Abstract
Persistent primitive hypoglossal artery (PPHA) is a rare internal carotid-vertebrobasilar anatomic variant. Awareness of this anomaly and its propensity for atherosclerotic disease is important to avoid misinterpretation of diagnostic studies and to allow appropriate interventional planning. As the predominant vascular supply to the anterior and posterior cerebral circulation, its luminal compromise can lead to devastating ischemic complications. Carotid endarterectomy and carotid stenting have both been performed to treat lesions involving a PPHA. Herein, we report a case of carotid endarterectomy involving a PPHA and discuss the clinical and surgical implications of a carotid lesion in the presence of a PPHA.
Keywords: Hypoglossal artery, Carotid artery disease, Atherosclerosis, Endarterectomy
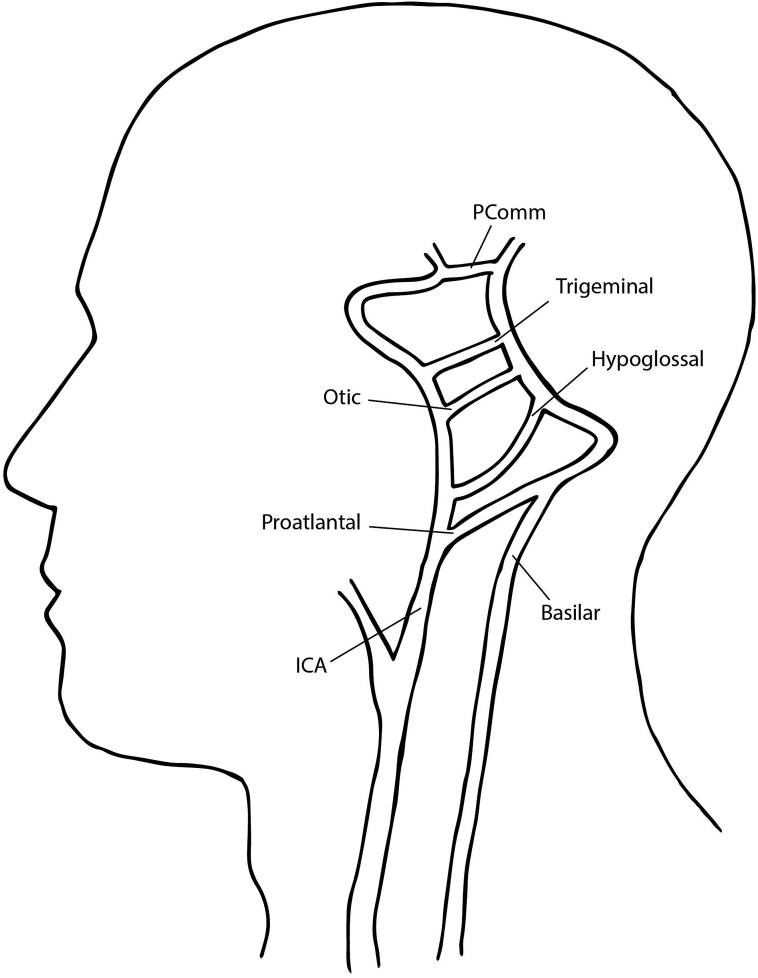
Four arterial anastomoses between the carotid and the developing vertebrobasilar system exist in fetal development. The presence of any of these arterial connections in adulthood is uncommon (Fig 1). The persistent primitive hypoglossal artery (PPHA) exists between the internal carotid artery (ICA) and basilar artery and is the second most frequently identified, with an estimated incidence of 0.02% to 0.26%.1 Because of its rarity, data regarding best management for carotid stenosis with ipsilateral PPHA are sparse. Herein, we present a case of a patient with a symptomatic high-degree lesion of the ICA involving a PPHA in which carotid endarterectomy (CEA) was chosen as the revascularization method.
Fig 1.
Schematic of persistent fetal carotid-vertebrobasilar segmental arteries. ICA, Internal carotid artery.
The patient has provided written informed consent to present the case.
Case report
A 68-year-old woman with hypertension, hyperlipidemia, and a remote history of tobacco use initially presented to our practice in 2009. She was referred by her primary care physician for evaluation after an episode of self-limited diplopia. Aspirin and rosuvastatin had been prescribed. A carotid duplex ultrasound examination performed at the initial visit demonstrated 16% to 49% stenosis of the right ICA and 1% to 15% stenosis of the left. Continued surveillance showed gradual progression in the degree of the carotid stenoses. On a routine visit in February 2019, she was found to have a hemodynamically significant 80% to 99% stenosis of the right ICA, distal to a 50% to 79% stenosis in the proximal ICA. During this visit, she reported having a second episode of diplopia 2 months earlier. She was started on clopidogrel 75 mg daily, given concern for a symptomatic lesion.
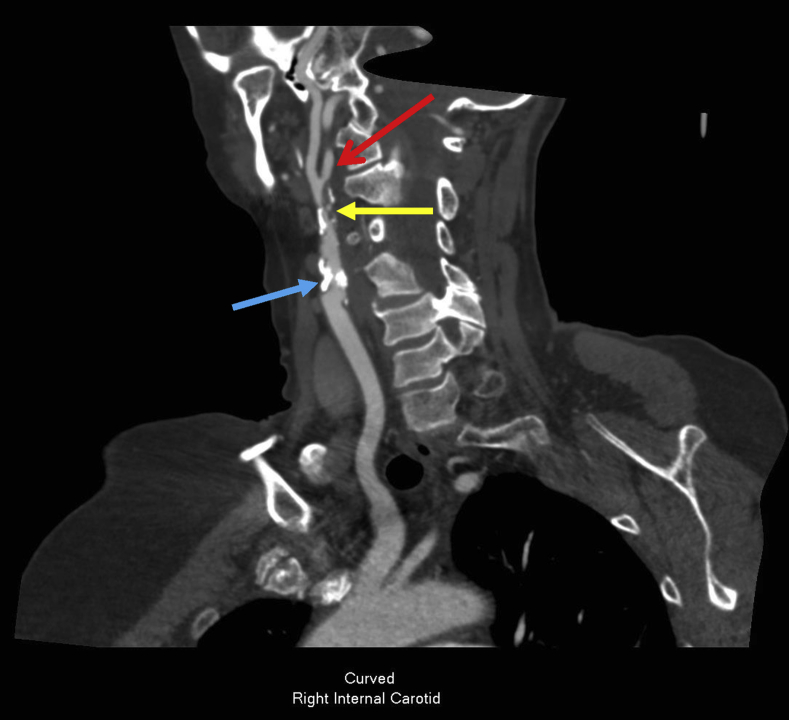
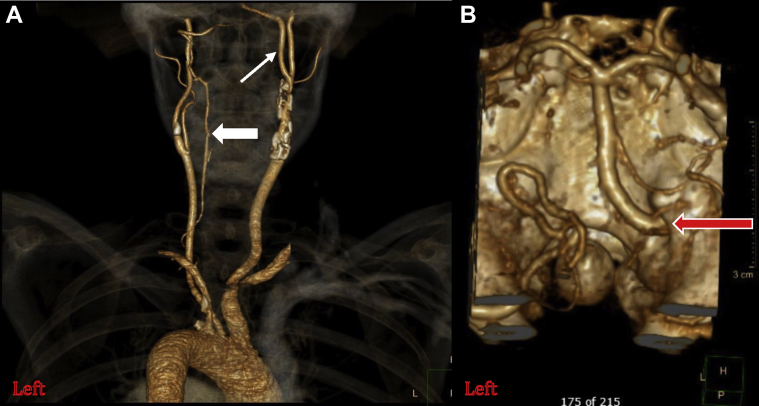
Computed tomography (CT) angiography was performed and demonstrated two distinct areas of severe stenosis with mixed calcific and soft-type plaque morphology. A proximal ICA lesion was identified just beyond the carotid bulb, and a more distal, ulcerated lesion was noted proximal to a bifurcation with a PPHA at the C1-C2 intervertebral space (Fig 2). Additional radiographic findings included an aplastic right vertebral artery and a diminutive left vertebral artery that supplied the posterior inferior cerebellar artery (Fig 3). The circle of Willis was intact.
Fig 2.
Computed tomography (CT) angiography of the head and neck demonstrating two distinct severe right carotid lesions. Blue arrow, Calcific stenosis above external carotid artery-internal carotid artery (ICA) bifurcation. Yellow arrow, Ulcerated soft plaque just proximal to ICA-persistent primitive hypoglossal artery (PPHA) bifurcation. Red arrow, PPHA.
Fig 3.
A, Three-dimensional reconstruction of computed tomography (CT) angiography image demonstrating no appreciable right-sided vertebral artery. Thick arrow, Small left vertebral artery that becomes the posterior inferior cerebellar artery. Thin arrow, Persistent primitive hypoglossal artery (PPHA). B, PPHA entering skull through the hypoglossal canal (red arrow) as the sole supply to the basilar artery.
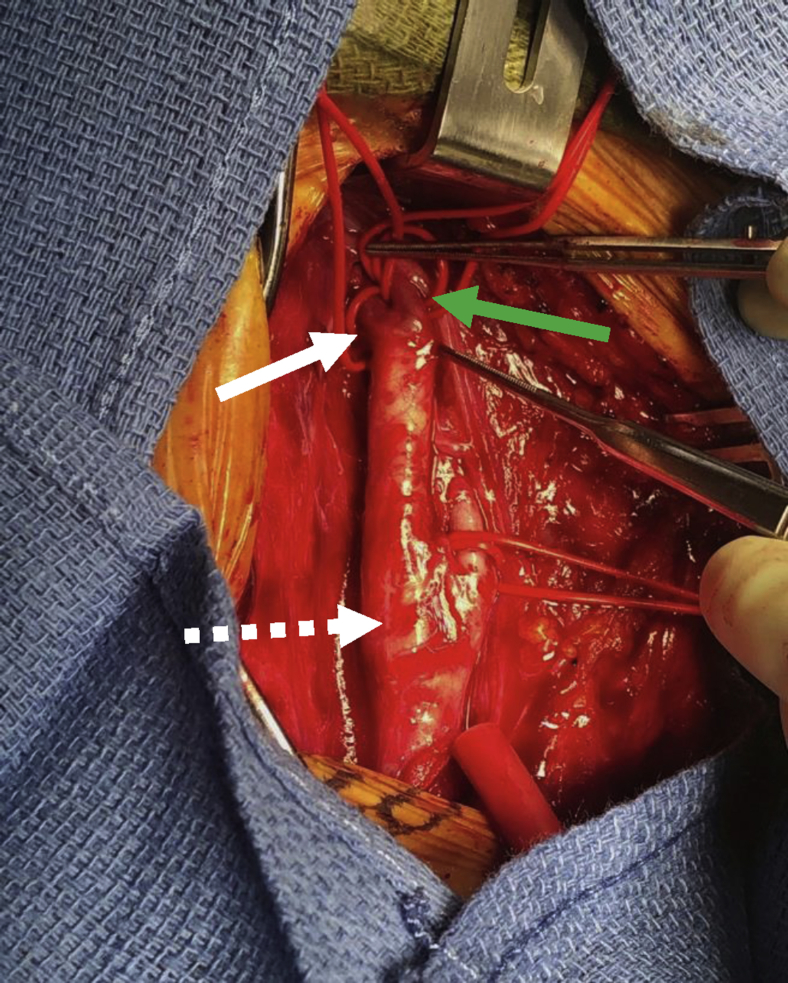
Given the high-degree stenosis with a presumed symptomatic lesion involving the ICA-PPHA bifurcation, the decision was made to treat our patient. We chose CEA rather than an endovascular approach. She was administered general anesthesia, and standard endarterectomy was performed with intraoperative shunting to the PPHA (Fig 4) as this was the sole vessel supplying the posterior circulation. The artery was repaired with bovine pericardial patch extending onto the PPHA. Intraoperative duplex ultrasound confirmed an excellent result. The patient awoke from anesthesia neurologically intact and was admitted overnight for strict blood pressure control. She had no postoperative complications. She was discharged in excellent condition on the first postoperative day. At 3-month follow-up, she remained in good health, with ultrasound imaging demonstrating continued normalization of velocities and waveform morphology.
Fig 4.
Intraoperative image. Dashed white arrow, Carotid bulb. Solid white arrow, Persistent primitive hypoglossal artery (PPHA). Green arrow, Internal carotid artery (ICA).
Discussion
The hypoglossal artery is one of the four segmental arteries that temporarily connect the anterior and posterior cerebral circulation in fetal development. They are named by their anatomic course and include the trigeminal artery, the otic artery, the hypoglossal artery, and the pro-atlantal segmental artery (Fig 1).2 The involution of the trigeminal, hypoglossal, and otic arteries coincides with the development of the posterior communicating artery,1,3 whereas the pro-atlantal artery continues to supply the hindbrain until the vertebral arteries develop.2 Consequently, persistence of these arteries beyond fetal development is often accompanied by the absence or hypoplasia of the posterior communicating and vertebral arteries.4
PPHA is more common in female patients and occurs primarily on the left side.5 Diagnostic criteria for a PPHA are fourfold: the artery arises from the cervical ICA typically at the level of the C1-C3 vertebral body; it enters the skull through the hypoglossal canal along with cranial nerve XII; the basilar artery is filled only beyond its anastomosis with the PPHA; and the posterior communicating artery and vertebral artery are hypoplastic or absent.5
PPHA is the second most common of these fetal anastomoses to persist into adulthood. Its presence is typically asymptomatic and identified incidentally on diagnostic imaging, although it may be missed on ultrasound because of its location in the high cervical region. A symptomatic lesion involving a PPHA may be manifested as combined carotid and vertebrobasilar territory infarct, mimicking a central embolic source6 and leading to missed or delayed diagnosis. PPHA is of particular clinical relevance, given its association with vascular disease including intracranial aneurysm, arteriovenous malformation, and atherosclerosis.7 The propensity for concomitant vascular anomalies was the basis for performing CT angiography before intervention in our patient. This is not routinely done in our practice as duplex ultrasound imaging is often sufficient for preoperative planning. This brings into question the added benefit from routine preoperative CT imaging before any carotid intervention.
The presence of a PPHA creates an aberrant bifurcation in the cervical ICA, leading to hemodynamic stress. This is hypothesized to increase the risk of local atherosclerosis as it does in the carotid bulb.1,7 In the absence of a posterior communicating artery or vertebral artery, as is commonly seen with PPHA, the brain stem, cerebellum, and ipsilateral hemisphere are entirely dependent on supply through the ICA.6 Because of the dependence on flow through the ICA, CEA with intraoperative shunting can provide continued intraprocedural perfusion to the cerebral circulation that may otherwise be compromised with flow reversal or balloon occlusion used during carotid artery stenting (CAS). Although limited data exist for CAS involving a PPHA, it has been used as a successful interventional option and may be favored in high lesions or in patients with limitations in adequate positioning.1,8,9
The decision to perform CEA over CAS was ultimately reached after extensive review of imaging and deliberation with our neurointerventional colleagues. Risk of plaque shift and potential to jail the ipsilateral ICA during CAS was believed to be exceedingly high. In favor of open surgery was the patient's otherwise good health and presumed low risk for perioperative cardiovascular events. She had no limitations in neck extension or rotation, allowing optimal positioning. On preoperative CT angiography, the ICA plaque predominated proximal to the bifurcation with a PPHA and was not thought to be exceedingly high. Finally, it was thought that intraoperative shunting would allow minimal cerebral ischemia time. In our case, the PPHA was shunted rather than the ICA because of the presence of an intact circle of Willis and single-vessel supply of the posterior circulation through the PPHA. In other reports of CEA in the presence of PPHA, additional techniques for cerebral perfusion monitoring were used and can be considered.3,4 Other surgical considerations include to which vessel to extend the arteriotomy and patch and whether to use a bifurcating patch or shunt.
Conclusions
Although PPHA is rare and typically clinically insignificant, its identification is essential for surgical planning before CEA, carotid stenting, or skull base surgery to avoid injury or potentially catastrophic cerebral ischemia. PPHA is associated with an increased propensity for local atherosclerotic disease and increased incidence of intracranial vascular disease, such as aneurysm and arteriovenous malformations. Data on optimal management of atherosclerotic lesions associated with this rare anatomic anomaly are limited because of its low incidence. Its presence carries with it significant clinical implications, making PPHA a continued topic of interest.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Zhang L., Song G., Chen L., Jiao L., Chen Y., Wang Y. Concomitant asymptomatic internal carotid artery and persistent primitive hypoglossal artery stenosis treated by endovascular stenting with proximal embolic protection. J Vasc Surg. 2016;63:237–240. doi: 10.1016/j.jvs.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 2.Coulier B. Persistent hypoglossal artery. J Belg Soc Radiol. 2018;102:28. doi: 10.5334/jbsr.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantini G.A., Reilly L.M., Stoney R.J. Persistent hypoglossal artery: diagnostic and therapeutic considerations concerning carotid thromboendarterectomy. J Vasc Surg. 1994;20:995–999. doi: 10.1016/0741-5214(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 4.Thayer W.P., Gaughen J.R., Harthun N.L. Surgical revascularization in the presence of a preserved primitive carotid-basilar communication. J Vasc Surg. 2005;41:1066–1069. doi: 10.1016/j.jvs.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas M.R., Vedaraju K.S., Manjappa B.H., Nagaraj B.R. Persistent primitive hypoglossal artery (PPHA)—a rare anomaly with literature review. J Clin Diagn Res. 2016;10:TD13–T14. doi: 10.7860/JCDR/2016/15556.7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elhammady M.S., Başkaya M.K., Sonmez O.F., Morcos J.J. Persistent primitive hypoglossal artery with retrograde flow from the vertebrobasilar system: a case report. Neurosurg Rev. 2007;30:345–349. doi: 10.1007/s10143-007-0092-6. discussion: 349. [DOI] [PubMed] [Google Scholar]
- 7.Vlychou M., Georganas M., Spanomichos G., Kanavaros P., Artinopoulos P., Zavras G.M. Angiographic findings and clinical implications of persistent primitive hypoglossal artery. BMC Med Imaging. 2003;3:2. doi: 10.1186/1471-2342-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murai S., Kusaka N., Umakoshi M., Itami H., Otsuka S., Nishiura T. Stenting for internal carotid artery stenosis associated with persistent primitive hypoglossal artery using proximal flow blockade and distal protection system: a technical case report and literature review. J Stroke Cerebrovasc Dis. 2016;25:e98–102. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Huang M., Moisi M., Zwillman M.E., Volpi J.J., Diaz O., Klucznik R. Transient ischemic attack in the setting of carotid atheromatous disease with a persistent primitive hypoglossal artery successfully treated with stenting: a case report. Cureus. 2016;8:e464. doi: 10.7759/cureus.464. [DOI] [PMC free article] [PubMed] [Google Scholar]