Abstract
Aims
The Treatment of Sleep‐Disordered Breathing with Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients with Heart Failure trial investigated the effects of adaptive servo‐ventilation (ASV) (vs. control) on outcomes of 1325 patients with heart failure and reduced ejection fraction (HFrEF) and central sleep apnoea (CSA). The primary outcome (a composite of all‐cause death or unplanned HF hospitalization) did not differ between the two groups. However, all‐cause and cardiovascular (CV) mortality were higher in the ASV group. Circulating biomarkers may help in better ascertain patients' risk, and this is the first study applying a large set of circulating biomarkers in patients with both HFrEF and CSA.
Methods and results
Circulating protein‐biomarkers (n = 276) ontologically involved in CV pathways, were studied in 749 (57% of the trial population) patients (biomarker substudy), to investigate their association with the study outcomes (primary outcome, CV death and all‐cause death). The mean age was 69 ± 10 years, and > 90% were male. The groups (ASV vs. control and biomarker substudy vs. no biomarker) were well balanced. The “best” clinical prognostic model included male sex, systolic blood pressure < 120 mmHg, diabetes, loop diuretic, cardiac device, 6‐min walking test distance, and N‐terminal pro BNP as the strongest prognosticators. On top of the “best” clinical prognostic model, the biomarkers that significantly improved both the discrimination (c‐index) and the net reclassification index (NRI) of the model were soluble suppression of tumorigenicity 2 for the primary outcome; neurogenic locus notch homolog protein 3 (Notch‐3) for CV‐death and all‐cause death; and growth differentiation factor 15 (GDF‐15) for all‐cause death only.
Conclusions
We studied 276 circulating biomarkers in patients with HFrEF and central sleep apnoea; of these biomarkers, three added significant prognostic information on top of the best clinical model: soluble suppression of tumorigenicity 2 (primary outcome), Notch‐3 (CV and all‐cause death), and GDF‐15 (all‐cause death).
Keywords: Heart failure, Adaptive servo‐ventilation, Circulating biomarkers, Prognosis
Introduction
Sleep disordered breathing is prevalent in patients with heart failure and reduced ejection fraction (HFrEF).1 In particular, central sleep apnoea (CSA) may be found in up to 40% of these patients.2 Patients with HFrEF and CSA represent a subset of patients with poor prognosis.3, 4 However, no ventilatory support treatment (to date) has shown to provide benefit in this subset of patients. In the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnoea and Heart Failure study, 258 patients with HFrEF and CSA were randomly assigned to receive continuous positive airway pressure plus guideline‐based medical treatment or guideline‐based medical treatment alone.5 After a median follow‐up of 18 months, no differences in the number of hospitalizations, rate of death, or heart transplantation were detected.
Adaptive servo‐ventilation (ASV) is a non‐invasive ventilatory therapy that effectively alleviates CSA by delivering servo‐controlled inspiratory pressure support on top of expiratory positive airway pressure potentially providing a more physiological treatment of CSA than continuous positive airway pressure.6 The Treatment of Sleep‐Disordered Breathing with Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE‐HF) trial investigated the effects of ASV (AutoSet CS, ResMed, Martinsried, Germany) plus guideline‐based medical treatment vs. guideline‐based medical treatment alone on survival and cardiovascular (CV) outcomes in 1325 patients who had HFrEF and predominantly CSA.7 After a median follow‐up of 31 months, the incidence of the primary outcome [a composite of first event of death from any cause, lifesaving CV intervention, or unplanned heart failure (HF) hospitalization] did not significantly differ between the two groups. However, all‐cause and CV mortality were significantly higher in the ASV group.8, 9 We hypothesized that circulating biomarkers of interest may help ascertaining patients' risk and provide insight on the underlying pathological pathways in this population.
Using a large set of circulating protein‐biomarkers (associated with cardiovascular and inflammatory processes) we sought to investigate the biomarkers that are associated with the study outcomes and improve the prognostic accuracy on top of a well calibrated ‘clinical model'.
Methods
Study design, randomization, and main results
The SERVE‐HF trial was an international, multicenter, randomized, parallel‐group, and event‐driven study. Detailed information about the trial design, procedures, outcomes, and results have been reported previously.7, 10 In short, enrolled patients had HF with a left ventricular ejection fraction ≤45%, New York Heart Association class ≥II, and predominant CSA [apnoea–hypopnea index (AHI) ≥15 events per hour, with >50% central events and a central AHI of ≥10 events per hour]. Patients were advised to use the ASV device for at least 5 h per night and 7 days per week. The target was to reduce the AHI to less than 10 events per hour within 14 days after starting ASV.
The primary outcome in the time‐to‐event analysis was the first event of the composite of death from any cause, a lifesaving CV intervention, or an unplanned hospitalization for worsening HF. Secondary outcomes included the time to death from any cause and the time to death from CV causes. The median (percentile25–75) follow up time was of 3.0 (1.9–4.5) years.
The incidence of the primary end point did not differ significantly between the ASV group and the control group, with event rates of 54.1% and 50.8%, respectively {hazard ratio [HR] [95% confidence interval (CI)] = 1.13 [0.97–1.31]; P = 0.10}. All‐cause and CV mortality were higher in the ASV group than in the control group with all‐cause mortality being 34.8% and 29.3%, respectively [HR (95%CI) = 1.28 (1.06–1.55); P = 0.01], and CV mortality of 29.9% and 24.0%, respectively [HR (95%CI) =1.34 (1.09‐1.65); P = 0.006].
Biomarker substudy population
In the present manuscript, we report the results of the SERVE‐HF biomarker substudy. This substudy includes 817 patients in whom the biomaterials had been taken at the time of randomization (baseline) and stored for later analyses/biomarker determination. These patients were mostly enrolled in sites in Germany (n = 776; 95%). The characteristics of these patients are similar to the overall study population (see Supporting Information, Table S1 ). From these 817 patients, 8 (1%) did not have clinical information on essential adjustment variables (blood pressure, creatinine, haemoglobin, and concomitant medications), and 60 (7%) did not have biomarker measurements. These patients were excluded, leaving 749 subjects available for the biomarker analyses, 381 in the ASV group and 368 in the control group.
Biomarker assessments
Baseline plasma samples were analysed for protein biomarkers using the Olink Proseek® Multiplex Cardiovascular II, Cardiovascular III, and inflammation panels (Olink Proteomics, Uppsala, Sweden). These were ‘clusters/panels' of biomarkers selected on the basis of being ontologically associated with mechanistic pathways involved in cardiovascular diseases and inflammation; they are processed together within each panel according to the Olink® methods. The assay use a proximity extension assay (PEA) technology,11 where 92 oligonucleotide‐labelled antibody probe pairs per panel are allowed to bind to their respective targets in the sample in 96‐well plate format. When binding to their correct targets, they give rise to new DNA amplicons with each ID‐barcoding their respective antigens. The amplicons are subsequently quantified using a Fluidigm BioMark™ HD real‐time PCR platform (Fluidigm Corp., South San Francisco, CA). The platform provides log2‐Normalized Protein eXpression (NPX) data (for further details please visit: https://www.olink.com/question/what-is-npx/).
A total of 276 protein biomarkers were assessed in baseline samples. The abbreviations, full names and respective Olink® multiplex panels of the studied proteins are described in the (see Supporting Information, Table S2 ).
The assays were performed in a ‘blind' fashion to treatment allocation. The proteomic results were then merged with the baseline clinical data.
In the SERVE‐HF biomarker substudy, growth differentiation factor 15 (GDF‐15) was measured by two methods, the ‘standard' electrochemiluminescence on a Roche cobas® platform (F. Hoffmann‐La Roche AG, Basel, Switzerland) and Normalized Protein eXpression (NPX) by Olink®. Both methods showed excellent correlation (>0.9) (see Supporting Information, Figure S1 ).
Statistical considerations
For the baseline clinical characteristics, continuous variables are expressed as means and respective standard deviation. Categorical variables are presented as frequencies and percentages. Patient baseline characteristics were compared between ASV and controls using t‐tests, Mann–Whitney, or χ 2 tests, as appropriate.
Time‐to‐event analysis was conducted using Cox regression models. Clinical variable log‐linearity was checked by plotting the beta estimates vs. the mean across deciles and then clinically relevant cut‐offs were chosen for the candidate variables. Proportional hazards assumptions were assessed by plotting the scaled Schoenfeld residuals vs. the log of time. No proportional hazards violations were found. Missing predictor values of the clinical variables (missing values <10%) were imputed using multi‐chain Monte Carlo methods with Gibbs sampling. We used the r package ‘mice'. We imputed missing data 10 times, performed the analysis over all 10 imputations, and averaged results using the Rubin's rule.12 Variables were then entered in the multivariable model in a backward stepwise regression analysis with the P value to enter and stay in the model set to a P value <=0.1 and < 0.05, respectively. In the multivariable models, all the covariates depicted on Table 1 were considered. Model discrimination was determined by calculation of the C‐statistic.13 Assessment of model calibration was performed by plotting the cumulative incidence of observed vs. expected primary outcome events derived from the Cox model across tertiles of the predicted risk. The ‘best clinical model' (determined by the clinical importance of the variables and the likelihood ratio test) included the variables depicted in Table 2. The model incorporated (and retained) N‐terminal pro BNP (NT‐proBNP) levels, recommended for prognostication in the current guidelines.14, 15 This model was developed for the primary outcome and then applied for CV and all‐cause death. The model performed even better for the deadly events, and therefore, the same clinical model was used for all the studied outcomes.
Table 1.
Characteristics of the study population
| Characteristics | Control n = 368 | ASV n = 381 | P value |
|---|---|---|---|
| Age, year | 69.1 ± 10.2 | 69.3 ± 9.4 | 0.72 |
| Male sex, n (%) | 334 (90.8%) | 347 (91.1%) | 0.88 |
| Body mass index, kg/m2 | 28.9 ± 5.4 | 28.5 ± 4.4 | 0.33 |
| NYHA class III–IV, n (%) | 272 (74.3%) | 272 (71.8%) | 0.43 |
| LVEF, % | 33.3 ± 7.7 | 33.2 ± 7.9 | 0.95 |
| Diabetes mellitus, n (%) | 143 (39.1%) | 152 (40.1%) | 0.77 |
| Ischemic HF, n (%) | 196 (54.9%) | 218 (58.1%) | 0.38 |
| Systolic blood pressure, mmHg | 123.5 ± 20.2 | 124.0 ± 19.4 | 0.69 |
| Left bundle–branch block, n (%) | 85 (23.5%) | 106 (28.4%) | 0.13 |
| Atrial fibrillation, n (%) | 98 (27.0%) | 117 (31.4%) | 0.19 |
| Cardiac device, n (%) | 201 (54.6%) | 204 (53.5%) | 0.77 |
| Haemoglobin, g/dL | 14.0 ± 1.6 | 13.9 ± 1.6 | 0.44 |
| eGFR, ml/min/1.73m2 | 58.5 ± 21.0 | 57.1 ± 21.1 | 0.39 |
| 6MWT distance, m | 333.5 ± 128.2 | 326.9 ± 121.7 | 0.48 |
| ACEi or ARB, n (%) | 341 (92.7%) | 351 (92.1%) | 0.78 |
| Beta–blocker, n (%) | 344 (93.5%) | 349 (91.6%) | 0.33 |
| Aldosterone antagonist, n (%) | 207 (56.3%) | 186 (48.8%) | 0.042 |
| Loop diuretic, n (%) | 329 (89.4%) | 322 (84.5%) | 0.047 |
| Cardiac glycoside, n (%) | 83 (22.6%) | 101 (26.5%) | 0.21 |
| Antiarrhythmic drug, n (%) | 45 (12.2%) | 85 (22.3%) | <0.001 |
| Epworth Sleep Scale, scale: 0–24 | 2.9 ± 5.9 | 2.6 ± 5.5 | 0.44 |
| AHI, n events/hr | 31.1 ± 13.2 | 29.9 ± 12.2 | 0.18 |
| Central apnoea index/total AHI, % | 51.4 ± 29.3 | 45.7 ± 28.7 | 0.007 |
| Central AHI/total AHI, % | 81.4 ± 15.3 | 80.8 ± 14.9 | 0.56 |
| Oxygen Desaturation index, mean ± SD | 34.0 ± 18.4 | 32.8 ± 17.5 | 0.38 |
| Average oxygen saturation (%), mean ± SD | 92.7 ± 2.6 | 92.7 ± 2.2 | 0.71 |
| Minimum oxygen saturation (%), mean ± SD | 80.3 ± 6.9 | 81.1 ± 6.5 | 0.12 |
| Oxygen desaturation index, n of events/hr | 52.7 ± 68.1 | 49.9 ± 63.7 | 0.57 |
| Cheyne–Stokes respiration | |||
| <20% | 68 (21.3%) | 71 (21.6%) | 0.22 |
| 20–50% | 114 (35.6%) | 136 (41.5%) | |
| >50% | 138 (43.1%) | 121 (36.9%) | |
| NT‐proBNP, pg/ml | 1474 (600–3232) | 1344 (613–2937) | 0.66 |
| Outcomes | |||
| Primary outcomea | 186 (50.5%) | 209 (54.9%) | 0.23 |
| CV death | 84 (22.8%) | 113 (29.7%) | 0.034 |
| All‐cause death | 107 (29.1%) | 130 (34.1%) | 0.14 |
6MWT, 6‐minute walking test; ACEi, angiotensin‐converting‐enzyme inhibitor; AHI, apnoea–hypopnea index; ARB, angiotensin II receptor blockers; ASV, adaptive servo‐ventilation; CV, cardiovascular; eGFR, estimated glomerular filtration rate calculated with the CKD‐EPI formula; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT‐proBNP, N‐termianl pro BNP; SD, standard deviation.
The primary outcome was a composite of death from any cause, a lifesaving CV intervention, or an unplanned hospitalization for worsening HF.
Table 2.
Best clinical risk model
| Variable | HR (95%CI) | P value |
|---|---|---|
| ASV (yes) | 1.13 (0.93–1.38) | 0.22 |
| Age (per year) | 0.99 (0.98–1.01) | 0.56 |
| Male sex | 2.27 (1.50–3.44) | <0.001 |
| SBP <120 mmHg | 1.43 (1.16–1.75) | 0.001 |
| Diabetes (yes) | 1.46 (1.19–1.78) | <0.001 |
| Loop diuretic (yes) | 1.94 (1.31–2.87) | 0.001 |
| Cardiac device (yes) | 1.38 (1.12–1.71) | 0.002 |
| 6MWT (per each −50 m) | 1.10 (1.05–1.15) | <0.001 |
| NT‐proBNP (per log increase) | 1.65 (1.51–1.81) | <0.001 |
ASV, adaptive servo‐ventilation; CI, confidence interval; SBP, systolic blood pressure; 6MWT, 6‐minute walking test distance; HR, hazard ratio; NT‐proBNP, N‐terminal pro BNP.
Harrel's C‐index =0.727 for the primary outcome; =0.750 for CV death; =0.737 for all‐cause death.
ASV, age and sex were ‘forced' into the model.
Cox regression models adjusting for the best clinical model were then used to identify protein biomarkers associated with the primary outcome corrected for multiple testing using a Bonferroni correction (0.05/276).16 Only the proteins that were found to be statistically significant at the set P value <0.0002 were considered as prognosticators. No hierarchy or further adjustments were performed for the outcomes of CV and all‐cause death, and these should be regarded as exploratory. Because proteins were measured using log2 normalized NPX values, the HR for each protein estimates the increase in the hazards of event associated with a doubling in the protein concentration. We assessed the added discriminatory value of each biomarker by comparing the C‐index of the clinical model with that of the clinical model plus the biomarker of interest (ΔC‐index)17 and the 1‐year net reclassification improvement (NRI)18, 19 of the biomarker of interest on top of the clinical model. This method assesses the ability of a new model to reclassify subjects with and without a clinical event during follow‐up. The ability of the new model to reclassify is summarized by the NRI statistics. The continuous NRI method does not require a prior definition of strata risk, thus considering the change in the estimation prediction as a continuous variable.19
The analyses were performed using stata version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP) and r® [R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria] software.
Results
Patients' characteristics
Patients' characteristics are depicted in Table 1. The mean age was 69 ± 10 years, and >90% were male. The groups (ASV vs. control) were well balanced, except for the use of antiarrhythmic drugs, which was more common in the ASV group (22% vs. 12%; P < 0.001) (similarly to the main report). Other statistically significant differences (aldosterone antagonists, loop diuretics, and total AHI) were small in absolute numbers.
Clinical risk model (best clinical model)
The best clinical model included male sex, systolic blood pressure <120 mmHg, diabetes, loop diuretic, cardiac device, 6‐min walking test distance, and NT‐proBNP as the strongest prognosticators. Age and treatment group allocation (ASV or control) were kept in the model. The C‐index of the model was 0.73 for the primary outcome, 0.75 for CV death, and 0.74 for all‐cause death (Table 2). The model was well calibrated with a steep increase of observed vs. predicted events by tertiles of patients' risk (see Supporting Information, Figure S2 ).
Top biomarkers
The results of the biomarkers associated with the studied outcomes on top of the best clinical model and adjusted for multiple testing are shown in Table 3. The discrimination improvement for the biomarkers that improve both the model discrimination and net reclassification is represented in Figure 1. The HRs (95%CIs) for all the available biomarkers are presented in Supporting Information Table S3 .
Table 3.
Multiple test‐corrected biomarkers
| Risk model + biomarker | HR (95%CI) | P value | C‐index | ΔC‐index P value | cNRI (95%CI) |
|---|---|---|---|---|---|
| Primary outcome | |||||
| sST2 | 1.50 (1.30–1.74) | <0.0001 | 0.736 | 0.033 | +0.23 (+0.04, +0.39) |
| TR | 1.27 (1.11–1.46) | 0.0002 | 0.736 | 0.005 | 0.10 (−0.15, 0.28) |
| ACE2 | 1.34 (1.17–1.53) | <0.0001 | 0.731 | 0.23 | 0.23 (−0.10, 0.40) |
| AMBP | 0.53 (0.38–0.74) | 0.0002 | 0.730 | 0.24 | 0.03 (−0.17, 0.21) |
| PON3 | 0.77 (0.68–0.88) | 0.0001 | 0.732 | 0.11 | 0.13 (−0.08, 0.27) |
| CV death | |||||
| Notch‐3 | 1.71 (1.31–2.23) | 0.0001 | 0.761 | 0.049 | +0.18 (+0.07, +0.28) |
| IL‐6 | 1.26 (2.32–3.40) | <0.0001 | 0.763 | 0.019 | 0.05 (−0.10, 0.13) |
| OPG | 2.18 (1.62–2.93) | <0.0001 | 0.762 | 0.21 | +0.13 (+0.03, +0.24) |
| OPN | 1.48 (1–20–1.84) | 0.0002 | 0.759 | 0.37 | +0.15 (+0.03, +0.26) |
| ACE2 | 1.55 (1.28–1.88) | <0.0001 | 0.755 | 0.96 | 0.02 (−0.14, 0.10) |
| GDF‐15 | 1.47 (1.22–1.77) | 0.0001 | 0.759 | 0.59 | 0.12 (−0.06, 0.21) |
| AP‐N | 1.87 (1.40–2.49) | <0.0001 | 0.760 | 0.12 | 0.07 (−0.05, 0.18) |
| sST2 | 1.73 (1.41–2.12) | <0.0001 | 0.760 | 0.35 | 0.01 (−0.01, 0.02) |
| IGFBP‐7 | 1.48 (1.22–1.79) | <0.0001 | 0.758 | 0.22 | 0.09 (−0.03, 0.19) |
| All‐cause death | |||||
| GDF‐15 | 1.59 (1.34–1.88) | <0.0001 | 0.753 | 0.026 | +0.12 (+0.02, +0.23) |
| Notch‐3 | 1.64 (1.29–2.09) | 0.0001 | 0.748 | 0.036 | +0.15 (+0.06, +0.26) |
| IL‐6 | 1.31 (1.18–1.44) | <0.0001 | 0.752 | 0.008 | 0.03 (−0.06, 0.15) |
| vWF | 1.23 (1.11–1.37) | 0.0001 | 0.748 | 0.037 | 0.06 (−0.03, 0.18) |
| FGF‐23 | 1.16 (1.08–1.25) | 0.0001 | 0.745 | 0.041 | 0.06 (−0.16, 0.06) |
| OPG | 2.09 (1.59–2.74) | <0.0001 | 0.748 | 0.29 | +0.13 (+0.03, +0.23) |
| IL‐1RT1 | 1.77 (1.33–2.36) | 0.0001 | 0.739 | 0.54 | +0.14 (+0.02, +0.23) |
| OPN | 1.43 (1.18–1.74) | 0.0002 | 0.746 | 0.36 | +0.16 (+0.04, +0.26) |
| IGFBP‐2 | 1.44 (1.18–1.76) | 0.0002 | 0.743 | 0.33 | +0.18 (+0.08, +0.29) |
| ACE2 | 1.50 (1.27–1.79) | <0.0001 | 0.743 | 0.80 | 0.01 (−0.10, 0.11) |
| sST2 | 1.68 (1.40–2.03) | <0.0001 | 0.746 | 0.51 | 0.04 (−0.05, 0.16) |
| IGFBP‐7 | 1.48 (1.24–1.77) | <0.0001 | 0.747 | 0.14 | 0.09 (−0.01, 0.03) |
| LIF‐R | 1.78 (1.34–2.36) | 0.0001 | 0.741 | 0.79 | 0.11 (−0.01, 0.20) |
| HGF | 1.43 (1.19–1.73) | 0.0002 | 0.743 | 0.53 | 0.01 (−0.01, 0.02) |
ACE2, angiotensin‐converting enzyme 2; AMBP, α1‐microglobulin/bikunin precursor; AP‐N, Aminopeptidase N; CI, confidence interval; FGF‐23, fibroblast growth factor 23; GDF‐15, growth differentiation factor 15; IGFBP‐7, insulin‐like growth factor‐binding protein 7; IGFBP‐2, insulin‐like growth factor‐binding protein 2; HGF, human growth factor; IL1RT1, interleukin 1 receptor type 1; IL‐6, interleukin‐6; LIF‐R, LIF receptor; NRI, net reclassification index; Notch‐3, neurogenic locus notch homolog protein 3; OPG, osteoprotegerin; OPN, osteopontin; PON3, paraoxonase‐3; PRELP, prolargin; sST2, soluble suppression of tumorigenicity 2; TR, transferrin receptor protein 1; vWF, von Willebrand factor.
ΔC‐index, c‐index change on the clinical risk model after the addition of the biomarker.
cNRI, continuous net reclassification index.
Dark green: both c‐index and NRI improvement; Green: c‐index improvement only; Light green: NRI improvement only.
Figure 1.
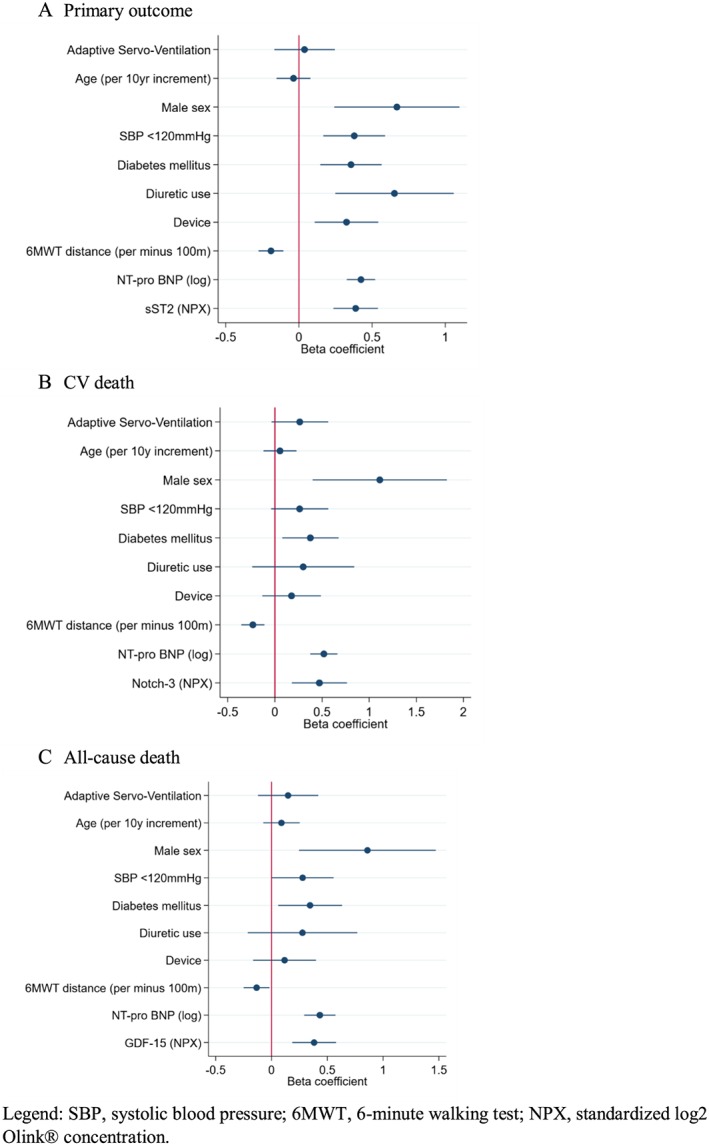
Selected biomarkers for each outcome on top of the clinical model (i.e. adjusted) (A) Primary outcome, (B) cardiovascular CV death, (C) All‐cause death. Legend: SBP, systolic blood pressure; 6MWT, 6‐minute walking test; NPX, standardized log2 Olink® concentration.
With regards to the primary outcome, soluble suppression of tumorigenicity 2 (sST2), improved both the model discrimination and event reclassification; transferrin receptor protein only improved the model discrimination (and not the model net reclassification). The primary outcome top biomarkers were poorly correlated (Spearman correlation <0.5 for all comparisons) (see Supporting Information, Table S4 ).
For CV death, neurogenic locus notch homolog protein 3 (Notch‐3) improved both the model discrimination and event reclassification; interleukin‐6 improved only the model discrimination; osteoprotegerin (OPG) and osteopontin (OPN) improved only the model reclassification. Notch‐3 was well correlated with insulin‐like growth factor‐binding protein 7 (IGFBP‐7) (see Supporting Information, Table S5 ).
For all‐cause death, GDF‐15 and Notch‐3 improved both the model discrimination and event reclassification; interleukin‐6, von‐Willebrand factor, and fibroblast growth factor‐23 (FGF‐23) improved only the discrimination of the model; whereas OPG, interleukin‐1 receptor type 1 (IL‐1RT1), OPN, and insulin like growth factor binding protein 2 improved only the model reclassification.
GDF‐15 was well correlated with IGFBP‐7 and moderately correlated with IL‐1RT1, OPN, sST2, IGFBP‐7, FGF‐23, prolargin, OPG, and Notch‐3 (that showed similar correlation profiles to GDF‐15) (see Supporting Information, Table S6 ). The correlation between the top biomarkers (sST2, Notch‐3, and GDF‐15), and age, plus the available electrocardiographic and echocardiographic parameters was weak (Spearman Rho <0.5 for all comparisons), suggesting that these biomarkers may represent systemic disease processes rather than cardiac‐specific ones (see Supporting Information, Table S7 ).
Discussion
From the 276 studied biomarkers, three added significant prognostic information on top of the best clinical model: sST2 (for the primary outcome), Notch‐3 (for CV and all‐cause death), and GDF‐15 (for all‐cause death).
Prognostic stratification in HF is relevant for therapeutic decisions, patient–family information, and follow‐up strategy.20 Current prognostic models including clinical variables plus natriuretic peptides (BNP and/or NT‐proBNP) offer good prognostication performance (C‐index >0.7)21; the discrimination gains with ‘new' biomarkers on top of these models is usually modest.22, 23, 24 Soluble ST2 (the circulating form of the receptor for interleukin‐33)25 is derived from the heart and peripheral tissues, and its production is promoted by tissue damage, inflammation, and extracellular matrix remodelling.26 Soluble ST2 has been one of the biomarkers often shown to offer additional prognostic information increment in HF.27, 28, 29, 30 In SERVE‐HF, sST2 also offered slight prognostic improvement for the primary outcome of death from any cause, lifesaving CV intervention, or unplanned HF hospitalization, but not for CV or all‐cause death alone.
Notch‐3 improved CV and all‐cause death models. For the CV death outcome, Notch‐3 was the only biomarker that improved both discrimination and event reclassification. Notch signalling is involved in the modulation of cardiomyocytes survival, cardiac stem cells differentiation, and angiogenesis that are factors known to determine the extent of pathological cardiac remodelling.31 Moreover, Notch‐3 knockout mice did not adapt to pressure overload (by not developing arterial media hypertrophy) and exhibited HF.32 These data suggest that Notch‐3 is important in the adaptation to pressure overload playing a major role in the angiogenic pathways. It should be noted that pressure overload‐associated endothelial changes have been previously described in sleep apnoea patients as well as in intermittent hypoxia models. Chronic exposure to biomechanical forces may alter mechanoreceptive molecules such as platelet endothelial cell adhesion molecule, a cell–cell adhesion molecule most abundantly expressed in endothelial cells. This may represent an important mechanism modulating endothelial cells sensitivity to mechanical stimuli.33 In conditions such as sleep apnoea or sleep apnoea‐associated intermittent hypoxia, where shear stress is indeed a critical determinant of cardiovascular homeostasis, regulating remodelling and atherogenesis, platelet endothelial cell adhesion molecule has been evidenced as being down‐regulated as well as associated with early vascular remodelling.34 How this is linked to Notch‐3 remains to be further studied. The association of Notch‐3 with cause‐specific and all‐cause death supports further investigation of this biomarker, assessing its potential role in HF with concomitant sleep‐disordered breathing, both as a prognosticator and therapeutic target.
In addition to Notch‐3, GDF‐15 also improved the all‐cause death model. GDF‐15 is a member of the TGF‐β superfamily involved in the regulation of body‐weight, inflammation, and apoptosis, all key mechanisms in cardiac remodelling and HF.35, 36 Elevated levels of GDF‐15 have been associated with worse prognosis of patients with HF regardless of ejection fraction and mode of presentation.24, 37, 38, 39 Our findings are confirmatory with regards to GDF‐15 in HF prognosis. However, whether GDF‐15 may be a potential target for HF treatment requires further investigation.40
The correlation between the biomarkers associated with CV and all‐cause death was important. Notch‐3 and GDF‐15 were moderately correlated (>0.5), and these biomarkers were also correlated with IGFBP‐7, IL‐1RT1, OPN, sST2, IGFBP‐7, FGF‐23, prolargin, and OPG that were associated with fatal outcomes in SERVE‐HF suggesting that inflammation, glucose metabolism, angiogenesis, and cardiac remodelling play a central role in HF.41, 42
In a previously published secondary analysis of the SERVE‐HF trial,43 using a more limited number of circulating biomarkers, there were no significant differences between treatment groups in changes in NT‐proBNP, troponin T, troponin I, sST2, galectin‐3, cystatin C, creatinine, neutrophil gelatinase‐associated lipocalin, high‐sensitivity C‐reactive protein, and tumor necrosis factor alpha, suggesting that treatment of predominant CSA in HFrEF with ASV therapy did not meaningfully change cardiac structure or function or biomarkers of heart function, renal function, or systemic inflammation. This is in keeping with the lack of effect of ASV on both general and disease‐specific quality of life in the main SERVE‐HF study, along with a lack of difference in HF‐related hospitalisations between the ASV and control groups.7, 43
Limitations
Several limitations should be noted in the present study. This is a post hoc analysis in a subpopulation of the SERVE‐HF trial; hence, these findings are subject to bias inherent to observational studies; however, the characteristic similarities between this subpopulation and the whole trial population support the generalization of these findings to patients with the characteristics of those in SERVE‐HF. The circulating biomarkers were measured using peripheral venous samples; therefore, the myocardium is only one potential source for the measured proteins. The exclusion criteria used in SERVE‐HF served to minimize the impact of many other potential organ sources of these biomarkers; for example, patients with chronic hepatic, bone, or skin disease, systemic inflammatory diseases, malignancies, and pregnancy were excluded. No external validation was performed to confirm these findings; however, the specificity of the SERVE‐HF population renders replication unlikely in a near future. These proteomics assays do not provide standard concentration units, making comparisons with clinically applied cut‐offs challenging. Finally, we only measured biomarkers at baseline; in consequence, we cannot comment on prognostic information provided by the changes in these biomarkers with time or with treatment.
Conclusions
From the studied 276 biomarkers available at baseline, three added significant prognostic information on top of the best clinical model: sST2 (for the primary outcome), Notch‐3 (for CV and all‐cause death), and GDF‐15 (for all‐cause death only). sST2 and GDF‐15 are well‐validated and extensively replicated biomarkers in HF. The role of Notch‐3 may be more specific of HF patients with sleep‐disordered breathing and could deserve further investigation.
Conflict of interest
None declared.
Funding information
J. P. F., P. R., and F. Z. are supported by a public grant overseen by the French National Research Agency (ANR) as part of the second ‘Investissements d'Avenir' programme (ANR‐15‐RHU‐0004).
Supporting information
Figure S1. GDF‐15 correlation: electrochemiluminesence vs normalized protein expression.
Figure S2. Observed vs. Predicted % of events at 2 years by tertiles of risk score.
Table S1. Baseline characteristics by inclusion (or not) in Biomarker study.
Table S2. Biomarker names and respective Olink® panels.
Table S3. Hazard ratios and 95% confidence intervals for all the studied biomarkers (n = 276) with regards to the primary outcome.
Table S4. Primary outcome biomarker correlation.
Table S5. Cardiovascular death biomarker correlation.
Table S6. All‐cause death biomarker correlation.
Table S7. Correlation of the top biomarkers (sST2, Notch‐3, and GDF‐15) with age, plus the available echocardiographic and electrocardiographic parameters.
Ferreira, J. P. , Duarte, K. , Woehrle, H. , Cowie, M. R. , Wegscheider, K. , Angermann, C. , d'Ortho, M.‐P. , Erdmann, E. , Levy, P. , Simonds, A. K. , Somers, V. K. , Teschler, H. , Rossignol, P. , Koenig, W. , and Zannad, F. (2020) Biomarkers in patients with heart failure and central sleep apnoea: findings from the SERVE‐HF trial. ESC Heart Failure, 7: 503–511. 10.1002/ehf2.12521.
References
- 1. Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep‐disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007; 9: 251–257. [DOI] [PubMed] [Google Scholar]
- 2. Grimm W, Sosnovskaya A, Timmesfeld N, Hildebrandt O, Koehler U. Prognostic impact of central sleep apnea in patients with heart failure. J Card Fail 2015; 21: 126–133. [DOI] [PubMed] [Google Scholar]
- 3. Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49: 2028–2034. [DOI] [PubMed] [Google Scholar]
- 4. Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O. Cheyne‐Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter‐defibrillator therapies in patients with congestive heart failure. Eur Heart J 2011; 32: 61–74. [DOI] [PubMed] [Google Scholar]
- 5. Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353: 2025–2033. [DOI] [PubMed] [Google Scholar]
- 6. Sharma BK, Bakker JP, McSharry DG, Desai AS, Javaheri S, Malhotra A. Adaptive servoventilation for treatment of sleep‐disordered breathing in heart failure: a systematic review and meta‐analysis. Chest 2012; 142: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magalang UJ, Pack AI. Heart failure and sleep‐disordered breathing—The plot thickens. N Engl J Med 2015; 373: 1166–1167. [DOI] [PubMed] [Google Scholar]
- 9. Eulenburg C, Wegscheider K, Woehrle H, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H, Cowie MR. Mechanisms underlying increased mortality risk in patients with heart failure and reduced ejection fraction randomly assigned to adaptive servoventilation in the SERVE‐HF study: results of a secondary multistate modelling analysis. Lancet Respir Med 2016; 4: 873–881. [DOI] [PubMed] [Google Scholar]
- 10. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds A, Somers VK, Zannad F, Teschler H. Rationale and design of the SERVE‐HF study: treatment of sleep‐disordered breathing with predominant central sleep apnoea with adaptive servo‐ventilation in patients with chronic heart failure. Eur J Heart Fail 2013; 15: 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody‐based proximity extension assays provide sensitive and specific detection of low‐abundant proteins in human blood. Nucleic Acids Res 2011; 39: e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Buuren S, Groothuis‐oudshoorn K. mice: multivariate imputation by chained equations in r . J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 13. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med, England 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 15. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 16. Johnson RC, Nelson GW, Troyer JL, Lautenberger JA, Kessing BD, Winkler CA, O'Brien SJ. Accounting for multiple comparisons in a genome‐wide association study (GWAS). BMC Genomics 2010; 11: 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right‐censored survival outcome: a one‐shot nonparametric approach. Stat Med 2015; 34: 685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172 discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 19. Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with surviva data. Stat Med 2013; 32: 2430–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibrahim N, Januzzi JL. The potential role of natriuretic peptides and other biomarkers in heart failure diagnosis, prognosis and management. Expert Rev Cardiovasc Ther 2015; 13: 1017–1030. [DOI] [PubMed] [Google Scholar]
- 21. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017; 19: 627–634. [DOI] [PubMed] [Google Scholar]
- 22. Yin WH, Chen JW, Feng AN, Lin SJ, Young S. Multimarker approach to risk stratification among patients with advanced chronic heart failure. Clin Cardiol 2007; 30: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bayes‐Genis A, Richards AM, Maisel AS, Mueller C, Ky B. Multimarker testing with ST2 in chronic heart failure. Am J Cardiol 2015; 115: 76b–80b. [DOI] [PubMed] [Google Scholar]
- 24. Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, Qian M, Teerlink JR, Metra M, Davison BA, Voors AA. A multimarker multi‐time point‐based risk stratification strategy in acute heart failure: results from the RELAX‐AHF trial. Eur J Heart Fail 2017; 19: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 25. Kakkar R, Lee RT. The IL‐33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 2008; 7: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pascual‐Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol 2015; 115: 3b–7b. [DOI] [PubMed] [Google Scholar]
- 27. Emdin M, Aimo A, Vergaro G, Bayes‐Genis A, Lupon J, Latini R, Meessen J, Anand IS, Cohn JN, Gravning J, Gullestad L, Broch K, Ueland T, Nymo SH, Brunner‐La Rocca HP, de Boer RA, Gaggin HK, Ripoli A, Passino C, Januzzi JL Jr. sST2 predicts outcome in chronic heart failure beyond NT‐proBNP and high‐sensitivity troponin T. J Am Coll Cardiol 2018; 72: 2309–2320. [DOI] [PubMed] [Google Scholar]
- 28. Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, Bayes‐Genis A, Anand I, Januzzi JL, Emdin M. Prognostic value of soluble suppression of tumorigenicity‐2 in chronic heart failure: a meta‐analysis. JACC Heart Fail 2017; 5: 280–286. [DOI] [PubMed] [Google Scholar]
- 29. Broch K, Ueland T, Nymo SH, Kjekshus J, Hulthe J, Muntendam P, McMurray JJ, Wikstrand J, Cleland JG, Aukrust P, Gullestad L. Soluble ST2 is associated with adverse outcome in patients with heart failure of ischaemic aetiology. Eur J Heart Fail 2012; 14: 268–277. [DOI] [PubMed] [Google Scholar]
- 30. Anand IS, Rector TS, Kuskowski M, Snider J, Cohn JN. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail 2014; 7: 418–426. [DOI] [PubMed] [Google Scholar]
- 31. Ferrari R, Rizzo P. The Notch pathway: a novel target for myocardial remodelling therapy? Eur Heart J 2014; 35: 2140–2145. [DOI] [PubMed] [Google Scholar]
- 32. Ragot H, Monfort A, Baudet M, Azibani F, Fazal L, Merval R, Polidano E, Cohen‐Solal A, Delcayre C, Vodovar N, Chatziantoniou C, Samuel JL. Loss of Notch3 signaling in vascular smooth muscle cells promotes severe heart failure upon hypertension. Hypertension 2016; 68: 392–400. [DOI] [PubMed] [Google Scholar]
- 33. Tzima E, Irani‐Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005; 437: 426–431. [DOI] [PubMed] [Google Scholar]
- 34. Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 2008; 177: 227–235. [DOI] [PubMed] [Google Scholar]
- 35. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017; 63: 140–151. [DOI] [PubMed] [Google Scholar]
- 36. Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti‐inflammatory strategies for ventricular remodeling following ST‐segment elevation acute myocardial infarction. J Am Coll Cardiol 2014; 63: 1593–1603. [DOI] [PubMed] [Google Scholar]
- 37. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation 2010; 122: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 38. Bouabdallaoui N, Claggett B, Zile MR, McMurray JJV, O'Meara E, Packer M, Prescott MF, Swedberg K, Solomon SD, Rouleau JL. Growth differentiation factor‐15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2018; 20: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 39. Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep 2012; 9: 337–345. [DOI] [PubMed] [Google Scholar]
- 40. George M, Jena A, Srivatsan V, Muthukumar R, Dhandapani VE. GDF 15—a novel biomarker in the offing for heart failure. Curr Cardiol Rev 2016; 12: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tromp J, Khan MA, Klip IT, Meyer S, de Boer RA, Jaarsma T, Hillege H, van Veldhuisen DJ, van der Meer P, Voors AA. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc 2017; 6: pii: e003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018; 72: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 43. Cowie MR, Woehrle H, Wegscheider K, Vettorazzi E, Lezius S, Koenig W, Weidemann F, Smith G, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnoea in systolic heart failure: results of the major substudy of SERVE‐HF. Eur J Heart Fail 2018; 20: 536–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. GDF‐15 correlation: electrochemiluminesence vs normalized protein expression.
Figure S2. Observed vs. Predicted % of events at 2 years by tertiles of risk score.
Table S1. Baseline characteristics by inclusion (or not) in Biomarker study.
Table S2. Biomarker names and respective Olink® panels.
Table S3. Hazard ratios and 95% confidence intervals for all the studied biomarkers (n = 276) with regards to the primary outcome.
Table S4. Primary outcome biomarker correlation.
Table S5. Cardiovascular death biomarker correlation.
Table S6. All‐cause death biomarker correlation.
Table S7. Correlation of the top biomarkers (sST2, Notch‐3, and GDF‐15) with age, plus the available echocardiographic and electrocardiographic parameters.


