Abstract
Aim
Sacubitril/valsartan is a first‐in‐class angiotensin receptor‐neprilysin inhibitor developed for the treatment of heart failure with reduced ejection fraction. Its benefits are achieved through the inhibition of neprilysin (NEP) and the specific blockade of the angiotensin receptor AT1. The many peptides metabolized by NEP suggest multifaceted potential consequences of its inhibition. We sought to evaluate the short‐term changes in serum endorphin (EP) values and their relation with patients' physical functioning after initiation of sacubitril/valsartan treatment.
Methods and results
A total of 105 patients with heart failure with reduced ejection fraction, who were candidates for sacubitril/valsartan treatment, were included in this prospective, observational, multicentre, and international study. In a first visit, and in agreement with current guidelines, treatment with angiotensin‐converting enzyme inhibitors or angiotensin receptor blocker was replaced by sacubitril/valsartan because of clinical indication by the responsible physician. By protocol, patients were reevaluated at 30 days after the start of sacubitril/valsartan. Serum levels of α‐ (α‐EP), γ‐Endorphin (γ‐EP), and soluble NEP (sNEP) were measured using enzyme‐linked immunoassays. New York Heart Association (NYHA) functional class was used as an indicator of patient's functional status. Baseline median levels of circulating α‐EP, γ‐EP, and sNEP were 582 (160–772), 101 (37–287), and 222 pg/mL (124–820), respectively. There was not a significant increase in α‐EP nor γ‐EP serum values after sacubitril/valsartan treatment (P value = 0.194 and 0.102, respectively). There were no significant differences in sNEP values between 30 days and baseline (P value = 0.103). Medians (IQR) of Δα‐EP, Δγ‐EP, and ΔsNEP between 30 days and baseline were 9.3 (−34 − 44), −3.0 (−46.0 − 18.9), and 0 units (−16.4 − 157.0), respectively. In a pre–post sacubitril/valsartan treatment comparison, there was a significant improvement in NYHA class, with 36 (34.3%) patients experiencing improvement by at least one NYHA class category. Δα‐EP and ΔsNEP showed to be significantly associated with NYHA class after 30 days of treatment (P = 0.014 and P < 0.001, respectively). Δα‐EP was linear and significantly associated with NYHA class improvement after 30 days of sacubitril/valsartan treatment.
Conclusions
These preliminary data suggest that beyond the haemodynamic benefits achieved with sacubitril/valsartan, the altered cleavage of endorphin peptides by NEP inhibition may participate in patients' symptoms improvement.
Keywords: Heart failure, Neprilysin, Sacubitril/valsartan, Endorphins, α‐Endorphin, γ‐Endorphin
Introduction
Sacubitril/valsartan is a first‐in‐class angiotensin receptor neprilysin inhibitor developed for the treatment of heart failure (HF) with reduced ejection fraction (HFrEF). The PARADIGM‐HF trial, which tested sacubitril/valsartan for treating HFrEF, showed that it was superior to enalapril in reducing the risk of cardiovascular death and HF hospitalization, as well as improving quality of life of surviving HFrEF patients.1, 2 The benefits of sacubitril/valsartan are achieved via two mechanisms: (i) the inhibition of neprilysin (NEP) causes an increase in the activity of its substrates, such as natriuretic peptides, and (ii) the specific blockade of the angiotensin receptor AT1 instead of the inhibition of the angiotensin‐converting enzyme.3
Neprilysin is an abundant enzyme that can also be found in its circulating soluble form, which has emerged as a new biomarker with promising implications for prognosis and therapy in patients with HF.4, 5, 6 Its ubiquity allows it to participate in the degradation path of multiple substrates, suggesting many multifaceted potential consequences of its inhibition beyond the natriuretic peptide system.7 Neuropeptides, including endorphins (EPs), are also recognized substrates that NEP is capable of degrading. α‐Endorphin (α‐EP) and γ‐Endorphin (γ‐EP), well‐known endogenous opioid peptides, have morphinomimetic behaviour, affect several physiologic activities, and are potential antipsychotic and analgesic drugs.8 EPs are also capable of producing feelings of welfare and tolerance to pain.9, 10
In the present work, we aimed to explore the dynamics of serum EPs values in patients with HFrEF upon treatment with sacubitril/valsartan and its relation to the improvement in functional class assessed by New York Heart Association (NYHA) class.
Methods
Study design and participants
A prospective, observational, multicentre, international study was conducted between 1 November 2016 and 1 December 2017. Patients were eligible if they had stable HF with left ventricular ejection fraction ≤40% and were under treatment with angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker (Figure 1 ).
Figure 1.
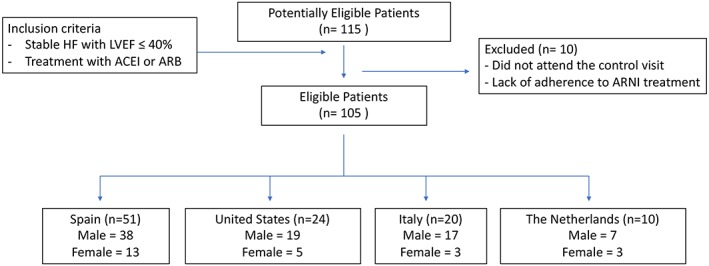
Patients flow diagram. ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor‐neprilysin inhibitor; HF, heart failure; LVEF, left ventricular ejection fraction.
In a first visit, and in agreement with current guidelines,11, 12 treatment with angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker was replaced by sacubitril/valsartan because of clinical indication by the responsible physician. Doses of sacubitril/valsartan treatment were individualized. By protocol, patients were reevaluated at 30 days after the start of sacubitril/valsartan, and no other therapeutic modifications were performed between both visits. Demographic information, medical history, vital signs, 12‐leads electrocardiogram, standard laboratory data, and pharmacological treatments were routinely assessed at visits. A trained cardiologist assessed the NYHA association class in the study visits. The estimated glomerular filtration rate (eGFR) was estimated by the Modification of Diet in Renal Disease Study equation, based on creatinine and other characteristics of the patients. Institutional committees of each participating centre approved the study protocol, local ethics committees approved the study, and all patients provided written informed consent prior to enrolment. This study was conducted in accordance with the Declaration of Helsinki. Patients with presence of extracardiac disease with an estimated life expectancy of less than 1 year, treated with narcotic analgesics, with moderate to severe alcoholism, severe renal (eGFR < 30 mL/min/1.73 m2) or severe hepatic insufficiency (Child‐Pugh C classification), hyperkalaemia (>5.4 mmol/L), systolic blood pressure < 100 mmHg, prior history of angioedema, pregnant women, or participating in randomized clinical trial, were excluded.
Measurement of serum endorphins levels
Blood samples were collected and serum was obtained by centrifugation for 10 min at 3000 rpm and stored at −80°C. All samples were obtained between 09:00 am and 12:00 pm using the same protocol at the first visit and at 30 days.
α‐EP was measured by Human α‐Endorphin ELISA kit (BlueGene Biotech; Shanghai, China; Code No. E01A3095; Lot No. 20161219). Serum aliquots were diluted 1:2 in PBS (pH 7.0–7.2). This assay has high sensitivity and excellent specificity for detecting α‐EP, and the manufacturer observed no significant cross‐reactivity or interference between α‐EP and analogues. The range of the assay is between 0 and 2500 pg/mL. The sensitivity in this assay is 1.0 pg/mL.
γ‐EP was analysed by a competitive binding enzyme‐linked immunosorbent assay technology, Human γ‐Endorphin ELISA kit (Abbexa Ltd.; Cambridge Science Park, Cambridge, UK; Code No. abx258229; Lot No. L201701A884). Assay's range is between 12.35 and 1000 pg/mL, and assay's sensitivity is 5.47 pg/mL.
Measurement of sNEP
Soluble neprilysin was measured by a sandwich immunoassay human neprilysin/CD10 ELISA kit (Aviscera Bioscience; Santa Clara, CA; Code No. SK00724‐01; Lot No. 20113187). Serum aliquots were diluted one quarter in dilution buffer provided by the manufacturer before incubation. Standard range and sensitivity are between 62.5–8000 and 30 pg/mL, respectively. Physicians in charge of clinical assessment were blinded to endorphins and sNEP levels.
Statistical analysis
Continuous variables were expressed as mean (±standard deviation) or median (interquartile range) as appropriate. Discrete variables were expressed as percentages. The primary outcome was the association between absolute changes in plasma Δα and Δγ‐endorphins (visit posttreatment − visit pretreatment) with the NYHA class at 30 days (NYHA30‐d class). Secondary outcome included the association between ΔsNEP (visit posttreatment − visit pretreatment) and NYHA30‐d class. The independent association among these markers and NYHA30‐d class was tested with analysis of covariance. NYHAbaseline class was included as a covariate in all models. Other covariates were selected by means of multivariable fractional polynomial method, which combines backward elimination with the selection of a fractional polynomial function. This ensures the right match between the functional form of continuous covariates and the outcome. A two‐sided P value of <0.05 was set as alpha for all analyses. All analyses were performed using Stata 14.1.
Results
Baseline characteristics of HF patients treated with sacubitril/valsartan are shown in Table 1. A total of 115 patients admitted in six sites in four countries (Spain, Italy, the Netherlands, and United States) were enrolled. However, we excluded 10 patients because they did not attend the follow‐up visits, or they admitted they did not adhere to treatment. The median (IQR) age was 68 years (60–75), and 81 (77.1%) were male. The most frequent aetiology was ischaemic heart disease (62.9%). Baseline medians of left ventricular ejection fraction, NT‐proBNP, and eGFR were 30% (25–35), 925 ng/L (374–1700), and 64 mL/min/1.73 m2 (50–82), respectively. The starting dose of sacubitril/valsartan was 24/26 mg in 67 (63.8%) and 49/51 mg in 38 (36.2%) patients according to their clinical status.
Table 1.
Baseline characteristics
| Age (years) | 66.7 ± 11.4 |
| Men, n (%) | 81 (77.1) |
| Aetiology, n (%) | |
| CM ischaemic | 66 (62.9) |
| CM hypertensive | 4 (3.8) |
| CM dilated | 35 (33.3) |
| NYHA functional class, n (%) | |
| I | 9 (8.6) |
| II | 77 (73.3) |
| III | 18 (17.1) |
| IV | 1 (1) |
| Hypertension, n (%) | 72 (68.6) |
| Dyslipidaemia, n (%) | 72 (68.6) |
| Prior admission for acute heart failure, n (%) | 54 (51.4) |
| Left ventricular ejection fraction (%) | 30 (25–35) (25–35) |
| Heart rate (bpm) | 65 (60–73) |
| Systolic blood pressure (mmHg) | 115 (107–130) |
| Diastolic blood pressure (mmHg) | 70 (62–78) |
| Urea (mg/dL) | 45 (32–64)) |
| Creatinine, mg/dL (IQR) | 1.15 (0.95–1.38) |
| Sodium, mmol/L (IQR) | 139 (137–141) |
| Haemoglobin, g/dl (IQR) | 13.8 (12.4–14.8) |
| NT‐proBNP, ng/L (IQR) | 925 (374–1700) |
| eGFR, m/min/m2 (IQR) | 64 (50–82) |
| α‐EP, pg/mL (IQR) | 582 (160–772) |
| γ‐EP, pg/mL (IQR) | 101 (37–287) |
| sNEP, pg/mL (IQR) | 222 (124–820) |
| Δα‐EP, units (IQR) | 9.3 (−34–44) |
| Δγ‐EP, units (IQR) | −3 (−46–18.9) |
| ΔsNEP, units (IQR) | 0 (−16.4–157) |
| Treatments | |
| Furosemide, n (%) | 67 (63.8) |
| Torasemide, n (%) | 15 (14.3) |
| Antialdosteronic, n (%) | 91 (86.7) |
| Beta blockers, n (%) | 101 (96.2) |
| Digoxin, n (%) | 13 (12.4) |
| Statin, n (%) | 71 (67.6) |
| Acetylsalicylic acid, n (%) | 55 (52.4) |
| Nitrates, n (%) | 15 (14.3) |
| Anticoagulants, n (%) | 55 (52.4) |
| Initial daily dose Sac/Val 24/26 mg, n (%) | 67 (63.8) |
| Initial daily dose Sac/Val 49/51 mg, n (%) | 38 (36.2) |
Values are expressed as mean ± SE or median (IQR).
CM, cardiomyopathy; EP, endorphin; eGFR, glomerular filtration rate; NEP, neprilysin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptides; NYHA, New York Heart Association; sNEP, soluble neprilysin.
Baseline median levels of circulating α‐EP, γ‐EP, and sNEP were 582 (160–772), 101 (37–287), and 222 pg/mL (124–820), respectively. There was not a significant increase of α‐EP nor γ‐EP serum values after sacubitril/valsartan treatment (P value = 0.194 and 0.102, respectively). There were no significant differences in sNEP values between 30 days and baseline (P value = 0.103). Medians (IQR) of Δα‐EP, Δγ‐EP, and ΔsNEP between 30 days and baseline were 9.3 (−34 − 44), −3.0 (−46.0 − 18.9), and 0 units (−16.4 − 157.0), respectively (Table 1). Baseline NYHA functional class is shown in Table 1. After 30 days treatment with sacubitril/valsartan, a significant improvement in NYHA class was found (Figure 2 ), with 36 (34.3%) patients experiencing improvement by at least one NYHA class category. Before sacubitril/valsartan treatment 8.6% of the patients were in NYHA class I, while after the treatment the percentage of patients in NYHA class I was 32.4%. A multivariable analysis revealed that NYHAbaseline class (Figure 3 A), NT‐proBNPbaseline (Figure 3 B), and eGFRbaseline (Figure 3 C) were significant predictors of NYHA30‐d class.
Figure 2.
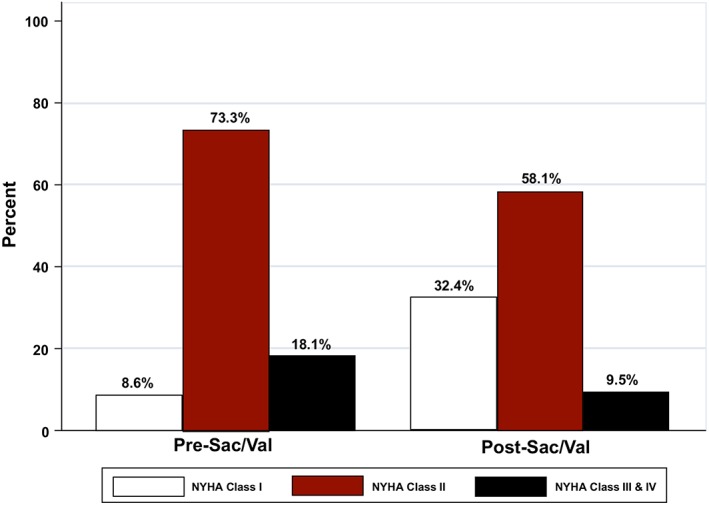
NYHA class changes after sacubitril/valsartan initiation. NYHA, New York Hear Association.
Figure 3.
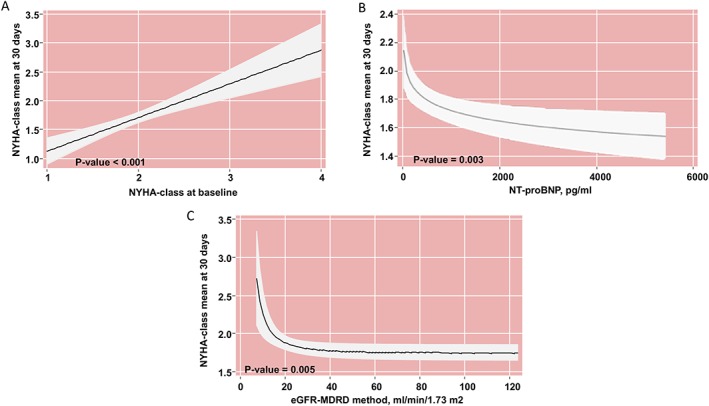
NYHAbaseline class (A), NT‐proBNPbaseline (B), and eGFRbaseline (C) were significantly predictors of the outcome. (A) Analysis adjusted by baseline values of eGFR‐MDRD method, and NT‐proBNP, and ∆α‐endorphin, ∆‐Log‐Ɣ‐endorphin, and ∆‐sNEP. (B) Analysis adjusted by baseline values of NYHA class and eGFR‐MDRD method, and ∆α‐endorphin, ∆‐Log‐Ɣ‐endorphin, and ∆‐sNEP. (C) Analysis adjusted by baseline values of NYHA class, and NT‐proBNP, and ∆α‐endorphin, ∆‐Log‐Ɣ‐endorphin, and ∆‐sNEP. eGFR, estimated glomerular filtration index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptides; NYHA, New York Heart Association; sNEP, soluble neprilysin; ∆, 30‐day minus baseline.
After forcing the three markers in the statistical model, Δα‐EP and ΔsNEP showed to be significantly associated with NYHA class after 30 days of treatment (NYHA30‐d class) (P = 0.014 and P < 0.001, respectively). For Δα‐EP, the association was linear and negative (Figure 4 A), where higher Δα‐EP values are indicative of lower NYHA30‐d class. For Δγ‐EP, the association was not significant (Figure 4 B). On the other hand, ΔsNEP was positive and linearly associated with higher NYHA30‐d class (Figure 4 C). In a sensitivity analysis, the sacubitril/valsartan dose [(24/26)/(49/51)] was not significant as predictor of NYHA30‐d class, either as main effect or as interacting with any of the markers (P value for interactions for α‐EP, γ‐EP, and sNEP were 0.201, 0.808, and 0.233, respectively).
Figure 4.
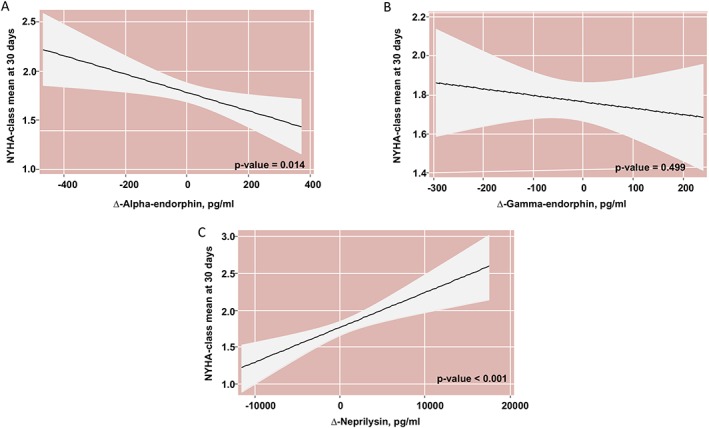
(A) α‐endorphin dynamics and NYHA class at 30 days; (B) Ɣ‐endorphin dynamics and NYHA class at 30 days; (C) sNEP dynamics and NYHA class at 30 days. *All estimates adjusted by NYHA class, eGFR, NT‐proBNP, ∆α‐endorphin, ∆Ɣ‐endorphin, and ∆‐sNEP. eGFR, estimated glomerular filtration index; NYHA, New York Heart Association; sNEP, soluble neprilysin; ∆, 30‐day minus baseline.
Discussion
In this contemporary cohort of patients with HFrEF, we found an important association between Δα‐EP and the early clinical improvement after the start of sacubitril/valsartan. These findings suggest that the altered cleavage of endorphin peptides by NEP inhibition may participate in patients' symptoms improvement beyond the haemodynamic benefits achieved with sacubitril/valsartan.
Heart failure is a chronic and progressive disease with multiple hospital readmissions and worse prognosis. Symptoms of HF worsen patients' quality of life substantially. Thus, changes in one NYHA functional class category lead to positive improvements in patient's quality of life.13, 14 Sacubitril/valsartan has proven to be a superior alternative to angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker to treat HF, reducing the risk of death and improving the condition of patients with HErEF.1, 2, 15 However, because of the large variety of substrates degraded by NEP, its pharmacological inhibition could lead to a significant increase in its substrates deriving in unknown effects.
Endogenous opioid peptides play an important role in the management of pain and several physiological activities.16, 17 They are known to be released with states of pleasure including emotions brought upon by laughter, love, sex, and sports. They are also involved in the development of cardiovascular diseases such as hypertension and congestive HF, although the mechanisms of action are not completely known.18, 19 Endogenous opioid peptides may modulate cardiac function at the hormonal level via coupling to mu opiate receptors, which have impact in the heart's performance and the vasculature.20 Endorphins such as α‐EP and γ‐EP act as functionally antagonistic neuropeptides in the brain, and their balanced expression is very important for behavioural homeostasis.21 Its implication in diverse physiological activities determines its importance to maintain a correct balance between them.
α‐EP and γ‐EP are both originated from the same precursor22, 23; the proteolytic activity cleaves β‐EP into γ‐EP and convert it into α‐EP.24 However, our results show that an increase in α‐EP was associated with an early clinical improvement as determined by NYHA functional class. In spite that α‐EP and γ‐EP are equivalent substrates for NEP,25 they seem to evolve differently. The baseline values of α‐EP were higher than the basal values of γ‐EP (582 vs. 101 pg/mL). This difference may be one of the reasons why there is an association between NYHA class and α‐EP and not with γ‐EP. Serum values of β‐EP are well known in congestive26 and acute27 HF, but α‐EP and γ‐EP pathways are little studied in HF patients.
Opioid peptides are susceptible to rapid enzymatic degradation,28 and it is known that a lot of peptidases are involved in their degradation, such as aminopeptidases, angiotensin‐converting enzyme, insulin‐degrading enzyme, and dipeptidyl peptidases, among others.29, 30, 31 Previous studies reported a significant decrease in NHYA class after sacubitril/valsartan treatment,31 and our data provide preliminary evidence of the association between Δα‐EP and the early clinical improvement after sacubitril/valsartan treatment.
We did not find significant differences between baseline and posttreatment α‐EP levels in the whole sample. However, we found a significant relationship between Δα‐EP and NYHA improvement. These findings suggest a heterogeneous and idiosyncratic endorphins response to sacubitril/valsartan that may partially explain the short‐term clinical improvement found in some patients.31, 32, 33, 34, 35 The absence of increased α‐EP and γ‐EP after treatment with sacubitril/valsartan in all patients may be due to the existence of these degradation pathways, because of the importance of maintaining its equilibrium for behavioural homeostasis.
This study has several limitations. The NYHA is based on the subjective assessment made by the physician and a more objective determination of the patients' functional status may be of interest. However, we should point out that NYHA evaluation was blinded to clinicians. In addition, because of the importance of the balance between α‐EP and γ‐EP, the homeostatic mechanisms for this maintenance could be the reason for not finding significant increases of these substrates after the treatment with sacubitril/valsartan; however, to corroborate this theory, further studies are necessary.
In conclusion, from an HF standpoint, endorphins and their effects and interactions are yet partially understood and studied. It is likely that endorphin elevation after NEP inhibition may have autocrine, paracrine, or endocrine functions; however, the duration and effects of endorphin elevation with sacubitril/valsartan is unclear. Our data suggest that beyond the haemodynamic benefits achieved with sacubitril/valsartan, the altered cleavage of endorphin peptides by NEP inhibition may participate in patients' symptoms improvement.
Conflict of interest
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Funding
This work was supported in part by Fundació La Marató de TV3 (201516‐10, 201502‐30), Generalitat de Catalunya (Departament de Salut) PERIS Acció Instrumental de Programes de Recerca Orientats (SLT002/16/00234) and AGAUR (2017‐SGR‐483), Societat Catalana de Cardiologia, “la Caixa” Banking Foundation, grants from the Spanish Ministry of Economy and Competitiveness‐MINECO (SAF2017‐84324‐C2‐1‐R), Instituto de Salud Carlos III (PI17/01487, PI18/00256, and PIC18/00014), Red de Terapia Celular‐TerCel (RD16/00111/0006), CIBER Cardiovascular (CB16/11/00403) projects, as a part of the Plan Nacional de I + D + I, and it was cofunded by ISCIII‐Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER). This work has been developed in the context of CERCA Programme (Generalitat de Catalunya) and AdvanceCat with the support of ACCIÓ (Catalonia Trade & Investment; Generalitat de Catalunya) under the Catalan ERDF operational program (European Regional Development Fund) 2014–2020.
Acknowledgements
We would like to acknowledge the work of the HF units nurses in collecting the data and their invaluable patient care. And we are very grateful to the patients who decided to participate in this study.
Revuelta‐López, E. , Núñez, J. , Gastelurrutia, P. , Cediel, G. , Januzzi, J. L. , Ibrahim, N. E. , Emdin, M. , VanKimmenade, R. , Pascual‐Figal, D. , Núñez, E. , Gommans, F. , Lupón, J. , and Bayés‐Genís, A. (2020) Neprilysin inhibition, endorphin dynamics, and early symptomatic improvement in heart failure: a pilot study. ESC Heart Failure, 7: 559–566. 10.1002/ehf2.12607.
References
- 1. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Bělohlávek J, Böhm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzàlez‐Medina A, Hagège AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan Ö, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC, PARADIGM‐HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54–61. [DOI] [PubMed] [Google Scholar]
- 3. Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin‐angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014; 2: 663–670. [DOI] [PubMed] [Google Scholar]
- 4. Bayés‐Genís A, Barallat J, Galán A, de Antonio M, Domingo M, Zamora E, Gastelurrutia P, Vila J, Peñafiel J, Gálvez‐Montón C, Lupón J. Multimarker strategy for heart failure prognostication. Value of neurohormonal biomarkers: neprilysin vs NT‐proBNP. Rev Esp Cardiol (Engl Ed) 2015. Dec; 68: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 5. Bayés‐Genís A, Barallat J, Pascual‐Figal D, Nuñez J, Miñana G, Sánchez‐Mas J, Galan A, Sanchis J, Zamora E, Pérez‐Martínez MT, Lupón J. Prognostic value and kinetics of soluble neprilysin in acute heart failure: a pilot study. JACC Heart Fail 2015; 3: 641–644. [DOI] [PubMed] [Google Scholar]
- 6. Bayés‐Genís A, Barallat J, Galán A, de Antonio M, Domingo M, Zamora E, Urrutia A, Lupón J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol 2015; 65: 657–665. [DOI] [PubMed] [Google Scholar]
- 7. Bayes‐Genis A, Lupón J. Neprilysin: indications, expectations, and challenges. Rev Esp Cardiol (Engl Ed) 2016; 69: 647–649. [DOI] [PubMed] [Google Scholar]
- 8. Kosanam H, Ramagiri S, Dass C. Quantification of endogenous α‐ and γ‐endorphins in rat brain by liquid chromatography‐tandem mass spectrometry. Anal Biochem 2009; 392: 83–89. [DOI] [PubMed] [Google Scholar]
- 9. de Wied D. Behavioural actions of neurohypophysial peptides. Proc R Soc Lond B Biol Sci 1980; 210: 183–195. [DOI] [PubMed] [Google Scholar]
- 10. Baker AK, Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther 2002; 302: 1253–1264. [DOI] [PubMed] [Google Scholar]
- 11. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members , Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey de Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride P, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the management of heart failure. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 13. Gastelurrutia P, Lupón J, Altimir S, de Antonio M, González B, Cabanes R, Cano L, Urrutia A, Domingo M, Zamora E, Díez C, Coll R, Bayes‐Genis A. Effect of fragility on quality of life in patients with heart failure. Am J Cardiol 2013; 112: 1785–1789. [DOI] [PubMed] [Google Scholar]
- 14. Lupón J, Gastelurrutia P, de Antonio M, González B, Cano L, Cabanes R, Urruria A, Díez C, Coll R, Altimir S, Bayes‐Genis A. Quality of life monitoring in ambulatory heart failure patients: temporal changes and prognostic value. Eur J Heart Fail 2013; 15: 103–109. [DOI] [PubMed] [Google Scholar]
- 15. Marques da Silva P, Aguiar C. Sacubitril/valsartan: An important piece in the therapeutic puzzle of heart failure. Rev Port Cardiol 2017; 36: 655–668. [DOI] [PubMed] [Google Scholar]
- 16. Stein C, Hassan AH, Lehrberger K, Giefing J, Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet 1993; 342: 321–324. [DOI] [PubMed] [Google Scholar]
- 17. Koneru A, Satyanarayana S, Rizman S. Endogenous opioids: their physiological role and receptors. Global J Pharmacol 2009; 3: 149–153. [Google Scholar]
- 18. van den Brink OWV, Delbridge LM, Rosenfeldt FL, Penny D, Esmore DS, Quick D, Kaye DM, Pepe S. Endogenous cardiac opioids: enkephalins in adaptation and protection of the heart. Heart Lung Circ 2003; 12: 178–187. [DOI] [PubMed] [Google Scholar]
- 19. Rawal H, Patel BM. Opioids in cardiovascular disease: Therapeutic options. J Cardiovasc Pharmacol Ther 2018; 23: 279–291. [DOI] [PubMed] [Google Scholar]
- 20. Stefano GB, Zhu W, Cadet P, Mantione K, Bilfinger TV, Bianchi E, Guarna M. A hormonal role for endogenous opiate alkaloids: vascular tissues. Neuro Endocrinol Lett 2002; 23: 21–26 Review. [PubMed] [Google Scholar]
- 21. Wiegant VM, Ronken E, Kovács G, de Wied D. Endorphins and schizophrenia. Prog Brain Res 1992; 93: 433–453. [PubMed] [Google Scholar]
- 22. Ling N, Burgus R, Guillemin R. Isolation, primary structure, and synthesis of alpha‐endorphin and gamma‐endorphin, two peptides of hypothalamic‐hypophysial origin with morphinomimetic activity. Proc Natl Acad Sci U S A 1976; 73: 3942–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bloom FE. The endorphins: a growing family of pharmacologically pertinent peptides. Annu Rev Pharmacol Toxicol 1983; 23: 151–170. [DOI] [PubMed] [Google Scholar]
- 24. Burbach JP, Loeber JG, Verhoef J, Wiegant VM, de Kloet ER, de Wied D. Selective conversion of beta‐endorphin into peptides related to gamma‐ and alpha‐endorphin. Nature 1980; 283: 96–97. [DOI] [PubMed] [Google Scholar]
- 25. Webster CI, Burrell M, Olsson LL, Fowler SB, Digby S, Sandercock A, Snijder A, Tebbe J, Haupts U, Grudzinska J, Jermutus L, Andersson C. Engineering neprilysin activity and specificity to create a novel therapeutic for Alzheimer's disease. PLoS ONE 2014; 9: e104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cozzolino D, Sasso FC, Salvatore T, Torella M, Gentile S, Torella R, Giugliano D. Acute effects of beta‐endorphin on cardiovascular function in patients with mild to moderate chronic heart failure. Am Heart J 2004; 148: E13. [DOI] [PubMed] [Google Scholar]
- 27. Feng SD, Jiang Y, Lin ZH, Lin PH, Lin SM, Liu QC. Diagnostic value of brain natriuretic peptide and β‐endorphin plasma concentration changes in patients with acute left heart failure and atrial fibrillation. Medicine (Baltimore) 2017; 96: e7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKnight AT, Corbett AD, Kosterlitz HW. Increase in potencies of opioid peptides after peptidase inhibition. Eur J Pharmacol 1983; 86: 393–402. [DOI] [PubMed] [Google Scholar]
- 29. Augustyns K, Van der Veken P, Senten K, Haemers A. The therapeutic potential of inhibitors of dipeptidyl peptidase IV (DPP IV) and related proline‐specific dipeptidyl aminopeptidases. Curr Med Chem 2005; 12: 971–998. [DOI] [PubMed] [Google Scholar]
- 30. Asvadi NH, Morgan M, Hewavitharana AK, Shaw PN, Cabot PJ. Biotransformation of beta‐endorphin and possible therapeutic implications. Front Pharmacol 2014; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cameli M, Pastore MC, Pagliaro A, di Tommaso C, Reccia R, Curci V, Mandoli GE, Mondillo S. Sacubitril/valsartan in an elderly patient with heart failure: a case report. Cardiology 2017; 138: 3–6. [DOI] [PubMed] [Google Scholar]
- 32. Paolini C, Perrone C, Pellizzari CA, Randon ML, Bilato C. Management of a patient with heart failure by sacubitril/valsartan: improvement of functional capacity. Curr Med Res Opin 2019; 35: 7–8. [DOI] [PubMed] [Google Scholar]
- 33. Khariton Y, Fonarow GC, Arnold SV, Hellkamp A, Nassif ME, Sharma PP, Butler J, Thomas L, Duffy CI, DeVore AD, Albert NM, Patterson JH, Williams FB, McCague K, Spertus JA. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail 2019; 7: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beltrán P, Palau P, Domínguez E, Faraudo M, Núñez E, Guri O, Mollar A, Sanchis J, Bayés‐Genís A, Núñez J. Sacubitril/valsartan and short‐term changes in the 6‐minute walk test: a pilot study. Int J Cardiol 2018; 252: 136–139. [DOI] [PubMed] [Google Scholar]
- 35. Palau P, Mollar A, Domínguez E, Sanchis J, Bayés‐Genís A, Núñez J. Early Sacubitril/valsartan‐driven benefit on exercise capacity in heart failure with reduced ejection fraction: a pilot study. Rev Esp Cardiol (Engl Ed) 2019; 72: 167–169. [DOI] [PubMed] [Google Scholar]


