Summary
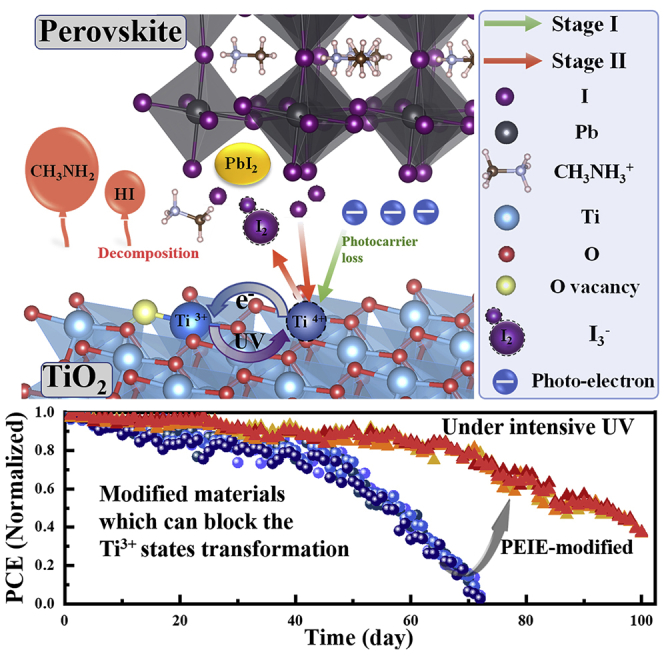
The failure of perovskite solar cells (PSCs) under ultraviolet (UV) irradiation is a serious barrier of commercial utilization. Here, a two-stage degradation process of TiO2-based PSCs is discovered under continuous UV irradiation in an inert atmosphere. In the first decay stage, oxygen vacancy-Ti3+ (Ti3+-VO) transform into active Ti4+-VO trap states under UV excitation and cause photocarrier loss. Furthermore, Ti4+-VO states can convert back into Ti3+-VO states through oxidizing I−, which result in the accumulation of I3−. Sequentially, the rapid decomposition of perovskite accelerated by increasing I3− replaces the photocarrier loss as the dominant mechanism leading to the second decay stage. Then, a universal method is proposed to improve the UV stability by blocking the transformation of Ti3+-VO states, which can be realized by polyethyleneimine ethoxylated (PEIE) modified layer. The optimized devices remain ∼75% of its initial efficiency (20.51%) under UV irradiation at 72 days, whereas the normal devices fail completely.
Subject Areas: Inorganic Materials, Optical Materials, Materials Chemistry
Graphical Abstract
Highlights
-
•
A two-stage degradation process of TiO2-based PSCs under continuous UV irradiation
-
•
The transformation of Ti3+-VO to Ti4+-VO states is responsible for the UV degradation
-
•
A universal method to enhance the UV stability of PSCs was proposed
Inorganic Materials; Optical Materials; Materials Chemistry
Introduction
Organic-inorganic lead halide perovskite solar cells (PSCs) show great potential to become the promising candidate for future energy conversion device (Tsai et al., 2018, Jeon et al., 2015, Yang et al., 2019, Bai et al., 2019, Cui et al., 2019), with the latest certification efficiency of 25.2% (Best, 2019). The most popular electron transport layer (ETL) in the high-performance PSCs is the inorganic compact TiO2 owing to its suitable band alignment and high transmittance (Kim et al., 2016, Niu et al., 2015). But the poor stability of TiO2-based PSCs under working conditions still restricts its commercialization. Especially, the intrinsic instability of TiO2-based PSCs under ultraviolet (UV) irradiation has attracted extensive attention.
To overcome UV instability of TiO2-based PSCs, Al2O3, MgO, Sb2S3, etc. were used to suppress the continuous degradation by modifying, separating, or replacing TiO2 ETL, which remained 70%–90% of the initial efficiency after hundreds of hours of UV exposure (Ito et al., 2014, Wei et al., 2016, Wei et al., 2019a, Han et al., 2015, Lee et al., 2018, Wei et al., 2019b, Wan et al., 2018). Meanwhile, the study of degradation mechanism is necessary to further improve the UV stability of PSCs. It is well known that the TiO2 is photocatalytic, which can supply electrons to accelerate organic decomposition reactions. It can help to explain the decomposition of perovskite material under UV illumination with moisture or oxygen, which is responsible for the instability of PSCs (Ito et al., 2014, Aristidou et al., 2015, Abdelmageed et al., 2016, Shlenskaya et al., 2018). In terms of carrier transfer, the recombination loss induced by UV irradiation can also lead to the decay of PSCs performance with the assistance of oxygen vacancies in TiO2 and oxygen molecules in air (Leijtens et al., 2013, Bryant et al., 2016). In addition, at the interface between TiO2 and perovskite layers, the voltage loss due to the charge accumulation under light soaking can also cause the performance decrease of PSCs (Gottesman et al., 2016). However, the UV degradation of TiO2-based PSCs performance still exists in the absence of moisture, oxygen, and visible light (Shin et al., 2017, Wang et al., 2019, Ahn et al., 2016), which is future work condition of PSCs. Therefore, the exploration of the UV-degradation process and the underlying mechanism of TiO2-based PSCs in inert atmosphere become an important and practical research topic.
Here, long-term monitoring of PSCs performance under continuous UV irradiation in glovebox was conducted. We discovered the two-stage UV degradation of TiO2-based PSCs and explored the underlying mechanism. Then, we proposed a universal method to inhibit the UV degradation of PSCs. And, polyethyleneimine ethoxylated (PEIE)-modified layer was introduced on TiO2 ETL, obtaining UV stable PSCs with high champion efficiency of up to 20.51%, which held ∼75% of the initial efficiency when the normal PSCs failed completely after ∼72 days intensive UV irradiation. This method provides a promising way to develop UV-stable and high-performance perovskite solar cells.
Results and Discussion
The Two-Stage Degradation under UV Irradiation
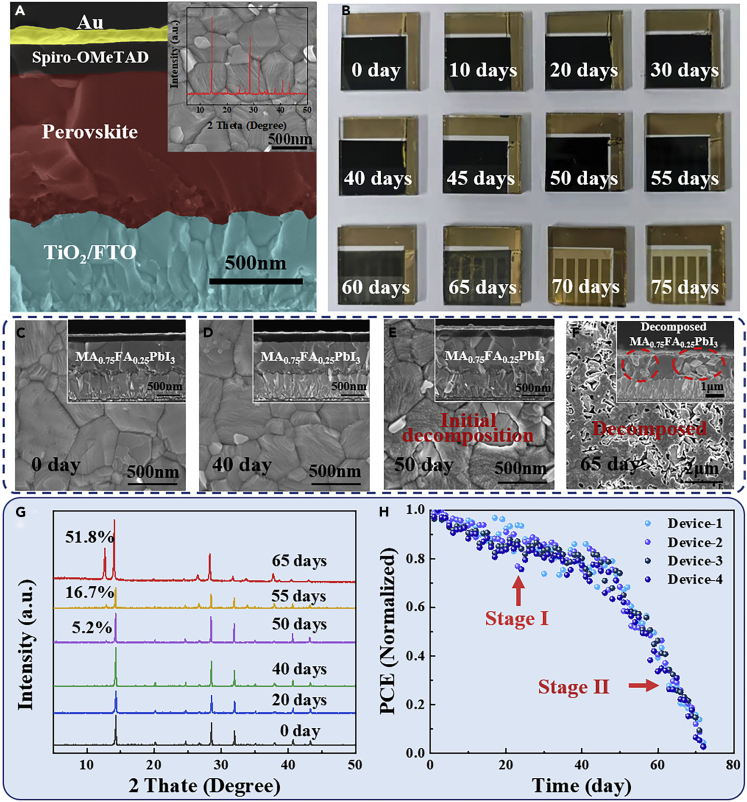
In this work, we prepared planar perovskite solar cells (PSCs) structured as glass/FTO/c-TiO2/perovskite/spiro-OMeTAD/Au using the convenient one-step solution method. The detailed fabrication procedures are shown in the Transparent Methods section of Supplemental Information. Figure 1A shows the cross-sectional scanning electron microscopy (SEM) image of the PSCs, wherein MA0.75FA0.25PbI3 was used as the perovskite absorber material. Meanwhile, the compact TiO2 and the 2,2′,7,7′-tetrakis (N, N-di-p-methoxyphenylamine)-9,9′ spirobifluorene (spiro-OMeTAD) serve as the electron transport layer (ETL) and hole transport layer (HTL), respectively. As seen, the thickness of the perovskite layer is ∼700 nm. The top-view SEM image and XRD pattern inserted in Figure 1A indicate the uniform and high-quality perovskite film, which is similar to our previous reports (Song et al., 2016, Cui et al., 2017, Wei et al., 2019a, Wei et al., 2019b).
Figure 1.
Degradation of the Perovskite Solar Cells under UV Irradiation
(A) SEM cross-sectional image of PSCs. The layers from the bottom up are: FTO, TiO2, MA0.75FA0.25PbI3, spiro-OMeTAD (doped with Li-TFSI, FK 209, and TBP), and Au. And the inserted images are top-view SEM image and XRD patterns of the corresponding perovskite film.
(B) The photographs of PSCs for different UV exposure time.
(C–F) The SEM images of the MA0.75FA0.25PbI3 films had been prepared in solar cells after UV irradiation for 0, 40, 50, and 65 days.
(G) XRD patterns for MA0.75FA0.25PbI3 in PSCs under UV irradiation for different times.
(H) The evolution of the normalized PCE of the PSCs under UV irradiation for 72 days. For the perovskite film measurements, the Au electrodes were removed with tapes and the Spiro-based HTL were rinsed with chlorobenzene.
In order to explore the influence of UV irradiation on PSCs, we first observed the deterioration of perovskite films in PSCs after continuous UV exposure. Here, we used Philip UV lamp (λ = 254 nm) with an intensity of ∼50 mW·cm−2 (equivalent to 11 suns of UV light below 400 nm) to irradiate the PSCs from the TiO2 ETL side at room temperature in an argon-filled glovebox. All UV irradiation treatments of PSCs for this work were carried out under such UV radiation conditions, unless otherwise specified. This is a rather brutal test that helps to quickly identify the degradation rule of PSCs under UV irradiation. Figure 1B shows the photographs of PSCs for different UV exposure times. We found that perovskite films prepared on TiO2 remained uniform black phase for ∼50 days, until dramatic yellow phase transition began around 55 days. In general, the black phase of the absorber material is recognized as perovskite structure and the yellow phase can be recognized as PbI2. Hence, it is speculated that the decomposition of perovskite film could occur rapidly after a lag time of ∼50 days under such a UV irradiation condition. Furthermore, the top-view SEM images of perovskite film in UV-irradiated PSCs are shown in Figures 1C–1F, which reflect the evolution of perovskite film morphologies. Apparently, the film morphology did not change significantly at the early stage (within 50 days). After this time threshold, the perovskite films began to collapse and a new cleavage surface appeared, indicating the initial decomposition. On the 65th day, a large number of holes appeared in the perovskite films, demonstrating the serious decomposition and deterioration of the perovskite film. In addition, the perovskite film without underlying TiO2 ETL shows excellent stability under the same UV irradiation condition and did not decompose significantly within 150 days, as shown in Figure S1.
The phase purity and crystal structure of the perovskite films in UV-irradiated PSCs were characterized by X-ray diffraction (XRD) to confirm the component transmutation behind the morphology evolution. Figure 1G exhibits the XRD patterns of perovskite film under UV irradiation for 0, 20, 40, 50, 55, and 65 days. As we can see, all the XRD patterns of perovskite film on TiO2 ETL under UV exposure within 40 days are almost identical, where the main diffraction peaks centered at 13.99° and 28.19° can be attributed to the (110) and (220) planes of perovskite films. And there is no obvious diffraction peak of PbI2 centered at 12.60° until UV exposure for 50 days, which is corresponding to the turning point of film morphology. With the extension of illumination time, the proportion of PbI2 diffraction peak increases rapidly from 5.2% to 51.8% (the intensity ratio compared with the strongest peak) within 15 days. The content of lead iodide (PbI2) is used to characterize the decomposition degree of perovskite film because PbI2 is often observed as the final degradation product from lead iodide-based perovskites. Therefore, we can infer that the perovskite film remains unchanged in ∼50 days under such a UV irradiation condition. Afterward, it begins to decompose dramatically in a short time.
Based on the deterioration phenomenon of perovskite films, we investigated the stability of the complete PSCs under continuous UV irradiation (as mentioned above) without encapsulation using an apparatus of stability test. The cells were removed every 24 h to measure the current voltage curves in reverse scan direction under simulated AM 1.5G 100 mW·cm−2 irradiance. Figure 1H shows the decay process of device photoelectric conversion efficiency (PCE) calculated from the J-V curves. In the earlier stage, the decay rate is relatively low, wherein the efficiency of PSCs remains 70% of the initial value by ∼50 days. In the later stage, the decay rate suddenly increases, wherein the efficiency drops to ∼1% of the initial value from 50 to 72 days. Apparently, the time node of the PCE abrupt decrease is consistent with the perovskite film decomposition. It can be inferred that the sharp decrease of the device efficiency in the second stage is caused by the rapid decomposition of perovskite absorber. Therefore, we can draw a conclusion that the UV irradiation can lead to two-stage degradation of TiO2-based PSCs. The earlier decay stage (stage I) and the later decay stage (stage II) constitute the whole degradation process of PSCs. It is also noted that the different decay rates of stage I and stage II indicate different dominating inducements. The underlying trigger factor and the mechanism of the two-stage PSCs UV-degradation process are discussed below.
The Mechanism of Two-Stage UV Degradation
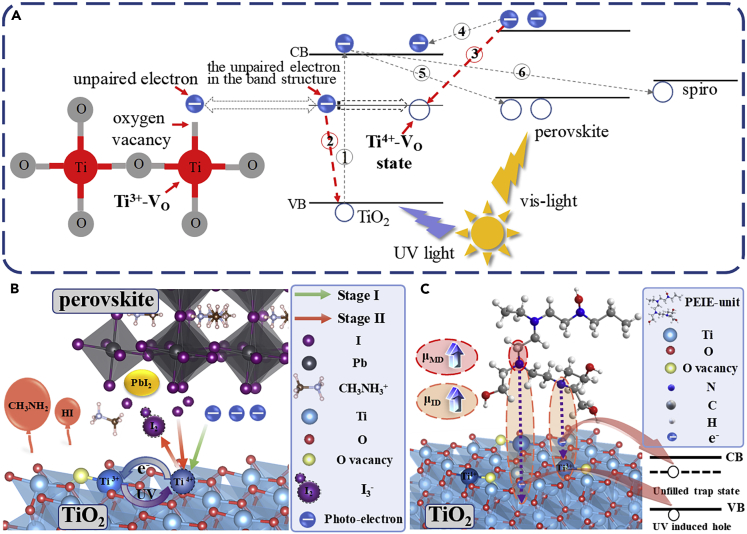
It is well known that there exist inherent oxygen vacancies in TiO2 ETL, which correspond to Ti3+ defects (Ti3+-VO) with unpaired outer electrons. And the Ti3+-VO tend to transform into Ti4+ defects (Ti4+-VO) serving as deep traps. Figure 2A shows the detailed generation process of Ti4+-VO and the carrier recombination channel from the perspective of energy band. In the prepared TiO2 film, the inherent oxygen vacancies result in Ti3+-VO with an unpaired electron at ∼1 eV below conduction band minimum (Naldoni et al., 2012, Wang et al., 2009). Upon band excitation of TiO2 induced by UV light, valence band electrons transit to the conduction band (step ①), leaving free holes. Unpaired electrons at the Ti3+-VO site tend to recombine the holes in the valence band (step ②). This process leaves free electrons in the conduction band and Ti4+-VO defect states. The Ti4+-VO states capture photo-generated electrons from perovskite layer (step ③) actively, reverting Ti4+-VO to Ti3+-VO, as indicated by the thin green arrow in Figure 2B. And the accumulation of photoelectrons induced by the Ti4+-VO provide extra electron recombination accesses to holes in the perovskite layer (step ⑤) and HTL layer (step ⑥) (Leijtens et al., 2013), which results in performance decay of PSCs. With the prolongation of UV irradiation time, the concentration of Ti4+-VO defect states increases continuously, which is resulted from the reaction equilibrium of defects transformation and the increase of Ti3+-VO (Figure S2) (Xiong et al., 2012, Bennett et al., 2015, Yu et al., 2011, Zhang et al., 2013), leading to the continuous decay of device performance in stage I.
Figure 2.
Degradation Mechanism of PSCs under Continuous UV Irradiation
(A) The energy band of TiO2 ETL and perovskite absorber.
(B) The mechanism of PSCs degradation under continuous UV irradiation.
(C) The mechanism of PEIE blocking Ti3+-VO transformation through donated electrons.
Meanwhile, the UV-excited Ti4+-VO states can lead to rapid decomposition of perovskite, corresponding to the sharp performance decay. Ti4+-VO defects provide a large number of oxidizing sites that can oxidize I− leading to the deposition of I2 or rather I3−, as indicated by the thin red arrow in Figure 2B. Along with the accumulation of polyiodide I3−, perovskite films decompose rapidly after a certain delay time under UV illumination, as demonstrated in Figure 1, resulting in the sharp performance decay of PSCs until complete failure. We suppose that it is determined by the kinetics of the decomposition reaction based on the mass action law (Hinrichs and Dreijer-van der Glas, 2015). The decomposition process of perovskite materials under UV irradiation can be described by the following four chemical equations. As is mentioned above, Ti3+-VO defects in TiO2 can be converted to oxidizing Ti4+-VO states after UV exposure (Leijtens et al., 2013), which serve as electron accepters (Equation 1). And the I− surrounded by electron accepters tend to be oxidized turning to I2, leaving PbI2 (Abdelmageed et al., 2016, Shlenskaya et al., 2018) (Equation 2). Disengagement of I− from the lattice caused the collapse of the octahedral structure, releasing bound CH3NH3+ and causing CH3NH3+ migration to the interface between TiO2 ETL and perovskite layer. Meanwhile, the generated I2 will combine with the free I− reversibly (free I− is abundant in perovskite material [Eames et al., 2015, Haruyama et al., 2015, Azpiroz et al., 2015]), producing polyiodide species I3− with strong reduction (Shlenskaya et al., 2018). According to the Pearson hard and soft acid base concept (Shlenskaya et al., 2018, Kim et al., 2012), the neutralization reaction of free soft acid CH3NH3+ and free soft base I3− happens spontaneously at the interface with the participation of photoelectrons. And this process, shown in Equation 4, generates volatile CH3NH2 and HI, exposing PbI2 product as illustrated in XRD data. Moreover, according to the Equation 2, oxidizing Ti4+-VO states can trigger the oxidation of I− circularly excitation, resulting in the accumulation of I3− and I2. Actually, the consumption of CH3NH3+ and I− lead to the deposition of PbI2, which ultimately completed the perovskite decomposition process. Equation 4 can be recognized as the direct procedure that determines the decomposition rate of perovskite materials. Therefore, the decomposition rate of perovskite material can be approximated by the reaction rate of Equation 4.
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
| (Equation 4) |
According to the mass action law (Equation S1), the concentration of CH3NH3+ and I3− is the main factor that determines the reaction rate of Equation 4. In other words, the accumulation amount of CH3NH3+ and I3− determines the decomposition rate of perovskite material. The intrinsic free CH3NH3+ is negligible (Eames et al., 2015, Haruyama et al., 2015, Azpiroz et al., 2015); the amount of CH3NH3+ that participates in the decomposition reaction is proportional to the amount of deposited I2 and I3−. According to the stoichiometry of Equation 2 and Equation 3, it can be concluded: , where , , and are the qualitative concentration of CH3NH3+, I2, and I3−, respectively. Combining with the Equation S1, the formula of perovskite decomposition rate can be derived:
where is the decomposition rate of perovskite, is the chemical reaction rate constant, is the qualitative concentration of I3−, is the chemical equilibrium constant, and is the qualitative concentration of I−, which can be considered as a constant. The detailed derivation process of this formula can be seen in Supplemental Information. As shown, the decomposition rate of perovskite is proportional to the fourth power of I3− concentration. Consequently, perovskite material will decompose rapidly after a certain I3− concentration threshold (Figure S3), corresponding to the lagging sharp decay of device performance in the stage II. The required accumulation time of the threshold concentration of polyiodide I3−, namely, the duration time of stage I, is the root of the time difference between stage I and stage II. In addition, we have carried out an intensifying experiment to confirm the origin of the lagging rapid decomposition. External PbI2, MAI, and I2 were added into perovskite precursors to prepare PSCs with different perovskite films containing excess suspected decomposition inducers. (Figure S4) This can simulate the reaction environment of the rapid decomposition. Then, we exposed these three groups PSCs to intensive UV irradiation under the above-mentioned test conditions to observe the degradation process. The experimental results demonstrated that the excessive I2, which could represent the content of I3−, led to rapid decomposition of the perovskite film after 5 days UV irradiation (Figure S5). The detailed experimental procedures can be seen in the Supplemental Information.
The transformation of Ti3+-VO states to Ti4+-VO states in TiO2 ETL under UV irradiation serves as the driving force of PSCs UV degradation. In stage I, the photocarrier loss induced by Ti4+-VO states results in the slow performance decay of PSCs. Afterward, the rapid decomposition of perovskite material initialized by Ti4+-VO states replaces photocarrier loss to be the domain inducement of degradation, leading to the sharp performance decay in stage II. Therefore, blocking the transformation of Ti3+-VO states to Ti4+-VO states becomes the essential approach to inhibit the UV degradation of TiO2-based PSCs.
PEIE has been proved to be an effective electron donor material (Zhou et al., 2012, Zhou et al., 2014, Rasool et al., 2019). Thus, PEIE film can serve as the modified layer of TiO2 ETL to block the transformation of Ti3+-VO states. Figure 2C shows the mechanism of PEIE blocking the transformation and passivating the active Ti4+-VO trap states. The molecular dipole moment (Rasool et al., 2019) and the interface dipole moment (Zhou et al., 2012, Heimel et al., 2006, Heimel et al., 2008, Hong et al., 2010) together result in the electron donation of the amine group. As mentioned above, the UV-excited holes in TiO2 can capture the outer electron of Ti3+-VO, turning it into Ti4+-VO states. Hence, the donated electrons can deplete the UV-excited holes, blocking the transformation of Ti3+-VO states (Rasool et al., 2019, Zhang et al., 2019, Saracco et al., 2013, Ishii et al., 1999). Moreover, the external electrons can directly passivate Ti4+-VO trap states, avoiding the capture of photoelectrons and the oxidation of I−. Therefore, we can infer that PEIE serving as modified layer of TiO2 ETL can enhance the UV resistance of PSCs significantly.
Evidence for the Transformation of Ti3+-VO States and the Blocking Effect of PEIE
We prepared planar TiO2-based PSCs with and without PEIE modified layer on TiO2 ETL to explore the transformation of Ti3+-VO states and the blocking effect of PEIE layer. The PSCs construction and the molecular structure of PEIE are shown in Figure S6A. The detailed fabrication procedures of the PEIE-modified PSCs are shown in the Experimental section of Supplemental Information. Figure S6B displays the X-ray photoelectron spectroscopy (XPS) results of the PEIE-TiO2 and pure-TiO2 films deposited on FTO/glass. The presence of PEIE can be verified by the N1s peak at 399.8 eV, which is negligible in pure-TiO2. The surface morphology of PEIE-modified TiO2 ETL was measured by atomic force microscopy (AFM), as shown in Figure S7. The transformation of Ti3+-VO defect states is discussed below.
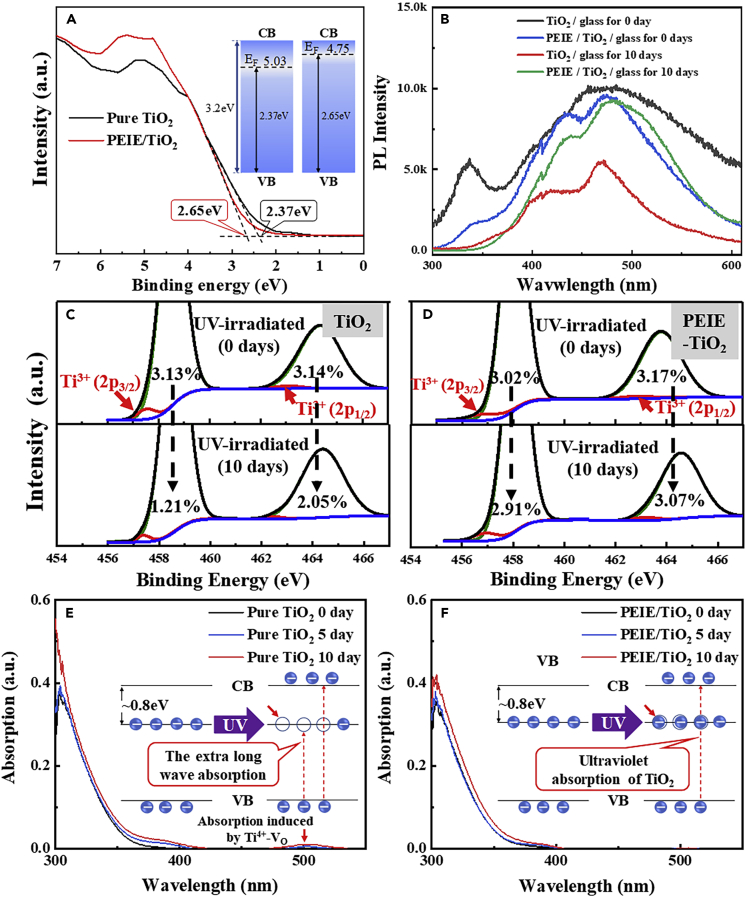
In order to confirm the blocking effect of PEIE on the transformation of Ti3+-VO states, which is achieved by the donated electrons, the Fermi level of TiO2 films with and without PEIE layer were estimated by UV photoelectron spectroscopy (UPS) patterns through the tangential extension method. As shown in Figure 3A, the point at which the tangent line intersects the horizontal line on the x-coordinate in UPS curves represents the distance from the valence band maximum to the Fermi energy level. The energy distance of pure-TiO2 is 2.37 eV compared with 2.65 eV of PEIE-TiO2, which confirmed the 0.28-eV increment in Fermi level of PEIE-TiO2. This result proves that external electrons can be injected into the TiO2 efficiently from the PEIE layer by processes such as tunneling or thermionic injection (Kim et al., 2012). The donated electrons tend to deplete the UV exited holes.
Figure 3.
Evidence of the Ti3+-VO States Transformation and the Blocking Effect of PEIE
(A) UPS measurements of pure-TiO2 and PEIE-TiO2 films on glass substrate.
(B) Low temperature (80 K) steady-state PL spectra (250-nm xenon lamp excitation) of pure-TiO2 and PEIE-TiO2 film on glass substrate under UV irradiation for 0 and 10 days.
(C and D) XPS Ti(2p) spectra of pure-TiO2 film (C) and PEIE-TiO2 film (D) on glass under UV irradiation for 0 and 10 days.
(E and F) UV-visible absorption spectra of (E) pure-TiO2 film and (F) PEIE-TiO2 film under continuous UV irradiation for 0, 5, and 10 days.
See also Figure S8.
The defect states density in TiO2 ETL under UV irradiation was characterized by steady photoluminescence (PL) measurement. The samples of TiO2 films with and without PEIE layer on glass substrate, which had been exposed in UV irradiation for 0, 10 days in an argon-filled glovebox, were operated in liquid nitrogen at 80 K for steady PL measurement. (The dominant recombination processes of anatase TiO2 at room temperature are nonradiative [Knorr et al., 2008, Li et al., 2016].) As shown in Figure 3B, the PL spectrum reveals a broad emission centered at around 480 nm. And the corresponding radiation energy is 2.58 eV, demonstrating that the photoluminescence is mainly produced by Ti3+ defect level (∼2.5 eV above the valence band maximum) at 80 K. Under UV irradiation for 10 days, the PL intensity of pure-TiO2 film is declined markedly. In general, the PL intensity of TiO2 film is related to the concentration of defect states that can serve as the nonradiative carrier recombination centers. Hence, the lower PL intensity of the irradiated pure-TiO2 film (the red line) indicates that the density of defect states in pure-TiO2 increased obviously after UV irradiation. However, the PL intensity of PEIE-TiO2 film is almost unchanged, demonstrating that the PEIE modification layer on TiO2 can significantly suppress the UV-induced defect states.
To confirm the nature source of UV-induced defect states, the TiO2 films on glass with and without PEIE modification layer under UV irradiation for 0 and 10 days was also measured by XPS, as depicted in Figures S8A and S8B. The peak located at 458.7 eV is assigned to the Ti (2p3/2), and the peak located at 464.3 eV is assigned to Ti (2p1/2). For the 458.7 eV peak, two titanium containing components are clearly resolved through peak fitting by Origin. The main peak at 458.7 eV (marked by the green line) is assigned to the lattice Ti4+ (2p3/2), and the shoulder peak at ∼457.5 eV (marked by the red line) is assigned to the oxygen vacancy-Ti3+ (2p3/2) (Sandell et al., 2003, Jiang et al., 2012). This ∼1.2-eV energy difference is consistent with previous reports (Wang et al., 2009, Pan et al., 2013, Wetzelaer et al., 2015). It is well known that the area of characteristic peaks can indicate the content of corresponding elements. Through the peak area integral calculation, as shown, the content of Ti3+(2p3/2) in pure-TiO2 film was reduced from 3.13% to 1.21% after 10 days of UV exposure, as indicated in Figure 3C. This occurs when unpaired electrons in Ti3+-VO recombine with holes in valence band excited by UV radiation, indicating the transformation of Ti3+-VO states to Ti4+-VO states. Moreover, the 464.3 eV peak, which corresponds to Ti (2p1/2), shows a similar phenomenon. Therefore, it can be concluded that the generated defect states in TiO2 under UV irradiation are Ti4+-VO states, which are transformed from Ti3+-VO states. Figure 3D shows the Ti3+-VO decrement of PEIE-TiO2 from 3.02% to 2.91% for Ti3+(2p3/2) and from 3.17% to 3.07% for Ti3+ (2p1/2) under UV irradiation for 10 days, which is significantly less than pure-TiO2, demonstrating the effective blocking effect of PEIE on Ti3+-VO transformation, consistent with PL result.
Furthermore, the UV-vis spectra were obtained to measure the generation of Ti4+-VO states. As the inserted diagram in Figure 3E shows, the Ti3+-VO defect states with unpaired electrons have no significant effect on the absorption spectrum of TiO2 film. Under UV irradiation, Ti3+-VO states tend to transform into Ti4+-VO states leaving unfilled defect energy levels in TiO2, which can cause long wave absorption of TiO2 films. Figures 3E and 3F exhibit the UV-vis absorption spectra of TiO2 films with and without PEIE modification layer on glass substrate under UV illumination for 0, 5, and 10 days. As seen, after 5 and 10 days UV exposure, the pure-TiO2 film generated a weak defect absorption peak at the wavelength of ∼500 nm, which corresponds to the Ti4+-VO states at ∼1 eV below the conduction band minimum. This result confirms the presence of Ti4+-VO states in TiO2 transformed from Ti3+-VO after UV irradiation. As discussed earlier, the PEIE layer can inject electrons into the TiO2 film filling the UV-induced defect states, which is the passivation process of Ti4+-VO states, as is depicted in the inserted image in Figure 3F. This process can suppress the defect absorption effectively, so there is no obvious defect peak in the absorption spectrum of PEIE-TiO2 films.
Performance Decrease Induced by Ti4+-VO States
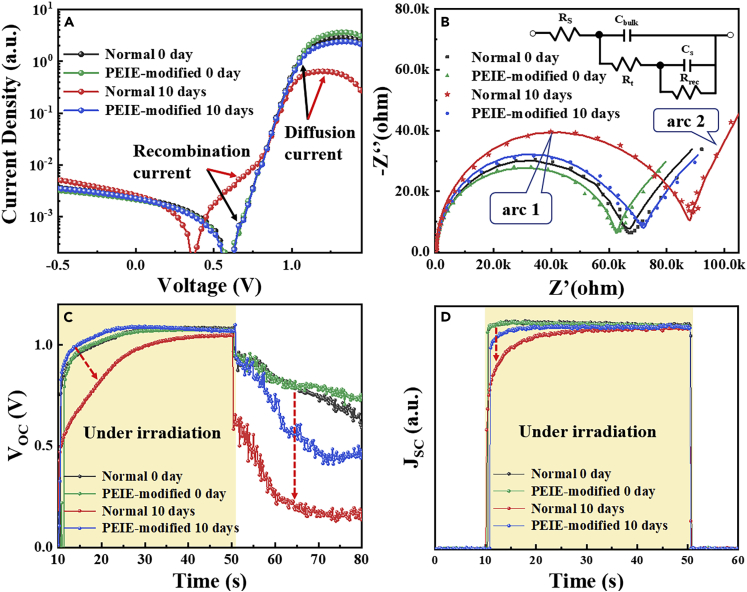
In an attempt to explore the effect of Ti4+-VO trap states on device performance, we have performed multiple electrical tests for PSCs after 0 and 10 days UV exposure. The dark current curves of the PSCs based on pure-TiO2 ETL and PEIE-TiO2 ETL are characterized and shown in Figure 4A. As seen, the dark current at the low voltage scale, mainly determined by the recombination current, is higher in UV-degraded PSC with pure-TiO2 ETL (the red line), whereas the current after the threshold voltage, mainly determined by the diffusion current, is lower. As the recombination of the PSCs is evidenced to be governed by the trap states (Song et al., 2016, Wetzelaer et al., 2015), the higher recombination current in the degraded PSC indicates more severe recombination induced by Ti4+-VO states, reflecting the photocarrier loss. And the lower diffusion current in the UV-degraded PSC corresponds to the high resistance to photoelectron transport raised by Ti4+-VO states. On the contrary, the PSCs based on PEIE-TiO2 ETL have no apparent performance variation in dark current curve after UV exposure, demonstrating that the PEIE layer does reduce the carrier recombination caused by Ti4+-VO state and enhance electron transport.
Figure 4.
Performance Decrease of Perovskite Solar Cells
(A)The evolution of dark current characteristic curves.
(B) The evolution of electrochemical impedance.
(C) The evolution of VOC decay.
(D) The evolution of Transient JSC (normalized at maxima). These PSCs were exposed to UV irradiation in an argon-filled glovebox at room temperature. Complex impedance plot for PSCs measured in dark condition at short circuit conditions.
The interfacial electron transfer between ETL and perovskite layer was investigated by electrochemical impedance spectrum (EIS) to observe the degradation caused by UV-induced Ti4+-VO states. EIS is used as an effective tool to certify the charge transport and recombination processes. Figure 4B shows the Nyquist plots of PSCs with pure-TiO2 ETL and PEIE-TiO2 ETL under UV irradiation for 0 and 10 days measured at 0 V bias in dark. Rt (transport resistance) and Rrec (recombination resistance) can be obtained by fitting Nyquist plots using the equivalent circuit shown in Figure 4B inserted image. The lower frequency circle (arc 2) is related to the charge recombination, and the higher frequency circle (arc 1) belongs to charge transfer at ETL/perovskite interfaces because the perovskite/HTL interfaces were identical in both cases. For the UV-degraded PSCs based on pure-TiO2, the Rrec is significantly smaller than the fresh PSCs, whereas the transfer resistance Rt is larger (the fitting values are shown in the Table S1). This consequence indicates that UV-induced Ti4+-VO states can increase photocarrier recombination and transfer resistance, leading to the performance decrease. However, the Rrec and Rt of the PSCs modified with PEIE layer have no remarkable change after UV exposure, consistent with dark current data.
Open circuit voltage decay (OCVD) was performed to study the effect of Ti4+-VO states on electron extraction. As shown in Figure 4C, the VOC decay curves of UV-degraded PSCs with pure-TiO2 ETL (the red line) exhibit lower voltage than the fresh one, which correspond to the accumulation of photocarrier at the interface between the absorb layer and transport layer. At the initial descent point (immediately after the irradiation off) of the VOC decay curve, the decrease of VOC is obviously faster in the UV-degraded PSCs compared with the fresh one. And the voltage increment of the UV-degraded PSC immediately after the irradiation on is slower. In our previous work, we show that the more severe decay in OCVD curves corresponds to the presence of more trap states and the poor performance of PSCs (Song et al., 2015, Song et al., 2016, Cui et al., 2015), which are consistent with the case of UV-degraded PSCs. These results illustrate that the UV-induced Ti4+-VO states, proved earlier, can lead to serious electron recombination and poor electron extraction, resulting in photocarrier loss. Furthermore, the UV-induced Ti4+-VO states also lead to the slow response of the photocurrent. As shown in Figure 4D, JSC of the UV-degraded PSCs with pure-TiO2 ETL increases more slowly after the light on than that of the fresh PSCs, further proving the photocarrier loss caused by UV-induced Ti4+-VO states. The VOC decay curve and JSC increase curve of UV-degraded PSCs with PEIE-TiO2 ETL (the blue line) retain slow decay and fast increase, respectively. This proves that the PEIE layer can effectively reduce the interface carrier recombination, indicating the improved UV stability of modified PSCs.
Ti4+-VO states can also trigger the decomposition of perovskite film causing performance decrease. After 50 days of UV irradiation, the perovskite film began to decompose rapidly. This can be proved by the XRD patterns and SEM images in Figures 1C–1G. Notably, the PEIE-modified layer on TiO2 ETL can effectively inhibit the decomposition of perovskite film. Figure S9A and S9B show the XRD spectra of the perovskite films in normal and PEIE-modified PSCs under UV irradiation for 0, 20, 40, 50, 55, and 65 days in an argon-filled glovebox at room temperature. As mentioned above, the perovskite films in normal PSCs decomposed significantly after 50 days UV exposure. However, there is no apparent decomposition of the perovskite films in PEIE-modified PSCs for 65 days UV irradiation. Moreover, the morphology evolution of perovskite films is depicted in Figure S10. The broken perovskite films in normal PSCs after 50 days UV exposure indicate the serious decomposition. But the PEIE-modified samples exhibit no obvious morphology change. In conclusion, perovskite films decompose seriously after 50 days, but the introduction of the PEIE modified layer can effectively suppress the decomposition process, suggesting the excellent UV resistance.
The Improved Device Stability
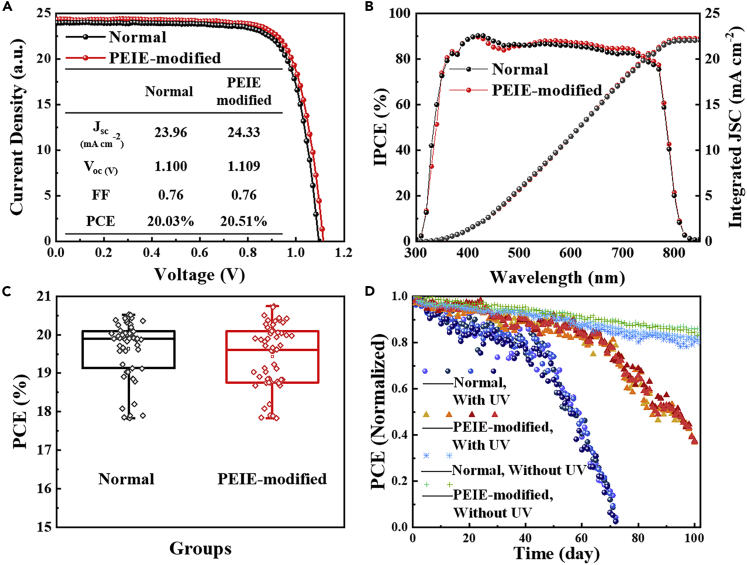
The photovoltaic performance of the perovskite solar cells consisting of pure-TiO2 ETL and PEIE-modified TiO2 ETL was measured under AM 1.5G, 1 sun illumination in reverse direction. Figure 5A exhibits the current-voltage plots of the devices with (red) and without (black) PEIE modification layer. The detailed photovoltaic parameters of short-circuit current density (JSC), open-circuit voltage (VOC), fill factor (FF), and photoelectric conversion efficiency values (PCE) are summarized in the inserted table. The best device among the normal PSCs achieved a PCE of 20.03% with a JSC of 23.96 mA·cm−2, a VOC of 1.100 V, and a fill factor (FF) of 76%. And the PEIE-modified device achieved a PCE of 20.51% with a JSC of 24.33 mA·cm−2, a VOC of 1.109 V, and a fill factor (FF) of 76%. The corresponding external quantum efficiency (EQE) and integrated short-circuit current density are presented in Figure 5B. The EQE measurements showing a high photo-to-current conversion over 90% at 430 nm indicates good utilization of photons in the short-wavelength (360–560 nm) region of sunlight. And the integrated current density of 22.08 and 22.30 mA·cm−2 for normal and PEIE-modified devices, respectively, is in good agreement with J-V results. In addition, both normal and PEIE-modified PSCs show a narrow distribution of PCE with the median value of 19.8% and 19.6%, as depicted in Figure 5C. Notably, the introduction of PEIE has slightly reduced the repeatability of device performance. These performance results prove a fact that the introduction of PEIE modification layer has no significant impact on the photoelectric performance of PSCs; on the contrary, it even has the potential to improve the device efficiency due to the enhanced electron extraction of TiO2 ETL (Zhou et al., 2012, Zhou et al., 2014). This can also be demonstrated by PL spectrums of perovskite film, as shown in Figure S11. In addition, the introduction of PEIE layer did not improve the hysteresis effect of PSCs, which was still obvious, as shown in Figure S12.
Figure 5.
PSCs Performance and Stability
(A) J-V curves of the best PSCs with (red) and without (black) PEIE modification layer under AM 1.5G 100 mW·cm−2 illumination. Inset: Device performance parameters calculated from the curves.
(B) External quantum efficiency (EQE) spectrum of the normal and PEIE-modified PSCs.
(C) Device performance distribution for 55 normal devices and 55 PEIE-modified devices in one batch.
(D) The normalized PCE decay of the normal and PEIE-modified PSCs under 254-nm UV irradiation with an intensity of 50 mW·cm−2 at room temperature in an argon-filled glovebox.
The more important goal of this work is to investigate the effect of blocking transformation by PEIE on UV stability. Here, we used UV lamp (λ = 254 nm) to irradiate the PSCs at room temperature in an argon-filled glovebox. Figure 5D displays the efficiency decay curves of the PSCs with and without PEIE modification layer on TiO2 ETL under UV irradiation for 100 days. As a comparison, the decay curves of normal PSCs and PEIE modified PSCs without UV irradiation are also exhibited in Figure 5D. Significant two-stage UV degradation of all the PSCs is clearly observed. However, the efficiency decay of the PEIE-modified PSCs is slower. On the whole, the PEIE-modified PSCs remained ∼75% of its initial efficiency when the normal PSCs failed completely at 75 days under continuous intensive UV irradiation. Furthermore, in stage I, the decay rate of PEIE-modified PSCs is reduced from 0.60 to 0.42, when compared with normal PSCs. In stage II, the decay rate of PEIE-modified PSCs is reduced from 3.50 to 2.33. (This rate value is calculated by the equation: , where r is decay rate, is percentage decrease of efficiency, and t is irradiation time). In addition, the time threshold of rapid perovskite decomposition is postponed from 50 to 70 days. In this work, a large number of devices (including normal PSCs and PEIE-modified PSCs) were monitored under the same UV irradiation condition. The error of the transition point in decay curves is ±5 days. The outcomes suggest that blocking the Ti3+ transformation by PEIE-modified layer is an effective way to enhance the UV stability of the TiO2-based PSCs.
Conclusion
In summary, a two-stage UV degradation process of TiO2-based PSCs was discovered. The Ti4+-VO states transformed from inherent Ti3+-VO under UV irradiation cause the photocarrier loss, resulting in the first slower decay process (stage I), whereas the rapid decomposition of perovskite film after a period of UV exposure triggered by Ti4+-VO states leads to the second sharp performance decay (stage II). In essence, the transformation of Ti3+-VO states drives the two-stage UV degradation. Based on this mechanism, a universal method was proposed that blocking the transformation of Ti3+-VO states inhibits the UV degradation of TiO2-based PSCs. Herein, PEIE-modified layer was introduced to block the transformation enhancing the UV stability significantly. The two-stage UV-degradation mechanism can help address the UV instability issue of TiO2-based PSCs, offering a promising path to achieve high-efficiency devices with excellent UV resistance for commercialization.
Limitations of the Study
In this study, an ultrathin polymer interfacial layer (PEIE) is introduced to improve the UV stability of PSCs. However, this layer may not be uniform and compact, which limits its UV resistance and the repeatability of device performance. The deposition process of the PEIE layer should be further optimized.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work is supported partially by National Natural Science Foundation of China (Grant nos. 51772096 and 51972110), Beijing Science and Technology Project (Z181100005118002), Par-Eu Scholars Program, Science and Technology Beijing 100 Leading Talent Training Project, the Fundamental Research Funds for the Central Universities (2017ZZD02, 2019QN060), and the NCEPU "Double First-Class" Program.
Author Contributions
M.L. and J.J. conceived the idea and designed the experiments. J.J. and X.L. contributed equally to this work. J.J. and X.L. fabricated the devices and conducted stability test. H.J., M.D, B.L., and H.H performed the optical characterizations (XPS, steady-state PL, XRD, and absorption spectrums) of the TiO2 ETL. H.J., H.H., D.W., and Y.L. performed the electrical characterizations (dark current, EIS, and OCVD) of the PSCs. All authors contributed to the data analysis and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101013.
Data and Code Availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental Information. Additional data related to this paper may be requested from M.L. (mcli@ncepu.edu.cn).
Supplemental Information
References
- Abdelmageed G., Jewell L., Hellier K., Seymour L., Luo B., Bridges F., Zhang J.Z., Carter S. Mechanisms for light induced degradation in MAPbI3 perovskite thin films and solar cells. Appl. Phys. Lett. 2016;109:233905. [Google Scholar]
- Ahn N., Kwak K., Jang M.S., Yoon H., Lee B.Y., Lee J.-K., Pikhitsa P.V., Byun J., Choi M. Trapped charge-driven degradation of perovskite solar cells. Nat. Commun. 2016;7:13422. doi: 10.1038/ncomms13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristidou N., Sanchez-Molina I., Chotchuangchutchaval T., Brown M., Martinez L., Rath T., Haque A.A. The role of oxygen in the degradation of methylammonium lead trihalide perovskite photoactive layers. Angew. Chem. Int. Ed. 2015;54:8208–8212. doi: 10.1002/anie.201503153. [DOI] [PubMed] [Google Scholar]
- Azpiroz J.M., Mosconi E., Bisquert J., De Angelis F. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation. Energy Environ. Sci. 2015;8:2118–2127. [Google Scholar]
- Bai S., Da P., Li C., Wang Z., Yuan Z., Fu F., Kawecki M., Liu X., Sakai N., Wang J.T.-W. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature. 2019;571:245–250. doi: 10.1038/s41586-019-1357-2. [DOI] [PubMed] [Google Scholar]
- Bennett T., Adnan R.H., Alvino J.F., Kler R., Golovko V.B., Metha G.F., Andersson G.G. Effect of gold nanoclusters on the production of Ti3+ defect sites in titanium dioxide nanoparticles under ultraviolet and soft X-ray radiation. J. Phys. Chem. C. 2015;119:11171–11177. [Google Scholar]
- Best, NREL., 2019. https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20191106.pdf.
- Bryant D., Aristidou N., Pont S., Sanchez-Molina I., Chotchunangatchaval T., Wheeler S., Durrant J.R., Haque S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016;9:1655–1660. [Google Scholar]
- Cui P., Fu P., Wei D., Li M., Song D., Yue X., Li Y., Zhang Z., Li Y., Mbengue J.M. Reduced surface defects of organometallic perovskite by thermal annealing for highly efficient perovskite solar cells. RSC Adv. 2015;5:75622–75629. [Google Scholar]
- Cui P., Wei D., Ji J., Song D., Li Y., Liu X., Huang J., Wang T., You J., Li M. Highly efficient electron-selective layer free perovskite solar cells by constructing effective p-n heterojunction. Solar RRL. 2017;1:1600027. [Google Scholar]
- Cui P., Wei D., Ji J., Huang H., Jia E., Dou S., Wang T., Wang W., Li M. Planar p-n homojunction perovskite solar cells with efficiency exceeding 21.3% Nat. Energy. 2019;4:150–159. [Google Scholar]
- Eames C., Frost J.M., Barnes P.R., O’regan B.C., Walsh A., Islam M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015;6:7497. doi: 10.1038/ncomms8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman R., Lopez-Varo P., Gouda L., Jimenez-Tejada J.A., Hu J., Tirosh S., Zaban A., Bisquert J. Dynamic phenomena at perovskite/electron-selective contact interface as interpreted from photovoltage decays. Chem. 2016;1:776–789. [Google Scholar]
- Han G.S., Chung H.S., Kim B.J., Kim D.H., Lee J.W., Swain B.S., Mahmood K., Yoo J.S., Park N.-G., Lee J.H. Retarding charge recombination in perovskite solar cells using ultrathin MgO-coated TiO2 nanoparticulate films. J. Mater. Chem. A. 2015;3:9160–9164. [Google Scholar]
- Haruyama J., Sodeyama K., Han L., Tateyama Y. First-principles study of ion diffusion in perovskite solar cell sensitizers. J. Am. Chem. Soc. 2015;137:10048–10051. doi: 10.1021/jacs.5b03615. [DOI] [PubMed] [Google Scholar]
- Heimel G., Romaner L., Brédas J.L., Zojer E. Interface energetics and level alignment at covalent metal-molecule junctions: π-conjugated thiols on gold. Phys. Rev. Lett. 2006;96:196806. doi: 10.1103/PhysRevLett.96.196806. [DOI] [PubMed] [Google Scholar]
- Heimel G., Romaner L., Zojer E., Bredas J.-L. The interface energetics of self-assembled monolayers on metals. Acc. Chem. Res. 2008;41:721–729. doi: 10.1021/ar700284q. [DOI] [PubMed] [Google Scholar]
- Hinrichs W., Dreijer-van der Glas S. Springer International Publishing; 2015. Physical Chemistry. [Google Scholar]
- Hong L., Paramonov P., Bredas J.L. Theoretical study of the surface modification of indium tin oxide with trifluorophenyl phosphonic acid molecules: impact of coverage density and binding geometry. J. Mater. Chem. 2010;20:2630–2637. [Google Scholar]
- Ishii H., Sugiyama K., Ito E., Seki K. Energy level alignment and interfacial electronic structures at organic/metal and organic/organic interfaces. Adv. Mater. 1999;11:605–625. [Google Scholar]
- Ito S., Tanaka S., Manabe K., Nishino H. Effects of surface blocking layer of Sb2S3 on nanocrystalline TiO2 for CH3NH3PbI3 perovskite solar cells. J. Phys. Chem. C. 2014;118:16995–17000. [Google Scholar]
- Jeon N.J., Noh J.H., Yang W.S., Kim Y.C., Ryu S., Seo J., Seok S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature. 2015;517:476–480. doi: 10.1038/nature14133. [DOI] [PubMed] [Google Scholar]
- Jiang X., Zhang Y., Jing J., Rong Y., Pan C. Characterization of oxygen vacancy associates within hydrogenated TiO2: a positron annihilation study. J. Phys. Chem. C. 2012;116:22619–22624. [Google Scholar]
- Kim S.H., Kim E.M., Lee C.M., Dong W.K., Lim S.T., Sohn M.H., Jeong H.J. Synthesis of PEG-iodine-Capped gold nanoparticles and their Contrast enhancement in in vitro and in vivo for X-ray/CT. J. Nanomater. 2012;2012:344–353. [Google Scholar]
- Kim H., Lim K.G., Lee T.W. Planar heterojunction organometal halide perovskite solar cells: roles of interfacial layers. Energy Environ. Sci. 2016;9:12–30. [Google Scholar]
- Knorr F.J., Mercado C.C., McHale J.L. Trap-state distributions and carrier transport in pure and mixed-phase TiO2: influence of contacting solvent and interphasial electron transfer. J. Phys. Chem. C. 2008;112:12786–12794. [Google Scholar]
- Lee S.-W., Kim S., Bae S., Cho K., Chung T., Hwang J.-K., Song I., Lee W., Park S., Jung J. Enhanced UV stability of perovskite solar cells with a SrO interlayer. Org. Electron. 2018;63:343–348. [Google Scholar]
- Leijtens T., Eperon G.E., Pathak S., Abate A., Lee M.M., Snaith H.J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 2013;4:2885. doi: 10.1038/ncomms3885. [DOI] [PubMed] [Google Scholar]
- Li Y., Cooper J.K., Liu W., Sutter-Fella C.M., Amani M., Beeman J.W., Javey A., Ager J.W., Liu Y., Toma F.M. Defective TiO2 with high photoconductive gain for efficient and stable planar heterojunction perovskite solar cells. Nat. Commun. 2016;7:12446. doi: 10.1038/ncomms12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldoni A., Allieta M., Santangelo S., Marelli M., Fabbri F., Cappelli S., Bianchi C.L., Psaro R., Dal Santo V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012;134:7600–7603. doi: 10.1021/ja3012676. [DOI] [PubMed] [Google Scholar]
- Niu G., Guo X., Wang L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A. 2015;3:8970–8980. [Google Scholar]
- Pan X., Yang M.-Q., Fu X., Zhang N., Xu Y.-J. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale. 2013;5:3601–3614. doi: 10.1039/c3nr00476g. [DOI] [PubMed] [Google Scholar]
- Rasool S., Khan N., Jahankhan M., Kim D.H., Ho T.T., Do L.T., Song C.E., Lee H.K., Lee S.K., Lee J.C. Amine-based interfacial engineering in solution-processed organic and perovskite solar cells. ACS Appl. Mater. Inter. 2019;11:16785–16794. doi: 10.1021/acsami.9b03298. [DOI] [PubMed] [Google Scholar]
- Sandell A., Andersson M.P., Johansson K.J., Karlsson P.G., Uvdal P. Metalorganic chemical vapor deposition of anatase titanium dioxide on Si: modifying the interface by pre-oxidation. Surf. Sci. 2003;530:63–70. [Google Scholar]
- Saracco E., Bouthinon B., Verilhac J.M., Celle C., Chevalier N., Mariolle D., Dhez O., Simonato J.P. Work function tuning for high-performance solution-processed organic photodetectors with inverted structure. Adv. Mater. 2013;25:6534–6538. doi: 10.1002/adma.201302338. [DOI] [PubMed] [Google Scholar]
- Shin S.S., Yeom E.J., Yang W.S., Hur S., Kim M.G., Im J., Seo J., Noh J.H., Seok S.I. Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science. 2017;356:167–171. doi: 10.1126/science.aam6620. [DOI] [PubMed] [Google Scholar]
- Shlenskaya N.N., Belich N.A., Grätzel M., Goodilin E.A., Tarasov A.B. Light-induced reactivity of gold and hybrid perovskite as a new possible degradation mechanism in perovskite solar cells. J. Mater. Chem. A. 2018;6:1780–1786. [Google Scholar]
- Song D., Cui P., Wang T., Wei D., Li M., Cao F., Yue X., Fu P., Li Y., He Y. Managing carrier lifetime and doping property of lead halide perovskite by postannealing processes for highly efficient perovskite solar cells. J. Phys. Chem. C. 2015;119:22812–22819. [Google Scholar]
- Song D., Ji J., Li Y., Li G., Li M., Wang T., Wei D., Cui P., He Y., Mbengue J.M. Degradation of organometallic perovskite solar cells induced by trap states. Appl. Phys. Lett. 2016;108:093901. [Google Scholar]
- Tsai H., Asadpour R., Blancon J.-C., Stoumpos C.C., Durand O., Strzalka J.W., Chen B., Verduzco R., Ajayan P.M., Tretiak S. Light-induced lattice expansion leads to high-efficiency perovskite solar cells. Science. 2018;360:67–70. doi: 10.1126/science.aap8671. [DOI] [PubMed] [Google Scholar]
- Wan F., Qiu X., Chen H., Liu Y., Xie H., Shi J., Huang H., Yuan Y., Gao Y., Zhou C. Accelerated electron extraction and improved UV stability of TiO2 based perovskite solar cells by SnO2 based surface passivation. Org. Electron. 2018;59:184–189. [Google Scholar]
- Wang J., Tafen D.N., Lewis J.P., Hong Z., Manivannan A., Zhi M., Li M., Wu N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009;131:12290–12297. doi: 10.1021/ja903781h. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhou H., Hu J., Huang B., Sun M., Dong B., Zheng G., Huang Y., Chen Y., Li L. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science. 2019;363:265–270. doi: 10.1126/science.aau5701. [DOI] [PubMed] [Google Scholar]
- Wei J., Li H., Zhao Y., Zhou W., Fu R., Pan H., Zhao Q. Flexible perovskite solar cells based on the metal-cinsulator-semiconductor structure. Chem. Commun. 2016;52:10791–10794. doi: 10.1039/c6cc04840d. [DOI] [PubMed] [Google Scholar]
- Wei J., Guo F., Liu B., Sun X., Wang X., Yang Z., Xu K., Lei M., Zhao Y., Xu D. UV-inert ZnTiO3 electron selective layer for photostable perovskite solar cells. Adv. Energy Mater. 2019;9:1901620. [Google Scholar]
- Wei D., Huang H., Cui P., Ji J., Dou S., Jia E., Sajid S., Cui M., Chu L., Li Y. Moisture-tolerant supermolecule for the stability enhancement of organic-inorganic perovskite solar cells in ambient air. Nanoscale. 2019;11:1228–1235. doi: 10.1039/c8nr07638c. [DOI] [PubMed] [Google Scholar]
- Wetzelaer G.J.A.H., Scheepers M., Sempere A.M., Momblona C., Ávila J., Bolink H.J. Trap-assisted non-radiative recombination in organic-inorganic perovskite solar cells. Adv. Mater. 2015;27:1837–1841. doi: 10.1002/adma.201405372. [DOI] [PubMed] [Google Scholar]
- Xiong L.-B., Li J.-L., Yang B., Yu Y.J. Ti3+ in the surface of titanium dioxide: generation, properties and photocatalytic application. Nanomater. 2012;9:831524. [Google Scholar]
- Yang S., Chen S., Mosconi E., Fang Y., Xiao X., Wang C., Zhou Y., Yu Z., Zhao J., Gao Y. Stabilizing halide perovskite surfaces for solar cell operation with wide-bandgap lead oxysalts. Science. 2019;365:473–478. doi: 10.1126/science.aax3294. [DOI] [PubMed] [Google Scholar]
- Yu Y., Wu K., Wang D. Dye-sensitized solar cells with modified TiO2 surface chemical states: the role of Ti3+ Appl. Phys. Lett. 2011;99:192104. [Google Scholar]
- Zhang X., Tian H., Wang X., Xue G., Tian Z., Zhang J., Yuan S., Yu T., Zou Z. The role of oxygen vacancy-Ti3+ states on TiO2 nanotubes' surface in dye-sensitized solar cells. Mater. Lett. 2013;100:51–53. [Google Scholar]
- Zhang B., Song Z., Jin J., Bi W., Li H., Chen C., Dai Q., Xu L., Song H. Efficient rare earth Co-doped TiO2 electron transport layer for high-performance perovskite solar cells. J. Colloid Interf. Sci. 2019;533:14–21. doi: 10.1016/j.jcis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Fuentes-Hernandez C., Shim J., Meyer J., Giordano A.J., Li H., Winget P., Papadopoulos T., Cheun H., Kim J. A universal method to produce low-work function electrodes for organic electronics. Science. 2012;336:327–332. doi: 10.1126/science.1218829. [DOI] [PubMed] [Google Scholar]
- Zhou H., Chen Q., Li G., Luo S., Song T.-b., Duan H.-S., Hong Z., You J., Liu Y., Yang Y. Interface engineering of highly efficient perovskite solar cells. Science. 2014;345:542–546. doi: 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental Information. Additional data related to this paper may be requested from M.L. (mcli@ncepu.edu.cn).