide greater prognostic differentiation, help for more precise targeting of therapies to patients, and stimulate the discovery of new more effective treatments. It is reasonable to consider an alternative and more reliable classification, including other imaging parameters or their changes over time and taking into account biomarkers and the phenotype migration. HFmrEF may occur either as a recovery from HFrEF or, less often, as a progression from HFpEF. It may also be the first presentation of HF, although it may move to HFrEF or HFpEF in its clinical course. In each instance, the HFmrEF phenotype has a different clinical makeup and prognosis. LVEF alone is insufficient to capture which phenotype HFmrEF belongs to and what the trajectory/prognosis of the phenotype is (Table 8).
Table 8.
Pros and cons of an LVEF‐based classification for patients with HFmrEF
| Pros | Cons |
|---|---|
|
Standardize the clinical approach Standardize the care The LVEF is a simple criterion to plan and design randomized trials Acknowledgement of a subgroup with a specific bio‐clinical profile that is different from HFrEF and HFpEF Lack of other classifiers and targets that are reproducible and/or treatable |
Large mobility of patient with HF between LVEF categories Lack of consideration of the underlying HF aetiology Poor information about the ventricular remodelling and/or ventricular dyssynchrony Intra‐variability and inter‐variability of the echo LVEF measurement Intrinsic limitations of LVEF alone HFmrEF patient cohort is a heterogeneous population with many phenotypes Loss of prognostic power of LVEF proportionally to the increase of LVEF values |
Conflict of interest
None declared.
Funding
Dr Fudim is supported by an American Heart Association (grant 17MCPRP33460225) and National Institutes of Health T32 post‐doctoral training (grant 5T32HL007101‐42) and consults for Axon Therapies and Galvani.
Branca, L. , Sbolli, M. , Metra, M. , and Fudim, M. (2020) Heart failure with mid‐range ejection fraction: pro and cons of the new classification of Heart Failure by European Society of Cardiology guidelines. ESC Heart Failure, 7: 381–399. 10.1002/ehf2.12586.
References
- 1. Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JGF, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O'Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV, Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail 2014; 2: 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 32013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 3. Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, Hopper I, Kistler PM, Briffa T, Wong J, Abhayaratna W, Thomas L. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018; 27: 1123–1208. [DOI] [PubMed] [Google Scholar]
- 4. Rickenbacher P, Kaufmann BA, Maeder MT, Bernheim A, Goetschalckx K, Pfister O, Pfisterer M, Brunner‐la Rocca HP, TIME‐CHF Investigators . Heart failure with mid‐range ejection fraction: a distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME‐CHF). Eur J Heart Fail 2017; 19: 1586–1596. [DOI] [PubMed] [Google Scholar]
- 5. Toma M, Ezekowitz JA, Bakal JA, O'Connor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW, Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND‐HF Trial. Eur J Heart Fail 2014; 16: 334–341. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 7. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray J, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; 20: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 9. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 10. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, Savarese G, Lam CSP, Lund LH. A comprehensive population‐based characterization of heart failure with mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1624–1634. [DOI] [PubMed] [Google Scholar]
- 11. Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014; 168: 721–730. [DOI] [PubMed] [Google Scholar]
- 13. He KL, Burkhoff D, Leng WX, Liang ZR, Fan L, Wang J, Maurer MS. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40%. Am J Cardiol 2009; 103: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sweitzer NK, Lopatin M, Yancy CW, Mills RMSL. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or = 55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol 2008; 101: 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nadruz W, West E, Santos M, Skali H, Groarke JD, Forman DE, Shah AM. Heart failure and midrange ejection fraction: implications of recovered ejection fraction for exercise tolerance and outcomes. Circ Heart Fail 2016; 9: e002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Löfman I, Szummer K, Dahlstrom U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail 2017; 19: 1606–1614. [DOI] [PubMed] [Google Scholar]
- 17. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail 2016; 4: 464–472. [DOI] [PubMed] [Google Scholar]
- 18. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, CHART‐2 Investigators . Characterization of heart failure patients with mid‐range left ventricular ejection fraction‐a report from the CHART‐2 study. Eur J Heart Fail 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 19. Lupón J, Diez‐Lopez C, de Antonio M, Domingo M, Zamora E, Moliner P, Gonzalez B, Santesmases J, Troya MI, Bayés‐Genís A. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail 2017; 19: 1615–1623. [DOI] [PubMed] [Google Scholar]
- 20. Coles AH, Fisher K, Darling C, Yarzebski J, McManus D, Gore JM, Lessard D, Goldberg RJ. Long‐term survival for patients with acute decompensated heart failure according to ejection fraction findings. Am J Cardiol 2014; 114: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coles AH, Tisminetzky M, Yarzebski J, Lessard D, Gore JM, Darling CE, Goldberg RJ. Magnitude of and prognostic factors associated with 1‐year mortality after hospital discharge for acute decompensated heart failure based on ejection fraction findings. J Am Heart Assoc 2015; 4: e002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lund LH, Vedin O, Savarese G. Is ejection fraction in heart failure a limitation or an opportunity? Eur J Heart Fail 2018. Mar; 20: 431–432. [DOI] [PubMed] [Google Scholar]
- 23. Packer M. The room where it happens: A skeptic's analysis of the new heart failure guidelines. J Card Fail 2016; 22: 726–730. [DOI] [PubMed] [Google Scholar]
- 24. Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B. Arrhythmia‐induced cardiomyopathies mechanisms, recognition, and management. J Am Coll Cardiol 2015; 66: 1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan A, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA‐MRI study. J Am Coll Cardiol 2017; 70: 1949–1961. [DOI] [PubMed] [Google Scholar]
- 26. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 379: 492. [DOI] [PubMed] [Google Scholar]
- 27. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC Hear Fail 2019; 7: 447–456. [DOI] [PubMed] [Google Scholar]
- 28. Marwick TH. Ejection fraction pros and cons: JACC state‐of‐the‐art review. J Am Coll Cardiol 2018; 72: 2360–2379. [DOI] [PubMed] [Google Scholar]
- 29. Nauta JF, Hummel YM, van Melle JP, van der Meer P, Lam CSP, Ponikowski P, Voors AA. What have we learned about heart failure with mid‐range ejection fraction one year after its introduction? Eur J Heart Fail 2017; 19: 1569–1573. [DOI] [PubMed] [Google Scholar]
- 30. Vedin O, Lam CSP, Koh AS, Benson L, Teng TH, Tay WT, Braun OÖ. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail 2017; 10: pii: e003875. [DOI] [PubMed] [Google Scholar]
- 31. Savarese G, Vedin O, D'Amario D, Uijl A, Dahlström U, Rosano G, Lam CS, Lund LH. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail 2019; 7: 306–317. [DOI] [PubMed] [Google Scholar]
- 32. Mele D, Nardozza M, Ferrari R. Left ventricular ejection fraction and heart failure: an indissoluble marriage? Eur J Heart Fail 2018; 20: 427–430. [DOI] [PubMed] [Google Scholar]
- 33. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol 2012; 60: 2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fudim M, Fathallah M, Shaw LK, Liu PR, James O, Samad Z, Piccini JP, Hess PL, Borges‐Neto S. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary heart disease. JACC Cardiovasc Imaging 2019; 12: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 35. Fudim M, Dalgaard F, Fathallah M, Iskandrian AE, Borges‐Neto S. Mechanical dyssynchrony: how do we measure it, what it means, and what we can do about it. J Nucl Cardiol 2019. [DOI] [PubMed] [Google Scholar]
- 36. Čelutkienė J, Plymen CM, Flachskampf FA, de Boer RA, Grapsa J, Manka R, Anderson L, Garbi M, Barberis V, Filardi PP, Gargiulo P, Zamorano JL, Lainscak M, Seferovic P, Ruschitzka F, Rosano GMC, Nihoyannopoulos P. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1615–1633. [DOI] [PubMed] [Google Scholar]
- 37. Hensen LCR, Goossens K, Delgado V, Abou R, Rotmans JI, Jukema JW, Bax JJ. Prevalence of left ventricular systolic dysfunction in pre‐dialysis and dialysis patients with preserved left ventricular ejection fraction. Eur J Heart Fail 2018; 20: 560–568. [DOI] [PubMed] [Google Scholar]
- 38. DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, Lin G, Redfield MM, Lewis G, Semigran MJ, Anstrom KJ. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail 2017; 19: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 2018; 11: 260–274. [DOI] [PubMed] [Google Scholar]
- 40. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009; 2: 356–364. [DOI] [PubMed] [Google Scholar]
- 41. Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray J, Velazquez EJ, Kober L, Pfeffer MA, Solomon SD, VALIANT investigators . Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol 2010; 56: 1812–1822. [DOI] [PubMed] [Google Scholar]
- 42. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 2018; 71: 1947–1957. [DOI] [PubMed] [Google Scholar]
- 43. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1078. [DOI] [PubMed] [Google Scholar]
- 44. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1559–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McNally EM, Amaral AP. Predicting arrhythmia risk in dilated cardiomyopathy using genetic mutation status. J Am Coll Cardiol 2019; 74: 1491–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fukuta H, Goto T, Wakami K, Ohte N. Effects of drug and exercise intervention on functional capacity and quality of life in heart failure with preserved ejection fraction: a meta‐analysis of randomized controlled trials. Eur J Prev Cardiol 2016; 23: 78–85. [DOI] [PubMed] [Google Scholar]
- 47. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, de Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019; 40: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 2016; 37: 1642–1650. [DOI] [PubMed] [Google Scholar]
- 49. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Savarese G, Hage C, Orsini N, Dahlström U, Perrone‐Filardi P, Rosano GMC, Lund LH Reductions in N‐terminal pro‐brain natriuretic peptide levels are associated with lower mortality and heart failure hospitalization rates in patients with heart failure with mid‐range and preserved ejection fraction. Circ Hear Fail 2016; 9: pii: e003105. [DOI] [PubMed] [Google Scholar]
- 51. Savarese G, Orsini N, Hage C, Dahlström U, Vedin O, Rosano GMC, Lund LH. Associations with and prognostic and discriminatory role of N‐terminal pro‐B‐type natriuretic peptide in heart failure with preserved versus mid‐range versus reduced ejection fraction. J Card Fail 2018; 24: 365–374. [DOI] [PubMed] [Google Scholar]
- 52. Greene SJ, Fonarow GC, Solomon SD, Subacius HP, Ambrosy AP, Vaduganathan M, Maggioni AP, Böhm M, Lewis EF, Zannad F, Butler J, Gheorghiade M, ASTRONAUT Investigators and Coordinators . Influence of atrial fibrillation on post‐discharge natriuretic peptide trajectory and clinical outcomes among patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur J Heart Fail 2017; 19: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gohar A, Chong JPC, Liew OW, den Ruijter H, de Kleijn DPV, Sim D, Yeo DPS, Ong HY, Jaufeerally F, Leong GKT, Ling LH, Lam CSP, Richards AM. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur J Heart Fail 2017; 19: 1638–1647. [DOI] [PubMed] [Google Scholar]
- 54. Tromp J, Khan MAF, Mentz RJ, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JGF, Givertz MM, Bloomfield DM, van Veldhuisen D, Hillege HL, Voors AA, van der Meer P. Biomarker profiles of acute heart failure patients with a mid‐range ejection fraction. JACC Heart Fail 2017; 5: 507–517. [DOI] [PubMed] [Google Scholar]
- 55. Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, Norhammar A. Type 2 diabetes and heart failure: characteristics and prognosis in preserved, mid‐range and reduced ventricular function. Diab Vasc Dis Res 2018; 15: 494–503. [DOI] [PubMed] [Google Scholar]
- 56. Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid‐range, and reduced ejection fraction. JACC Hear Fail 2017. Aug; 5: 565–574. [DOI] [PubMed] [Google Scholar]
- 57. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 58. Lam CSP, Solomon SD. The middle child in heart failure: heart failure with mid‐range ejection fraction (40‐50%). Eur J Heart Fail 2014; 16: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 59. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 60. Abdul‐Rahim AH, Shen L, Rush CJ, Jhund PS, Lees KR, McMurray JJV. Effect of digoxin in patients with heart failure and mid‐range (borderline) left ventricular ejection fraction. Eur J Heart Fail 2018; 20: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 61. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SV, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. PARAGON‐HF Investigators and Committees. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 62. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray J, Ruschitzka F, van Veldhuisen D, von Lueder T, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D, Beta‐blockers in Heart Failure Collaborative Group . Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 64. Xiang Y, Shi W, Li Z, Yang Y, Wang SY, Xiang R, Feng P, Wen L, Huang W. Efficacy and safety of spironolactone in the heart failure with mid‐range ejection fraction and heart failure with preserved ejection fraction: a meta‐analysis of randomized clinical trials. Medicine (Baltimore) 2019; 98: e14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. A report from the OPTIMIZE‐HF registry. J Am Coll Cardiol 2007; 50: 768–777. [DOI] [PubMed] [Google Scholar]
- 66. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray J, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐preserved trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 67. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction. JACC Hear Fail 2017; 5: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JG, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186 [DOI] [PubMed] [Google Scholar]
- 69. Pieske B, Maggioni AP, Lam CSP, Pieske‐Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES‐PRESERVED) study. Eur Heart J 2017; 38: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 1881–1882. [DOI] [PubMed] [Google Scholar]
- 71. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]


