Abstract
Aims
Childhood cancer therapy is associated with a significant risk of therapy‐related cardiotoxicity. This meta‐analysis aims to evaluate cardiac biomarkers for the detection of cancer therapy‐related left ventricular (LV) dysfunction in childhood cancer patients.
Methods and results
PubMed, Cochrane Library, Wiley Library, and Web of Science were screened for studies investigating brain natriuretic peptide (BNP)/N‐terminal proBNP (NT‐proBNP) or cardiac troponin in childhood cancer patients. The odds ratios (OR) for elevation of cardiac biomarkers and association with LV dysfunction were calculated using a random‐effects model. Data from 27 studies with 1651 subjects were included. BNP/NT‐proBNP levels were higher post‐treatment compared with controls or pre‐treatment values [standardized mean difference = 1.0; 95% confidence interval (CI) = 0.6–1.4; n = 320; P < 0.001]. LV dysfunction was present in 11.76% of included patients, and risk for LV dysfunction was increased in patients with elevated BNP/NT‐proBNP (OR = 7.1; 95% CI = 2.0–25.5; n = 350; P = 0.003). The sensitivity of BNP/NT‐proBNP for the detection of LV dysfunction was 33.3%, and the specificity was 91.5%. Sensitivity increased when selecting for studies that assessed patients < 5 years after anthracycline exposure and for studies including high cumulative anthracycline doses. Anthracycline chemotherapy was associated with an increased frequency of elevated troponin (OR = 3.7; 95% CI = 2.1–6.5; n = 348; P < 0.001). The available evidence on the association between elevated troponin and LV dysfunction was insufficient for an adequate analysis. In five included studies, the frequency of LV dysfunction was not increased in patients with elevated troponin (OR = 2.5; 95% CI = 0.5–13.2; n = 179; P = 0.53).
Conclusions
BNP/NT‐proBNP is associated with cardiotoxicity in paediatric cancer patients receiving anthracycline therapy, but owing to low sensitivity, BNP/NT‐proBNP has to be evaluated in the context of further parameters including clinical assessment and echocardiography. Future studies are needed to determine whether troponin serves as a marker for cardiotoxicity in children. Standardized recommendations for the application of cardiac biomarkers in children undergoing cardiotoxic cancer therapy may benefit management and clinical outcome.
Keywords: Anthracycline, Brain natriuretic peptide, Cancer, Cardiotoxicity, Children, Troponin
1. Introduction
Cancer therapy in children has greatly improved over the last decades with an increased number of long‐term survivors. It is estimated that ~379 000 survivors of childhood cancer currently live in the USA.1 This development is, however, associated with significant cancer therapy‐related cardiovascular diseases.2, 3 Cardiovascular side effects include heart failure, coronary artery disease, arrhythmia, valvular disease, and thromboembolism.4, 5 The rate of heart failure is at 4.8% by the age of 45 years, representing a major impact on morbidity and quality of life.1 Anthracyclines (e.g. doxorubicin, daunorubicin, and epirubicin) are components of the treatment of nearly 60% of children with cancer and account for the majority of therapy‐related cardiotoxicity. Anthracyclines have a known cardiotoxic effect that is mediated by generation of reactive oxygen species and inhibition of topoisomerase II inducing dose‐dependent acute myocardial injury.4 Anthracycline‐induced myocardial injury can lead to acute or chronic left ventricular (LV) dysfunction with subsequent development of apparent heart failure.4 Children are particularly susceptible to anthracycline cardiotoxicity with subclinical signs for myocardial injury in up to 47% of patients and subsequent development of cardiomyopathy in 10%.6, 7, 8 Therefore, identification and monitoring of patients at risk in both the acute setting and survivors of childhood cancer is critical. Reliable diagnostic parameters are therefore needed.5, 8
Cardiac biomarkers are cornerstones in the diagnosis of major cardiovascular diseases. Elevated cardiac troponin is the main criterion for the diagnosis for non‐ST segment elevation myocardial infarction in the presence of signs of ischaemia.9 Brain natriuretic peptide (BNP) and the N‐terminal fragment of its prohormone N‐terminal proBNP (NT‐proBNP) are established as markers for heart failure with high sensitivity but low specificity.10 Both biomarkers were identified as potential markers for chemotherapy‐related LV dysfunction and concomitant manifest heart failure in adult cancer patients. They are recommended as an auxiliary measure for diagnosis and monitoring by the European and American cardio‐oncology position papers.11, 12, 13 Cardiac biomarkers also have the potential to identify childhood cancer patients who are likely to have cancer therapy‐related LV dysfunction and require further workup including echocardiography, but systematic evidence is sparse.5, 8
This meta‐analysis therefore aims to evaluate the diagnostic value of troponin and BNP/NT‐proBNP for the detection of anthracycline‐related LV dysfunction in paediatric cancer patients and survivors of childhood cancer.
2. Methods
The methodology of this meta‐analysis was in accordance with the ‘Meta‐analysis of Observational Studies in Epidemiology (MOOSE)’ recommendations.14 Reporting was documented via the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses reporting guidelines flow diagram and checklist (Tables S1 and S2 ).15
The study was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42018106616). The data that support the findings of this study are available within the article or from the corresponding author upon reasonable request.
2.1. Data acquisition
PubMed, Cochrane Library, Wiley Library, and Web of Science were screened for cross‐sectional and prospective studies investigating BNP, NT‐proBNP, and cardiac troponin I or T in cancer therapy‐related cardiotoxicity in paediatric patients or survivors of childhood cancer as defined as onset of cancer at age under 18 years. The search was not restricted for date of publication. The search terms included ‘cancer’, ‘cardiotoxicity’, ‘children’, ‘childhood’, ‘pediatric’, ‘troponin’, and ‘brain natriuretic peptide’. The detailed search strategy can be found within the Supporting Information. Full‐text articles in English language and n ≥ 10 patients were included. For interventional studies investigating potential cardioprotective measures, only the control groups were included. Inclusion was not restricted for study design or type of cancer therapy. The following parameters were extracted: type of study; number of participants; type of therapy; type of biomarker; biomarker values pre‐treatment, post‐treatment, and control; and biomarker levels in patients with and without LV dysfunction.
LV dysfunction was defined as the primary endpoint. For the definition of LV dysfunction, decreased LV ejection fraction (LVEF) or fractional shortening (FS) as defined by the study's protocol was used. Strain analysis, static LV parameters, and diastolic dysfunction as definitions of LV dysfunction were excluded. As secondary endpoints, absolute biomarker levels in patients before treatment or control group compared with patients after treatment were studied. In addition, cardiac biomarkers patients with LV dysfunction compared with patients without LV dysfunction were examined. Prospective studies and cross‐sectional studies with control groups were included for the assessment of all appropriate endpoints. Cross‐sectional studies without control groups were not included in the analyses of pre‐treatment data. Continuous variables presented as median and inter‐quartile range were not included in the quantitative synthesis. Biomarker cut‐offs were used as defined by the study's protocol. Two researchers (L. M. and R. I. M.) conducted screening and extraction of data independently. In case of disagreement, a consensus was negotiated (M. T.).
2.2. Statistical analysis
Data are presented in a forest plot analysis using odds ratio (OR) and 95% confidence interval (CI) for dichotomous variables. For continuous variables, a meta‐analysis is presented using standardized mean difference (SMD) and 95% CI. Individual data are shown as mean and standard deviation. A random‐effects model was used to account for potential discrepancies in study settings. Descriptive statistics were performed using Revman 5.3 software (The Cochrane Collaboration).
Heterogeneity between studies was assessed using the Q statistic, and I 2 statistic was used to quantify heterogeneity according to published thresholds.16 Funnel plot test (Egger's test) was performed in all analyses including 10 or more studies.16 Bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 revised criteria17 and the Newcastle‐Ottawa Scale.18 Criteria for high risk of bias are detailed within the Supporting Information.
3. Results
3.1. Study characteristics
Screening revealed 94 potentially relevant articles. Following exclusion of non‐eligible articles, 27 studies with 1651 subjects met the inclusion criteria and were included in the meta‐analysis (Figure 1). A prospective design was present in 13 studies. The analysed population consisted of 1331 cancer patients and 320 healthy control subjects who were included for the analysis of biomarker dynamics in response to chemotherapy. All studies referred to anthracycline therapy as the main cause of cardiotoxicity. The most commonly used substance was doxorubicin (95% of studies), and the median cumulative anthracycline dose was 223 mg/m2 (Table S3). BNP or NT‐proBNP was assessed in 19 studies (1153 subjects) and troponin in 19 studies (1182 subjects) (Table 1). All studies were included for the analysis of cardiac biomarkers in response to cancer therapy, while only studies using a cut‐off‐based identification of LV dysfunction via echocardiography after or at the same time of biomarker evaluation (11 studies, 652 subjects) were included for the analysis of the association between LV dysfunction and cardiac biomarkers. The overall incidence of LV dysfunction was 11.80%.
Figure 1.
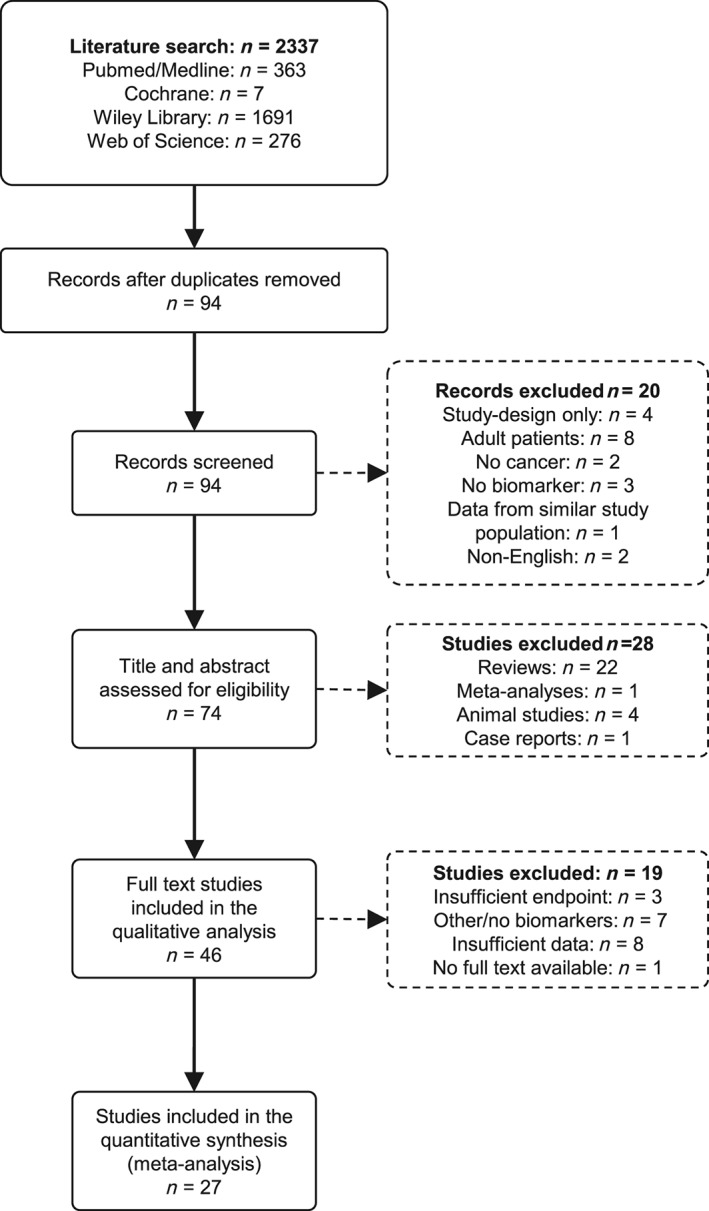
PRISMA flow diagram showing the process of study inclusion. After 94 potentially relevant articles were screened, 27 studies were included in the quantitative synthesis. n, number of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.
Table 1.
Study characteristics summary
| Variable | Subjects (n) | Included studies (n) | |
|---|---|---|---|
| Total number of patients | 1651 | 27 | |
| Biomarker | Troponin I | 217 | 5 |
| Troponin T | 889 | 13 | |
| Troponin I + T | 76 | 1 | |
| BNP | 427 | 8 | |
| NT‐proBNP | 726 | 11 | |
| Study design | Prospective | 784 | 13 |
| Cross‐sectional/retrospective | 867 | 14 | |
| Type of cancer | ALL | 326 | 5 |
| Various acute leukaemia | 141 | 3 | |
| Various haematological | 423 | 5 | |
| Various haematological + solid | 669 | 12 | |
| Various solid | 24 | 1 | |
| Not available | 68 | 1 |
ALL, acute lymphoblastic leukaemia; BNP, brain natriuretic peptide; n, number; NT‐proBNP, N‐terminal proBNP.
3.2. Brain natriuretic peptide/N‐terminal pro‐brain natriuretic peptide
The frequency of BNP/NT‐proBNP elevation above the cut‐off for normal values did not show an increase post‐treatment (OR = 2.0; 95% CI = 0.3–13.3; n = 137; P = 0.47) (Figure 2 A). However, an analysis of absolute BNP/NT‐proBNP revealed 2.6‐fold (median) (25th–75th percentile: 2.2–3.6) increased biomarker concentrations in the post‐treatment group (SMD = 1.0; 95% CI = 0.6–1.4; n = 320; P < 0.001) (Figure 2 B). This was individually recapitulated when compared with non‐treated, healthy control subjects and compared with pre‐treatment values with patients serving as their own control (SMD = 0.7; 95% CI = 0.5–0.9; n = 208; P < 0.001 compared with non‐treated control subjects and SMD = 1.8; 95% CI = 0.4–3.2; n = 112; P = 0.01 compared with pre‐treatment values).
Figure 2.
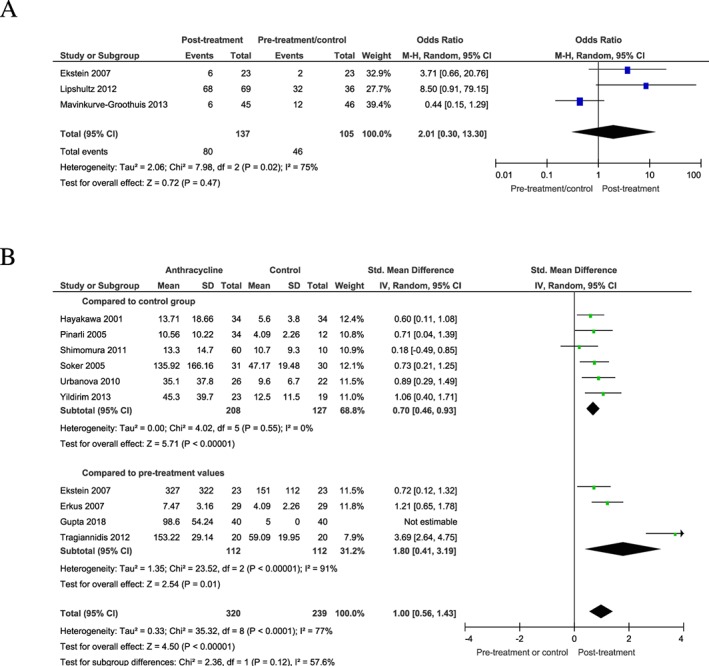
BNP/NT‐proBNP pre‐treatment and post‐treatment. (A) frequency of BNP/NT‐proBNP elevation post‐treatment compared with pre‐treatment or control cohort. (B) absolute BNP/NT‐proBNP levels in patients post‐treatment compared with pre‐treatment or control cohort. Parallelogram boxes denote the odds ratio or standardized mean differences, and horizontal lines represent the 95% confidence interval.
Data on the association between BNP/NT‐proBNP and LV dysfunction were available from six studies investigating the frequency of LV dysfunction in patients with elevated biomarker compared with normal biomarker (dichotomous) and four studies assessing the absolute biomarker levels in patients with and without LV dysfunction (continuous). A cross‐sectional study design was found in all studies except one study with prospective design.19 The median cut‐offs used for the definition of LV dysfunction were FS < 29% (range: 27–30%) and LVEF < 55% (range: 50–64%). High cut‐offs for LVEF were associated with a higher incidence of LV dysfunction (LVEF 60–64%: 17.61% vs. LVEF 50–55%: 10.26%). The frequency of LV dysfunction was significantly higher in patients with elevated BNP/NT‐proBNP (OR = 7.1; 95% CI = 2.0–25.5; n = 350; P = 0.003) (Figure 3 A). A significant effect was only observed for acute/subacute cardiotoxicity (OR = 41.4; 95% CI = 6.1–280.3; n = 88; P < 0.001), but not for long‐term cardiotoxicity in survivors of childhood cancer with cancer diagnosis before ≥5 years prior to biomarker assessment (OR = 3.1; 95% CI = 0.99–9.7; n = 262; P = 0.05 for survivors of childhood cancer). Owing to a high range of age of the included studies (2001–2015), studies were allocated to subgroups showing studies before 2011 and studies published from and after 2011. The analysis revealed that a benefit of BNP/NT‐proBNP for the detection of cardiotoxicity was mainly driven by recent studies (2011 and younger) (OR = 8.0; 95% CI = 0.4–154.8; n = 180; P = 0.17 for studies prior to 2011 and OR = 7.3; 95% CI = 1.6–33.4; n = 170; P = 0.01 for studies 2011 and younger) (Figure S1 ). High cumulative anthracycline doses as defined by 240–600 mg/m2 of doxorubicin or doxorubicin‐equivalent dose were associated with an increased frequency of LV dysfunction compared with studies including lower doses < 240 mg/m2 (20.31% vs. 12.40%). A subgroup analysis indicated that the association between elevated BNP/NT‐proBNP and LV dysfunction was stronger in patients treated with high cumulative anthracycline doses compared with lower doses (OR 3.1; 95% CI = 0.99–9.7; n = 286; P = 0.05 for <240 mg/m2 of cumulative dose and OR = 41.4; 95% CI = 6.1–280.3; n = 64; P < 0.001 for 240–600 mg/m2 of cumulative dose) (Figure S2).
Figure 3.
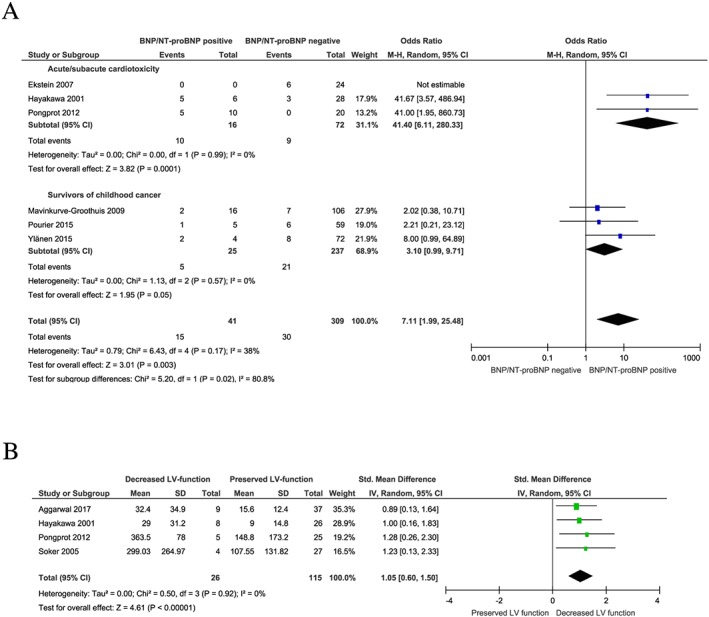
BNP/NT‐proBNP for the detection of LV dysfunction. (A) Frequency of LV dysfunction in patients with elevated BNP/NT‐proBNP compared with patients without elevated BNP/NT‐proBNP post‐treatment. (B) Absolute BNP/NT‐proBNP levels in patients with LV dysfunction compared with patients without LV dysfunction post‐treatment. Parallelogram boxes denote the odds ratio or standardized mean difference, and horizontal lines represent the 95% confidence interval. LV, left ventricular; Std, standardized.
BNP/NT‐proBNP levels were increased in patients with anthracycline‐related LV dysfunction than in patients with preserved LV function (SMD = 1.1; 95% CI: 0.6–1.5; n = 141; P < 0.001) (Figure 3 B). There was no difference between BNP and NT‐proBNP (SMD = 0.9; 95% CI = 0.4–1.5; n = 80; P = 0.001 for BNP and OR = 1.3; 95% CI = 0.5–2.0; n = 61; P = 0.001 for NT‐proBNP) (Figure S3 ). Sensitivity for the detection LV dysfunction by elevated BNP/NT‐proBNP was 33.3%, and specificity was at 91.5% (Table 2). The diagnostic value of BNP/NT‐proBNP for the detection of LV dysfunction was superior in acute/subacute compared with long‐term cardiotoxicity with a sensitivity of 52.6% and 23.8%, respectively (Tables S4 and S5). Additionally, sensitivity increased to 76.9% when selecting for high cumulative anthracycline doses, but the applicability of this observation was limited owing to the low number of included patients within this subgroup.
Table 2.
Diagnostic accuracy of brain natriuretic peptide/N‐terminal pro‐brain natriuretic peptide in detecting overall left ventricular dysfunction
| LV dysf. | No LV dysf. | |||
|---|---|---|---|---|
| Positive test | 15 | 26 | PPV | 0.366 |
| Negative test | 30 | 279 | NPV | 0.903 |
| Sensitivity | Specificity | |||
| 0.333 | 0.915 |
LV dysf., left ventricular dysfunction; NPV, negative predictive value; PPV, positive predictive value.
3.3. Troponin
The analysis of troponin release after anthracycline therapy was available for absolute values and for the frequency of exceeding predefined cut‐offs for significant release compared with pre‐treatment values or control subjects in 19 studies. Troponins I and T from conventional and high‐sensitivity assays were included. The frequency of troponin elevations was higher in patients post‐treatment (OR = 3.7; 95% CI = 2.1–6.5; n = 348; P < 0.001) (Figure 4 A), but within three included studies providing absolute values, troponin levels remained unchanged (SMD = 0.2; 95% CI = −0.2 to 0.5; n = 129; P = 0.32) (Figure 4 B). There was no detectable difference between absolute troponins I and T, but this observation was limited owing to a small number of included patients [dichotomous data: OR = 2.9 for troponin I and OR = 3.8 for troponin T (test for subgroup differences: χ 2 = 0.10 and P = 0.75); absolute troponin levels: SMD = 0.2 for troponin I and SMD = 0.0 for troponin T (test for subgroup differences: χ 2 = 0.38 and P = 0.54)] (Figure S4A, B ). No troponin elevation was demonstrated in studies that performed assessment of troponin ≥3 months after the end of anthracycline therapy.
Figure 4.

Troponin pre‐treatment and post‐treatment. (A) Frequency of troponin elevation post‐treatment compared with pre‐treatment or control cohort. (B) Absolute troponin level in patients post‐treatment compared with pre‐treatment or control cohort. Parallelogram boxes denote the odds ratio or standardized mean difference, and horizontal lines represent the 95% confidence interval.
The association of troponin elevation with LV dysfunction as indicated by decreased LVEF or FS was examined in five studies with 179 patients. For the definition of LV dysfunction, FS < 29% (range: 27–30%) and LVEF < 55% were included. A significant association of troponin and LV dysfunction was not observed in a cut‐off‐based (dichotomous) analysis (OR = 2.5; 95% CI = 0.5–13.2; n = 179; P = 0.29) (Figure 5).
Figure 5.

Troponin for the detection of left ventricular (LV) dysfunction. Frequency of LV dysfunction in patients with elevated troponin compared with patients without elevation post‐treatment. Parallelogram boxes denote the odds ratio, and horizontal lines represent the 95% confidence interval.
3.4. Assessment of bias and heterogeneity
Risk of patient selection bias, index test bias, and flow and timing bias was assessed in all included studies. Reference standard bias was only assessed in studies that included a correlation with echocardiography and biomarkers (Figure S5 ). The highest risk of bias was found for patient selection bias, and flow and timing bias (Figure 6). A median of six stars on a scale of nine stars maximum was reached using the Newcastle‐Ottawa‐Scale (Table S6). Non‐blinded biomarker assessment and inadequate follow‐up were most commonly found.
Figure 6.

Bias of included studies. Bias assessment of all included studies using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) revised criteria. Risk of bias is depicted as low risk of bias (green), unclear risk of bias (yellow), and high risk of bias (red).
I 2 statistic revealed moderate to substantial heterogeneity for the assessment of BNP/NT‐proBNP post‐treatment compared with pre‐treatment or control (I 2 = 75% for dichotomous data and I 2 = 77% for continuous data). The analysis of the likelihood for LV dysfunction in patients with elevated biomarker and the analysis of biomarker levels in patients with or without LV dysfunction showed moderate heterogeneity (I 2 = 38% for dichotomous data and I 2 = 0% for continuous data). Funnel plot test (Egger's test) identified one outlier20 for BNP/NT‐proBNP post‐treatment compared with pre‐treatment or controls (Figure S6 ).
4. Discussion
This meta‐analysis aimed to evaluate cardiac troponin and BNP/NT‐proBNP in diagnosis and screening of cancer therapy‐related cardiotoxicity in paediatric cancer patients and survivors of childhood cancer. The study indicated that anthracycline chemotherapy in children is associated with increased BNP/NT‐proBNP levels. Elevated BNP/NT‐proBNP indicated an increased risk for LV dysfunction in children receiving anthracycline chemotherapy with a low sensitivity. Troponin increased during anthracycline chemotherapy, but the available evidence did not support an associated with LV dysfunction.
Timely detection of cardiotoxicity in children undergoing anthracycline chemotherapy is crucial because early cardioprotective therapy may prevent or mitigate cardiotoxicity.6, 21, 22 Echocardiography serves as the primary tool for the detection of LV dysfunction, but clinically apparent changes can be a delayed phenomenon and are therefore limited as an early indicator for the need of cardioprotective interventions.1, 23, 24 Additionally, reference values for the assessment of LV function are less validated for paediatric patients than in adults. The strain analysis may benefit the early identification of cardiotoxicity in childhood cancer patients, but only few studies covered myocardial strain for an early diagnosis of LV dysfunction.25, 26 At present, systematic evidence on the role of biomarkers for cardiotoxicity in childhood cancer patients is limited with one available systematic review that did not assess acute/subacute cardiotoxicity seen within <1 year after the beginning of cancer therapy.27
With an overall low sensitivity of 52.6%, the value of BNP/NT‐proBNP for the detection of acute/subacute cardiotoxicity is limited. A stronger association of BNP/NT‐proBNP with LV dysfunction and an increased sensitivity of 76.9% was observed within the small group of patients who received high‐dose anthracycline treatment (≥240 mg/m2 of doxorubicin or doxorubicin‐equivalent dose) compared with those who received lower doses. Still, it is not justified to use BNP/NT‐proBNP as a standalone marker in this collective. All except one study had a cross‐sectional study design. Therefore, a role of BNP/NT‐proBNP in detecting cardiotoxicity was demonstrated, but there is no sufficient evidence for BNP/NT‐proBNP in predicting future cardiac dysfunction. Despite its potential as an auxiliary diagnostic parameter, the evaluation of BNP/NT‐proBNP is challenging owing to variations in reference values during childhood. Particularly, BNP/NT‐proBNP decreases with age and is increased in girls within the second decade of life.28 Therefore, an age‐adjusted interpretation of BNP/NT‐proBNP plasma levels is recommended in children, and potential further effects on BNP/NT‐proBNP plasma levels may remain uncovered.28 Therefore, assessment of BNP/NT‐proBNP may be embedded in a cardio‐oncology workup that further includes assessment of risk factors, physical examination, and echocardiography, rather than being used as a standalone marker.
High prevalence of late cardiotoxicity in survivors of childhood cancer indicates the need for lifelong cardiomyopathy surveillance.5 Echocardiography is the primary surveillance modality, but cardiac biomarkers may serve as an additional tool for screening and monitoring of trends.1, 5, 8 The absence of interobserver variability and the simple and cost‐effective execution of biomarker assessment may be beneficial for this purpose.5 However, cardiac biomarker assessment is not recommended within the current clinical practice guidelines owing to lack of evidence.5, 8 The present systematic analysis aimed to assess BNP/NT‐proBNP and troponin within this collective. The meta‐analysis did not demonstrate a robust association between elevated BNP/NT‐proBNP and the prevalence of LV dysfunction in survivors of childhood cancer. Considering the low sensitivity, our results indicate that there is not yet sufficient evidence to support the routine use of BNP/NT‐proBNP as a parameter for screening of patients at risk for late anthracycline‐related LV dysfunction. However, elevated BNP/NT‐proBNP in childhood cancer patients indicates high likelihood of LV dysfunction with a specificity of 91.5% and negative predictive value of 90.3%. Therefore, elevated BNP/NT‐proBNP should prompt further cardio‐oncologic workup. Additionally, assessment of BNP/NT‐proBNP may benefit evaluation of LV function in the presence of inconclusive echocardiography, bad imaging quality, or borderline LV function. Again, further diagnostic measures are necessary to achieve sufficient diagnostic accuracy.
Large studies investigating cardiac troponin as marker for cardiotoxicity in adult patients have shown robust data on troponin as a predictor of chemotherapy‐related cardiotoxicity.12, 29 It is hypothesized that troponin indicates early myocardial injury in response to anthracycline exposure and thereby predicts development of manifest heart failure in the further course. Regarding promising results in single studies on troponin for cardiotoxicity in childhood cancer therapy, troponin was clearly expected to be associated with anthracycline cardiotoxicity in paediatric cancer patients, but no such association was demonstrated. Several confounding factors may have contributed to this unexpected result. At first, the analysis of troponin for the detection of LV dysfunction was underpowered, and the small number of included patients and the low frequency of elevated troponin within included studies limited interpretation of the results. Secondly, anthracycline‐related cardiotoxicity typically induces a low‐level increase of troponin compared with troponin dynamics in acute myocardial infarction6 and use of high‐sensitivity‐/ultra‐sensitivity troponin assays may benefit the value of troponin for detection of anthracycline‐related cardiotoxicity. Of note, only one study included for assessment of troponin for the detection of LV dysfunction stated the use of a high‐sensitivity troponin assay. Finally, the optimum timing of troponin assessment after anthracycline exposure has not yet been systematically evaluated in childhood cancer patients and may improve diagnostic and prognostic performance of troponin. With troponin indicating acute myocardial injury in response to anthracycline exposure, assessment within the first days after therapy is expected to display peak values as seen in adult patients.30 Within included studies, no biomarker elevation was found ≥3 months after the end of anthracycline therapy, further supporting the use of troponin in the acute setting. However, the available evidence is insufficient to confirm or refute a benefit of troponin for the detection and/or prediction of anthracycline‐related cardiotoxicity in children.
Only few studies cover the response of cardiac biomarkers to cardioprotective therapy in children receiving anthracyclines.6, 21, 22 The frequency of troponin elevations was reduced when children received concomitant therapy with the cardioprotective iron chelator dexrazoxane.6 Heart failure therapy with ACE inhibitors or beta‐blockers has also shown to reduce troponin levels in children treated with anthracyclines,21, 22 but an association between troponin and LV function in this setting has not yet been demonstrated.6, 21, 22 In adult cancer patients, elevated troponin was successfully used as a marker for high‐risk patients to initiate cardioprotective treatment for the prevention of LV dysfunction.31, 32 Future studies are needed to validate cardiac biomarkers to identify children at risk for cardiotoxicity that benefit from cardioprotective therapy.
Several limitations were found within the present meta‐analysis. At first, the definitions for LV dysfunction were not uniformly applied. Within most of studies, a combined endpoint with decreased LVEF or decreased FS was used. FS for the assessment of LV dysfunction is judged as a valid method for the assessment of cardiotoxicity in children33 but may still be less valid than ejection fraction. Additionally, unusual cut‐offs for FS and LVEF that were used in three of eight studies included for the analysis of BNP/NT‐proBNP for LV dysfunction may have caused misclassification bias owing to false‐positive results. Secondly, several studies used fixed biomarker cut‐offs for cardiac biomarkers, while others utilized age‐dependent cut‐offs. These differences may have caused heterogeneity and bias. Thirdly, studies on cardiac biomarkers regarding other relevant cancer therapy‐related side effects such as an increased risk for coronary artery disease or other forms of cardiotoxic cancer therapy such as radiotherapy were not available for the present analysis. Patients who received high‐dose anthracyclines and chest radiotherapy have an up to 61.5‐fold increased risk and a cumulative incidence for overall cardiac disease of 11.0% at the age of 40 years.2 Therefore, evaluation of cardiac biomarkers in the detection of cardiovascular adverse events other than heart failure is needed in future studies.
5. Conclusions
In conclusion, BNP/NT‐proBNP increases in response to anthracycline chemotherapy in children and is associated with acute therapy‐related LV dysfunction in children with cancer who are receiving anthracycline chemotherapy. With a low sensitivity, assessment of BNP/NT‐proBNP should be embedded in a cardio‐oncology workup including clinical examination and echocardiography. Our data do not support the use of BNP/NT‐proBNP for screening of late cardiotoxicity in survivors of childhood cancer. Randomized studies are needed to provide evidence if monitoring of BNP/NT‐proBNP decreases the risk for manifest heart failure and mortality in childhood cancer patients. Standardized recommendations on the application of cardiac biomarkers for the assessment of cardiotoxicity in paediatric cancer patients are fundamental to facilitate optimum cardio‐oncology care of this collective.
Conflict of interest
Lars Michel, Raluca I. Mincu, Simone M. Mrotzek, Sebastian Korste, Ulrich Neudorf, Tienush Rassaf, and Matthias Totzeck declare that they have no conflict of interest.
Funding
This work was supported by the IFORES research grant from the Medical Faculty, University of Duisburg‐Essen (Universität Duisburg‐Essen), Hufelandstrasse 55, 45147 Essen, Germany to LM. The funder had no involvement in the implementation of the study.
Supporting information
Data S1. Supporting information.
Data S2. Supporting information.
Michel, L. , Mincu, R. I. , Mrotzek, S. M. , Korste, S. , Neudorf, U. , Rassaf, T. , and Totzeck, M. (2020) Cardiac biomarkers for the detection of cardiotoxicity in childhood cancer—a meta‐analysis. ESC Heart Failure, 7: 423–433. 10.1002/ehf2.12589.
References
- 1. Lipshultz SE, Sambatakos P, Maguire M, Karnik R, Ross SW, Franco VI, Miller TL. Cardiotoxicity and cardioprotection in childhood cancer. Acta Haematol 2014; 132: 391–399. [DOI] [PubMed] [Google Scholar]
- 2. Haddy N, Diallo S, El‐Fayech C, Schwartz B, Pein F, Hawkins M, Veres C, Oberlin O, Guibout C, Pacquement H, Munzer M. Cardiac diseases following childhood cancer treatment: cohort study. Circulation 2016; 133: 31–38. [DOI] [PubMed] [Google Scholar]
- 3. Kremer LC, Caron HN. Anthracycline cardiotoxicity in children. N Engl J Med 2004; 351: 120–121. [DOI] [PubMed] [Google Scholar]
- 4. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio‐oncology—strategies for management of cancer‐therapy related cardiovascular disease. Int J Cardiol 2019; 280: 163–175. [DOI] [PubMed] [Google Scholar]
- 5. Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJ, Shankar S, Sieswerda E, Skinner R, Steinberger J, Van Dalen E, Van der Pal H, Wallace WH, Levitt G, Kremer LC, International Late Effects of Childhood Cancer Guideline Harmonization Group . Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015; 16: e123–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, Colan SD, Neuberg DS, Dahlberg SE, Henkel JM, Asselin BL, Athale UH, Clavell LA, Laverdière C, Michon B, Schorin MA, Sallan SE. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high‐risk acute lymphoblastic leukemia: associations with long‐term echocardiographic outcomes. J Clin Oncol 2012; 30: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michel L, Rassaf T. Cardio‐oncology: need for novel structures. Eur J Med Res 2019; 24: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, Hudson MM, Kremer LC, Landy DC, Miller TL, Oeffinger KC, Rosenthal DN, Sable CA, Sallan SE, Singh GK, Steinberger J, Cochran TR, Wilkinson JD, American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Basic Cardiovascular Sciences, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Radiolo . Long‐term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 2013; 128: 1927–1995. [DOI] [PubMed] [Google Scholar]
- 9. Roffi M. Patrono C1, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 11. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, Piña IL, Volgman AS, American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research . Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation 2018; 137: e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy‐related cardiotoxicity. J Thorac Dis 2018; 10: S4282–s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768–2801. [DOI] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of Observational Studies in Epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://handbook.cochrane.org (01 January 2019).
- 17. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS‐2 Group . QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 18. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (01 January 2019).
- 19. Ekstein S, Nir A, Rein AJ, Perles Z, Bar‐Oz B, Salpeter L, Algur N, Weintraub M. N‐terminal‐proB‐type natriuretic peptide as a marker for acute anthracycline cardiotoxicity in children. J Pediatr Hematol Oncol 2007; 29: 440–444. [DOI] [PubMed] [Google Scholar]
- 20. Tragiannidis A, Dokos C, Tsotoulidou V, Giannopoulos A, Pana ZD, Papageorgiou T, Karamouzis M, Athanassiadou F. Brain natriuretic peptide as a cardiotoxicity biomarker in children with hematological malignancies. Minerva Pediatr 2012; 64: 307–312. [PubMed] [Google Scholar]
- 21. El‐Shitany NA, Tolba OA, El‐Shanshory MR, El‐Hawary EE. Protective effect of carvedilol on adriamycin‐induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail 2012; 18: 607–613. [DOI] [PubMed] [Google Scholar]
- 22. Gupta V, Kumar Singh S, Agrawal V, Bali ST. Role of ACE inhibitors in anthracycline‐induced cardiotoxicity: a randomized, double‐blind, placebo‐controlled trial. Pediatr Blood Cancer 2018; 65: e27308. [DOI] [PubMed] [Google Scholar]
- 23. Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, Sandor G, Benson L, Williams R. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Children's Cancer Study Group. Pediatrics 1992; 89: 942–949. [PubMed] [Google Scholar]
- 24. Fallah‐Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II‐positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol 2011; 57: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 25. Mawad W, Drolet C, Dahdah N, Dallaire F. A review and critique of the statistical methods used to generate reference values in pediatric echocardiography. J Am Soc Echocardiogr 2013; 26: 29–37. [DOI] [PubMed] [Google Scholar]
- 26. Pignatelli RH, Ghazi P, Reddy SC, Thompson P, Cui Q, Castro J, Okcu MF, Jefferies JL. Abnormal myocardial strain indices in children receiving anthracycline chemotherapy. Pediatr Cardiol 2015; 36: 1610–1616. [DOI] [PubMed] [Google Scholar]
- 27. Leerink JM, Verkleij SJ, Feijen EA, Mavinkurve‐Groothuis AM, Pourier MS, Ylänen K, Tissing WJ, Louwerens M, Van Den Heuvel MM, van Dulmen‐den Broeder E, de Vries AC. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart 2019; 105: 210–216. [DOI] [PubMed] [Google Scholar]
- 28. Nir A, Lindinger A, Rauh M, Bar‐Oz B, Laer S, Schwachtgen L, Koch A, Falkenberg J, Mir TS. NT‐pro‐B‐type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009; 30: 3–8. [DOI] [PubMed] [Google Scholar]
- 29. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high‐dose chemotherapy. Circulation 2004; 109: 2749–2754. [DOI] [PubMed] [Google Scholar]
- 30. Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, Cinieri S, Martinelli G, Fiorentini C, Cipolla CM. Myocardial injury revealed by plasma troponin I in breast cancer treated with high‐dose chemotherapy. Ann Oncol 2002; 13: 710–715. [DOI] [PubMed] [Google Scholar]
- 31. Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, Barbieri E, Gori S, Colombo A, Curigliano G, Salvatici M, Rizzo A, Ghisoni F, Bianchi A, Falci C, Aquilina M, Rocca A, Monopoli A, Milandri C, Rossetti G, Bregni M, Sicuro M, Malossi A, Nassiacos D, Verusio C, Giordano M, Staszewsky L, Barlera S, Nicolis EB, Magnoli M, Masson S, Cipolla CM, ICOS‐ONE Study Investigators . Anthracycline‐induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society‐one trial. Eur J Cancer 2018; 94: 126–137. [DOI] [PubMed] [Google Scholar]
- 32. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation 2006; 114: 2474–2481. [DOI] [PubMed] [Google Scholar]
- 33. Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006; 19: 1413–1430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data S2. Supporting information.


