Abstract
A tumor contains special types of cells that have characteristics similar to stem cells that aid in tumor initiation, evasion and proliferation and are often resistant to chemotherapy. These cancer stem cells can be differentiated to eradicate their stemness and proliferative capacity by differentiating agents. This study investigated the effect of differentiation on the expression of two immune checkpoint inhibitors, human leukocyte antigen-G (HLA-G) and programmed death ligand-1 (PD-L1). Two cancer cell lines (OVCAR-3-NIH and KATO-III) were treated with adipocyte and neurocyte differentiation media for 14 days. Bone-marrow derived mesenchymal stem cells (BM-MSCs) were used as control healthy stem cells. We found that the cancer cell lines (OVCAR-3-NIH and KATO-III) when subjected to differentiation lost their proliferation ability. BM-MSC proliferation was not halted but was decreased in the adipocyte differentiation media. There was no decrease in the CD90 stem cell marker in the BM-MSCs; however, both cancer cell lines showed decreased CD90 stem cell marker. A significant increase in HLA-G was noted for both the cancer cell lines following adipocyte differentiation. No effect was found for BM-MSCs. Moreover, an increase in PD-L1 in cancer cell lines was found following neurocyte differentiation. Moreover, we found that differentiation resulted in decreased PD-L1 expression in BM-MSCs. Differentiation therapy of cancer stem cells may result in increased immunosuppression ability, hence causing hindrance in the removal of cancer cells. Moreover, the differentiation of healthy stem cells can result in increased immunogenic reactivity owing to a decrease in PD-L1 expression.
Keywords: differentiation therapy, immune resistance, human leukocyte antigen-G, HLA-G, programmed death ligand-1, PD-L1, cancer stem cells, bone marrow stem cells
Introduction
The traditional approach towards cancer treatment is to kill the tumor cells, yet there exists a parallel idea to transform/revert cancer cells into normal cells, thereby destroying their ability to proliferate (1). It has been reported that blood cancers and solid tumors contain special type of cancer stem cells (CSCs), also known as tumor-initiating cells. These cells assist in tumor progression and metastasis and are resistant to chemotherapy (2). Poor cellular differentiation is one of the main traits of cancer. Hence, differentiation therapy is focused on reactivating the endogenous differentiation pathways in CSCs (3). Differentiation therapy is a therapeutic strategy that transforms malignant cells to a more benign cell type by masking the proliferative abilities of these cells.
CSCs, like other stem cells exhibit low immunogenicity that helps them evade the immune response of the host, hence making them invulnerable not only to radiotherapy and chemotherapy but also towards immunotherapy (4). Immune system surveillance of tumor cells occurs through a process known as tumor immune editing. Tumor cells are also reported to highly express a T-cell inhibitory molecule i.e. PD-L1 in order to evade the immune response. Programmed death ligand-1 (PD-L1) is a well-known molecule in tumor evasion and has been documented in several studies (5). It helps cancer cells in immune evasion by binding to the PD-1 receptor on T cells thus inhibiting T-cell activity. There are several antibodies for PD-L1 which are in phase III clinical trials with the aim to suppress the immune evasion ability of cancer cells (6).
Another important molecule that is involved in each of these three phases is human leukocyte antigen-G (HLA-G). It renders cytotoxic cells ineffective towards tumor cells through trogocytosis and by inhibiting or decreasing the killer function of T and natural killer (NK) cells (7,8). The increased expression of both molecules i.e. HLA-G (9–11) and PD-L1 (12–17) are found to be correlated with a decreased survival rate.
One of the important examples of differentiation therapy is the use of all-trans retinoic acid (atRA) in acute promyelocytic leukemia (APL) that has shown promising results in APL but not for other cancer subtypes. The sensitivity of APL for atRA is dependent on the expression of the promyelocytic leukemia/retinoic acid receptor α (PML-RARα) protein (18). Even in APL, the major issue is of relapse with cells which are more aggressive in nature and are difficult to treat (19). Recently, we reported the impact of a cell differentiation inducer on epithelial-mesenchymal transition as well as on solid cancer stem cells (20). Here, the aim of the present study was to investigate the effect of differentiation in the perspective of cancer immune evasion through evaluation of HLA-G and PD-L1, both of which are involved in inactivation of T and NK cells.
Materials and methods
Cell culture
Cancer cell lines used included: OVCAR-3-NIH (ovarian) and KATO-III (gastric) purchased from ATCC®. Adult human mesenchymal stem cells (BM-MSCs; derived from bone marrow) obtained from ‘PromoCell GmbH’ were used as control (normal healthy stem cells). The cell lines were chosen based on expression of the CD90 stem cell marker, and immune checkpoint molecules, PD-L1 and HLA-G. Each cell line was maintained in medium specified by the provider at 37°C in a humidified chamber maintained with 5% CO2. The media used were RPMI-1640 for OVCAR-3-NIH, IMDM for KATO-III each supplemented with 10% fetal bovine serum (FBS), 1% pencillin-streptomycin and 1% L-glutamine (Gibco; Thermo Fisher Scientific, Inc.), while specialized Mesenchymal Stem Cell Medium from PromoCell was used for the BM-MSCs. All cell lines used were of low passages (less than 7 passages) to maintain pluripotency.
Differentiation
The cultured cells were washed with phosphate-buffered saline (PBS) to remove any medium constituents. To induce adipocyte and neurocyte differentiation, KATO-III, OVCAR-3-NIH and BM-MSCs were incubated for 14 days with the StemPro® Adipocyte, Differentiation Kit (Gibco Life Technologies™; Thermo Fisher Scientific, Inc.) and Neurobasal® medium containing 2 mM B-27 supplement and 1% glutamine (Thermo Fisher Scientific, Inc.) with regular medium feeding. For coloration, after 14 days the induced cells were fixed using 4% paraformaldehyde (PFA) for 10 min at room temperature and then washed with PBS to remove PFA. Adipocytes contain lipid droplets in the cytoplasm which were colored using 60% Oil red O (0.3 g Oil red O in 1 ml isopropanol) in distilled water. The cells were incubated in 60% isopropanol for 5 min before incubation with Oil red O solution. The differentiated neurocytes were stained for nissl bodies using nissl staining solution (0.5 g Cresyl violet in 100 ml of 0.6% glacial acetic acid) for 30 min. Cells were then imaged using EVOS FL Auto Imaging System (EVOS) microscope at ×20 magnification.
FACS
To analyze stem cell properties, the live cells cultured in normal media (control) and differentiation media (adipocyte and neurocyte differentiation medium) were stained using the CD90 antibody (Beckman Coulter; cat. no. IM1840U) at a dilution of 1:100. FACS was performed using BD FACSCanto™ II eight-color standard flow cymometer (Becton Dickinson Biosciences, France). The data were analyzed using Kaluza 2.0 Analysis software (Beckman Coulter).
Cell proliferation assay
In order to evaluate the proliferative ability of cells following differentiation, the cell viability assay was performed using the RealTime-Glo™ MT cell viability test (Promega France). KATO-III, OVCAR-3-NIH and MSC-BMs were cultured (500 cells in 96-well plates) using classic culture media. After 24 h, the media were replaced with differentiation media (StemPro® Adipocyte Differentiation Kit and Neurobasal® medium) containing luminescence agent and substrate. Bioluminescence was measured with a spectrofluorometer (SAFAS Xenius XC; SAFAS) at a specified interval. Cell proliferation curve was made using GraphPad Prism 6 (GraphPad Software, Inc.) and the difference in proliferation was analyzed using two-way analysis of variance (ANOVA).
Real-time qPCR
Relative gene expression of HLA-G and PD-L1 was compared for each cell line using ß-actin as the housekeeping gene through qPCR performed in Roche Lightcycler 96 (Roche Applied Science). The primers used were: ß-actin (sense, AGAGCTACGAGCTGCCTGAC and antisense, AGCACTGTGTTGGCGTACAG), PD-L1 (sense, CAAAGAATTTTGGTTGTGGA and antisense, AGCTTCTCCTCTCTCTTGGA), HLA-G (sense, GCGGCTACTACAACCAGAGC and anti-sense, GAGGTAATCCTTGCCATCGTAG). The relative expression was calculated using the ΔΔCq method (21).
Immunohistochemistry
The differentiated cells as well as controls were collected on slides using Cytospin centrifugation (Cytospin2; Shandon Inc.). The slides were then fixed for 10 min using 4% PFA solution. After washing with PBS-Tween (0.1%) for 5 min, the cells were incubated for 30 min in 1% BSA in PBS-Tween. After that the cells were incubated with anti-rabbit anti-HLA-G polyclonal antibody (Epigentek Group Inc.; cat. no. A53328) and anti-mouse anti-PD-L1 monoclonal antibody (Proteintech Group, Inc.; cat. no. 17952-1-AP) in dilution of 1:100 using 1% BSA (PBS-Tween) for 3 h at room temperature. The cells were washed with PBS three times before being incubated with a mixture of goat secondary antibodies anti-mouse Alexa Fluor 594 (cat. no. A-11005; Thermo Fischer Scientific, Inc.) and anti-rabbit Alexa Fluor 488 (cat. no. A32731; Thermo Fischer Scientific, Inc.) for 1 h. The slides were washed and mounted with an aqueous medium containing DAPI (VectaMount®; Vector Laboratories, Inc.) and allowed to dry in the dark. The labeling was then analyzed by immunofluorescence using an EVOS FL Auto Imaging System (EVOS) fluorescence microscope at ×20 magnification.
Statistical analysis
Proliferation data were analyzed using two-way ANOVA with post-hoc Bonferroni's test. The qPCR data were analyzed using ANOVA test followed by post-hoc Bonferroni's test. The statistical analysis was performed on the GraphPadPrism® software (GraphPad Software, Inc.). A P-value of <0.05 was considered significantly different. The bars and statistical values are represented as mean ± SEM (standard error of the mean).
Results
Differentiation of cancer cells
Decrease in stem cell properties after differentiation
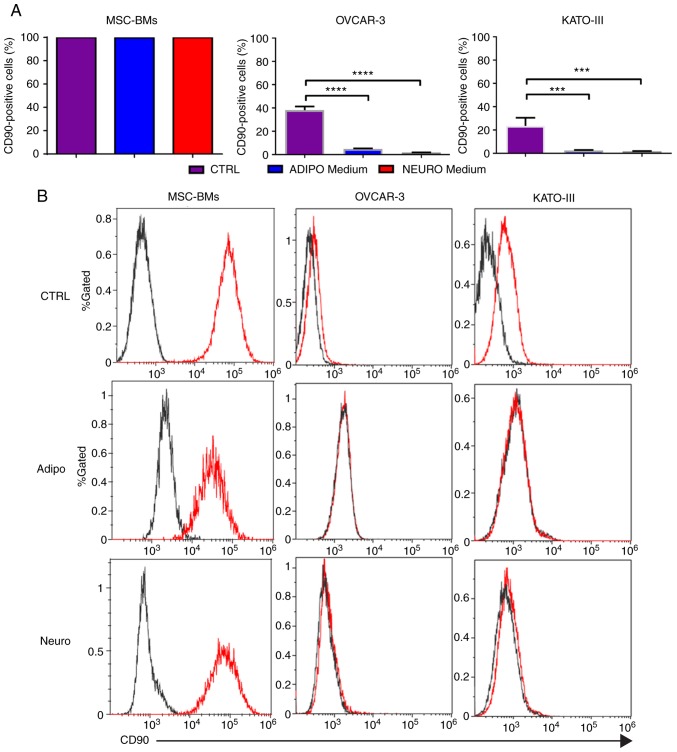
The CD90 stem cell marker was selected owing to its presence in each of our cell line. FACS analysis showed that each cell line exhibited the CD90 marker. The expression of the CD90 marker in MSC-BMs was 99.89% compared to 38.14% in OVCAR-3-NIH and 23.51% in KATO-III cells. A significant decrease in the CD90 marker was observed after treatment with differentiation medium in each of the cancer cell line. The percentage of OVCAR-3-NIH cells expressing the CD90 marker was 4.8 and 1.73% after adipocyte differentiation and neurocyte differentiation, respectively, compared to 38.1% in the OVCAR-3-NIH cells cultured in classic media. Similarly, adipocyte differentiated and neurocyte differentiated KATO-III cells showed 2.3 and 1.6% CD90 marker expression, respectively. No decrease in CD90 marker expression was noted in the BM-MSCs (Fig. 1).
Figure 1.
CD90 marker expression before and after differentiation. (A) CD90 marker expression in MSC-BMs, OVCAR-3-NIH and KATO-III cell lines before (CTRL) and after differentiation (adipocyte and neurocyte). (B) FACS peak graphs showing the CD90 marker expression [(red) and black (isotype control)]. Adipo, adipocyte differentiation medium; Neuro, neurocyte differentiation medium; MSC-BMs, bone-marrow derived mesenchymal stem cells. (***P<0.001, ****P<0.0001).
Decreased cell proliferation and change in the cytological aspects of cancer cells after differentiation
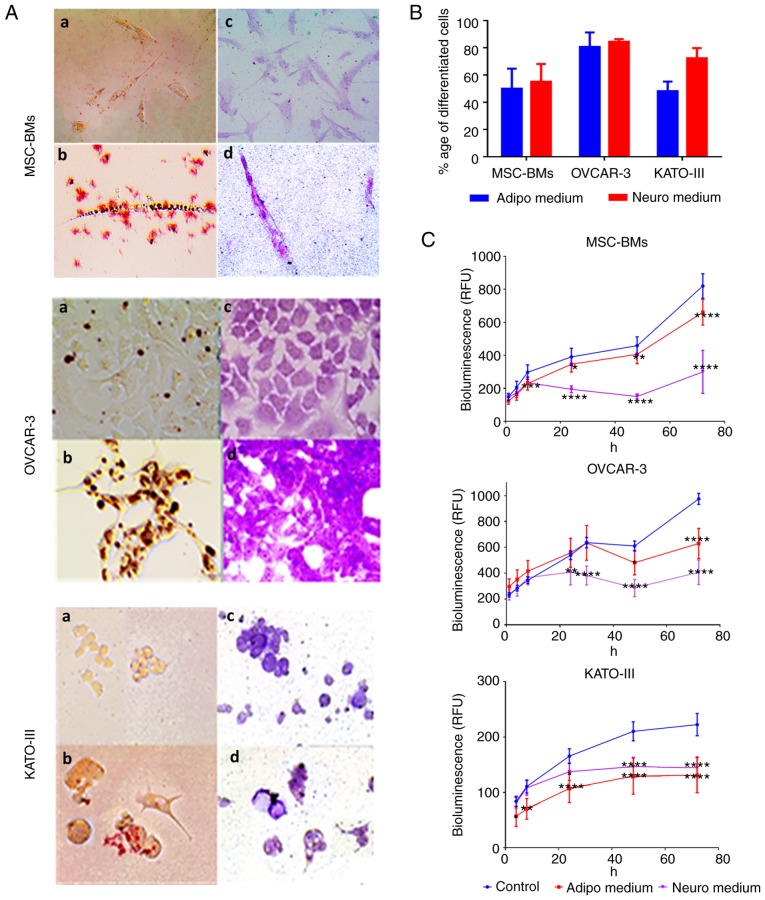
Adipogenic and neurogenic differentiation of cell lines was confirmed by the visualization of intracytoplasmic lipid drops stained in red using Oil red O (Fig. 2A-B) and dark black-violet (Fig. 2A-D) due to accumulations of nissl bodies respectively while the coloration was absent in the cells grown in classic culture media (Fig. 2A-a and C). We found 50 to 60% of differentiated cells in the BM-MSCs and KATO-III, and up to 80% in the OVCAR-3-NIH cells (Fig. 2B) after 14 days of incubation with differentiation induction media.
Figure 2.
Coloration (staining) of the differentiated cells and proliferation rate after differentiation. The differentiation into adipocytes and neurocytes was confirmed by visual staining. (A) OVCAR-3-NIH, KATO-III and MSC-BMs were evaluated for their differentiation potential into adipocytes and neurocytes when grown in inducing differentiation media. Oil Red O staining (a, control; b, differentiated) revealed lipid droplets (in red) and Nissl bodies were stained with Cresyl violet (c, control; d, differentiated; magnification, ×20). (B) The percentage of differentiation for each cell line into adipocytes and neurocytes after 14 days of incubation with differentiation media. (C) Impact of incubation of differentiation media on real-time cell line proliferation (*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001). Adipo, adipocyte differentiation medium; Neuro, neurocyte differentiation medium; MSC-BMs, bone-marrow derived mesenchymal stem cells.
When the cell lines were treated with differentiation induction media, a significant proliferation inhibition was observed for each cell line (P<0.005). This inhibition was maintained over time. At 72 h, the inhibition was highly significant for the two differentiation induction media when compared with the control for each cell line (P<0.0001; Fig. 2C).
Modified immune checkpoints
qPCR analysis
We observed a significant increase in HLA-G expression (Fig. 3A) after adipocyte differentiation for the two cancer cell lines, OVCAR-3-NIH (P<0.05) and KATO-III (P<0.0001). However, the BM-MSCs did not show any effect of differentiation on expression of HLA-G. In addition, among the three cell lines, only OVCAR-3-NIH showed a strong increase in HLA-G expression after neurocyte differentiation (P<0.0001). The expression of PD-L1 was upregulated by adipocyte differentiation in KATO-III cancer cells (P<0.05), and was upregulated in both cancer cell lines (OVCAR-3-NIH and KATO-III) following neurocyte differentiation (P<0.0001; Fig. 3B). Notably, the BM-MSCs showed a significant decrease in PD-L1 expression following adipocyte as well as neurocyte differentiation (P<0.0001).
Figure 3.
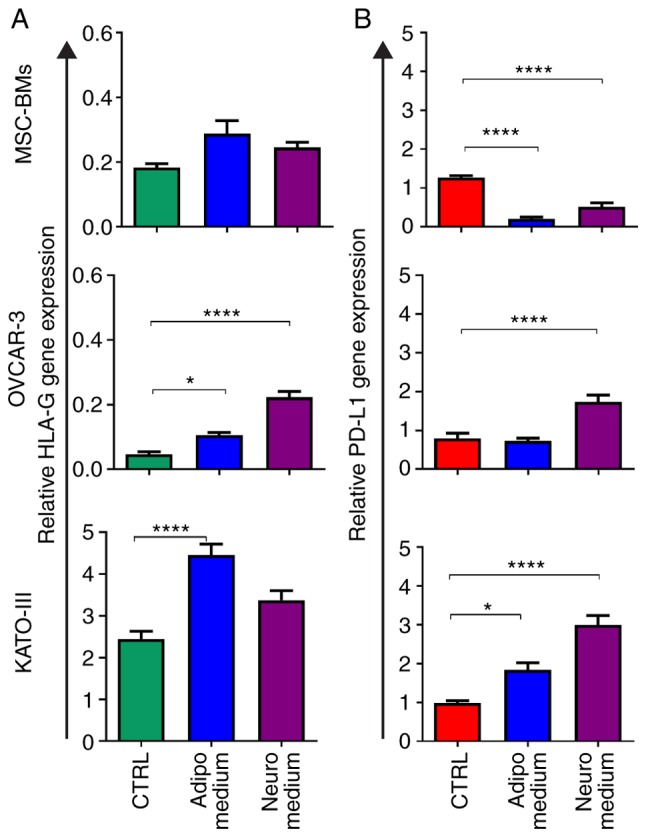
Relative expression of HLA-G and PD-L1 before and after differentiation. (A) HLA-G and (B) PD-L1 expression in MSC-BMs, OVCAR-3-NIH and KATO-III cells before differentiation (CTRL; green) and after differentiation (Adipocyte, blue; Neurocyte, purple) (*P<0.05 and ****P<0.0001). HLA-G, human leukocyte antigen-G; PD-L1, programmed death ligand-1; Adipo, adipocyte differentiation medium; Neuro, neurocyte differentiation medium; MSC-BMs, bone-marrow derived mesenchymal stem cells.
Immunocytochemical analysis
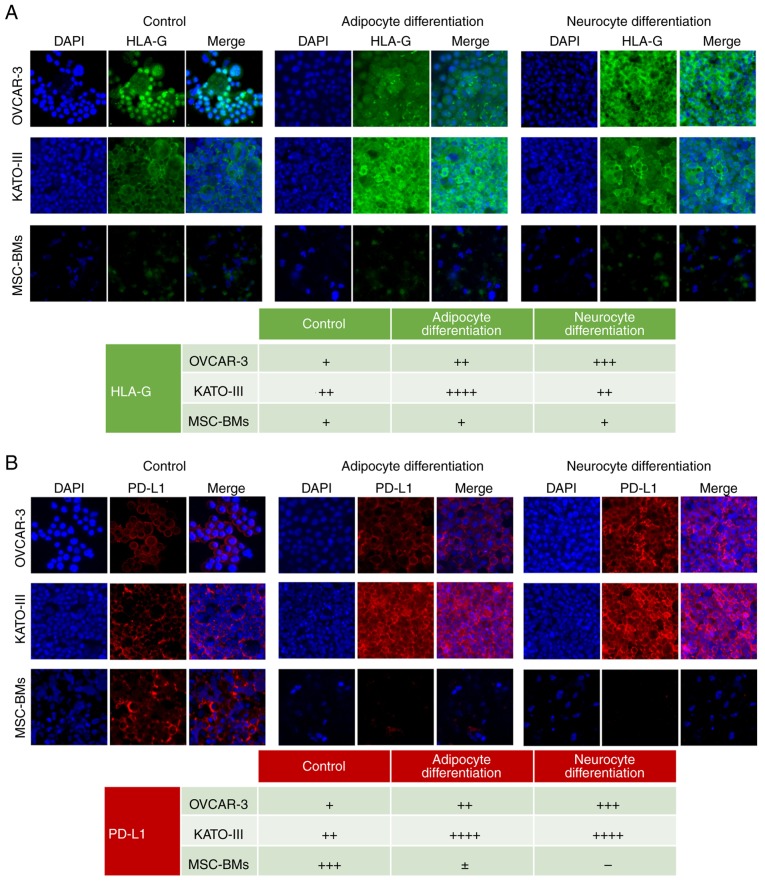
The qPCR results were confirmed through immunofluorescence microscopy. We observed a strong noticeable increased expression of HLA-G in OVCAR-3-NIH cells after neurocyte differentiation and after adipocyte differentiation in KATO-III cells. However, no observable difference was found in the expression of HLA-G in differentiated BM-MSCs compared to the control cells (Fig. 4A).
Figure 4.
Expression of HLA-G and PD-L1 in cell lines before and after differentiation through immunofluorescence. OVCAR-3-NIH, KATO-III and MSC-BMs without treatment as control (left), treatment with adipocyte-inducing medium (middle) or treatment with neurocyte-inducing medium (right). (A) HLA-G expression in OVCAR-3-NIH, KATO-III and MSC-BMs with nuclear stained using DAPI (blue) and HLA-G in green. (B) PD-L1 expression in OVCAR-3-NIH, KATO-III and MSC-BMs is shown as red staining with nuclei in blue. Magnification, ×20. Tables show semi-quantitative analysis of HLA-G and PD-L1 expression (− negative, + low expression, ++ intermediate expression, +++ high expression). HLA-G, human leukocyte antigen-G; PD-L1, programmed death ligand-1; MSC-BMs, bone-marrow derived mesenchymal stem cells.
PD-L1 expression was strongly upregulated only by adipocyte differentiation, although a slight upregulation was also observered in the OVCAR-3-NIH cell line following neurocyte differentiation. However, it was upregulated in KATO-III cells after adipocyte as well as neurocyte differentiation. Moreover, notably PD-L1 expression was downregulated in BM-MSCs after neurocyte and adipocyte differentiation (Fig. 4B).
Discussion
Malignant tumors contain specific type of cells which exhibit stem cell properties and are known as cancer stem cells (CSCs) (22). These cells are resistant to chemotherapy and are capable of self-renewing giving rise to different cell types and are invulnerable to immune cell attack. This ability makes CSCs an important target to fight against cancer relapse after remission. CSCs in tumors can be identified using stem cell marker expression such as CD90. CSCs follow several pathways for their renewal and proliferation, hence regular chemotherapy or targeting one pathway is not sufficient to limit their carcinogenicity. Another approach to target CSCs is to use differentiation therapy (23), which induces differentiation in CSCs in order to limit their proliferation and renewal capacity. Our group has previously demonstrated that differentiation of cancer cells induces mesenchymal to epithelial transition (20). Therefore, using the same model, we aimed to investigate how the differentiation of cancer cells impact the expression of immune checkpoints mainly human leukocyte antigen-G (HLA-G) and programmed death ligand-1 (PD-L1).
In order to confirm differentiation in vitro, we evaluated the CD90 stem cell marker expression in the cancer cell lines. We found a significant decrease in CD90 stem cell marker in the cancer cell lines. However, there was no difference in the CD90 expression marker in the bone marrow-derived mesenchymal stem cells (BM-MSCs). This demonstrated that BM-MSCs still retained their stem cell properties even after incubation in differentiation-inducing media for 14 days.
Furthermore, to confirm the differentiation of cancer cells in different cell lines, we performed histological staining. For cells differentiated into adipocytes we used Oil-red O staining that stains lipid droplets in red color. We found the presence of oil droplets in the majority of cells following differentiation in each of the cell lines. For neurocyte differentiated cells, we found the presence of dark violet nissl bodies present in cells after staining with Cresyl violet. This further confirmed the differentiation of cancer cell lines into respective cell types. As the ideal media for differentiation should block the proliferation of cancer cells, we performed a viability assay in order to assess the effect of differentiation on the growth and proliferation of cells. We found that the differentiation media halted the proliferation of cancer cells after 30 h, and there was a significant decrease in proliferation capacity of the MSC-BMs after 72 h of incubation in the differentiation media. This suggests the importance of differentiation therapy specifically for cancer cells that not only differentiates CSCs but can also block the proliferation of cancer cells. These results validate the differentiation model reported by Shah et al (20) in two additional cell lines.
In order to investigate the effects of differentiation on the immune profile of the cell lines, we evaluated the expression of HLA-G and PD-L1, both being known to suppress the immune system. We found that in cancer cell lines, adipocyte differentiation resulted in increased expression of both HLA-G as well as PD-L1 in the KATO-III cell line. while, only HLA-G expression was increased in the OVCAR-3-NIH cells following adipocyte differentiation. On the other hand, differentiation to neurocytes resulted in increased expression of HLA-G as well as PD-L1 in the OVCAR-3-NIH cell line, but in the KATO-III cells only an increase in PD-L1 expression was observed. These results suggest that the CSCs upon differentiation can lead to increased immunosuppression, which can make it difficult for the body's immune system to eliminate them (24). However, development of a mouse model is required to further investigate the oncogenic potential of the cancer cells after differentiation. Hence, we suggest combining the immune checkpoint inhibitor with differentiation therapy in order to avoid the immune evasion that may be attributed to increased expression of HLA-G or PD-L1 following differentiation therapy.
Interestingly, no change in HLA-G expression was found in BM-MSCs following adipocyte or neurocyte differentiation. This finding is in accordance with previously reported results, showing that there is no impact on the immunosuppressive property of MSCs after induction of osteocyte differentiation (25). However, an interesting observation was found for BM-MSCs where the PD-L1 levels were strongly decreased after adipocyte as well as neurocyte differentiation. Therefore, even when HLA-G expression was not altered by induction of differentiation, the BM-MSCs showed lower expression of PD-L1 that can result in increased immunogenic potential in the differentiated stem cells. These results were also confirmed through immunofluorescence. An interesting observation here, is that in an autoimmune disease of aplastic anemia, there is enhanced bone marrow adiposity (26–28). We found that the differentiation of bone marrow cells in adipocytes resulted in decreased PD-L1 expression, which may provoke the immune response against the bone marrow cells, causing aplastic anemia. Aplastic anemia is attributed to the adipogenesis in bone marrow. We, for the first time, explained in vitro that bone marrow cell differentiation into adipocytes leads to decreased PD-L1 expression that may result in the rejection of administered bone marrow stem cells.
In conclusion, we found that tumors in response to stress conditions such as differentiation alters their immunological characteristics. The present study suggests that the differentiated cancer cells can be more immunosuppressive through an increase in both the immune suppressive proteins, HLA-G and PD-L1.
Acknowledgements
The authors acknowledge the considerable effort spent by Professor Amu Therwath for improvement of the script.
Funding
No funding was received.
Availability of data and materials
The original data for all the experiments is available upon request.
Authors' contributions
MU drafted the manuscript and along with SM planned and carried out the experiments. RK carried out the immunocytochemistry. SS and CP helped in differentiation protocols. MP helped in the data interpretation and scientific understanding. MM conceived and supervised the overall direction of the work. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The study does not involve any animal or human experimentation, hence no ethical approval was required.
Patient consent for publication
Not applicable.
Competing interests
The author confirms no conflict of interest.
References
- 1.Yan M, Liu Q. Differentiation therapy: A promising strategy for cancer treatment. Chin J Cancer. 2016;35:3. doi: 10.1186/s40880-015-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinogradov S, Wei X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine (Lond) 2012;7:597–615. doi: 10.2217/nnm.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codony-Servat J, Rosell R. Cancer stem cells and immunoresistance: Clinical implications and solutions. Transl Lung Cancer Res. 2015;4:689–703. doi: 10.3978/j.issn.2218-6751.2015.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sultan M, Coyle KM, Vidovic D, Thomas ML, Gujar S, Marcato P. Hide-and-seek: The interplay between cancer stem cells and the immune system. Carcinogenesis. 2017;38:107–118. doi: 10.1093/carcin/bgw115. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Sunwoo J. PD-L1 is preferentially expressed on CD44+ tumor-initiating cells in head and neck squamous cell carcinoma. J Immunother Cancer. 2014;2(Suppl):270. doi: 10.1186/2051-1426-2-S3-P270. [DOI] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED. Immune regulation by pretenders: Cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 8.Ullah M, Azazzen D, Kaci R, Benabbou N, Pujade Lauraine E, Pocard M, Mirshahi M. High expression of HLA-G in ovarian carcinomatosis: The role of interleukin-1β. Neoplasia. 2019;21:331–342. doi: 10.1016/j.neo.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer. 2007;58:267–274. doi: 10.1016/j.lungcan.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Cai MB, Han HQ, Bei JX, Liu CC, Lei JJ, Cui Q, Feng QS, Wang HY, Zhang JX, Liang Y, et al. Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma. Int J Biol Sci. 2012;8:891–900. doi: 10.7150/ijbs.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirana C, Ruszkiewicz A, Stubbs RS, Hardingham JE, Hewett PJ, Maddern GJ, Hauben E. Soluble HLA-G is a differential prognostic marker in sequential colorectal cancer disease stages. Int J Cancer. 2017;140:2577–2586. doi: 10.1002/ijc.30667. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, Chen N, Zhan J, He X, Qin T, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–14219. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamura K, Yokouchi Y, Sakakibara R, Kobayashi M, Subat S, Ninomiya H, Nagano H, Nomura K, Okumura S, Ishikawa Y. Relationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol. 2016;46:935–941. doi: 10.1093/jjco/hyw087. [DOI] [PubMed] [Google Scholar]
- 14.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 15.Xiang X, Yu PC, Long D, Liao XL, Zhang S, You XM, Zhong JH, Li LQ. Prognostic value of PD-L1 expression in patients with primary solid tumors. Oncotarget. 2017;9:5058–5072. doi: 10.18632/oncotarget.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorensen SF, Zho W, Dolled-Filhart M, Georgsen JB, Wang Z, Emancipator K, Wu D, Busch-Sørensen M, Meldgaard P, Hager H. PD-L1 expression and survival among patients with advanced non-small cell lung cancer treated with chemotherapy. Transl Oncol. 2016;9:64–69. doi: 10.1016/j.tranon.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0182692. doi: 10.1371/journal.pone.0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grignani F, Ferrucci PF, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Grignani F, Peschle C, Nicoletti I, et al. The acute promyelocytic leukemia-specific PML-RARα fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74:423–431. doi: 10.1016/0092-8674(93)80044-F. [DOI] [PubMed] [Google Scholar]
- 19.Su M, Alonso S, Jones JW, Yu J, Kane MA, Jones RJ, Ghiaur G. All-trans retinoic acid activity in acute myeloid leukemia: Role of cytochrome P450 enzyme expression by the microenvironment. PLoS One. 2015;10:e0127790. doi: 10.1371/journal.pone.0127790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah S, Pocard M, Mirshahi M. Targeting the differentiation of gastric cancer cells (KATO-III) downregulates epithelial-mesenchymal and cancer stem cell markers. Oncol Rep. 2019;42:670–678. doi: 10.3892/or.2019.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Eaves CJ. Cancer stem cells: Here, there, everywhere? Nature. 2008;456:581–582. doi: 10.1038/456581a. [DOI] [PubMed] [Google Scholar]
- 23.Kawamata H, Tachibana M, Fujimori T, Imai Y. Differentiation-inducing therapy for solid tumors. Curr Pharm Des. 2006;12:379–385. doi: 10.2174/138161206775201947. [DOI] [PubMed] [Google Scholar]
- 24.Kaci R, Shahid S, Réa L, Philipe B, Amu T, Marc P, Massoud MJII. Neural signature expressed by cells from ovarian carcinoma (A Case Report) J Immunochem Immunopathol. 2015;1:2. [Google Scholar]
- 25.Montespan F, Deschaseaux F, Sensébé L, Carosella ED, Rouas-Freiss N. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: Implications in bone repair therapy. J Immunol Res. 2014;2014:230346. doi: 10.1155/2014/230346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhuis-Vlug AG, Rosen CJ. Clinical implications of bone marrow adiposity. J Intern Med. 2018;283:121–139. doi: 10.1111/joim.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam A. Do bone marrow fat cells or their precursors have a pathogenic role in idiopathic aplastic anaemia? Med Hypotheses. 1988;25:209–217. doi: 10.1016/0306-9877(88)90032-1. [DOI] [PubMed] [Google Scholar]
- 28.Lu W, Weng W, Zhu Q, Zhai Y, Wan Y, Liu H, Yang S, Yu Y, Wei Y, Shi J. Small bone marrow adipocytes predict poor prognosis in acute myeloid leukemia. Haematologica. 2018;103:e21–e24. doi: 10.3324/haematol.2017.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data for all the experiments is available upon request.