Abstract
Amyloidosis is associated with poor prognosis, and patients with cardiac involvement have especially poor outcomes. Cardiac amyloidosis leads to higher rates of atrial arrhythmia and an increased risk of intracardiac thrombus formation. However, atrial mechanical dysfunction due to protein deposition in amyloidosis may lead to thrombus formation in the absence of atrial arrhythmia. We present a 42‐year‐old male patient with familial transthyretin amyloidosis who suffered an embolic stroke that originated from a left atrial appendage thrombus in the absence of any documented atrial fibrillation. This case highlights atrial mechanical dysfunction in patients with cardiac amyloidosis and the need to better stratify thrombotic risk in this population with integration of echocardiographic parameters and transesophageal echocardiography.
Keywords: Cardiac amyloidosis, Thrombosis, Stroke, TTR amyloid, Atrial myopathy
1. Introduction
Thromboembolic risk in cardiac amyloidosis (CA) is known to be elevated.1 However, clinical application of anticoagulation in this population is fraught with difficulties. We illustrate the necessity of transesophageal imaging and assessment of atrial mechanical function to better stratify thrombotic risk.
2. Case report
A 42‐year‐old male patient presented to the hospital with an altered level of consciousness and right sided weakness that resulted in a motor vehicle accident. He was promptly diagnosed with a left middle cerebral artery infarction (Figure 1 ) and underwent successful endovascular treatment with mechanical thrombectomy. He was diagnosed with familial transthyretin (TTR) amyloidosis 8 years earlier when he developed autonomic neuropathy leading to a fat pad biopsy, which was positive. Genetic testing confirmed a rare glutamic acid mutation (TTR‐ASP 10 Glu) known to cause polyneuropathy and CA. His most recent echocardiogram prior to his stroke demonstrated marked biventricular hypertrophy, severe left ventricular (LV) diastolic dysfunction, moderate LV systolic dysfunction (LV ejection fraction 35%) and bi‐atrial enlargement (Figure 2 A).
Figure 1.
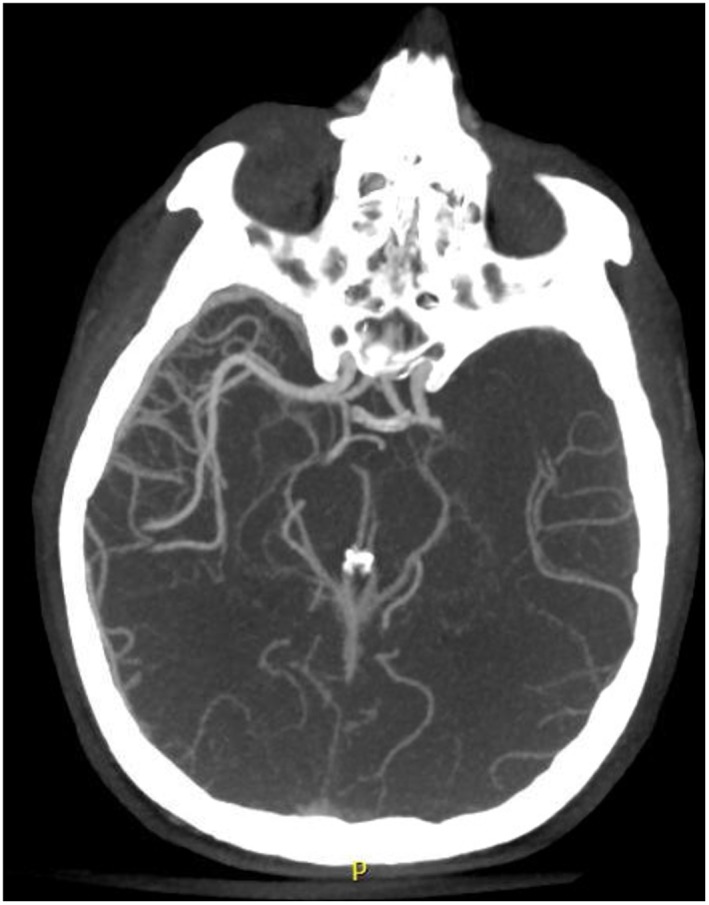
CT Head demonstrating left middle cerebral artery occlusion (red arrow).
Figure 2.
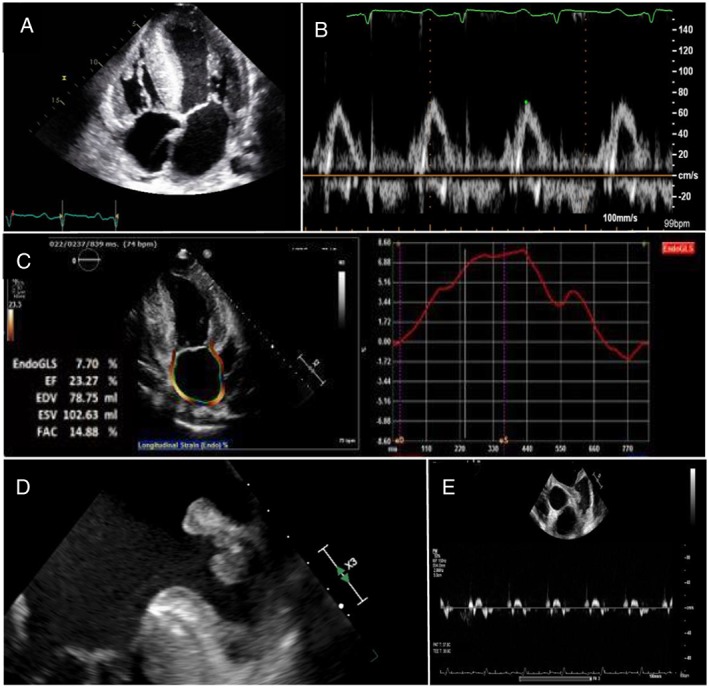
(A) Transthoracic echocardiography showing bi‐ventricular hypertrophy and bi‐atrial enlargement. (B) Transmitral Pulse Doppler across mitral valve with absent A wave despite sinus rhythm. (C) Reduced peak longitudinal left atrial strain. (D) Culprit left atrial appendage thrombus. (E) Significantly reduced contractility of left atrial appendage with very low pulse wave velocities.
As part of his stroke work‐up, he had a 12‐lead electrocardiogram and continuous cardiac monitoring for 10 days, which revealed occasional atrial ectopy, but no sustained atrial arrhythmia. Review of medical records and prior electrocardiograms in the 8 years preceding the stroke and 3 months following, consistently revealed sinus rhythm. Imaging of his head, neck, and lower limb vasculature did not reveal any source of embolism. A bubble transthoracic echocardiogram was performed, which demonstrated right to left shunt only with release of Valsalva maneuver, consistent with patent foramen ovale. Paradoxical emboli was initially considered, although less likely in the absence of deep vein thrombosis. Transesophageal echocardiography (TEE) and left atrial strain analysis were also performed. Spontaneous echo contrast (“smoke”) was noted in the left atrium consistent with a low‐flow state. The transmitral pulse wave Doppler signal showed an absent A wave despite sinus rhythm, suggesting loss of atrial contractility and electromechanical dissociation (Figure 2 B). Peak longitudinal left atrial strain was markedly reduced (Figure 2 C). Imaging of the left atrial appendage revealed reduced atrial emptying velocities and a large thrombus that was felt to be culprit for our patient's stroke (Figure 2 D and 2 E). Anticoagulation was initiated with full dose direct oral anticoagulant (apixaban 5 mg twice daily). A repeat TEE 6 weeks later demonstrated persistent appendage thrombus, prompting a change in anticoagulation to warfarin.
3. Discussion
Amyloidosis is a group of diseases characterized by extracellular deposition of amyloid protein—a misfolded, insoluble protein that can deposit in virtually any organ system. Cardiac involvement is a major determinant of survival and is associated with poor prognosis due to development of heart failure and arrhythmias. Atrial arrhythmia is a very prominent feature in CA.2 Described mostly in an AL amyloidosis population, a recent study demonstrated a high burden of atrial fibrillation (AF) in TTR amyloidosis,3 although this was not shown to impact mortality. Our case is unique, given the clinical presentation of stroke due to left atrial appendage thrombus without documented AF.
There is mounting evidence that prior to atrial arrhythmia, atrial mechanical dysfunction occurs due to protein deposition within the left atrium. Patients with CA in the absence of other cardiovascular disease have previously been shown to have impaired left atrial (LA) and LA appendage mechanical function compared with controls.2, 4 The degree of LA dysfunction also correlated with ventricular dysfunction, suggesting a natural history of amyloid deposition in atrial and ventricular myocardium.2
Patients with CA and atrial involvement are at high risk for the formation of atrial thrombi. The true incidence of intracardiac thrombus is likely underestimated in CA. Feng et al. showed in a large autopsy study that 33% of patients with various subtypes of amyloidosis had intracardiac thrombi.1 Patients with thrombi present had worse atrial mechanical activity and higher filling pressures on TEE before death. Overall, 26% of patients died of embolic complications. A follow up imaging study evaluated 80 patients with various subtypes of amyloidosis with TEE and found that 27% of patients had an identifiable thrombus. Interestingly, 20% of patients without documented AF had thrombi.5 A recent study retrospectively assessed patients with CA who underwent electrical cardioversion for atrial arrhythmia compared with a control population without CA. Over two thirds of patients had TEE‐guided procedures. Despite high rates of anticoagulation, the groups differed significantly for the presence of atrial thrombi (28% CA group versus 2.5% control group).6 Despite these results, there is a paucity of evidence examining the diagnosis of CA and cerebral ischaemia from thromboembolism, and as a result, guidance on anticoagulation is lacking.
This case demonstrates a growing concern in patients with CA for atrial mechanical dysfunction and resultant LA thrombus formation and cardioembolic stroke despite antecedent sinus rhythm. Atrial mechanical dysfunction may be suspected on transthoracic echocardiography by a loss of transmitral Doppler A wave or reduced left atrial strain. The true stroke risk in CA patients is unknown2 but established in recent reports.7 This case highlights the clinical need to have a low threshold for TEE for appendage imaging for patients with CA presenting with stroke to rule out a cardioembolic source.
Conflict of interest
None declared.
Ballantyne, B. , Manian, U. , Sheyin, O. , Davey, R. , and De, S. (2020) Stroke risk and atrial mechanical dysfunction in cardiac amyloidosis. ESC Heart Failure, 7: 705–707. 10.1002/ehf2.12602.
References
- 1. Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, Syed II, Hughes DA, Lust JA, Jaffe AS, Gertz MA. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007; 116: 2420–2426. [DOI] [PubMed] [Google Scholar]
- 2. Nochioka K, Quarta CC, Claggett B, Roca GQ, Rapezzi C, Falk RH, Solomon SD. Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2017; 18: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 3. Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild‐type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Failure 2018; 5: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Bella G, Minutoli F, Madaffari A, Mazzeo A, Russo M, Donato R, Zito C, Aquaro GD, Piccione MC, Pedri S, Vita G. Left atrial function in cardiac amyloidosis. J Cardiovasc Med 2016; 17: 113–121. [DOI] [PubMed] [Google Scholar]
- 5. Feng D, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, Edwards WD, Gertz MA, Klarich KW. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009; 119: 2490–2497. [DOI] [PubMed] [Google Scholar]
- 6. El‐Am EA, Dispenzieri A, Melduni RM, Ammash NM, White RD, Hodge DO, Noseworthy PA, Lin G, Pislaru SV, Egbe AC, Grogan M. Direct Current Cardioversion of Atrial Arrhythmias in Adults with Cardiac Amyloidosis. J Am Coll Cardiol 2019; 73: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russo D, Limite LR, Arcari L, Autore C, Musumeci MB. Predicting the unpredictable: how to score the risk of stroke in cardiac amyloidosis. J Am Coll Cardiol 2019; 73: 2910–2929. [DOI] [PubMed] [Google Scholar]


