Abstract
Proteins are molecular machines with the capacity to perform diverse physical work as response to signals from the environment. Proteins may be found as monomers or polymers, two states that represent an important subset of protein interactions and generate considerable functional diversity, leading to regulatory mechanisms closely akin to decision-making in service systems. Polymerization is not unique to proteins. Other cell compartments (e.g. secretory granules) or tissue states (e.g. miniature end plate potential) are associated with polymerization of some sort, leading to information transport. This data-processing mechanism has similarities with (and led us to the investigation of) granule homotypic polymerization kinetics. Using information theory, we demonstrate the role played by the heterogeneity induced by polymerization: granule size distribution and the stealthy machine behind granule life cycle increase system entropy, which modulates the source/receiver potential that affects communication between the cell and its environment. The granule inventory management by the same nano-machine is discussed.
Keywords: unit granule, homotypic fusion, SNARE, cellular communication
SUMMARY
Introduction
The granule alphabet
Secretion rate
Change-point recognition
Inventory management - tradeoff between inventory and information
Econo-biology of granule inventory management
Summary words on the surreptitious nano-machine
1. Introduction
Most biological systems engage in data processing. Data are transmitted by means of material movement, performed by loss of organization (e.g. secretory granules are degranulated, receptors aggregate) and stored by polymerization: the DNA stores information in linear sequence of four alternating basic letters; enzymes store processing information in a sequence of amino acids; receptors polymerize to transfer data into the cell. The well documented formal definition of biological language has as common basis a “string” from a list of restricted symbols, the alphabet code for DNA, RNA, proteins, etc. Another level of biological information is stored in polymers, in which the alphabet consists of a single letter, the monomer. Energy is stored as phosphate units [phosphate = the energy unit, AMP (monomer), ADP (dimer), ATP (trimer)]1. In neuronal transmission, miniature endplate potentials have been proved to be composed of polymers, multiples of monomers of a basic size2-4. Secretory granules have been demonstrated to be of quantal size (the unit granule aggregation model - Hammel, Lagunoff and Galli 2010)5 and single receptors are known to aggregate following binding to activator6,7. All of these different languages have a restricted “grammar” currently under investigation worldwide. In a number of biological systems investigated, single-letter polymers have size distributions that have been found to fit Geometric-like and Poisson-like distributions5, depending respectively on whether polymers of any size can expand by integration with any other polymer or growth is due to the addition of one single monomer at a time to any other polymer5.
Our working paradigm is that immature unit granules (broken line G1 in Figure 1), packaged in the Golgi and released at Poisson process moments, have for some restricted time the ability to fuse with granules. Beyond this transient period these immature unit granules become (at some rate η) mature unit granules (solid line G1 in Figure 1), but some immature unit granules fuse early enough with mature unit granules (homotypic fusion), that grow as a result by unit quantal incremental size, from some size Gn (i.e., granule of quantal size n) to the next size Gn+1. Each mature granule is eventually secreted from the cell, by (heterotypic) fusion with the cell membrane. We have developed8,9 a growth and elimination (G&E) Markovian model under which mature granules grow at size-dependent rate λnand exit the cell at size-dependent rate μn. These rates are modelled by statistical mechanics reasoning so as to represent the probability of formation of the SNARE protein rosette that facilitates fusion. Letting the rosette be a ring composed of K SNARE complexes, K-1 such complexes must form during some interval of time at some disk around some initial SNARE. The probability of this simultaneous event is of order
Figure 1. Scheme illustrating the dynamics of granule life in secretory cells.
(A) in which single steps in the G&E Markov model (B), based (via four global parameters β, γ, λ, µ) on two transition rates λn= λnβand µn =µnγ that describe the probability rate of a Gn-granule (granule of quantal size n) to move one level up and become a Gn+1-granule (i.e. Gn+G1àGn+1) or to move out of the system, respectively. The transition, which occurs after an exponentially distributed time with mean 1/(λn+µn), is an increase in size with probability λn/(λn+µn) and exit with the complementary probability µn/( λn+µn). “Birth” (Figure 1B) follows a Poisson process of formation of unit granules, each of which undergoes, independently, a Markov history as in Figure 1A. Newly formed immature secretory unit granules (broken line G1) can either mature (solid line G1) or homotypically fuse with another granule.
[(area-disk)/(surface-area-granule)]K-1=[const*(area-disk)/(volume-granule)2/3]K-1=[const*(area-disk)/n2/3]K-1=const*n-(2/3)(K-1).
We have accordingly modelled9 the growth rate as λn= λnβand the elimination rate as μn= μnγ, where β=-(2/3)(Kβ -1) and γ=-(2/3)(Kγ -1) are expressed in terms of the SNARE rosette sizes Kβ and Kγ necessary for homotypic and heterotypic fusion respectively (assumed constant and explained throughout the paper). These rates determine two natural quantal volume size distributions, the stationary distribution STAT of quantal volume of granules in the cell and the distribution EXIT of the quantal volume size of granules upon leaving the cell. These two distributions are readily evaluated from the effective kinetics factor μ/λ, the effective surface factor γ-β and the surface tension γ.
The above applies to cells in basal mode. A cell in occasional evoked mode will secrete granules according to STAT, playing the role of the typical spatial distribution near the membrane. Thus, we expect basally secreted granules to be EXIT distributed, and evokedly secreted granules to be STAT distributed. The literature abounds with carefully measured such pairs of distributions. These are invariably multimodal, with equally spaced peaks shared by both distributions, which differ in the probabilities assigned to the common skeleton. The STAT volume is stochastically bigger than the EXIT volume. The EXIT distribution only depends on the effective kinetics and surface factors, and the STAT distribution is parametrically 2-dimensional too in essence, but the pair of distributions (EXIT, STAT) solidly identifies the three composite parameters above. Maximum likelihood estimation of μ/λ, β and γ was performed based on 12 such pairs of distributions9. The (negative) values of β and γ came out outside the range of dimensions amenable to classical analysis (van der Waals forces, etc.), but the particle physics approach applied above provides a powerful viable alternative.
The values obtained for the rosette size pairs had Kγ ranging between 4 and 9 and Kβ usually equal to but in some cases exceeding Kγ by 1. K-values as high as 30 have been discussed in the literature9-11. Theoretical calculations show that the inequality Kγ ≤ Kβ+1 is necessary and sufficient for the existence of a stationary solution to the G&E model. Entropy considerations to be described in the sequel show that the information content of the change of mode from basal to evoked is high, for given Kβ, if Kγ is as high as possible, but the contribution of Kγ = Kβ+1 over that of Kγ = Kβ is not worth the instability of being borderline stable. We contend that the empirical relationship estimated betweenKβ and Kγ, under which Kβ is preferably equal to or exceeds Kγ by 1, is just what evolution should have aimed to achieve.
The estimated values of Kβ and Kγ are strongly increasingly correlated with granule size. The following Euclidean geometry argument supports a “square-root” rule: since SNAREs are very homogeneous, their length h is constant (Figure 2). Let us think of the rosette as a chordal disk in the spherical granule of radius r, at (the small) maximal distance h from the surface. Hence, the radius of this disk is 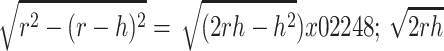 . In other words, the number of SNARE units, proportional to the circumference of this disk, should be proportional to the square root of granule diameter. Linear regression of K on the square root of reported average diameter applied to the 12 cells in the study, led to the empirical relation
. In other words, the number of SNARE units, proportional to the circumference of this disk, should be proportional to the square root of granule diameter. Linear regression of K on the square root of reported average diameter applied to the 12 cells in the study, led to the empirical relation  , (
, (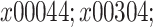 measured in nm).
measured in nm).
Figure 2. SNARE depth h is roughly constant, small with respect to granule radius (r).
Hence, SNARE size Kγ, proportional to the perimeter of the rosette (2πa), is proportional to the square root of granule diameter (D=2r).
Let ξ stand for the fraction of evoked secretion out of total secretion. In principle, the six parameters η, λ, β, μ, γ and ξ determine the dynamics and steady state of the entire process. We will now generalize the numerical methods to evaluate various features of the steady state of the system developed by Nitzany, Hammel and Meilijson8, and extend the scope of the methods to the study of transient behavior as well.
Digressing to Queuing Theory, an M/M/s queuing system is a service station with s servers (s≤∞) that serve a Poisson stream of arriving customers. Inter-arrival times are exponentially distributed with some rate α and service times are exponentially distributed with some rate δ, all of these times independent of each other. The basal G&E model may be viewed as a series of M/M/∞ stations in tandem, where the first station, mature monomers, has arrival rate α=η and service rate δ=λ+μ. A fraction μ/(λ+μ) of the served “customers” leave the station (secretion of mature monomers) and the remaining “customers”, dimer polymers, constitute the arrivals to the second station, with arrival rate α=ηλ/(λ+μ) and service rate δ=λ*2β+μ*2γ. A fraction μ*2γ/(λ*2β+μ*2γ) of the dimer polymers leave the station (secretion of dimers) and the remaining “customers”, trimer polymers, constitute the arrivals to the third station, with arrival rate α=η*(λ/(λ+μ))*( λ*2β/(λ*2β+μ*2γ)) and service rate δ=λ*3β+μ*3γ, etc. While each station reaches steady state (because the queuing system M/M/∞ is always stable), the system as a whole may not, in the sense that the steady state occupancy at the various stations may have infinite sum. As already mentioned, we have proved8 that global stationarity requires that γ>β+1.
This Markovian model lends itself to formal and/or numerical analysis, without or with the incorporation of evoked secretion, via the parameter ξ. The MATLAB program HM2014_mixedsecret.m admits as inputs the parameters β, γ, μ/λ, ξ, η and N, the desired expected total number of granules in the cell under steady state (all MATLAB programs are listed in the Supplementary Material, which can be accessed at https://discoveriesjournals.org/discoveries/discoveries-02-021-s001.pdf). As a first step, the program evaluates the particular values of λ and μ that achieve N. It then provides the stationary and exit granule quantal size distributions, as well as a menu of parameters, such as mean (stationary and exit) granule quantal size, mean and standard deviation of sojourn time in the cell, and information-theoretic KLD (Kullback-Leibler Divergence) measures. Although the mean stationary granule quantal size is considered a parameter of major interest, the product of η with the mean exit granule quantal size provides the production rate of unit granules at the Golgi.
There are questions of interest that defy formal or numerical analysis, about transient behavior of the G&E system. How long does it take for polymers of a give size to stabilize, and how long does it take for the total cell granule content and mean granule size to stabilize? How stable is steady state? To answer these questions as well as others the answers might raise, we have written the cell simulator Matlab program HM2014_simuLIFO.m. The name of the program is indicative of one of the salient observations, that evolutionary inventory management induces an almost Last-In First-Out discipline, where newly created monomers exit the cell soon, and occasionally created high-order polymers become trapped in the cell for longer times, as buffer for emergency needs to be filled by evoked secretion. The program can output in principle the entire history of a cell scenario for days or years, the average of 100 such scenarios, and almost any parameter the user might find of interest. The parameters are essentially provided by the output of HM2014_mixedsecret.m, with the addition of the mean batch size of evoked secretion, a parameter that does not affect steady state distributions but has some effect on the stability of steady state.
There is a good reason to study steady state stability, or fluctuations around steady state benchmark central values. As proposed, cells transmit information to the cellular environment8. This information, specifically about the current dichotomous mode (basal or evoked, quiescent or active neuron), is coded into the difference between the current intra-cellular and exit granule content distributions. If mean granule content N is large (and only then), these two distributions are most of the time close, respectively, to the (steady state) stationary and exit distributions. Besides the obvious requirement that the buffer size N must be large enough as backup inventory for evoked secretion, evolution must find a compromise between the need to reach steady state swiftly (by making N small) or maintaining stable content benchmarks (by making N large). It is of no wonder, then, that cells with large, few granules are only capable of supplying materials, while cells that need to impart information underwent a long process of miniaturization to achieve values of N in the thousands or tens of thousands, where empirical distributions typically mimic their thermodynamical limits.
2. The granule alphabet
The effects of changes affecting “language text” can be quantified using information theory1 in terms of entropy and mutual information, measures of the decrease of uncertainty at a receiver. The communication engineer asks how much information can be transmitted (or is lost) due to the loss of organization. The chemist asks for a measure of the extent to which the energy of the system is unavailable to work due to the loss of organization, and answers it by counting the number of microstates a system can occupy, to provide both a measure of the entropy of the system as well as a way of telling which processes are permissible under the Second Law of thermodynamics1,12. The ecologist views entropy as an index of system diversity13,14. The factor common to all of these definitions is that they describe ways to define the system state.
The paradigm that views polymerization as interacting monomer particles and the granule G&E life cycle as a fusion nano-machine, leads to a single-letter alphabet (the monomer) in which system state (or “word”) is polymer size. As in the best tradition of Information Theory15, the different distributions of secreted polymer size and secretion rates under basal and evoked secretion constitute a noisy communication channel through which the cell transmits to the environment the dichotomous information of whether it is in basal or evoked mode. Noisy channels achieve reliability of information by means of redundancy and repetition, the role played by burst size in evoked secretion.
Flory16,17differentiated between two major basic mechanisms of polymerization. The first is one in which a polymer of any quantal size (n) may react with any another polymer (say, of size m) to form a larger polymer (Gn+Gm --> Gn+m). Such mechanism of polymerization will lead to geometric-like distributed quantal volume in the case of non-reversible kinetics. Under such a distribution, the frequency of polymers decreases in their size, monomers being always most likely.
The alternative mechanism considered by Flory16,17 is the very organized and restricted process under which only a unit granule (monomer) at a time may be added to polymers of any size (Gn + G1 --> Gn+1). Such unit addition mechanism leads to Poisson-like distributions (in reversible or non-reversible kinetics), where the most probable polymer size is close to the mean polymer size5,16,17. We documented in early stages of our work that granule size distribution shows a better fit to the unit addition mechanism18,19. Later work8,9,11 established the necessity to move away from classical biochemical and biophysical approaches (that assumed all monomers to be initially present and restricted granule size distributions to the two families above)20 in favor of a particle Physics approach under which a "packaging machine" produces unit granules of monomer size that coalesce with mature granules by the unit addition mechanism. The resulting SNARE-driven G&E model is the basis of our hypothesized mode-dependent stochastic polymer-size single-letter communication language.
The optimization process needed to construct such a language involves the imposition of constraints on polymer random frequency to detect errors efficiently1,12. Error detection and correction in noisy channels are based on forbidden or at least stochastically restricted letter combinations. Redundant transmission plays a role in the build-up of grammar. That such protective, redundant information accumulation has a positive survival value was pointed out by Gatlin1 and Dancoff and Quastler12. To decrease entropy and increase the fidelity of the message, the number of microstates has to be kept under proper control. Of the two polymerization mechanisms described above, the unit addition mechanism has a smaller message variety and better fidelity of message transmission than random addition, which seems to involve more redundant information content and smaller capacity, or mutual information between mode and size. Gatlin1 noted that the syntax of different biological languages differs in the number of letters: to increase fidelity of energy usage, energy is stored in a polymer of one type of monomer (the phosphate unit) but for the purpose of processing the huge diversity of information transferred by different ligands, a language of versatile units - proteins - is needed.
Intracellular as well as extracellular chatter face a ceaseless offensive of information. Thus, in order to elucidate the correct information the cells frequently use a single-letter language to achieve a precise and effective communication using a mechanism in which the “string message” size is regulated. In order to establish confident neural communication the unit granule size had to be decreased and made more homogeneous8,9. The evolutionary price tag was high turnover8, with an additional benefit - the rate of secretion became part of the information gain.
3. Secretion rate
Cells communicate with each other primarily through secretion. We have recently proposed11,21 that basal (or constitutive, spontaneous) and evoked (or active) secretion, that differ in calcium concentration [Ca2+], follow comparable dependence pattern secretion rate = const1*E{(const2*[Ca2+])min(X,M)}*e-K/2 of secretion rate on [Ca2+] (with truncated stochastic number of cooperating ions X) and SNARE rosette size K. This relationship is a wide-spectrum generalization of the cell-specific maximal in vitro relationship secretion rate = const*[Ca2+]M proposed by Dodge and Rahamimoff22, where const varies from cell type to cell type, and M is the maximal Ca2+ cooperativity. The generalization is two-fold, encompassing all cell sizes via the dependence on rosette size K and all secretion modes (basal, in-vivo evoked, in-vitro evoked, etc.) via the dependence on the random number X of cooperating Ca ions, for which we applied a working paradigm making it Poisson-distributed with mean proportional to [Ca2+]. The regression coefficient of log secretion rate on K came out so close to -0.5 that we substituted the latter, with no implied intrinsic reason. However, the order of magnitude is consistent with the G&E model, considering that e-K/2=2.117-(2/3)K corresponds to a representative quantal size slightly above 2. There is at present not enough joint ([Ca2+], K)data in the literature to estimate the value of const1 and validate the proposed multiplicative model, but the single-variable models secretion rate = const3*E{(const2*[Ca2+])min(X,M)} and secretion rate = const4* e-K/2 provide good fits to data. The constant const2, unnecessary in Dodge and Rahamimoff’s model22, adjusts for the volume unit chosen for recording [Ca2+].
The first recorded evidence for slow secretion dealt with neurosecretory synapses. This type of secretion, coined minis (for miniature neurotransmission) and hypothesized as a trans-synaptic process where single synaptic vesicles spontaneously secrete from pre-synaptic neurons and induce miniature postsynaptic potentials23, does not induce neurons to fire and was thus considered as insignificant background noise. In contrast, basal secretion in evolutionarily older cell types has been found to be interrelated with physiological roles24. Thus, recent findings23,25of abnormal synapse development in Drosophila larvae neurosecretory cells when both basal and evoked neurotransmission are blocked, should come as no surprise: inhibiting or stimulating evoked neurotransmission alone had no effect on synaptic development, blocking minis failed to develop synapses but stimulating the secretion of more minis made synapses get bigger25. The information-theoretic approach introduced in the next Section, based quantitatively on KLD (Kullback-Leibler Divergence), broadens the view that the interplay between basal secretion (by itself nearly meaningless as a carrier of information) and evoked secretion (by itself an express supplier of material) constitutes the channel for efficient cellular communication.
Before embarking on a quantitative information theoretic analysis, we summarize here, and relate to the M-K relation, the main finding that the gradient of granule size distribution between the two modes becomes less and less informative as granules become progressively smaller, creating a need for increased differentiation of rate of secretion between the two modes. The positive dependence of secretion rate on maximal ion cooperativity M may be the evolutionary tool to compensate for the negative dependence on rosette size K. The sharp Calcium gradient between the high extracellular to the low intracellular concentration (≈0.1 μM), in the order of 103-104, allows for fine-tuning of evoked secretion response through Calcium gates and Calcium binding proteins, and may have evolved Calcium-triggered exocytosis of secretory granules as the main mechanism for cell-to-cell communication in animals. In the course of evolution this pattern of exocytosis has been subsequently optimized for speed: while large granules stay close to the plasma membrane in an unbound state, SNARE complexes form between small neurosecretory granules and the plasma membrane to facilitate secretion. Such bound neuro-vesicles are immediately available for secretion. Synaptotagmin, a phospholipid and Ca2+ binding vesicular protein, sets the Ca2+ dependence of the evoked-state fusion process in neurosecretory vesicles. Synaptotagmins function as the primary Ca2+-sensors for most of these forms of exocytosis, acting via Ca2+ dependent interactions with both the fusing phospholipid membranes and the membrane fusion machinery26. Although many properties of the acknowledged Ca2+ receptor for exocytosis at the synapse have been described in a number of electrophysiological and biochemical studies, the molecular mechanisms that couple influx of Ca2+ and secretion of neurotransmitters have remained elusive. It is accepted, however, that fast neurosecretion involves maximal Calcium cooperativity (M) in the range of 4-5, as compared to M≤2 for slow large secretory granules: Ca2+ triggers various forms of exocytosis in different types of eukaryotic cells, synaptic vesicle exocytosis in all forms of neurons (3≤M≤5), granule exocytosis in hematopoietic cells (M≤2), and hormone exocytosis in endocrine cells (2≤M≤4) (Table 1). It is well accepted that neuro-vesicle evoked secretion is initiated by quite high Ca2+ concentrations within microdomains, while short-term facilitation is strongly influenced by the global buildup of "residual calcium". Neher and Sakaba27 documented that the intracellular Ca2+ concentration, at which the rate constant is 10% of the maximal rate, is in the range of 10-80 µM (initial intracellular Calcium concentration is in the order of 0.1 µM) and 1-20 µM in endocrine cells. Thus, in case of evoked state a local microdomain of [Ca2+] which is 4-8 times of initial concentration and M=3 will result in evoked rate 43-83 (64-512).
Table 1. Ca2+ dependence of the rate of exocytosis in various secretory cells in correlation with morphometric data and inventory content9,11.
| Cell Type | Basal rate (per sec) | Initial maximal rate (per sec) | Granule diameter (nm) | Rosette size (K) | Granules per cell | Calcium cooperativity (M) |
|---|---|---|---|---|---|---|
| ANP | 6 | 100 | 350 | 16 | 240 | |
| Chromaffin cells | 1.5 | 1500 | 177 | 11 | 41000 | 3-5 |
| Enterochromaffin-like cells | 9 | 160 | 200 | 12 | 5200 | |
| Eosinophil | < 0.0008 | 0.6 | 500 | 20 | 400 | |
| Juxtaglomerular cell | 0.0005 | 0.017 | 1050 | 29 | 445 | |
| Mast cell (peritoneum) | 0.00024 | 0.8 | 500 | 20 | 1000 | 1-2 |
| Pancreatic acinar cell | 0.0033 | 0.62 | 600 | 22 | 1100 | 3 |
| Pancreatic β-cell | 0.01 | 14 | 305 | 15 | 11000 | 3-5 |
| Parotid | 0.0042 | 0.3-1 | 800 | 25 | 450 | 1-2 |
| Pituitary melanotroph | 0.45 | 25-450 | 160 | 11 | 20000 | 3 |
| Type II alveolar cell | 0.00037 | 0.04 | 1270 | 32 | 188 | 1-2 |
4. Change-point recognition
The receiver (receiving end in a communication channel) observes independent and identically distributed output symbols from some benchmark distribution (e.g., basally secreted granule volume at a Poisson basal secretion rate θ), and once in a while this distribution is replaced by another (e.g., evokedly secreted granule volume at a higher Poisson secretion rate θ*R) for some length of time. The nature of the evoked disturbance should be designed so that the receiver will detect the true advent of evoked mode with high probability, while keeping the rate of false alarms below some control limit. Clearly, this would be the case if evoked secretion rate was “infinite”, i.e., if a burst of 20 granules was secreted simultaneously. Under biological feasibility constraints that bind the evoked secretion multiplier R to be below 10 or 50, recognition of the change should be based on all data available – secretion rate and granule size. There is a statistical theory discipline dedicated almost precisely to this endeavour, detecting a change-point in distribution, where once the change has happened, observations will follow forever the new distribution. The CUSUM statistic method (introduced by Page28, proved optimal by Lorden29 and Moustakides30) minimizes the expected time to detect a change after it has happened, keeping false alarm rate under control. Since in our biological application secretion reverts to the pre-change state following the evoked burst, the CUSUM expected time to detection criterion should be replaced by some longer time-window of evoked burst (say, 2SD above the mean) to render the probability of detection close enough to unity.
Let f and g stand respectively for the (known) densities of the observed data (granule volume and time since the previous secretion) before and after the change, and let x1, x2, … xn … (with xi=(ni,ti)) stand for the successive observations. The commonly used Neyman-Pearson-type statistic31 that best differentiates between two hypothesized stochastic regimes is the random walk
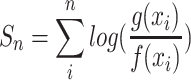
whose drift is negative as long as the first regime holds but turns positive after the change. The CUSUM statistic Dn is the draw-up at time n, Dn = Sn – min(S1, S2,… Sn), the incremental increase of the random walk from its minimal value so far. A detection is declared as soon as the draw-up exceeds some pre-assigned control limit, i.e., provides enough evidence that the trend turned upwards. Some details will be provided now, to quantify detection performance and compare the CUSUM method with neuronal integrate-and-fire management. It seems indeed that evolution got advanced notice of Page’s paper28.
The pre-and post-change drifts of the random walk, KLD(G,F)=Eg[log(g(X)/f(X))] and KLD(F,G)=-Ef[log(g(X)/f(X))], called Kullback-Leibler Divergence (KLD), constitute information theoretic measures of distance between the two distributions F and G. Each is the sum of two non-negative terms, the KLDc of secreted granule content and the KLDt of the (exponentially distributed) time interval from the previous secretion. It follows from the formula θ*exp(-θ*t) of the exponential density function (or rather its logarithm log(θ)-θ*t) that KLDt(G,F)=ln(R)-1+1/R and KLDt(F,G)=R-1-ln(R). These are increasing functions of the ratio R between evoked and basal secretion, with value obviously zero at R=1, a slow identical beginning ≈ (R-1)2 but growing like ln(R) and R respectively thereafter. The mean number MGD of evokedly secreted granules until detection of this secretion mode, for a desired mean number MGFA of basally secreted granules until a first false alarm8(see also Appendix) is approximately given by MGD ≈ ln(MGFA*KLD(F,G))/KLD(G,F). For MGFA=105 (about one mistake per day at secretion rate of 1Hz), MGD would be over 50 for R=2, 10 for R=10, 4.5 for R=100 and 3.1 at R=1000 if the STAT and EXIT distributions were indistinguishable. Since R has been assessed to be roughly 10 in vivo, an evoked burst of a few tens of undifferentiated granules would provide adequate detection. Considering that KLDt(G,F)=1.4 for R=10, if the granule content contribution KLDc(G,F) to the denominator KLD(G,F) of MGD was of double or triple size, burst size would be cut to half or a third respectively. Table 2 displays KLDc(G,F) for selected cell parameters covering the cells exhibited in Section 6. It is clear that for R>10, an evoked burst of ten granules is borderline adequate for detection as long as the rosettes consist of at least 8 SNARE units. Miniaturization brings about the need for longer and/or faster evoked bursts. Neuro-secretory vesicles seem to require R=100.
Table 2. Burst size needed for detection of mode change, for a wide choice of granule sizes.
Kβ – Number of SNAREs for homotypic fusion at basal state; Kγ – Number of SNAREs for heterotypic fusion at basal state; μ/λ - effective kinetics factor; KLDc - Kullback-Leibler Divergence of secreted granule content; MGS – Mean quantal granule size; MGD - Mean number of evokedly secreted granules until detection; STDGD – standard deviation of number of granules until detection; Burst – MGD+2*STDGD
| Kβ | Kγ | μ/λ | MGS evoked | MGS basal | KLDC(e,b) | KLDC(b,e) | MGD (R=10) | STDGD (R=10) | Burst (R=10) | MGD (R=100) | STDGD (R=100) | Burst (R=100) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 20 | 2 | 12.44 | 1.50 | 10.36 | 17.42 | 1.25 | 0.38 | 2.00 | 1.16 | 0.36 | 1.88 |
| 20 | 19 | 2 | 6.42 | 1.43 | 7.14 | 11.59 | 1.69 | 0.54 | 2.77 | 1.50 | 0.51 | 2.42 |
| 20 | 18 | 2 | 4.61 | 1.39 | 5.51 | 8.63 | 2.06 | 0.70 | 3.45 | 1.77 | 0.64 | 3.05 |
| 11 | 12 | 2 | 35.09 | 1.67 | 8.13 | 13.8 | 1.52 | 0.55 | 2.63 | 1.38 | 0.53 | 2.43 |
| 11 | 11 | 2 | 6.98 | 1.50 | 4.67 | 5.90 | 2.31 | 0.81 | 3.93 | 1.95 | 0.74 | 3.43 |
| 11 | 10 | 2 | 4.03 | 1.43 | 2.90 | 3.54 | 3.21 | 1.24 | 5.71 | 2.47 | 1.09 | 4.65 |
| 11 | 10 | 4 | 3.20 | 1.23 | 2.84 | 2.91 | 3.25 | 1.29 | 5.83 | 2.49 | 1.13 | 4.75 |
| 8 | 8 | 4 | 3.52 | 1.25 | 2.52 | 2.18 | 3.49 | 1.53 | 6.54 | 2.62 | 1.32 | 5.26 |
| 7 | 7 | 4 | 3.10 | 1.25 | 1.92 | 1.56 | 4.10 | 2.00 | 8.10 | 2.90 | 1.68 | 6.27 |
| 7 | 6 | 4 | 2.23 | 1.23 | 1.03 | 0.84 | 5.56 | 3.30 | 12.16 | 3.46 | 2.61 | 8.67 |
| 6 | 6 | 4 | 2.68 | 2.46 | 1.34 | 1.03 | 4.94 | 2.78 | 10.51 | 3.24 | 2.25 | 7.75 |
| 5 | 6 | 4 | 4.63 | 1.29 | 2.27 | 1.54 | 3.71 | 1.92 | 7.56 | 2.73 | 1.65 | 6.03 |
| 5 | 5 | 4 | 2.28 | 1.25 | 0.82 | 0.61 | 6.07 | 4.14 | 14.35 | 3.62 | 3.19 | 10.01 |
| 5 | 4 | 4 | 1.73 | 1.23 | 0.33 | 0.26 | 7.76 | 7.38 | 22.51 | 4.07 | 5.35 | 14.76 |
| 4 | 5 | 4 | 3.44 | 1.29 | 1.33 | 0.88 | 4.96 | 3.07 | 11.07 | 3.25 | 2.48 | 8.21 |
| 4 | 4 | 4 | 1.92 | 1.25 | 0.41 | 0.31 | 7.41 | 6.59 | 20.60 | 3.98 | 4.83 | 13.65 |
| 4 | 3 | 4 | 1.52 | 1.23 | 0.13 | 0.11 | 8.77 | 12.62 | 34.01 | 4.29 | 8.82 | 21.93 |
| 4 | 3 | 2 | 1.91 | 1.43 | 0.19 | 0.17 | 8.46 | 9.90 | 28.25 | 4.22 | 7.00 | 18.22 |
The draw-up Dn is stochastically smaller than the time Tn it takes the random walk to reach height n (because at that time Dn is at least n), somewhat easier to analyze than Dn. As a premature but motivating example (developed further in the Appendix), a random walk with increments +1 (with probability p>1/2) and -1 (with probability 1-p) will reach a positive integer threshold n at expected “time” (number of evokedly secreted vesicles) n/(2*p-1), variance n*4*p*(1-p)/(2*p-1)^3 and CV=SD/mean just below 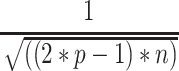 . If p=3/4 and expected time is 4 (see the bottom of Table 2, R=100) then n=2 and CV<1, so a burst size of 12 (2SD above the mean) seems adequate. If expected time is 8 (R=10) then n=4,
. If p=3/4 and expected time is 4 (see the bottom of Table 2, R=100) then n=2 and CV<1, so a burst size of 12 (2SD above the mean) seems adequate. If expected time is 8 (R=10) then n=4,  and the corresponding burst size would be
and the corresponding burst size would be 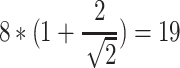 .
.
The increments of the log likelihood ratio random walk Sn are linear functions of log(granule size) (with positive coefficient) and time interval (with negative coefficient). Hence, the monitored draw-up behaves like an action potential that decays linearly in time, is prevented from becoming negative and gets as innovation inputs the logarithm of quantal granule content. This is very similar to integrate-and-fire neuronal management modelling, where the action potential decays exponentially rather than linearly with positivity constraint. The relationship between the CUSUM statistic and integrate-and-fire neuronal management has been documented elsewhere too32,33. Our approach proposes the identification of action potential input innovations as logarithms of secreted granule content.
5. Inventory management - trade-off between inventory and information
Granule packaging, the last step following content processing within the Golgi apparatus, protects macromolecules for storage and secretion, by creating a walled compartment. Granule packaging can be viewed as a synchronized system of organizing macromolecules assigned for transport and secretion within the cell, a warehouse in which the granule assembles act as inventory.
Within and attached to the granule membrane there is a variety of proteins of which some, designated as package-labeling, assign the granule for correct destination34-37. Granule packaging by membrane enclosure and membrane labeling can be rationalized in various ways. By enclosing granule content, the packed molecules are protected from biochemical and biophysical modifications, while at the same time shielding the environment from its content. In addition, since many of the secretory molecules have hydrolytic capacity, the cell cytoplasm is also protected. Keeping such barrier contents safeguards the cell and probably increases granule content shelf life38,39. The cell may contain different granule types, and each such granule type may contain some molecules that cannot share the same compartment with other molecules (e.g. storing an enzyme with its substrate). Namely, granule packaging accomplishes a vital role in reducing the security risks of long time storage.
It is evident from Table 1 that granules contain clusters/agglomerates of a number of major molecules. In all of these cases, the number of copies of the leading molecules is in the order of 103 and above. Since 1000 vesicles of unit volume will have 10 times more membrane as compared to a granule that contains the equivalent of 1000 vesicles, an increase in the macromolecule agglomerate brings about a lesser demand for content surface handling.
Granule inventory may be considered as an inventory of consumable goods, to be demanded by the environment. Shared information reduces uncertainty and reduces the need for safety stock. Thus, crosstalk between the cell and the environment must be established. The key to improved supply chain visibility, i.e. the capability of being readily perceived, is the sharing of information among supply chain members.
As a result, the organism becomes more responsive, and could ultimately become demand-driven rather than forecast-driven. Mason-Jones and Towill40 have demonstrated that “information-enriched” supply chains perform significantly better than those that do not have access to information beyond their corporate boundaries. Evolution fine-tunes the RER-Golgi pathway for packaging of goods to be supplied, to achieve agility - the ability to match supply more closely with demand. The key to agility is speed41 and a key characteristic of an agile organization is flexibility. If flow through the pipeline can be accelerated then unpredictable demand can be met more accurately.
Even better, less inventory makes the pipeline shorter – in effect Christopher and Towill41 have substituted information for inventory. The cross-talk between the cell and the environment (or the receiver) confirms knowledge of what goes on in other parts of the chain – e.g. granule inventory (=order status), inventory of supply for synthesis, work-in-process, pipeline inventory within the RER and the Golgi, actual demands, production plans and capacity of synthesis (=yields). These authors have established, in addition, the concept that success or failure of a supply chain is ultimately determined by the end consumer (i.e. the receiver). Getting the right product at the right time to the consumer "is not only the lynch pin to competitive success but also the key to survival". Uncertainty can’t be fully removed from the supply chain due to the stochastic type of product involved. For example, intrinsic properties of a pathogen that enters the tissue make demand and host survival unpredictable. Mast cell macrophage and neutrophil-specific granule inventory, confront with circumstances that require to accept uncertainty, need to develop a strategy that enables the cell to match supply and demand38,39. Accordingly, in many inventory systems of perishable items for consumption, the consumer controls the issuing of stock to meet demand in such a way that the movement of units through the system obeys a LIFO discipline42. Thus, upon pathogen invasion, the tissue activates the mast cell to secrete content upon demand.
6. Econo-biology of granule inventory management
Evolution has linked granule structure and function. Large granules (rosette size K>24) are mainly associated with adaptive tissue maintenance. The most studied cell in this class is the alveolar type-II cell of the lung (Table 1), whose main role is basal lubrication of the lung alveoli in order to reduce surface tension during breathing, about one granule per hour43. The next group of cells down the size ladder (rosette size K between 12 and 25) is represented by the adaptive immune system (e.g., mast cells, eosinophils) and the acinar cells of the gastro-intestinal system (GIT, e.g., pancreatic and parotid acinar cells). These cells, which vary in their evoked secretion frequency, respond to demand dictated by the environment, whether it is exposure to an antigen (immune system)38,39 or to food (GIT)44,45. Basal secretion is minimal in the immune system to prevent tissue destruction, whereas in GIT it is about 10 times faster due to its food processing role. The third group, the hormone secreting cells (rosette size K between 9 and 16), is represented by the pancreatic insulin-secreting β-cells and the chromaffin cells. These cells has well defined roles for both modes of secretion, which serve as regulators of tissues homeostatic states46,47.
The smallest secretory group consists of neuro-secretory vesicles (rosette size K between 4 and 8), with fast-excitatory hearing and visual sensory cells at the smallest end (basal secretion rate ≈ 1 Hz) and neuro-muscular synapses at the bigger end9,11. We have chosen to illustrate transient and steady state inventory maintenance for cells stabilizing at a content of at least 400 granules, with rosette sizes in the vicinity of 5, 11 and 20 SNARE units.
Figure 3 displays homotypic fusion rosette size Kβ=20 and heterotypic fusion rosette size Kγ between 19 and 21, for cells with μ=2λ that secrete 6 granules per hour, of which 0.1% are secreted evokedly, designed to stabilize at a total content of 1000 granules. The data demonstrates that even after 3 years the cells have not stabilized, reaching under 200 granules for Kγ=21 and just over 500 for Kγ=19 (Figure 3, inset), with mean granule size about 4 for Kγ=19 (consistent with Hammel et al.’s. experimental data48,49) and above 12 and still growing for Kγ=21 (Figure 3, inset). We see that monomers and dimers stabilize soon, trimers are the most frequent granules until stabilizing at about one month (for Kγ=19), after which bigger granule polymers take over. This display supports the classical observation by Padawer50that granule-associated thorium dioxide remains within the cell for at least six months. These observations are consistent with mast cell and eosinophil behavior. In contrast, pancreatic acinar cells seem to compensate by an initial accelerated synthesis rate about 20 times as fast5,51.
Figure 3. Simulations of granule inventory buildup.
Unit granule diameter is about 500±20 nm (Kβ= 20). One unit-granule (G1) is packed every ten minutes under the limitation of µ=2λ and heterotypic fusion of which 0.1% of the granules are secreted evokedly. Final inventory size is designed to stabilize at a total content of 1000 granules and takes significantly more than three years. Mean granule size (inset), independent of inventory size, correlates well with published data for rat mast cells47 eosinophil49 for the case of Kβ= Kγ+1=20.
Figure 4 displays the same parameters as Figure 3, with Kγ differing from Kβ by -1, 0 and 1, except that Kβ=11 instead of 20. The process is faster, mean granule size is smaller, but the pattern is similar. Dimers are the most frequent polymer in the first day, while two weeks later bigger polymers take over.
Figure 4. Simulations of granule inventory buildup in case of middle-sized granules.
Unit granule diameter is about 160±10 nm (Kβ= 11, with Kγ differing from Kβ by -1, 0 and 1). One unit-granule (G1) is packed every ten minutes under the limitation of µ=2λ and heterotypic fusion of which 0.1% of the granules are secreted evokedly. Final inventory size is designed to stabilize at a total content of 1000 granules and takes less than a year. Mean granule size (inset), display similar pattern of growth in case Kγ=10 or equal to Kβ.
What follows illustrates a different pattern of behavior following miniaturization. Figure 5 and 6 deal with Kβ=5 (and Kγ differing by -1, 0 and 1). Secretion rate is 1 Hz, evoked secretion constitutes 2% to 8% of total secretion, μ=4λ and cell content stabilizes at 400 to 8000 vesicles. Fixing Kβ and Kγ at 5, we can see the effect of total granule inventory. Low content (400 vesicles) brings the cell to stabilize within a little over one hour but generates notable size fluctuations, seemingly too erratic for accurate communication. Very high content (8000 vesicles) generates very stable size distribution but pays a heavy price in terms of relaxation time, stabilizing after roughly 100 hours.
Figure 5. Simulations of granule inventory buildup in case of small neurosecretory vesicles.
Unit vesicle diameter is within the range of 35-40 nm (Kβ= 5, with Kγ differing from Kβ by -1, 0 and 1, µ=4λ). Basal rate of secretion is 1 Hz and of which 2% of the granules are secreted evokedly. Final inventory size is designed to stabilize at a total content of 400-8000 vesicles.
It seems that content size 2000 is a reasonable compromise. It is worthwhile to note that steady state benchmark is identical in the three pictures. Keeping granule content as 2000 but reducing Kγ to 4 makes granule size stochastically smaller, with monomers becoming more frequent than dimers.
As for detection of change of mode and the impact of secretion rate magnification, by Table 2, the reduction of Kγ from 5 to 4 makes mean granule size decrease from 2.28 to 1.73, while KLDC(G,F), already as small as 0.82, decreases drastically to 0.33. However, these KLDC values are so small that granule size has only a minor impact, and detection of change of mode is primarily dictated by secretion rate amplification R: burst size increases from about 10 to about 12 (R=10) or from 5 to 5.4 (R=100). These figures have to be assessed in context - the last row of Table 2 shows that no granule size needs a burst size exceeding 14 (R=10) or 5.6 (R=100). In contrast, for granules with K ≥ 7, bursts of size 8 or smaller are adequate.
Shifting attention now to the fraction of evoked secretion, Figure 6 ascertains the minor impact of a 4-fold increase in evoked secretion on vesicle size distribution – the cell can maintain its information capacity under variable evoked demand. This complements the previous robustness finding that changes in secretion rate (equivalently, cell granule content) do not affect benchmark steady state.
Figure 6. Simulations of granule inventory buildup in case of small neurosecretory vesicles.
Unit vesicle diameter is within the range of 35-40 nm (Kβ= 5, with Kγ differing from Kβ by 0 and 1, µ=2λ). Secretion rate is 1 Hz, evoked secretion constitutes 2% to 8% of total secretion, μ=4λ and cell content stabilizes at 2000 vesicles.
An overview of the three granule size patterns displayed above shows that the proportion of monomers and dimers is very low for bigger granules and very high for smaller vesicles. As a result, turnover is high for neuro-secretory vesicles8, a price toll paid by miniaturization. A related heavy penalty associated with the similarity of evoked and basal vesicle size distribution brought about by miniaturization is the drastic reduction of KLDC. The contribution of size gradient to detection of change of mode is minor, and as a result the evoked burst must be bigger or faster.
The relative size of Kβ and Kγplays a decisive role. If Kγ=Kβ+1, the cell is unstable but information-efficient. If Kγ=Kβ-1, the cell is stable but less informative than it would be by either adding one SNARE unit to the Kγ rosette or removing one SNARE unit from the Kβ rosette. The simple evolutionary strategy of learning how to build one rosette size for both types of fusion seems also the most efficient.
7. Summary words on the surreptitious nano-machine
The most probable main role of basal secretion is to carry out biosynthesis and secretion of recently stored cargo to the cell plasma membrane, independently of extracellular signals. In the second pathway, regulated secretion, particular secretory products are sorted and stored within secretory granules, whose fusion with the plasma membrane takes effect only if prompted by extracellular signals. These secretory proteins are frequently synthesized as inactive precursors, cleaved into their functional forms during transport from the Golgi to the immature granule and upon granule maturation35-38. The secretory pathway thus serves two crucial functions: (i) physiological adaptation of secretory content in response to environmental changes and (ii) granule inventory management. The core components of the heterotrimeric apparatus that mediates secretion are three SNARE proteins, VAMP, SNAP and syntaxin, that assemble to form a rosette which serves as the minimal fusion nano-machine required for fusion52-58. The homotypic fusion rosette size at basal state is dictated by granule size (Kβ ≈ 0.9
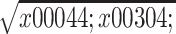 ). Further work lead us to propose a simple equation for rate of secretion as C*[Ca2+] min(X,M)*e-K/2 in which the heterotypic fusion rosette size K can be controlled to be within the range 1 ≤ K ≤ Kβ +1. Maximal evoked rate may implement K=18,9 while basal secretion seems to entail Kβ -1 ≤ K ≤ Kβ +1. In this report we have integrated some mathematical basics for such stealthy machine and a description of its administration of granule inventory.
). Further work lead us to propose a simple equation for rate of secretion as C*[Ca2+] min(X,M)*e-K/2 in which the heterotypic fusion rosette size K can be controlled to be within the range 1 ≤ K ≤ Kβ +1. Maximal evoked rate may implement K=18,9 while basal secretion seems to entail Kβ -1 ≤ K ≤ Kβ +1. In this report we have integrated some mathematical basics for such stealthy machine and a description of its administration of granule inventory.
The current knowledge on the structure of individual SNARE proteins has been summarized in a number of recent reviews, which present mechanistic insights into the question of how did the fusion machine evolve into such level of complexity52-58. Understanding the design principles and underpinning the function of such a dynamic modular protein system is a challenging task. Characterization of rate of secretion, a fundamental problem since the 1950s work of Sir Bernard Katz4, has been investigated using classical biophysics models or curve fitting to general equations (e.g. the Logistic curve, Hill plot etc.). The main drawback of these smooth approaches is that these rules "obey well" within a close range of data, for restricted cases. In a recent manuscript9 we proposed an alternative statistical-mechanics-based granule lifecycle G&E model. The current manuscript brings forward the wide-spectrum secretion rate relation C*[Ca2+]min(X,M) *e-K/2 as a facet of the mathematical G&E model11.
The validity of the statistical mechanics approach, that covers all biological range of [Ca2+], secretion rates (10-5–105 granules per millisecond)11 and granule dimensions, has been checked on 27 different cells, with granule volume range 8x103–1.1x108 nm3. This M-K relation is parsimoniously based on only two or three physical parameters: ion maximal (M) and actual (X) cooperativity, and the number of SNARE units (K) needed for fusion. We are aware of no earlier attempt to try on this subject discrete statistical mechanics approaches; classical biophysics and continuous space formulas sound more "scientific" than controversial probability assessments. However, within the small volume in which fusion reactions occur (≈1.4x10-26 m3), classical biophysics probably plays a minor contribution.
A significant part of this review dealt with granule inventory management and its evolution to the establishment of a simple communication machine. It emerges that such stealthy machine can manage granule hierarchy since rate (dictated by K) is highly correlated with granule size. While most cell types possess an inventory of granules of a single type, some secretory cells have a heterogeneous arsenal of granules of different type/content. Evidently, the emergence of a simple SNARE nano-machine that must manage secretion dictated by just very few parameters (the M-K relation) is a challenging evolutionary task. Since all granules "perceive" upon activation similar calcium concentration59, the cell’s option to monitor granule hierarchy is limited to the management of granule inventory by assigning to each granule type a rosette K-size. Namely, granule hierarchy is dictated by granule size11. Since secretion rate is linearly correlated with e-K/2, increasing K by four SNARE units leads to ≈7.4X acceleration of the secretion rate. However, since granule diameter is linearly correlated with K2, the price for a ∆K=4 increase in rosette SNARE size means a (K+4)2/K2X increase in granule diameter. In case K=8, 12, 16 and 20, granule diameter will increase (due to ∆K=4) by a factor of 2.25, 1.78, 1.56 and 1.44 respectively. There are two cells which were identified to have at least three granule types, polymorphonuclear neutrophil and platelets. Polymorphonuclear neutrophil (PMN) has two oblate D1≈85x209nm (K≈8-12), D2≈124x305nm (K≈9-15), and one spherical D3≈260nm (K≈14) granules60-62. Platelets have D1≈150nm (K≈11), D2≈200-250nm (K≈12-14) and D3≈200-400nm (K≈12-18)63-65. Both cells store active enzymes in the large granules and their substrates in the smallest granules. Thus, small granules will be queued first for secretion and the largest granules will be last. As such, the active enzymes will mainly process the substrates with less chances for tissue destruction. To decrease basal secretion of harmful proteins the PMN cells terminate granule synthesis (increase λ and thus decrease µ/λ) and generate ellipsoid-like granules (and thus increase K).
The co-existence of two secretory pathways within the same cell, basal and evoked, requires correct categorization of secretory content and vesicle machinery to each pathway66. Our model suggests that there may be no need for specialized sorting of cargo to each pathway: the cell selectively dictates synthesis of the new set of proteins to be secreted in response to environmental demands. The newly synthesized content is assigned to the newly formed granules and gets priority for secretion8,9. Newly formed granules which are not secreted, are incorporated to the granule inventory according to the stochastic pathway in Figure 1. Thus, the evolutionarily generated secretion machinery will assign mature granules to be secreted in the regulated pathway mostly upon stimulation.
We hope and predict that the statistical mechanics approach applied herein, in which a single simple machine emerges as a multitasking nano-machine, will have a key changing effect on the investigation of the pathophysiology of secretory mechanisms and on biophysical methodology for the investigation of secretion, beyond the electrophysiological, amperometric and fluorescence measurement approaches applied so far.
Open Questions
Which SNARE proteins dictate secretory granule pools with different secretion probabilities?
Since maturation of immature granules is under intracellular control and is integrated into higher regulatory networks, what are the proteins that dictate it?
Is there a unifying control mechanism for fusion?
What is the biophysical meaning of the effective kinetics factor μ/λ?
Acknowledgments
We are grateful to the many colleagues who have participated in the work described in this review, especially Ann Dvorak, Stephen J. Galli and David Lagunoff who collaborated on several of the early studies leading to the results presented in current work. The authors declare no conflict of interest. The authors work was supported by Tel Aviv University Intramural Research Program Funds.
Footnotes
Conflict of interests: The authors do not declare any conflict of interest.
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Information Theory and the Living System. Gatlin LL. Columbia University Press, New York. 1972 [Google Scholar]
- 2.Statistical nature of facilitation at a single nerve-muscle junction. DEL CASTILLO J, KATZ B. Nature. 1953;171(4362):1016–7. doi: 10.1038/1711016a0. [DOI] [PubMed] [Google Scholar]
- 3.Quantal components of the end-plate potential. DEL CASTILLO J, KATZ B. The Journal of physiology. 1954;124(3):560–73. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elementary components of synaptic transmission. Katz B. Die Naturwissenschaften. 1979;66(12):606–10. doi: 10.1007/BF00405119. [DOI] [PubMed] [Google Scholar]
- 5.Regulation of secretory granule size by the precise generation and fusion of unit granules. Hammel Ilan, Lagunoff David, Galli Stephen J. Journal of cellular and molecular medicine. 2010;14(7):1904–16. doi: 10.1111/j.1582-4934.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Switching Signals On or Off by Receptor Dimerization. Weiss Arthur, Schlessinger Joseph. Cell. 1998;94(3):277-280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 7.Chemokine receptor oligomerization: A further step toward chemokine function. Muñoz Laura Martínez, Holgado Borja López, Martínez-A Carlos, Rodríguez-Frade José Miguel, Mellado Mario. Immunology Letters. 2012;145(1-2):23-29. doi: 10.1016/j.imlet.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Quantal basis of vesicle growth and information content, a unified approach. Nitzany Eyal, Hammel Ilan, Meilijson Isaac. Journal of Theoretical Biology. 2010;266(1):202-209. doi: 10.1016/j.jtbi.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Function suggests nano-structure: electrophysiology supports that granule membranes play dice. Hammel I., Meilijson I. Journal of The Royal Society Interface. 2012;9(75):2516-2526. doi: 10.1098/rsif.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A freeze-fracture study of early membrane events during mast cell secretion. Burwen S. J. The Journal of Cell Biology. 1977;73(3):660-671. doi: 10.1083/jcb.73.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Function suggests nano-structure: towards a unified theory for secretion rate, a statistical mechanics approach. Hammel I., Meilijson I. Journal of The Royal Society Interface. 2013;10(88):20130640-20130640. doi: 10.1098/rsif.2013.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dancoff SM, Quastler H. The Information Content and Error Rate of Living Things In Henry Quastler (Ed.), Essays on the Use of Information Theory in Biology . University of Illinois Press; 1953. [Google Scholar]
- 13.The use of information theory in evolutionary biology. Adami Christoph. Annals of the New York Academy of Sciences. 2012;1256:49–65. doi: 10.1111/j.1749-6632.2011.06422.x. [DOI] [PubMed] [Google Scholar]
- 14.Information theory in ecology. Ulanowicz Robert E. The University of Illinois Press, Urbana, Illinois. 2001;25(4):393-399. [Google Scholar]
- 15.Shannon CE, Weaver W. The Mathematical Theory of Communication. University of Illinois Press; 1949. p. 117. [Google Scholar]
- 16.Flory P. Principles of Polymer Chemistry. Cornell University Press; 1953. [Google Scholar]
- 17.Statistical mechanics of chain molecules. P. J. Flory, pp. xix + 432, 1969. New York: Interscience. 164s. Gordon M. British Polymer Journal. 1970;2(5):302-303. [Google Scholar]
- 18.Theoretical Considerations on the Formation of Secretory Granules in the Rat Pancreas. Hammel I. Experimental Cell Research. 1993;204(1):1-5. doi: 10.1006/excr.1993.1001. [DOI] [PubMed] [Google Scholar]
- 19.Imaging of zymogen granules in fully wet cells. Evidence for restricted mechanism of granule growth. Hammel Ilan, Anaby Debbie. Microscopy Research and Technique. 2007;70(9):790-795. doi: 10.1002/jemt.20467. [DOI] [PubMed] [Google Scholar]
- 20.Molecular mechanisms of calcium-induced membrane fusion. Papahadjopoulos D, Nir S, Düzgünes N. Journal of bioenergetics and biomembranes. 1990;22(2):157–79. doi: 10.1007/BF00762944. [DOI] [PubMed] [Google Scholar]
- 21.The stealthy nano-machine behind mast cell granule size distribution. Hammel Ilan, Meilijson Isaac. Molecular immunology. 2015;63(1):45–54. doi: 10.1016/j.molimm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Co-operative action a calcium ions in transmitter release at the neuromuscular junction. Dodge F A, Rahamimoff R. The Journal of physiology. 1967;193(2):419–32. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evoked and spontaneous transmission favored by distinct sets of synapses. Peled Einat S, Newman Zachary L, Isacoff Ehud Y. Current biology : CB. 2014;24(5):484–93. doi: 10.1016/j.cub.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phylogenetic considerations of neurosecretory granule contents: role of nucleotides and basic hormone/transmitter packaging mechanisms. Payne C M. Archives of histology and cytology. 1989;52 Suppl:277–92. doi: 10.1679/aohc.52.suppl_277. [DOI] [PubMed] [Google Scholar]
- 25.Miniature neurotransmission regulates Drosophila synaptic structural maturation. Choi Ben Jiwon, Imlach Wendy L, Jiao Wei, Wolfram Verena, Wu Ying, Grbic Mark, Cela Carolina, Baines Richard A, Nitabach Michael N, McCabe Brian D. Neuron. 2014;82(3):618–34. doi: 10.1016/j.neuron.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cell biology of Ca2+-triggered exocytosis. Pang Zhiping P, Südhof Thomas C. Current opinion in cell biology. 2010;22(4):496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Multiple roles of calcium ions in the regulation of neurotransmitter release. Neher Erwin, Sakaba Takeshi. Neuron. 2008;59(6):861–72. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Continuous Inspection Schemes. Page E. S. Biometrika. 1954;41(1/2):100. [Google Scholar]
- 29.Procedures for Reacting to a Change in Distribution. Lorden G. The Annals of Mathematical Statistics. 1971;42(6):1897-1908. [Google Scholar]
- 30.Optimal Stopping Times for Detecting Changes in Distributions. Moustakides George V. The Annals of Statistics. 1986;14(4):1379-1387. [Google Scholar]
- 31.On the Problem of the Most Efficient Tests of Statistical Hypotheses. Neyman J., Pearson E. S. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 1933;231(694-706):289-337. [Google Scholar]
- 32.Detection of onset of neuronal activity by allowing for heterogeneity in the change points. Ritov Yaacov, Raz A., Bergman H. Journal of Neuroscience Methods. 2002;122(1):25-42. doi: 10.1016/s0165-0270(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 33.Model-Based Decoding, Information Estimation, and Change-Point Detection Techniques for Multineuron Spike Trains. Pillow Jonathan W., Ahmadian Yashar, Paninski Liam. Neural Computation. 2011;23(1):1-45. doi: 10.1162/NECO_a_00058. [DOI] [PubMed] [Google Scholar]
- 34.Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Case R M. Biological reviews of the Cambridge Philosophical Society. 1978;53(2):211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 35.Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Arvan P, Castle D. The Biochemical journal. 1998;332 ( Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maturation of secretory granules. Kögel Tanja, Gerdes Hans-Hermann. Results and problems in cell differentiation. 2010;50:1–20. doi: 10.1007/400_2009_31. [DOI] [PubMed] [Google Scholar]
- 37.Rab5 is a novel regulator of mast cell secretory granules: impact on size, cargo, and exocytosis. Azouz Nurit P, Zur Neta, Efergan Adi, Ohbayashi Norihiko, Fukuda Mitsunori, Amihai Dina, Hammel Ilan, Rothenberg Marc E, Sagi-Eisenberg Ronit. Journal of immunology (Baltimore, Md. : 1950) 2014;192(9):4043–53. doi: 10.4049/jimmunol.1302196. [DOI] [PubMed] [Google Scholar]
- 38.Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. Galli Stephen J, Tsai Mindy. Journal of dermatological science. 2008;49(1):7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Galli Stephen J, Borregaard Niels, Wynn Thomas A. Nature immunology. 2011;12(11):1035–44. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Information enrichment: designing the supply chain for competitive advantage. Mason‐Jones R., Towill D.R. Supply Chain Management: An International Journal. 1997;2(4):137-148. [Google Scholar]
- 41.Developing Market Specific Supply Chain Strategies. Christopher Martin, Towill Denis R. The International Journal of Logistics Management. 2002;13(1):1-14. [Google Scholar]
- 42.LIFO Inventory Systems. Cohen Morris A., Pekelman Dov. Management Science. 1978;24(11):1150-1162. [Google Scholar]
- 43.EXOCYTOSIS OF LUNG SURFACTANT: From the Secretory Vesicle to the Air-Liquid Interface. Dietl Paul, Haller Thomas. Annual Review of Physiology. 2005;67(1):595-621. doi: 10.1146/annurev.physiol.67.040403.102553. [DOI] [PubMed] [Google Scholar]
- 44.Heterogeneity of the exocrine pancreas. Adelson J. W., Miller P. E. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1989;256(5):G817-G825. doi: 10.1152/ajpgi.1989.256.5.G817. [DOI] [PubMed] [Google Scholar]
- 45.Pancreatic digestive enzyme secretion in the rabbit: rapid cyclic variations in enzyme composition. Adelson J. W., Clarizio R., Coutu J. A. Proceedings of the National Academy of Sciences. 1995;92(7):2553-2557. doi: 10.1073/pnas.92.7.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Secretory granules: and the last shall be first…. Solimena Michele, Gerdes Hans-Hermann. Trends in Cell Biology. 2003;13(8):399-402. doi: 10.1016/s0962-8924(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 47.Regulation of Insulin Secretion in Human Pancreatic Islets. Rorsman Patrik, Braun Matthias. Annual Review of Physiology. 2013;75(1):155-179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 48.Recovery of rat mast cells after secretion: A morphometric study. Hammel I., Lagunoff D., Krüger P.-G. Experimental Cell Research. 1989;184(2):518-523. doi: 10.1016/0014-4827(89)90349-2. [DOI] [PubMed] [Google Scholar]
- 49.Morphometric evidence that the maturation of the eosinophil granules is independent of volume change. Elmalek M, Hammel I. Journal of submicroscopic cytology. 1987;19(2):265–8. [PubMed] [Google Scholar]
- 50.UPTAKE OF COLLOIDAL THORIUM DIOXIDE BY MAST CELLS. Padawer J. The Journal of Cell Biology. 1969;40(3):747-760. doi: 10.1083/jcb.40.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cytoplasmic granule formation in mouse pancreatic acinar cells. Evidence for formation of immature granules (condensing vacuoles) by aggregation and fusion of progranules of unit size. Cell Tissue Res. 1994;278:327–336. doi: 10.1007/BF00414176. [DOI] [PubMed] [Google Scholar]
- 52.Functional Organization of the Porosome Complex and Associated Structures Facilitating Cellular Secretion. Jena Bhanu P. Physiology. 2009;24(6):367-376. doi: 10.1152/physiol.00021.2009. [DOI] [PubMed] [Google Scholar]
- 53.Role of SNAREs in membrane fusion. Jena Bhanu P. Advances in experimental medicine and biology. 2011;713:13–32. doi: 10.1007/978-94-007-0763-4_3. [DOI] [PubMed] [Google Scholar]
- 54.Mechanics of membrane fusion. Chernomordik Leonid V, Kozlov Michael M. Nature structural & molecular biology. 2008;15(7):675–83. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Three SNARE complexes cooperate to mediate membrane fusion. Hua Y., Scheller R. H. Proceedings of the National Academy of Sciences. 2001;98(14):8065-8070. doi: 10.1073/pnas.131214798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Principles of Exocytosis and Membrane Fusion. JAHN REINHARD. Annals of the New York Academy of Sciences. 2004;1014(1):170-178. doi: 10.1196/annals.1294.018. [DOI] [PubMed] [Google Scholar]
- 57.SNARE complexes and neuroexocytosis: how many, how close? Montecucco Cesare, Schiavo Giampietro, Pantano Sergio. Trends in biochemical sciences. 2005;30(7):367–72. doi: 10.1016/j.tibs.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Wickner William. Annual review of cell and developmental biology. 2010;26:115–36. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 59.Regulated granule trafficking in platelets and neurons: a common molecular machinery. Goubau Christophe, Buyse Gunnar M, Di Michele Michela, Van Geet Chris, Freson Kathleen. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2013;17(2):117–25. doi: 10.1016/j.ejpn.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Granules of the human neutrophilic polymorphonuclear leukocyte. Borregaard N, Cowland J B. Blood. 1997;89(10):3503–21. [PubMed] [Google Scholar]
- 61.Neutrophil granules: a library of innate immunity proteins. Borregaard Niels, Sørensen Ole E, Theilgaard-Mönch Kim. Trends in immunology. 2007;28(8):340–5. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Neutrophil granules in health and disease. Häger M, Cowland J B, Borregaard N. Journal of internal medicine. 2010;268(1):25–34. doi: 10.1111/j.1365-2796.2010.02237.x. [DOI] [PubMed] [Google Scholar]
- 63.The platelet release reaction: granules' constituents, secretion and functions. Rendu F, Brohard-Bohn B. Platelets. 2001;12(5):261–73. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 64.Platelet secretory mechanisms. Reed Guy L. Seminars in thrombosis and hemostasis. 2004;30(4):441–50. doi: 10.1055/s-2004-833479. [DOI] [PubMed] [Google Scholar]
- 65.Advances in platelet granule biology. Koseoglu Secil, Flaumenhaft Robert. Current opinion in hematology. 2013;20(5):464–71. doi: 10.1097/MOH.0b013e3283632e6b. [DOI] [PubMed] [Google Scholar]
- 66.Spontaneous neurotransmission: an independent pathway for neuronal signaling? Kavalali Ege T, Chung Chihye, Khvotchev Mikhail, Leitz Jeremy, Nosyreva Elena, Raingo Jesica, Ramirez Denise M O. Physiology (Bethesda, Md.) 2011;26(1):45–53. doi: 10.1152/physiol.00040.2010. [DOI] [PubMed] [Google Scholar]








