Abstract
Aims
In patients with aortic stenosis (AS), B‐type natriuretic peptide (BNP) is a prognostic marker. However, there is little information on the association between BNP and invasive haemodynamics in AS. The aim of the present study was to assess the hitherto not well‐defined relationship between BNP and invasive haemodynamics in patients with severe AS undergoing aortic valve replacement (AVR) with a view to understand the link between high BNP and poor prognosis in these patients. In particular, we were interested in the association between BNP and combined pre‐capillary and post‐capillary pulmonary hypertension (CpcPH).
Methods and results
BNP was measured in 252 patients (age 74 ± 10 years, 58% male patients) with severe AS [indexed aortic valve area 0.4 ± 0.1 cm2/m2 and left ventricular ejection fraction (LVEF) 57 ± 12%] the day before cardiac catheterization. Patients were followed for a median (interquartile range) period of 3.1 (2.3–4.3) years after surgical (n = 157) or transcatheter (n = 95) AVR. The prevalence of CpcPH (mean pulmonary artery pressure ≥ 25 mmHg, mean pulmonary artery wedge pressure > 15 mmHg, and pulmonary vascular resistance > 3 Wood units) was 13%. The median BNP plasma concentration was 188 (78–452) ng/L. The indexed aortic valve area was similar across BNP quartiles (P = 0.21). Independent predictors of higher BNP (ln transformed) included lower haemoglobin (beta = −0.18; P < 0.001), lower LVEF (beta = −0.20; P < 0.001), more severe mitral regurgitation (beta = 0.20; P < 0.001), higher mean pulmonary artery wedge pressure (beta = −0.37; P < 0.001), and higher pulmonary vascular resistance (beta = 0.21; P < 0.001). In a multivariate model with CpcPH rather than its haemodynamic components, CpcPH was independently associated with higher BNP (0.21; P < 0.001). Higher ln BNP was associated with higher mortality [hazard ratio 1.90 (95% confidence interval 1.33–2.71); P < 0.001] in the univariate analysis. Patients in the third and fourth BNP quartiles had a more than six‐fold risk of death compared with patients in the first and second quartiles [hazard ratio 6.29 (95% confidence interval 1.86–21.27); P = 0.003]. In the multivariate analysis, lower LVEF [hazard ratio 0.96 (95% confidence interval 0.94–0.99) per 1% increase; P = 0.01] and CpcPH [hazard ratio 4.58 (95% confidence interval 1.89–11.09); P = 0.001] but not BNP were independently associated with mortality. The areas under the receiver operator characteristics curve for BNP for the prediction of CpcPH and mortality were 0.88 and 0.74, respectively.
Conclusions
In patients with severe AS, higher BNP is a marker of the presence of CpcPH and its contributors. The association between BNP and such an adverse haemodynamic profile at least in part explains the ability of BNP to predict long‐term post‐AVR mortality.
Keywords: Natriuretic peptide, Haemodynamics, Pulmonary hypertension, Aortic stenosis, Prognosis, Valve replacement
Introduction
In patients with aortic stenosis (AS), B‐type natriuretic peptide (BNP) and the N‐terminal part of its precursor peptide, N terminal proBNP (NT‐proBNP) are markers of prognosis.1, 2, 3, 4 This applies for both asymptomatic patients managed conservatively in whom BNP predicts the occurrence of symptoms and the need for aortic valve replacement (AVR)5 and patients undergoing AVR in whom BNP and NT‐proBNP predict clinical outcomes after surgical AVR (SAVR)6 and transcatheter AVR (TAVR), respectively.2 Higher BNP in AS is associated with lower left ventricular ejection fraction (LVEF) and higher left ventricular mass.4 However, other information on the pathophysiological correlates of high BNP in AS is sparse. In general, the myocardial release of BNP is thought to be triggered primarily by stretch of left ventricular myocytes7 and high wall stress.8, 9 This concept underlies the idea that BNP might identify asymptomatic patients with AS in whom the compensatory mechanisms of the left ventricle start to become maladaptive with the consecutive development of left ventricular diastolic dysfunction and subtle systolic dysfunction leading to an increase in left ventricular end‐diastolic pressure (LVEDP). Current guidelines state that a markedly elevated BNP value represents a IIa indication for AVR in asymptomatic subjects with severe AS.10 However, the relationship between BNP and invasive haemodynamics in patients with severe AS has only been investigated in early small studies looking at a limited set of parameters.8, 11, 12 Given the recently reported strong prognostic impact of invasive haemodynamics in patients with AS,13, 14 its relationship with BNP is clinically relevant however.
Therefore, the aim of this study was to assess the hitherto not well‐defined relationship between BNP and invasive haemodynamics in patients with severe AS undergoing AVR with a view to understand the link between high BNP and poor prognosis in these patients. In particular, we investigated whether BNP is a marker of pulmonary hypertension and combined pre‐capillary and post‐capillary pulmonary hypertension (CpcPH), respectively, which both are markers of poor prognosis in AS.13, 15
Methods
Study population
We studied 252 consecutive patients with severe AS undergoing cardiac catheterization in a single centre between January 2011 and January 2016 prior to AVR. This is a retrospective analysis of prospectively and systematically collected haemodynamic data. The study complies with the Declaration of Helsinki. The study was approved by the local ethics committee. The present study population is a subgroup of a larger cohort we have previously reported on.13
B‐type natriuretic peptide measurement
On the day prior to cardiac catheterization, blood was drawn from an antecubital vein and collected in in plastic tubes containing ethylene‐diamine‐tetra‐acetate. BNP was measured using a commercially available and well characterized fluorescence immunoassay (Biosite Triage, Biosite Inc., San Diego, CA, USA). All analyses were performed in the clinical laboratory of the Kantonsspital St. Gallen by technicians unaware of any clinical data.
Cardiac catheterization
Patients underwent coronary angiography using five or six French catheters by femoral or radial access and right heart catheterization using six French Swan Ganz catheters by femoral or brachial access. The mean pulmonary artery pressure (mPAP) and pulmonary artery wedge pressure (mPAWP) were measured. Measurements were obtained at end expiration; the mPAWP was calculated over the entire cardiac cycle, and V waves were included to determine mPAWP. In patients with atrial fibrillation, at least five cardiac cycles were used to assess mPAP and mPAWP. Cardiac output was assessed by the indirect Fick method. The transpulmonary gradient was calculated as mPAP (mPAP) − mPAWP. Pulmonary vascular resistance (PVR) was calculated as transpulmonary gradient/cardiac output. If the aortic valve was crossed, which was at the discretion of the invasive cardiologist, the LVEDP was recorded by a pigtail catheter (n = 159). Pulmonary hypertension was defined as mPAP ≥ 25 mmHg, and CpcPH was defined as mPAP ≥ 25 mmHg, mPAWP > 15 mmHg, and PVR > 3 Wood units.16
Follow‐up
All patients underwent SAVR (n = 157) or TAVR (n = 95). Information on long‐term follow up was obtained from patients, general practitioner, and hospital or practice cardiologists. The clinical endpoint was all‐cause mortality.
Statistical analysis
Categorical data are presented as numbers and percentages, and continuous data are given as mean ± standard deviation or median (interquartile range) as appropriate. The population was divided into BNP quartiles. Patient characteristics and non‐invasive and invasive haemodynamic measurements were compared across BNP quartiles using χ 2‐tests, analysis of variance, or Kruskal–Wallis tests. For correlations of interest, Pearson correlation coefficients were calculated (with ln‐transformed BNP values given its skewed distribution). Multivariate linear regression was performed to identify independent predictors of plasma ln BNP. Survival of patients in different BNP quartiles was compared using Kaplan–Meier plots and log rank tests. Multivariate Cox regression was performed to assess independent predictors of mortality. Covariates associated with mortality in the univariate analysis (P value < 0.1) were entered into the multivariate model. We also performed a multivariate logistic regression analysis with BNP in the highest quartiles as the dependent variable. Receiver operator characteristic curves were constructed for the ability of BNP and the BNP ratio to predict the presence of pulmonary hypertension and CpcPH and as well as mortality. Given that BNP depends on age and gender,17 we also calculated the BNP ratio, i.e. the ratio of the measured BNP divided by the maximal normal value for age and gender.1 The BNP ratio has previously shown to predict outcomes in patients with asymptomatic AS.1 Therefore we report results for both BNP and the BNP ratio. A P value < 0.05 was considered statistically significant. All analyses were performed using SPSS statistical package version 20.0 (SPSS Inc, Chicago, Illinois).
Results
Study population
The mean age of the study population (n = 252) was 74 ± 10 years, and 58% were male patients. The mean indexed aortic valve area was 0.4 ± 0.1 cm2/m2, and the mean LVEF was 57 ± 12%. The prevalence of pulmonary hypertension was 44% (111/252 patients), and 13% (32/252) of the entire population had CpcPH.
Clinical characteristics and haemodynamics according to B‐type natriuretic peptide quartiles
The median (interquartile) BNP plasma concentration in the entire population was 188 (78–452) ng/L. With increasing BNP quartile, patients were older, had lower estimated glomerular filtration rate, haemoglobin, and forced expiratory volume within the first second; were more symptomatic and more likely to have atrial fibrillation and had higher heart rate; and were more likely to use oral anticoagulants, loop diuretics, spironolactone, and digoxin (Table 1).
Table 1.
Clinical characteristics according to B‐type natriuretic peptide quartiles
| Q1 (n = 63) | Q2 (n = 63) | Q3 (n = 63) | Q4 (n = 63) | P value | |
|---|---|---|---|---|---|
| Age (years) | 68 ± 11 | 76 ± 8 | 78 ± 8 | 79 ± 10 | <0.001 |
| Gender (male) | 38 (60%) | 37 (59%) | 29 (46%) | 34 (54%) | 0.37 |
| Body mass index (kg/m2) | 28.4 ± 4.5 | 28.7 ± 5.8 | 27.4 ± 5.4 | 27.0 ± 4.8 | 0.19 |
| eGFR (mL/min/1.73m2) | 85 ± 24 | 75 ± 26 | 69 ± 28 | 63 ± 30 | <0.001 |
| Haemoglobin (g/L) | 142 ± 14 | 135 ± 15 | 135 ± 17 | 126 ± 20 | <0.001 |
| Diabetes | 10 (16%) | 13 (21%) | 13 (21%) | 17 (30%) | 0.50 |
| Stroke | 4 (6%) | 3 (5%) | 6 (10%) | 5 (8%) | 0.75 |
| Chronic obstructive lung disease | 9 (14%) | 7 (11%) | 10 (16%) | 11 (17%) | 0.78 |
| FEV1 (% predicted) | 88 ± 23 | 94 ± 19 | 86 ± 20 | 76 ± 20 | <0.001 |
| Heart rhythm | 0.01 | ||||
| Sinus rhythm | 63 (100%) | 53 (84%) | 50 (79%) | 48 (76%) | |
| Atrial fibrillation | 0 | 6 (10%) | 7 (11%) | 12 (19%) | |
| Pacing | 0 | 4 (6%) | 5 (8%) | 3 (5%) | |
| Other | 0 | 0 | 1 (2%) | 0 | |
| Heart rate (bpm) | 70 ± 12 | 70 ± 11 | 68 ± 13 | 75 ± 15 | 0.02 |
| Medication | |||||
| Oral anticoagulation | 5 (8%) | 10 (16%) | 17 (27%) | 22 (35%) | 0.001 |
| Aspirin | 39 (62%) | 40 (63%) | 38 (60%) | 36 (57%) | 0.9 |
| Loop diuretics | 21 (33%) | 28 (44%) | 32 (51%) | 46 (73%) | <0.001 |
| Beta‐blocker | 21 (33%) | 32 (51%) | 30 (48%) | 29 (46%) | 0.21 |
| ACEI/ARB | 34 (54%) | 39 (62%) | 28 (44%) | 31 (49%) | 0.24 |
| Digoxin | 0 | 1 (2%) | 7 (11%) | 13 (21%) | <0.001 |
| Spironolactone | 0 | 2 (3%) | 3 (5%) | 7 (11%) | 0.03 |
| Symptoms | <0.001 | ||||
| Dyspnoea NYHA class | |||||
| I | 15 (24%) | 17 (27%) | 11 (17%) | 6 (10%) | |
| II | 35 (56%) | 34 (54%) | 30 (48%) | 23 (37%) | |
| III | 13 (21%) | 11 (17%) | 20 (32%) | 20 (32%) | |
| IV | 0 | 1 (2%) | 2 (3%) | 14 (22%) | |
| Mode of AVR | <0.001 | ||||
| SAVR | 54 (86%) | 43 (68%) | 36 (57%) | 24 (38%) | |
| TAVR | 9 (14%) | 20 (32%) | 27 (43%) | 39 (62%) |
ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; AVR, aortic valve replacement; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume within the first second; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Data are given as numbers and percentages, mean ± standard deviation, or median (interquartile range).
The indexed aortic valve area was similar across BNP quartiles (Table 2). However, with higher BNP quartile, left ventricular end‐diastolic diameter was larger, LVEF was lower, left atrial area was larger, the prevalence of more severe mitral regurgitation was higher, and tricuspid annular plane systolic excursion was lower (Table 2). With increasing BNP quartile, patients had a worse haemodynamic profile as reflected by higher right atrial pressure, right ventricular end‐diastolic pressure, mPAP, mPAWP, LVEDP, and PVR, lower cardiac index, and stroke volume index, as well as higher prevalence of pulmonary hypertension and CpcPH (Table 2).
Table 2.
Data from echocardiography and cardiac catheterization according to B‐type natriuretic peptide quartiles
| Q1 (n = 63) | Q2 (n = 63) | Q3 (n = 63) | Q4 (n = 63) | P value | |
|---|---|---|---|---|---|
| Echocardiography | |||||
| Left ventricular end‐diastolic diameter (mm) | 44 ± 6 | 47 ± 9 | 47 ± 8 | 49 ± 7 | 0.005 |
| Left ventricular ejection fraction (%) | 64 ± 7 | 59 ± 11 | 58 ± 12 | 47 ± 13 | <0.001 |
| Left atrial area (cm2) | 20 ± 3 | 22 ± 6 | 25 ± 5 | 31 ± 10 | <0.001 |
| Tricuspid annular plane systolic excursion (mm) | 23 ± 4 | 22 ± 5 | 22 ± 5 | 19 ± 4 | 0.006 |
| Mean aortic valve gradient (mmHg) | 43 ± 12 | 44 ± 17 | 48 ± 17 | 46 ± 18 | 0.37 |
| Aortic valve area (cm2) | 0.85 ± 0.22 | 0.87 ± 0.26 | 0.77 ± 0.23 | 0.75 ± 0.23 | 0.01 |
| Indexed aortic valve area (cm2/m2) | 0.43 ± 0.10 | 0.44 ± 0.15 | 0.41 ± 0.13 | 0.40 ± 0.12 | 0.21 |
| Mitral regurgitation | <0.001 | ||||
| No | 48 (76%) | 27 (43%) | 20 (32%) | 8 (13%) | |
| Mild | 8 (13%) | 25 (40%) | 36 (57%) | 38 (60%) | |
| Moderate | 2 (3%) | 3 (5%) | 4 (6%) | 12 (19%) | |
| Severe | 0 | 1 (2%) | 3 (5%) | 2 (3%) | |
| Coronary artery disease | 0.07 | ||||
| None | 38 (60%) | 29 (46%) | 28 (44%) | 26 (41%) | |
| 1‐vessel disease | 15 (24%) | 10 (16%) | 10 (16%) | 9 (14%) | |
| 2‐vessel disease | 5 (8%) | 8 (13%) | 13 (21%) | 14 (22%) | |
| 3‐vessel disease | 5 (8%) | 16 (25%) | 11 (17%) | 13 (21%) | |
| Invasive haemodynamics | |||||
| Mean right atrial pressure (mmHg) | 5 ± 2 | 6 ± 3 | 6 ± 3 | 9 ± 5 | <0.001 |
| Right ventricular end‐diastolic pressure (mmHg) | 7 ± 3 | 7 ± 3 | 8 ± 3 | 11 ± 6 | <0.001 |
| mPAP (mmHg) | 18 ± 5 | 22 ± 6 | 25 ± 7 | 36 ± 12 | <0.001 |
| mPAWP (mmHg) | 10 ± 4 | 13 ± 6 | 16 ± 6 | 24 ± 8 | <0.001 |
| Pulmonary vascular resistance (Wood units) | 1.7 ± 0.7 | 1.8 ± 0.7 | 2.2 ± 1.4 | 3.2 ± 1.9 | <0.001 |
| Left ventricular end‐diastolic pressure (mmHg) (n = 159) | 17 ± 6 | 21 ± 6 | 22 ± 7 | 25 ± 8 | <0.001 |
| Pulmonary hypertension | 9 (14%) | 21 (33%) | 30 (48%) | 51 (81%) | <0.001 |
| Combined pre‐capillary and post‐capillary pulmonary hypertension | 1 (2%) | 1 (2%) | 5 (8%) | 25 (40%) | <0.001 |
| Mean aortic pressure (mmHg) | 99 ± 15 | 100 ± 15 | 97 ± 12 | 94 ± 15 | 0.09 |
| Systemic vascular resistance (Wood units) | 18.8 ± 3.8 | 20.1 ± 5.2 | 21.5 ± 5.5 | 21.6 ± 5.3 | 0.004 |
| Cardiac output (L/min) | 5.2 ± 0.8 | 4.9 ± 1.0 | 4.4 ± 1.0 | 4.0 ± 0.9 | <0.001 |
| Cardiac index (L/min/m2) | 2.6 ± 0.4 | 2.5 ± 0.5 | 2.3 ± 0.7 | 2.2 ± 0.6 | 0.001 |
| Stroke volume (mL) | 76 ± 15 | 72 ± 17 | 66 ± 19 | 57 ± 17 | <0.001 |
| Stroke volume index (mL/m2) | 38 ± 8 | 36 ± 8 | 35 ± 11 | 30 ± 10 | <0.001 |
mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure.
Data are given as numbers and percentages, mean ± standard deviation, and/or median (interquartile range).
Predictors of high B‐type natriuretic peptide
Lower haemoglobin, lower LVEF, more severe mitral regurgitation, higher mPAWP, and higher PVR were independently associated with higher BNP (Table 3). The correlations between BNP and key haemodynamic variables are shown in Figure 1. If the presence of CpcPH rather than the single contributing haemodynamic parameters (mPAP, mPAWP, and PVR) was entered into the model, higher age (β = 0.21), lower haemoglobin (β = −0.15), lower forced expiratory volume within the first second (β = −0.16), lower LVEF (β = −0.33), more severe mitral regurgitation (β = 0.22), and presence of CpcPH (β = 0.21; P < 0.01 for all) were independently associated with higher ln BNP.
Table 3.
Univariate and multivariate linear regression analyses with B‐type natriuretic peptide (ln transformed) as the dependent variable (r 2 = 0.59)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| β | P value | β | P value | |
| Age | 0.36 | 0.001 | ||
| eGFR | −0.29 | <0.001 | ||
| Haemoglobin | −0.31 | <0.001 | −0.18 | <0.001 |
| FEV1 | −0.24 | <0.001 | ||
| Heart rhythm | 0.21 | 0.001 | ||
| Heart rate | 0.15 | 0.02 | ||
| Left ventricular ejection fraction | −0.49 | <0.001 | −0.20 | <0.001 |
| Tricuspid annular plane systolic excursion | −0.31 | 0.001 | ||
| Indexed aortic valve area | −0.17 | 0.01 | ||
| Mitral regurgitation severity | 0.48 | <0.001 | 0.21 | <0.001 |
| Coronary artery disease severity | 0.14 | 0.03 | ||
| Mean right atrial pressure | 0.40 | <0.001 | ||
| Right ventricular end‐diastolic pressure | 0.36 | <0.001 | ||
| mPAP | 0.66 | <0.001 | ||
| mPAWP | 0.64 | <0.001 | 0.37 | <0.001 |
| Pulmonary vascular resistance | 0.49 | <0.001 | 0.21 | <0.001 |
| Mean aortic pressure | −0.18 | 0.005 | ||
| Systemic vascular resistance | 0.22 | 0.001 | ||
| Stroke volume index | −0.30 | <0.001 | ||
| Cardiac index | −0.30 | <0.001 | ||
eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume within the first second; mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure.
Figure 1.
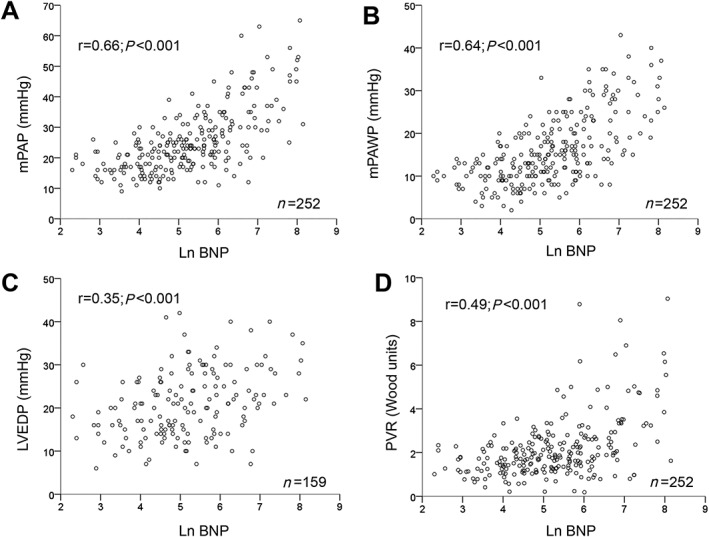
Scatter plots showing the correlations (Pearson correlation coefficients) between B‐type natriuretic peptide (BNP; logarithmic scale) and mean pulmonary artery pressure (mPAP; panel A), mean pulmonary artery wedge pressure (mPAWP; panel B), left ventricular end‐diastolic pressure (LVEDP; panel C), and pulmonary vascular resistance (PVR; panel D).
If BNP was used as a categorical variable, lower haemoglobin, lower LVEF, and higher mPAWP were independently associated with BNP in the highest quartile (see Supporting Information, Table S1). If the presence of CpcPH rather than the single haemodynamic parameters was entered into the model, lower haemoglobin [odds ratio (OR) 0.95, 95% confidence interval (CI) 0.93–0.98) per 1‐g/L increase; P < 0.001], lower LVEF [OR 0.91 (95% CI 0.88–0.94) per 1% increase; P < 0.001], and presence of CpcPH [OR 17.5 (6.2–49.6); P < 0.001] were independently associated with BNP in the highest quartile.
Clinical outcomes
After a median follow‐up of 3.1 (interquartile range 2.3–4.3) years after AVR, there were 22 deaths. In the univariate analysis, BNP as a continuous (Table 4) or categorical (Figure 2) variable was associated with mortality. As shown in Figure 2, patients in the third and fourth BNP quartiles had a more than six‐fold higher risk of death than patients in the first and second quartiles [hazard ratio (HR) 6.29 (95% CI 1.86–21.27); P = 0.003].
Table 4.
Univariate and multivariate Cox regression analyses with mortality as the dependent variable
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P value | Hazard ration (95% confidence interval) | P value | |
| Age | 1.051 (0.999–1.106) per year | 0.06 | ||
| Chronic obstructive lung disease | 2.99 (1.22–7.34) | 0.02 | ||
| Oral anticoagulation | 2.73 (1.17–6.39) | 0.02 | ||
| eGFR | 0.98 (0.97–0.99) per mL/min/1.73 m2 | 0.048 | ||
| Left ventricular ejection fraction | 0.95 (0.93–0.98) per % | <0.001 | 0.96 (0.94–0.99) per % | 0.01 |
| Combined pre‐capillary and post‐capillary pulmonary hypertension | 6.51 (2.81–15.08) | <0.001 | 4.58 (1.89–11.09) | 0.001 |
| Mean aortic valve gradient | 0.97 (0.94–1.00) per mmHg | 0.06 | ||
| Mitral regurgitation | 2.27 (1.41–3.67) per grade | 0.001 | ||
| FEV1 | 0.97 (0.95–0.99) per % | 0.003 | ||
| TAVR vs. SAVR | 2.70 (1.15–6.36) | 0.02 | ||
| ln BNP | 1.90 (1.33–2.71) | <0.001 | ||
BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume within the first second; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Figure 2.
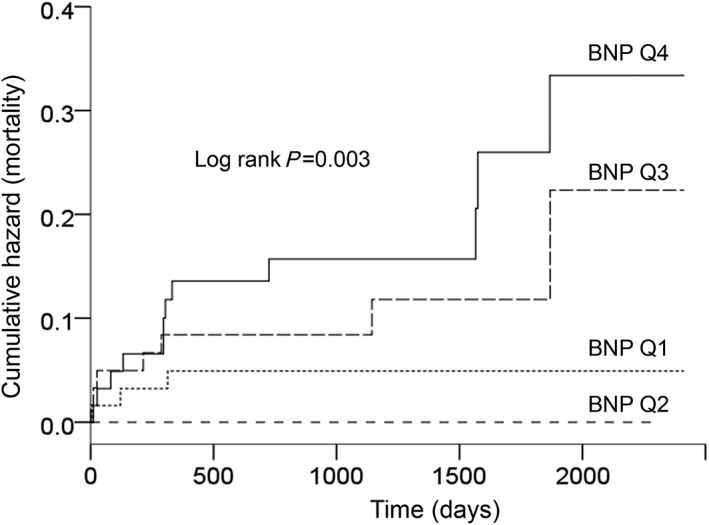
Kaplan–Meier plots showing the cumulative incidence of death in the four B‐type natriuretic peptide quartiles (BNP; Q1, lowest BNP values; and Q4, highest BNP values).
In the multivariate analysis without invasive haemodynamic parameters, more severe mitral regurgitation [HR 1.88 (95% CI 1.04–3.40); P = 0.04], presence of chronic obstructive lung disease [HR 3.13 (95% CI 1.26–7.81); P = 0.01], and higher ln BNP [HR 1.74 (95% CI 1.16–2.61); P = 0.01] were independently associated with higher mortality. In the multivariate analysis including invasive haemodynamic parameters, lower LVEF and CpcPH but not BNP were independently associated with mortality (Table 4).
Accuracy of B‐type natriuretic peptide for the prediction of pre‐capillary and post‐capillary pulmonary hypertension and death
The areas under the receiver operator characteristics curve (AUC) for BNP for the prediction of pulmonary hypertension and CpcPH were 0.80 and 0.88, respectively (Figure 3). The optimal BNP cut‐off for the prediction of pulmonary hypertension of 180 ng/L had a sensitivity of 77% and a specificity of 67%. The optimal cut‐off for the prediction of CpcPH of 557 ng/L had a sensitivity of 75% and a specificity of 89%. The AUC for BNP for the prediction of death was 0.74 (Figure 3). The optimal BNP cut‐off for the prediction of death of 361 ng/L had sensitivity of 73% and a specificity of 72%.
Figure 3.
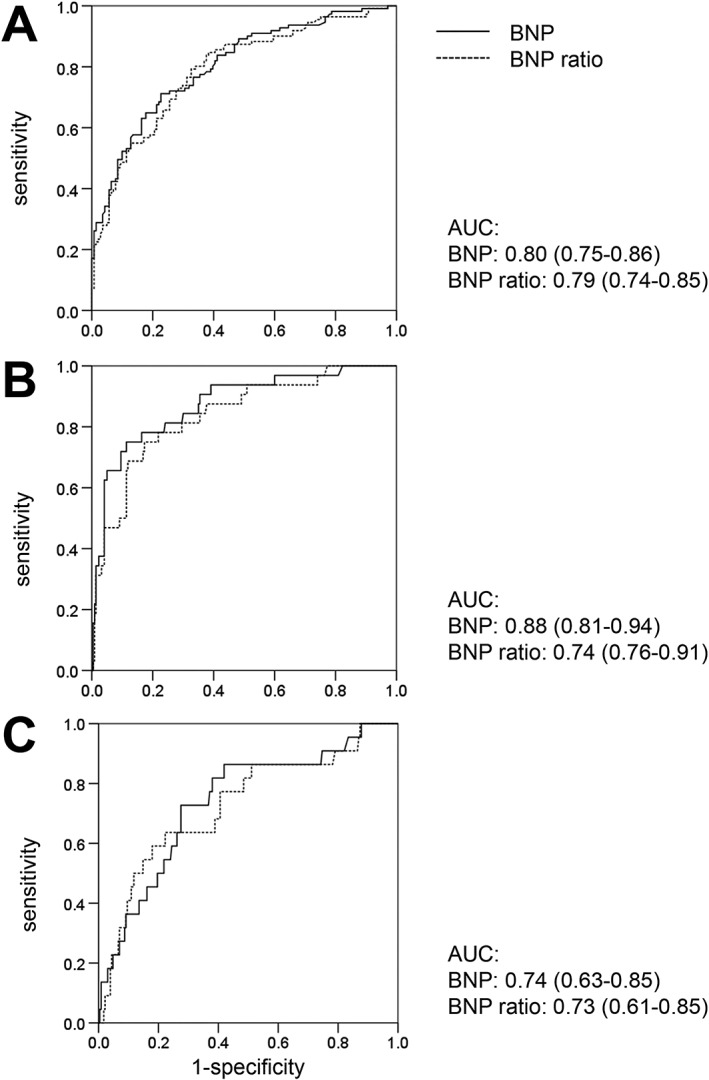
Receiver operator characteristics curves for B‐type natriuretic peptide (BNP) and the BNP ratio for the prediction of pulmonary hypertension (panel A), combined pre‐capillary and post‐capillary pulmonary hypertension (panel B), and mortality (panel C). The areas under the curve (AUC) are reported.
B‐type natriuretic peptide ratio
The BNP ratio quartiles were as follows: 0.47 ± 0.19, 1.35 ± 0.28, 2.87 ± 0.64, and 13.3 ± 11.1. In contrast to the analysis with absolute values, there were no statistically significant differences in age, estimated glomerular filtration rate, and haemoglobin across BNP ratio quartiles (Table S2 ). However, differences in symptoms and medication were similarly present across BNP ratio quartiles as for absolute BNP quartiles. The indexed aortic valve area was similar in the four BNP ratio quartiles. With increasing BNP ratio quartile, patients had larger left ventricular and left atrial dimensions, worse LVEF und right ventricular function, and a worse haemodynamic profile (Table S3 ). Independent predictors of higher BNP ratio included lower LVEF, more severe mitral regurgitation, and higher mPAP (Table S4 ). In the analysis with CpcPH, instead of single haemodynamic parameters as a co‐variate, lower LVEF and presence of CpcPH emerged as independent predictors of higher BNP ratio (data not shown). In the univariate analysis but not in the multivariate analysis, the BNP ratio was a predictor of mortality (Table S5 ). Patients in the highest BNP ratio quartile had a more than four‐fold risk of death compared with those in quartiles 1 to 3 [HR 4.03 (95% CI 1.84–10.09); P = 0.001]. As shown in Figure 3, the AUCs for the BNP ratio for the prediction of pulmonary hypertension, CpcPH, and death were similar to BNP.
Discussion
The present study provides the first detailed analysis of the invasive haemodynamic correlates of plasma BNP concentrations in a larger population of patients with severe AS. We showed that BNP is not a marker of the severity of AS per se but its consequences on the left ventricle and left atrium and the resulting effects on mPAWP and the pulmonary vasculature. Lower LVEF, more severe mitral regurgitation, higher mPAWP, and higher PVR were independently associated with higher BNP. While the correlations between BNP and single haemodynamic parameters were moderately strong, BNP was closely associated with the presence of CpcPH. We also confirmed the strong prognostic value of BNP as a predictor of long‐term mortality after AVR. The present data suggest that the association between BNP and mortality is at least in part mediated by the fact that BNP is a marker of haemodynamics (Figure 4).
Figure 4.
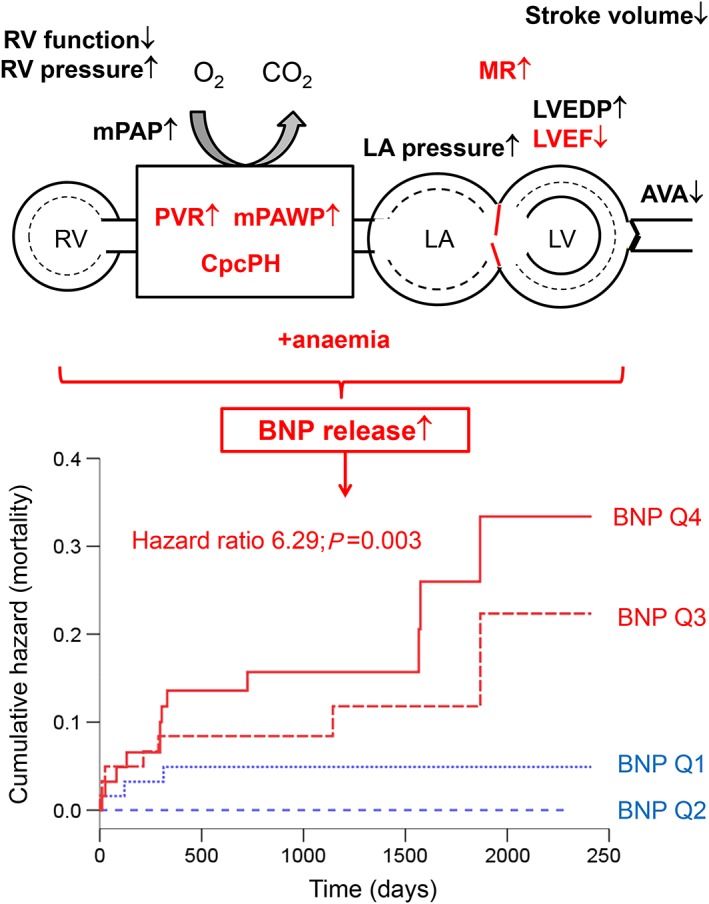
Schematic representation of the key haemodynamic measures (red) reflecting the maladaptive effects of severe aortic stenosis associated with increased plasma concentrations of B‐type natriuretic peptide (BNP; top) and the strong long‐term prognostic impact of high BNP (bottom). The hazard ratio refers to the comparison of quartiles (Q) 4 and 3 (red, poor prognosis) vs. Q2 and 1 (blue, favourable prognosis).
Natriuretic peptides are very frequently used biomarkers in clinical practice. Still, relatively little is known about the detailed mechanisms leading to the cardiac production and release of BNP and NT‐proBNP. Myocyte stretch has, among other stimuli, been shown to lead to BNP release in experimental studies.7 Human studies with measurements of BNP in arterial and coronary sinus plasma in patients with heart failure with reduced and preserved LVEF and healthy controls have revealed a relationship between the transcardiac BNP gradient and left ventricular end‐diastolic and end‐systolic wall stress.9 For patients with AS associations between higher BNP and higher New York Heart Association class, higher AS severity, higher left ventricular mass, and lower LVEF have been reported previously (summarized by Parikh et al.4). Small invasive studies in patients with AS had revealed associations between BNP and left ventricular end‐systolic wall stress11 and mPAWP.8 Thus, despite a guideline recommendation for high BNP as trigger for AVR in asymptomatic patients with severe AS,10 there has been very limited information as to whether BNP in AS really reflects haemodynamic parameters of interest. In the first larger and detailed invasive haemodynamic study on BNP in AS, we have shown that BNP in patients with severe AS is indeed a marker of the most important maladaptive effects of severe AS including reduced LVEF, significant mitral regurgitation, and elevated mPAWP and PVR.
While the associations between BNP and single haemodynamic parameters were moderately strong, the presence of CpcPH outperformed all these parameters in the multivariate analysis. The AUC for BNP for the prediction of CpcPH was 0.88, which is attractive for a blood biomarker because the non‐invasive assessment of pulmonary hypertension and the underlying haemodynamic mechanisms are notoriously challenging,15 and right heart catheterization only can definitely reveal the haemodynamic constellation. The definition of CpcPH is based on several parameters, i.e. mPAP, mPAWP, and PVR, and the presence CpcPH in AS represents an advanced disease stage with left ventricular and left atrial dysfunction, pulmonary vascular remodelling, and right ventricular dysfunction.13 Because pulmonary hypertension and CpcPH, respectively, do not directly impose stress on the left ventricle, the right ventricle is likely to contribute to cardiac BNP release in patients with AS and CpcPH as suggested for patients with pulmonary arterial hypertension.18, 19 In patients with pulmonary arterial hypertension, BNP and NT‐proBNP are established prognostic markers,16, 20 and several studies in patients with pulmonary arterial hypertension have shown a direct relationship between BNP and/or NT‐proBNP and PVR,21, 22 which is in line with the present findings. A study from our research group has shown correlations between higher transcardiac BNP and NT‐proBNP gradients (i.e. the difference between the natriuretic plasma concentrations in the coronary sinus and the natriuretic peptide plasma concentrations in the aorta, a measure of the cardiac release of BNP and NT‐proBNP) and higher PVR, lower tricuspid annular plane systolic excursion, smaller left ventricular end‐diastolic volume index, and higher left ventricular eccentricity index.19 Although the coronary sinus does not receive the complete venous drainage of the right ventricle, increased BNP and NT‐proBNP release from the right ventricle seems to be very likely. Taken together, these data collectively suggest that increased plasma concentrations of natriuretic peptides in pulmonary arterial hypertension and potentially also in CpcPH are due to increased release from the right ventricle due to increased right ventricular wall stress.
In accordance with previous studies,1, 2, 3, 4, 5, 6 we found a strong association between BNP and mortality after AVR. However, if invasive haemodynamic parameters were entered into the multivariate model, BNP was not an independent predictor of death anymore. This suggests that the prognostic value of BNP in this setting is to a relevant degree mediated by the fact that BNP is a marker of haemodynamics. It is well known that pulmonary hypertension can persist after AVR and that such a constellation is associated with poor prognosis.15 It is unknown but may be speculated that these are patients with a constellation of CpcPH prior to AVR. In such patients, AVR may not always lead to the desired clinical benefit, and importantly, persistent CpcPH after AVR does not respond to pulmonary arterial hypertension therapeutics.23 Thus, high BNP in a patient with AS may trigger a more detailed assessment including left and right heart catheterizations, which are currently not recommended as a routine procedure in patients evaluated for AVR. Although AVR reduces the left ventricular afterload, natriuretic peptides are not always reduced after AVR.24 In a recent study among 704 patients undergoing TAVR, there was a rise in NT‐proBNP from pre‐TAVR to discharge in nearly 50% of patients. Both high pre‐TAVR NT‐proBNP and a post‐TAVR rise in NT‐proBNP predicted mortality.24 The haemodynamic correlate of the post‐TAVR rise in NT‐proBNP remains unknown, but this observation clearly highlights that a more detailed non‐invasive and invasive evaluation pre‐AVR and post‐AVR is required to understand the impact of AVR on haemodynamics and who will benefit most from AVR and/or additional medical therapy.
Several non‐cardiac factors are known to affect plasma concentrations of circulating BNP including age, gender, body mass index, renal function, and haemoglobin.25 It has therefore been suggested not to use absolute BNP values but the so‐called BNP ratio, i.e. the ratio between the effective BNP and the maximal normal BNP value for a given age category and sex.1 In a previous study in a large population of patients with at least moderate AS, the BNP ratio was a strong predictor of mortality.1 We therefore repeated the statistical analysis with the BNP ratio instead of absolute BNP values, and although this eliminated differences in age and estimated glomerular filtration rate across quartiles, the strong association between higher BNP quartile and worse haemodynamics persisted. We found an independent association between higher BNP and lower haemoglobin, which was not present anymore in the analysis with the BNP ratio. Thus, while interpreting absolute BNP values in patients with AS, extracardiac factors have to be taken into account, which is in line with the guideline recommendation where the use of age‐corrected and sex‐corrected BNP values is proposed for decision making regarding AVR in asymptomatic patients with severe AS.10 However, the BNP ratio was not superior to absolute BNP for the prediction of CpcPH and mortality.
Limitations
First, although this represents the first larger study on BNP and haemodynamics in AS patients, the number of patients was still moderate. In addition, selection of the mode of AVR was not randomized, and thus the prognostic impact of TAVR vs. SAVR (Table 4) must be interpreted carefully because the TAVR group was much sicker. Second, although all patients had an echocardiogram, not all parameters of interest were measured in a systematic manner. In particular, information on left ventricular dimensions (wall thickness and diameter) was not available in all patients; and therefore, wall stress could not be calculated. Third, there is an established impact of atrial fibrillation on BNP,26 and this is also reflected by the present data (Tables 1 and 3). However, because we only labelled patients as having atrial fibrillation, if they had atrial fibrillation on admission and during cardiac catheterization, we probably have missed patients with paroxysmal atrial fibrillation. This speculation is supported by the fact that there were patients on oral anticoagulation but without atrial fibrillation. Patients in sinus rhythm and on oral anticoagulation had higher BNP levels than those in sinus rhythm without oral anticoagulation (data not shown), which is highlighting this limitation.
Conclusions
In patients with severe AS, higher BNP is a marker of the presence of CpcPH and its contributors. The association between BNP and such an adverse haemodynamic profile at least in part explains the ability of BNP to predict long‐term post‐AVR mortality.
Conflict of interest
None declared.
Funding
None
Supporting information
Table S1. Univariate and multivariate logistic regression analysis with BNP in Q4 as the dependent variable.
Table S2. Clinical characteristics according to B‐type natriuretic peptide (BNP) ratio quartiles
Table S3. Data from echocardiography and cardiac catheterization according to B‐type natriuretic peptide (BNP) quartiles
Table S4. Univariate and multivariate linear regression analysis with BNP ratio (ln‐transformed) as the dependent variable (r2 = 0.52).
Table S5. Univariate and multivariate Cox regression analysis with mortality as the dependent variable.
Acknowledgement
The great administrative support of Irene Schneider is very much appreciated.
Maeder, M. T. , Weber, L. , Ammann, P. , Buser, M. , Ehl, N. F. , Gerhard, M. , Brenner, R. , Haager, P. K. , Maisano, F. , and Rickli, H. (2020) Relationship between B‐type natriuretic peptide and invasive haemodynamics in patients with severe aortic valve stenosis. ESC Heart Failure, 7: 577–587. 10.1002/ehf2.12614.
References
- 1. Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez‐Sarano M. B‐type natriuretic peptide clinical activation in aortic stenosis: impact on long‐term survival. J Am Coll Cardiol 2014; 63: 2016–2025. [DOI] [PubMed] [Google Scholar]
- 2. Takagi H, Hari Y, Kawai N, Kuno T, Ando T. Meta‐analysis of impact of baseline N‐Terminal pro‐brain natriuretic peptide levels on survival after transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol 2019; 123: 820–826. [DOI] [PubMed] [Google Scholar]
- 3. Gardezi SK, Coffey S, Prendergast BD, Myerson SG. Serum biomarkers in valvular heart disease. Heart 2018; 104: 349–358. [DOI] [PubMed] [Google Scholar]
- 4. Parikh V, Kim C, Siegel RJ, Arsanjani R, Rader F. Natriuretic peptides for risk stratification of patients with valvular aortic stenosis. Circ Heart Fail 2015; 8: 373–380. [DOI] [PubMed] [Google Scholar]
- 5. Bergler‐Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom‐free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109: 2302–2308. [DOI] [PubMed] [Google Scholar]
- 6. Bergler‐Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, Fuchs C, Mohty D, Beanlands RS, Hachicha Z, Walter‐Publig N, Rader F, Baumgartner H. B‐type natriuretic peptide in low‐flow, low‐gradient aortic stenosis: relationship to hemodynamics and clinical outcome: results from the Multicenter Truly or Pseudo‐Severe Aortic Stenosis (TOPAS) study. Circulation 2007; 115: 2848–2855. [DOI] [PubMed] [Google Scholar]
- 7. Wiese S, Breyer T, Dragu A, Wakili R, Burkard T, Schmidt‐Schweda S, Fuchtbauer EM, Dohrmann U, Beyersdorf F, Radicke D, Holubarsch CJ. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation 2000; 102: 3074–3079. [DOI] [PubMed] [Google Scholar]
- 8. Vanderheyden M, Goethals M, Verstreken S, De Bruyne B, Muller K, Van Schuerbeeck E, Bartunek J. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 9. Maeder MT, Mariani JA, Kaye DM. Hemodynamic determinants of myocardial B‐type natriuretic peptide release: relative contributions of systolic and diastolic wall stress. Hypertension 2010; 56: 682–689. [DOI] [PubMed] [Google Scholar]
- 10. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 11. Ikeda T, Matsuda K, Itoh H, Shirakami G, Miyamoto Y, Yoshimasa T, Nakao K, Ban T. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end‐systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133: 307–314. [DOI] [PubMed] [Google Scholar]
- 12. Qi W, Mathisen P, Kjekshus J, Simonsen S, Bjornerheim R, Endresen K, Hall C. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142: 725–732. [DOI] [PubMed] [Google Scholar]
- 13. Weber L, Rickli H, Haager PK, Joerg L, Weilenmann D, Brenner R, Taramasso M, Baier P, Maisano F, Maeder MT. Haemodynamic mechanisms and long‐term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. Eur J Heart Fail 2019; 21: 172–181. [DOI] [PubMed] [Google Scholar]
- 14. O'Sullivan CJ, Wenaweser P, Ceylan O, Rat‐Wirtzler J, Stortecky S, Heg D, Spitzer E, Zanchin T, Praz F, Tuller D, Huber C, Pilgrim T, Nietlispach F, Khattab AA, Carrel T, Meier B, Windecker S, Buellesfeld L. Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: insights from the new proposed pulmonary hypertension classification. Circ Cardiovasc Interv 2015; 8: e002358. [DOI] [PubMed] [Google Scholar]
- 15. Maeder MT, Weber L, Buser M, Gerhard M, Haager PK, Maisano F, Rickli H. Pulmonary hypertension in aortic and mitral valve disease. Front Cardiovasc Med 2018; 5: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck‐Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Zamorano LJ. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 17. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40: 976–982. [DOI] [PubMed] [Google Scholar]
- 18. Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, Kuribayashi S, Hamada S, Kakishita M, Nakanishi N, Takamiya M, Kunieda T, Matsuo H, Kangawa K. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol 1998; 31: 202–208. [DOI] [PubMed] [Google Scholar]
- 19. Maeder MT, Kaye DM. Transcardiac gradients of B‐type natriuretic peptides are increased in human pulmonary arterial hypertension. Int J Cardiol 2011; 151: 117–119. [DOI] [PubMed] [Google Scholar]
- 20. Chin KM, Rubin LJ, Channick R, Di Scala L, Gaine S, Galie N, Ghofrani HA, Hoeper MM, Lang IM, McLaughlin VV, Preiss R, Simonneau G, Sitbon O, Tapson VF. Association of N‐terminal pro brain natriuretic peptide and long‐term outcome in patients with pulmonary arterial hypertension. Circulation 2019; 139: 2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, Behr J. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43: 764–770. [DOI] [PubMed] [Google Scholar]
- 22. Souza R, Jardim C, Julio Cesar Fernandes C, Silveira Lapa M, Rabelo R, Humbert M. NT‐proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir Med 2007; 101: 69–75. [DOI] [PubMed] [Google Scholar]
- 23. Bermejo J, Yotti R, Garcia‐Orta R, Sanchez‐Fernandez PL, Castano M, Segovia‐Cubero J, Escribano‐Subias P, San Roman JA, Borras X, Alonso‐Gomez A, Botas J, Crespo‐Leiro MG, Velasco S, Bayes‐Genis A, Lopez A, Munoz‐Aguilera R, de Teresa E, Gonzalez‐Juanatey JR, Evangelista A, Mombiela T, Gonzalez‐Mansilla A, Elizaga J, Martin‐Moreiras J, Gonzalez‐Santos JM, Moreno‐Escobar E, Fernandez‐Aviles F. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. Eur Heart J 2017; 39: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seoudy H, Frank J, Neu M, Gussefeld N, Klaus Y, Freitag‐Wolf S, Lambers M, Lutter G, Dempfle A, Rangrez AY, Kuhn C, Frey N, Frank D. Periprocedural changes of NT‐proBNP are associated with survival after transcatheter aortic valve implantation. J Am Heart Assoc 2019; 8: e010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maeder MT, Mueller C, Pfisterer ME, Buser PT, Brunner‐La Rocca HP. Use of B‐type natriuretic peptide outside of the emergency department. Int J Cardiol 2008; 127: 5–16. [DOI] [PubMed] [Google Scholar]
- 26. Gould PA, Gula LJ, Bhayana V, Subbiah RN, Bentley C, Yee R, Klein GJ, Krahn AD, Skanes AC. Characterization of cardiac brain natriuretic peptide release in patients with paroxysmal atrial fibrillation undergoing left atrial ablation. Circ Arrhythm Electrophysiol 2010; 3: 18–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate and multivariate logistic regression analysis with BNP in Q4 as the dependent variable.
Table S2. Clinical characteristics according to B‐type natriuretic peptide (BNP) ratio quartiles
Table S3. Data from echocardiography and cardiac catheterization according to B‐type natriuretic peptide (BNP) quartiles
Table S4. Univariate and multivariate linear regression analysis with BNP ratio (ln‐transformed) as the dependent variable (r2 = 0.52).
Table S5. Univariate and multivariate Cox regression analysis with mortality as the dependent variable.


