Abstract
Extranodal nasal natural killer (NK)/T cell lymphoma (ENKTCL) is a rare but highly aggressive subtype of non-Hodgkin lymphoma (NHL). Nevertheless, despite extensive research, the estimated 5-year overall survival of affected patients remains low. Therefore, new treatment strategies are needed urgently. Recent advances in immunotherapy have the potential to broaden the applications of chimeric antigen receptor-modified T (CAR-T) cells and the bispecific T-cell engaging (BiTE) antibody. Here, we screened a panel of biomarkers including the B7-H3, CD70, TIM-3, VISTA, ICAM-1, and PD-1 in NKTCL cell lines. As a result, we found for the first time that B7-H3 was highly and homogeneously expressed in these cells. Consequently, we constructed a novel anti-B7-H3/CD3 BiTE antibody and B7-H3-redirected CAR-T cells, and evaluated their efficacy against NKTCL cel lines both in vitro and in vivo. Notably, we found that both anti-B7-H3/CD3 BiTE and B7-H3-redirected CAR-T cells effectively targeted and killed NKTCL cells in vitro, and suppressed the growth of NKTCL tumors in NSG mouse models. Thus, B7-H3 might be a promising therapeutic target for treating patients with NKTCL tumors.
Introduction
Extranodal nasal natural killer (NK)/T cell lymphoma (ENKTCL) is a rare but highly aggressive subtype of non-Hodgkin lymphoma (NHL), with up to 10% of NHLs occurring in East Asia and South America [1]. It is characterized by its immunophenotypic expression of cytoplasmic CD3ε+, CD56+, and cytotoxic molecules, with no surface CD3 or T-cell receptor expressions [1,2]. The Epstein–Barr virus (EBV) is thought to play an essential role in its pathogenesis and regional differences, and its expression is a key for diagnosis [1,3]. Multitherapy including chemotherapy and local radiotherapy is used concurrently or sequentially for NKTCL treatment. Even so, the complete response rates are only 5% to 60% [4], and the estimated 5-year overall survival remains low. Therefore, novel therapies are urgently needed for patients with NKTCL.
Immunotherapy, representing a shift from the traditional approach to treat cancers, promises to meet the need to improve clinical outcomes for patients with ENKTCL, because immune suppression is tightly related to the occurrence, progression, and poor prognosis of such tumors [5,6]. Currently, there are various forms of cancer immunotherapy. Of these, redirecting T cells to target malignancies is an attractive strategy including antitumor agents designed to directly activate T cells against tumors, such as chimeric antigen receptor-modified T (CAR-T) cells and the bispecific T-cell engaging (BiTE) antibody. Both CAR-T cells and BiTE can target tumors without depending on the cytotoxic mechanisms of conventional therapies and can target tumor-specific antigens and active T cells simultaneously [7,8]. Nevertheless, clinical development of redirecting T cells therapy for solid tumors is still hindered by the lack of an appropriate antigen that has high tumor specificity [9].
Until now, various surface markers of NKTCL including CD30, CD38, PDL1, and EBV antigens (LMP1 and LMP2) have been used for immunotherapy [3]. Antibody drugs targeting ENKTCL cellular membrane proteins such as brentuximab [10], daratumumab [11], pembrolizumab, and nivolumab [12] have shown clinical benefits in patients with relapsed/refractory ENKTCL. Although some of the trials have shown responses, the overall outcomes are not impressive compared with other forms of immunotherapy used in treating hematological malignancies. One reason lies in heterogeneous antigen expression with regard to distribution and intensity [13]. Therefore, for both BiTE and CAR-T cell therapies, to avoid on-target/off-tumor toxicity and tumor escape, the ideal target antigens should be highly and homogeneously expressed by all tumor cells but not healthy tissues [8].
To develop a more potent therapy for patients with ENKTCL, we screened a panel of biomarkers including the B7-H3, CD70, TIM-3, VISTA, ICAM-1, and PD-1 in NKTCL cell lines. Notably, we found that B7-H3 was highly and homogeneously expressed. Then we constructed a novel anti-B7-H3/CD3 BiTE antibody and B7-H3-redirected CAR-T cells, and evaluated their efficacy in vitro and in vivo. Our results demonstrated that the B7-H3 molecule might be a promising therapeutic target for treating patients with NKTCL tumors.
Methods
Cell Lines and Mice
The NKTCL cell line (SNK-6) [14] was established from primary lesions of patients with NK/T cell lymphomas and cultured in RPMI 1640 medium (GIBCO, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum, 1.0 mmol/L penicillin–streptomycin combination (Hyclone, South Logan, UT, USA) and 700 U/mL recombinant human IL-2. Raji human Burkitt's lymphoma cells (purchased from the American Type Culture Collection, ATCC) were cultured in RPMI 1640 medium (GIBCO) containing 10% fetal bovine serum and 1.0 mmol/L penicillin–streptomycin combination (Hyclone). HEK293T cells (purchased from the ATCC) were maintained in DMEM (GIBCO) supplemented with 10% fetal bovine serum and 1.0 mmol/L penicillin–streptomycin combination (Hyclone). All cell lines were incubated in a humidified atmosphere at 37°C containing 5% CO2. To evaluate the efficacy of cell treatments, we generated SNK-6 cells stably expressing firefly luciferase (SNK-6- FFLuc). Six- to eight-week-old female NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were purchased from the Model Animal Resource Information Platform of Nanjing University, P. R. China. Mice were housed and handled in strict accordance with the requirements of the National Institutes of Health and our Institutional Animal Care and Use Committee. Ethical approval was given by the West China Hospital of Sichuan University Laboratory Animal Ethics Committee (approval number 2018212A).
Construction of B7-H3/CD3 BiTE
The anti-B7-H3 single-chain variable fragment (scFv) sequence used in BiTE and CAR vectors was derived from a highly specific monoclonal antibody (mAb) against B7-H3 (clone mAb-J42) generated by our group using a standard hybridoma technique. cDNA encoding the B7-H3-specific scFv (J42-scFv) and CD3-specific scFv (synthesized by Genewiz (https://www.genewiz.com) according to published amino acid sequences) were linked by a G4S linker to construct a recombinant single-chain BiTE. The recombinant cDNA was subcloned into a eukaryotic expression vector with a His tag at the C-terminal to facilitate protein purification. HEK293T cells were transduced with the expression vectors described above and cultured in FreeStyle serum-free medium (Thermo Fisher Scientific, Waltham, MA, USA). The supernatant was collected twice over 1 week, and the recombinant B7-H3/CD3 BiTE was purified on Ni-NTA affinity columns and subsequently subjected to size exclusion chromatography.
Production of B7-H3-Redirected CAR-T Cells
The anti-B7-H3 CAR consisting of a CD8α signal peptide, anti-B7-H3 scFv, a hinge region, a CD8 transmembrane domain, the 4-1BB and CD3ζ cytoplasmic domains, a P2A and an mCherry red fluorescent protein-encoding region were synthesized by a vendor (Genecreate company, Wuhan, China) as described previously [15]. The B7-H3-CAR and vehicle lentiviral vectors were produced using HEK293T cells. In brief, cells were plated 24 h before transfection, then cotransfected with lentiviral constructs (B7-H3-CAR or vehicle-treated control vectors) and packaging plasmids (psPAX2 and pMD2.G vectors) using polyetherimide. Supernatants were harvested at 48 h and 72 h after transfection and then further concentrated 50-fold by centrifugation at 15,000 rpm for 2 h at 4°C. The pellet was resuspended in serum-free RPMI medium and stored at −80°C immediately.
Normal human donor peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation and were further activated by 200 ng/mL of anti-CD3 mAb (BioLegend, San Diego, CA, USA; OKT3), 100 ng/mL CD28 mAb (BioLegend; CD28.2), and 100 U/mL recombinant human interleukin (IL)-2 (Life Sciences) at 37°C in a humidified atmosphere with 5% CO2 for 48 h. Then, activated T lymphocytes were transduced with lentivirus supernatants (multiplicity of infection, MOI = 3–10) to express anti-B7-H3 CAR in the presence of IL2. After 12 h, T cells were cultured in X-VIVO Medium (Lonza) in the presence of 100 U/mL IL2, 10 ng/mL IL7 (PeproTech), and 5 ng/mL IL15 (PeproTech). Vehicle-treated control T cells and nontransduced T (NT) cells were established using the same conditions.
Immunofluorescence Staining Analysis
Cells were incubated in 24-well plates with coverslips at 37°C for 24 h. Specimens were stained with the primary antibody for 60 min at 4°C and then stained with an FITC-conjugated secondary antibody (Proteintech, Rosemont, IL, USA) and DAPI (Beyotime Institute of Biotechnology, Shanghai, P. R. China). The primary mAbs used for staining included those for B7-H3 (Cell Signaling Technology, CST, Danvers, MA, USA; D9M2L), CD70 (Abcam, Cambridge, MA, USA; ab175389), TIM-3 (Abcam; ab47997), VISTA (CST; D5L5T), B7-H3 mAb (J42), and J42-scFv-Fc. The images were captured using confocal microscopy. For RNA-seq and survival analysis, the data were downloaded from were downloaded from the Gene Expression Profiling Interactive Analysis (GE-PIA) (http://gepia.cancer-pku.cn/), which has access to The Cancer Genome Atlas (TCGA).
Flow Cytometry
Cell surface expression levels of B7-H3, CD70, TIM-3, VISTA, ICAM-1, and PD-1 were analyzed by flow cytometry. Briefly, cells were washed in ice-cold phosphate-buffered saline (PBS), and collected by centrifugation. The cells were incubated with mAbs specific to human B7-H3, CD70, TIM-3, PD-1, ICAM-1 (BioLegend, San Diego, CA, USA; DCN.70, 113–16, F38-2E2, EH12.2H7, and HA58 respectively) and VISTA (BD Biosciences, Franklin Lakes, NJ, USA; MIH65) for 30 min in the dark on ice. After washing twice with ice-cold flow cytometry buffer, the cells were resuspended in 300 μL of the same buffer and analyzed using a Fortessa flow cytometer (BD Biosciences) according to the manufacturer's protocols. Anti-human CD4 and CD8 mAbs (BD Biosciences; RPA-T4 and RPA-T8), CD3, CD45RO, CCR7, CD25, CD69, TIM-3, and PD-1 (BioLegend; HIT3a, UCHL1, G043H7, 3C7, FN50, F38-2E2, and EH12.2H7) were used for T cell phenotype and coculture analyses. For each sample, at least 20,000 events were acquired and analyzed using FlowJo software (v. 10.6.0; https://www.flowjo.com).
Cytotoxicity Assays
Cytotoxicity of B7-H3-redirected CAR-T cells and B7-H3/CD3 BiTE mAb was analyzed using a 51Cr assay as described [16]. To evaluate the effect of B7-H3-redirected CAR-T cells, SNK-6 cells (1 × 105/mL) labeled with sodium chromate (Na251CrO4) were incubated with effector cells (CAR-T cells and vehicle-treated control T cells) at effector-to-target (E/T) ratios of 16:1, 8:1, 4:1, and 1:1 for 4 h. In addition, B7-H3-negative Raji cells were incubated with effector cells (CAR-T cells and vehicle-treated control T cells) at the different E/T ratios outlined above for 4 h. For B7-H3/CD3 BiTE, 1 × 105 cells/mL of tumor cells (SNK-6 and Raji) were preincubated with serially purified B7-H3/CD3 BiTE at different concentrations or in PBS for 1 h at 37°C. PBMCs from healthy donors were added at different E/T ratios and incubated for 4 h at 37°C in 5% CO2. The radioactivity of the supernatants was measured using a gamma counter. The percentage of specific lysis was calculated by the following formula: (test release – spontaneous release) / (maximal release – spontaneous release) × 100.
T Cell Function Assays
SNK-6 cells and Raji cells were seeded in 96-well plates at a concentration of 1 × 104 cells/well. CAR-T cells were added to the culture at an E/T ratio of 4:1 without the addition of exogenous cytokines. After 12 h, the supernatant was collected and cytokines (interferon gamma, IFN-γ, IL-2, and tumor necrosis factor alpha, TNF-α) were measured using specific enzyme-linked immunosorbent assay (ELISA) kits (BioLegend), respectively. For coculture assay, 1 × 105 tumor cells (SNK-6 and Raji) were cocultured with 4 × 105 NT cells, B7-H3-redirected CAR-T cells, or vehicle-treated control T cells in 24-well plates at 37°C without the addition of exogenous cytokines. Cells were harvested for flow cytometry analysis 24 h later, and residual tumor cells were counted.
Cell-Derived Xenograft Model in Mice
Six- to eight-week-old female NSG mice were injected subcutaneously into the right flank 10 days in advance with 2 × 106 SNK-6- FFLuc cells. The mice were divided into four groups randomly treated with PBS, vehicle control, 1 × 107 B7-H3 CAR-T cells, or B7-H3/CD3 BiTE (2 mg/kg, every 3–4 days) intravenously (via the tail vein) at indicated time points. One hour before BiTE was injected, mice were injected intravenously with 1 × 107 T cells. For antitumor efficacy analyses, tumor progression was monitored by whole-body imaging using an IVIS system (Caliper Life Sciences, Hopkinton, MA, USA) every 3 days beginning on day 0. Animals were euthanized when the tumor volume exceeded 1800 mm3.
Immunohistochemistry (IHC)
Tumor tissues were analyzed for B7-H3 expression. All samples were fixed in 10% formalin and embedded in paraffin wax for staining with a commercial anti-B7-H3 rabbit mAb (CST; 1:200). In brief, tissue sections were incubated at 65°C for 1 h and blocked with PBS containing 10% normal goat serum (Boster, Wuhan, P. R. China) for 30 min at room temperature, followed by incubation with a respective primary antibody at 4°C overnight. Bound primary antibodies were incubated with goat anti-rabbit secondary antibodies, followed by DAB detection (ZSGB-BIO, Beijing, P. R. China).
Statistical Analysis
In vitro experiments were repeated at least three times. All statistical analyses were performed using GraphPad Prism (version 8.02; http://www.graphpad.com). Data are presented as the mean ± standard deviation (SD) with statistically significant differences determined by tests as indicated in the figure legends; P values <.05 were considered statistically significant.
Results
Surface Expression of Diverse Molecules on SNK-6 Cells
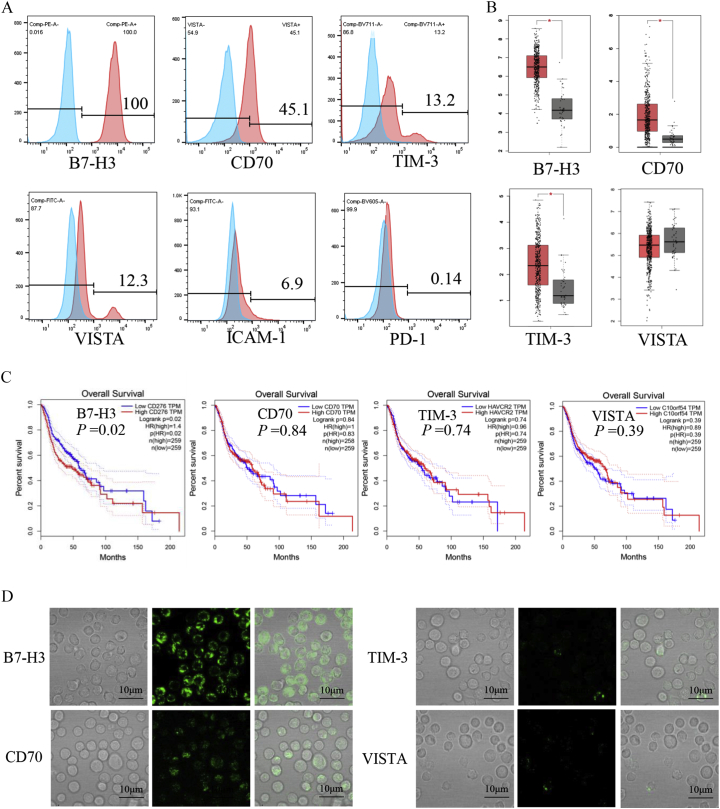
The expression levels of B7-H3, CD70, TIM-3, VISTA, ICAM-1, and PD-1 in SNK-6 cells were analyzed by flow cytometry using fluorescence-activated cell sorting (FACS). This showed that SNK-6 cells had high surface expression levels of B7-H3, while CD70, TIM-3, and VISTA were expressed at lower levels (Figure 1A). Because there are insufficient clinical data on patients with NKTCL in the Cancer Genome Atlas database (TCGA; http://cancergenome.nih.gov), we analyzed the mRNA levels of B7-H3, CD70, TIM-3, and VISTA in head and neck carcinomas based on data from the TCGA (Figure 1B). The expression levels of mRNAs for B7-H3, CD70, and TIM-3 were significantly higher in head and neck cancers compared with normal tissues. More importantly, the higher expression of B7-H3 in tumors correlated with worse overall survival in patients (P = .02) (Figure 1C). In addition, we further confirmed the expression levels of B7-H3, CD70, TIM-3, and VISTA on SNK-6 cells by immunofluorescence (Figure 1D). Together, these results indicate that the B7-H3 protein marker might serve as a valuable clinical target for the treatment of patients with ENKTCL.
Figure 1.
Surface expression of diverse molecules. (A) Cell-surface expression of B7-H, CD70, TIM-3, VISTA, ICAM-1, and PD-1 on human NKTCL cell lines (SNK-6) was evaluated by FACS analysis. (B) The mRNA expression levels of B7-H3, CD70, TIM-3, and VISTA in tumors and normal tissues were analyzed using the TCGA database. (D) Survival analysis of patients with head and neck cancers taken from the TCGA database. (E) Immunofluorescence staining indicates the surface expression of B7-H3, CD70, TIM-3, and VISTA (green) on SNK-6 cells, Scale bars = 10 μm.
Construction of B7-H3/CD3 BiTE and B7-H3-Redirected CAR-T Cells
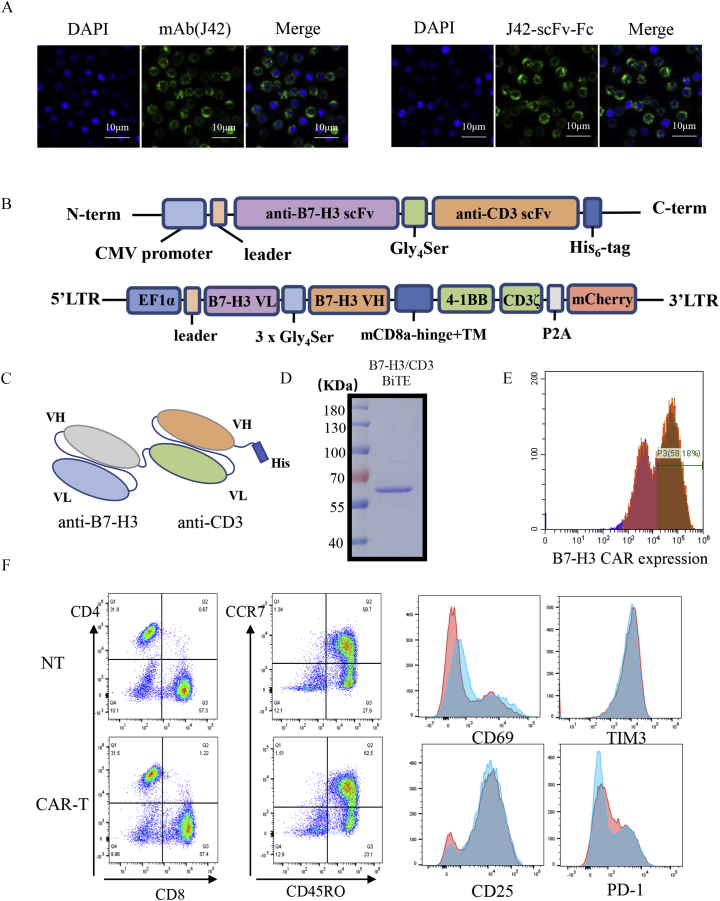
The binding properties of the J42 mAb and its derived B7-H3-scFv-Fc to SNK-6 cells were confirmed by immunofluorescence (Figure 2A). B7-H3/CD3 BiTE was engineered by combining a B7-H3 scFv derived from the mAb-J42 clone with a CD3 scFv. Each scFv contained a corresponding light chain (VL) and heavy chain (VH) joined together by a 5-amino-acid (G4S) linker (Figure 2, B and C). Figure 2D shows the SDS–PAGE analysis of the purified B7-H3 BiTE. For the B7-H3-redirected CAR-T cells, schematic diagrams of the construction of B7-H3 CAR are shown in Figure 2B. The transduction efficiency of CAR-T cells was confirmed using flow cytometry by detection of the coexpressed mCherry red fluorescent protein (PE Texas-Red in Figure 2E). The results demonstrated a 40–60% CAR expression compared with NT cells. Ten days after transduction, analysis of the phenotype of B7-H3 CAR-T cells using flow cytometry showed that they were 57–61% CD8+ T cells and 28–32% CD4+ T cells (the ratio of CD4+/CD8+ T cells was about 1:2), without significant differences in CD45RO, CCR7, CD25, CD69, PD-1, or TIM-3 between CAR-T cells and NT cell groups (Figure 2F).
Figure 2.
Construction of B7-H3/CD3 BiTE and B7-H3-redirected CAR-T cells. (A) The binding properties of mAb (J42) and B7-H3 scFv to SNK-6 cells were confirmed by immunofluorescence, Scale bars = 10 μm. (B) Schematic representation of B7-H3/CD3 BiTE and B7-H3-CAR constructs. (C) Schematic representation of B7-H3/CD3 BiTE. (D) SDS–PAGE results for purified B7-H3/CD3 BiTE. (E) The B7-H3-CAR expression on human T cells was analyzed by detecting mCherry red fluorescence expression with flow cytometry. (F) Ten days after transduction, the subsets and phenotypes of nontransduced T cells (NT) and CAR-T cells were analyzed using flow cytometry.
T Cell-Mediated Cytotoxicity Induced by B7-H3-Redirected BiTE and CAR-T Cells In Vitro
The activities of B7-H3/CD3 BiTE and B7-H3 CAR-T cells were evaluated using a coculture assay. Dose-dependent specific lysis was observed in SNK-6 cells in the presence of B7-H3/CD3 BiTE, and the IC50 of B7-H3/CD3 BiTE on SNK-6 cells was 77.96 ng/mL (Figure 3A). In the Cr51 release cytotoxic assay, the results of the specific antitumor effect of B7-H3/CD3 BiTE and B7-H3 CAR-T cells under different E/T ratios are shown in Figure 3B. No cytotoxicity was observed for B7-H3-negative Raji cells, even in the presence of T cells. Moreover, the vehicle-transfected control T cells did not mediate killing. After coculturing, the residual cells were analyzed by FACS, and the results are shown in Figure 3C with representative flow cytometry plots and the statistics for residual tumor cells are displayed in Figure 3D. As shown, the B7-H3 CAR-T cells and the B7-H3/CD3 BiTE could significantly decrease the viability of SNK-6 cells, but no significant difference was observed between BiTE and CAR-T treatment groups. In addition, the B7-H3-redirected T cells secreted >10-fold more IFN-γ, 14-fold more IL-2, and approximately 20-fold more TNF-α compared with vehicle-transfected control T cells (all P ≤ .05). Similarly, the B7-H3/CD3 BiTE cells significantly increased the levels of cytokines compared with the PBS-treated control cells (Figure 3E). These data suggest that the cytolytic activity of B7-H3 CAR-T cells and B7-H3/CD3 BiTE was accompanied by cytokine release consistent with T cell-induced cytotoxicity, but not in Raji cells (Supplementary Figure S1).
Figure 3.
T cell-mediated cytotoxicity induced by B7-H3-redirected BiTE and CAR-T cells in vitro. (A) Cell growth inhibition curves for SNK-6 cell lines with different concentrations of B7-H3/CD3 BiTE. The IC50 values are shown on the curve. (B) 51Cr-release assays of B7-H3/CD3 BiTE and B7-H3 CAR-T cells against SNK-6 and Raji cell lines at different E/T ratios. (C) Representative flow cytometry plots of SNK-6 and Raji cell lines after 24 h coculture with PBS, B7-H3/CD3 BiTE, vehicle control T cells, or CAR-T cells at an E/T ratio of 4:1. (D) Survival rates of residual tumor cells. (E) The secretion rates of IFN-γ, IL2, and TNF-α were measured using ELISA kits. Each experiment was repeated at least three times with similar results. For statistical analysis, unpaired two-tailed Student's t tests were applied. *P < .05, **P < .01, ***P < .001.
Supplementary Figure S1.

T cell-mediated cytotoxicity induced by B7-H3-redirected BiTE and CAR-T cells in vitro. The secretion rates of IFN-γ, IL2, and TNF-α were measured using ELISA kits.
Antitumor Effect of B7-H3-Redirected BiTE and CAR-T Cells In Vivo
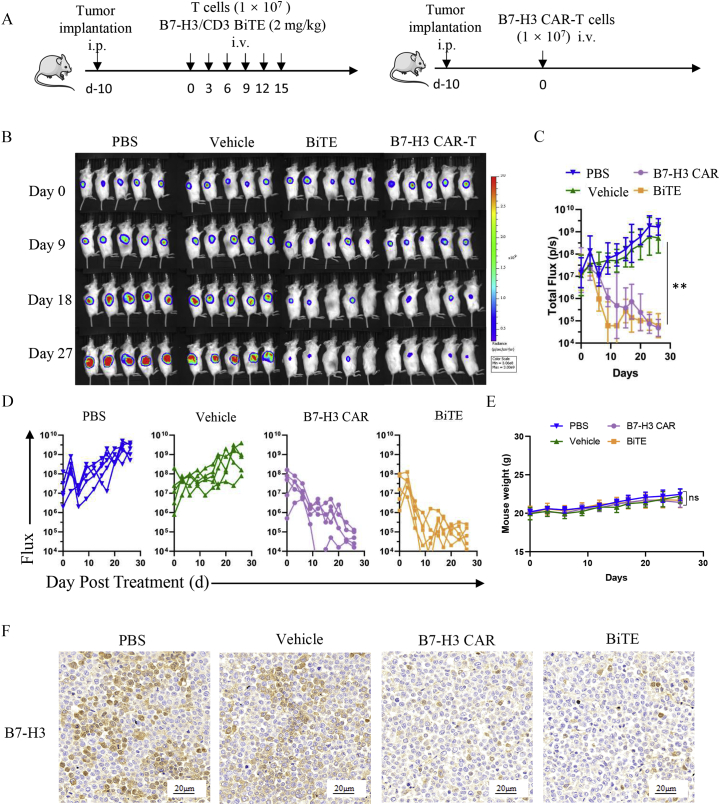
The potent in vitro cytotoxicity of B7-H3/CD3 BiTE and B7-H3-redirected CAR-T cells prompted us to assess the antitumor killing efficacy of these two potential immunotherapy agents in vivo. The schema is presented in Figure 4A. A bioluminescence analysis of mixed tumor growth over time is displayed in Figure 4B. Tumor total or individual flux was calculated using Living Image software (https://www.perkinelmer.com; Figure 4, C and D). As shown, significant antitumor activity was observed in both the B7-H3/CD3 BiTE cells and the B7-H3 CAR-T cells (P ≤ .01). Conversely, mice treated with PBS, or vehicle alone demonstrated progressive tumor burdens compared with the two B7-H3-targeted treatment groups. Of interest, B7-H3 BiTE treatment was found to mediate a rapid reduction in tumor burden on days 6 and 9 compared with the B7-H3 CAR-T group (P ≤ .05; Figure 4C). Compared with the control groups, no obvious weight change was observed for both the B7-H3-redirected BiTE and CAR-T treatment groups (P > 05; Figure 4E). IHC demonstrated obvious decreases in B7-H3 expression in both the B7-H3-redirected BiTE and CAR-T treatment groups (Figure 4F).
Figure 4.
Antitumor effect of B7-H3-redirected BiTE and CAR-T cells in vivo. (A) The treatment scheme of SNK-6-FFluc NSG mouse models. (B) Bioluminescence analysis of mixed tumor growth over time; n = 5. (C, D) Tumor total or individual flux data (in p/s) were calculated using Living Image software. Tumor growth rates are shown as mean values (unpaired two-tailed Student's t tests, **P < .01). (E) Mouse body weight curves. (unpaired two-tailed Student's t tests, P > .05). (F) Representative images of B7-H3 IHC staining in tumor sections after treatment. Scale bars = 20 μm. ns: Nonsignificant.
Discussion
Here, we screened a panel of biomarkers including B7-H3, CD70, TIM-3, VISTA, ICAM-1, and PD-1. We found that B7-H3 was highly and homogeneously expressed in NKTCL cell lines. We further described the construction and functional assessment of anti-B7-H3/CD3 BiTE and B7-H3 CAR-T cells. Both of these immunotherapy agents could suppress the proliferation and viability of ENKTCL cells in vitro and in vivo, highlighting B7-H3 as a potentially promising target for NKTCL immunotherapy.
The B7-H3 protein has now been identified as broadly overexpressed in multiple tumor cells, tumor-associated stromal tissues, angiogenic tumor vasculature, tumor-infiltrating dendritic cells, and macrophages [[17], [18], [19]], but has limited expression in normal tissues [20,21]. The function of B7-H3 is still controversial, in part because the molecular mechanisms, the signaling pathways, and the receptor for B7-H3 remain largely unknown. Originally, B7-H3 was reported as a positive costimulator of T cells in humans [22]. However, growing evidence has indicated that B7-H3 also serves as a potent negative regulator, which decreases type I IFN and IL-2 release by T cells [18]. Moreover, it was shown that the expression of B7-H3 in tumor cells was correlated with poorer patient prognosis, shorter survival, and higher recurrence rates [18,23]. All these characteristics make B7-H3 a potential clinical target for immunotherapies. Consequently, several B7-H3-specific mAbs and antibody–drug conjugates have shown potent and specific antitumor activity in early phase clinical trials, including some solid tumors [24,25]. For example, an antibody targeting B7-H3 (8H9) has been evaluated in clinical trials for pediatric patients with central nervous system (CNS) malignancies [26], and a B7-H3/CD3 bispecific molecule (MGD009) is in clinical trials for solid tumors expressing B7-H3 (NCT02628535) [17,27]. Moreover, several clinical trials involving B7-H3-redirected CAR-T cells have been registered at ClinicalTrials.gov. Of these, most have focused on recurrent or refractory central nervous system tumors (NCT04077866, NCT04185038). In the present study, we generated both BiTE and CAR-T cells based on a novel B7-H3-targeted scFv derived from the J42 monoclonal antibody and for the first time validated their antitumor effects on NKTCL cells. For the target-binding scFv of CAR-T cells, different antigen binding epitopes or affinity may have different antitumor effects [28]. Therefore, further studies are necessary to compare the antitumor effects of our J42-based CAR with other reported B7-H3-targeted CAR.
As outlined above, B7-H3 might serve as a potent and viable target for tumor immunotherapy. Nevertheless, its role in immunotherapy for patients with NKTCL is unknown. Here, we tested B7-H3-redirected BiTE and CAR-T approaches against NKTCL cells. Both B7-H3 CAR-T cells and anti-B7-H3 BiTE therapies showed promising results for the treatment of NKTCL compared with the PBS- and vehicle-treated control groups. The antitumor therapeutic potential of B7-H3-redirected BiTE and CAR-T cells was supported by evidence of tumor cell killing, cytokine production, and suppression of in vivo tumor burden in a mouse model. Of note, there were differences between the B7-H3 CAR-T and anti-B7-H3 BiTE treatment groups in terms of drug administration. As shown above, 9 days after the mice received different treatments, the total flux in the BiTE group was significantly lower than in the B7-H3 CAR-T group (P < .05), which indicated that BiTE cells might mediate rapid reductions in tumor burden compared with CAR-T cells. As previously reported, in part this is because the BiTE approach is less reliant on T cell expansion, while CAR-T cells need to undergo expansion in vivo to achieve sustained function [29]. In this study, the mice received six doses of BiTE compared with one dose of CAR-T cells. One key reason for the requirement of continuous administration of BiTE cells is their short half-life in serum [30]. To overcome these limitations, several methods including diabodies, bispecific immunoglobulins, and conjugates have been developed to increase the circulation time. Thus, CAR-T cells and BiTEs have been combined into a single platform for tumor immunotherapy. For example, Choi et al. constructed enhanced green fluorescence (EGFR)-specific BiTE-secreting CAR-T cells, and demonstrated that such cells could display potent killing activity against multiple tumors [31]. However, the strategies outlined above highlight the vast expanse of additional studies that are yet to be explored.
Not all the NSG mice showed tumor regression in our experiments, in part because of the antigen loss after B7-H3 BiTE and CAR-T treatments as shown by IHC, which is considered to be the main cause of tumor escape and treatment failure [13]. Diminished presentation of targeted antigens after T cell therapy has been widely reported in previous trials [32]. Strategies, such as targeting multiple specific tumor antigens or using combination therapies, have been developed to enhance treatment efficiency. For example, several preclinical studies have reported that bi- or trispecific CAR-T cells are highly effective in preventing tumor escape [33,34].
However, there were some limitations in this study. Because chemotherapy and local radiotherapy are the first-line therapies for patients with NKTCL, it is difficult to collect tumor specimens. Consequently, dedicated in vivo imaging strategies are urgently needed for identifying cancerous lesions by targeting B7-H3 on tumor cells. For example, the anti-B7-H3 mAb DS-5573a labeled with zirconium-89 (89Zr-DS-5573a) demonstrates specific and prolonged targeting of B7-H3-expressing tumors in vivo [35]. Furthermore, spectroscopic photoacoustic molecular imaging and contrast-enhanced ultrasound imaging have high potential for the identification of exogenous contrast agents targeted to specific markers [36,37]. Therefore, further study is needed to analyze the feasibility of clinical in vivo imaging for B7-H3 detection in patients with NKTCL.
Conclusions
We found that B7-H3 was highly expressed in an NKTCL cell line. Both of the anti-B7-H3 BiTE antibody and B7-H3-redirected CAR-T cells showed significant cytotoxic effects in vitro and obviously induced tumor regression in vivo. Our results indicate that B7-H3-redirected CAR-T cells and BiTE might provide new and viable therapeutic options for patients with NKTCL.
The following are the supplementary data related to this article.
Declaration of Competing Interests
A.T., Z.Z., G.G. and H.Y. have filed patents related to this work.. The other authors declare no competing interests.
Ethics Approval
This study was reviewed and approved by the Ethics Board at Sichuan University (approval number 2018212A).
Consent for Publication
All authors give consent for publication.
Acknowledgments
Acknowledgments
This work was supported by two grants from the Science and Technology Department of Sichuan Province, P. R. China (2017SZ0015 & 2019YFS0108).
Author Contributions
MJ. Z., and LY. Y. carried out the experiments, analyzed the data, and drafted the manuscript. H. Y. and AP. T. conceived and designed the experiments. JJ. H., HY. W., and D. L. participated in experiments, supported the study, and edited the manuscript. X.T, JH.H., ZL.Z., KH.Z. and Z. W. participated in IHC staining and FACS. YS. L., SX. L. and G. G. provided feedback and edited the manuscript.
Contributor Information
Aiping Tong, Email: aipingtong@scu.edu.cn.
Hui Yang, Email: yh8806@163.com.
References
- 1.Haverkos B.M., Pan Z., Gru A.A., Freud A.G., Rabinovitch R., Xu-Welliver M., Otto B., Barrionuevo C., Baiocchi R.A., Rochford R., Porcu P. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European Cases. Curr Hematol Malig Rep. 2016;11(6):514–527. doi: 10.1007/s11899-016-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tse E., Kwong Y.L. The diagnosis and management of NK/T-cell lymphomas. J. Hematol. Oncol. 2017;10(1):85. doi: 10.1186/s13045-017-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B., Oki Y. Novel immunotherapy options for extranodal NK/T-Cell Lymphoma. Front. Oncol. 2018;8:139. doi: 10.3389/fonc.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaccard A., Gachard N., Marin B., Rogez S., Audrain M., Suarez F., Tilly H., Morschhauser F., Thieblemont C., Ysebaert L., Devidas A., Petit B., de Leval L., Gaulard P., Feuillard J., Bordessoule D., Hermine O. Gela; Intergroup, G., Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 5.Buatois V., Johnson Z., Salgado-Pires S., Papaioannou A., Hatterer E., Chauchet X., Richard F., Barba L., Daubeuf B., Cons L., Broyer L., D'Asaro M., Matthes T., LeGallou S., Fest T., Tarte K., Clarke Hinojosa R.K., Genesca Ferrer E., Ribera J.M., Dey A., Bailey K., Fielding A.K., Eissenberg L., Ritchey J., Rettig M., DiPersio J.F., Kosco-Vilbois M.H., Masternak K., Fischer N., Shang L., Ferlin W.G. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018;17(8):1739–1751. doi: 10.1158/1535-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandya P.H., Murray M.E., Pollok K.E., Renbarger J.L. The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res. 2016:1–13. doi: 10.1155/2016/4273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madduri D., Dhodapkar M.V., Lonial S., Jagannath S., Cho H.J. SOHO state of the art updates and next questions: T-cell-directed immune therapies for multiple myeloma: chimeric antigen receptor-modified t cells and bispecific t-cell-engaging agents. Clin Lymphoma Myeloma Leuk. 2019;19(9):537–544. doi: 10.1016/j.clml.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Velasquez M.P., Bonifant C.L., Gottschalk S.J.B. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2017 doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An anti-glypican 3/CD3 bispecific T cell–redirecting antibody for treatment of solid tumors. Science Translational Medicine. 2017;9(410) doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 10.Poon L.M., Kwong Y.L. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann. Hematol. 2016;95(5):847–849. doi: 10.1007/s00277-016-2627-9. [DOI] [PubMed] [Google Scholar]
- 11.Hari P., Raj R.V., Olteanu H. Targeting CD38 in refractory extranodal natural killer cell-T-cell lymphoma. N. Engl. J. Med. 2016;375(15):1501–1502. doi: 10.1056/NEJMc1605684. [DOI] [PubMed] [Google Scholar]
- 12.Kwong Y.L., Chan T.S.Y., Tan D., Kim S.J., Poon L.M., Mow B., Khong P.L., Loong F., Au-Yeung R., Iqbal J., Phipps C., Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 13.Sonia, Guedane, Marco Emerging cellular therapies for cancer. Annual Review of Immunology. 2018 doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata H., Konno A., Kimura N. Characterization of novel natural killer (NK)-cell and gamma delta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood. 2001;97(3):708–713. doi: 10.1182/blood.V97.3.708. [DOI] [PubMed] [Google Scholar]
- 15.Tang X., Zhao S., Zhang Y. B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncolytics. 2019;14:279–287. doi: 10.1016/j.omto.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschalk S., Edwards O.L., Sili U., Huls M.H., Goltsova T., Davis A.R., Heslop H.E., Rooney C.M. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101(5):1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 17.Seaman S., Zhu Z., Saha S., Zhang X.M., Yang M.Y., Hilton M.B., Morris K., Szot C., Morris H., Swing D.A., Tessarollo L., Smith S.W., Degrado S., Borkin D., Jain N., Scheiermann J., Feng Y., Wang Y., Li J., Welsch D., DeCrescenzo G., Chaudhary A., Zudaire E., Klarmann K.D., Keller J.R., Dimitrov D.S., St Croix B. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31(4):501–515. doi: 10.1016/j.ccell.2017.03.005. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y.H., Martin-Orozco N., Zheng P., Li J., Zhang P., Tan H., Park H.J., Jeong M., Chang S.H., Kim B.S., Xiong W., Zang W., Guo L., Liu Y., Dong Z.J., Overwijk W.W., Hwu P., Yi Q., Kwak L., Yang Z., Mak T.W., Li W., Radvanyi L.G., Ni L., Liu D., Dong C. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27(8):1034–1045. doi: 10.1038/cr.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inamura K., Yokouchi Y., Kobayashi M., Sakakibara R., Ninomiya H., Subat S., Nagano H., Nomura K., Okumura S., Shibutani T., Ishikawa Y. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer. 2017;103:44–51. doi: 10.1016/j.lungcan.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Hofmeyer K.A., Ray A., Zang X. The contrasting role of B7-H3. Proc. Natl. Acad. Sci. 2008;105(30):10277–10278. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du H., Hirabayashi K., Ahn S., Kren N.P., Montgomery S.A., Wang X., Tiruthani K., Mirlekar B., Michaud D., Greene K., Herrera S.G., Xu Y., Sun C., Chen Y., Ma X., Ferrone C.R., Pylayeva-Gupta Y., Yeh J.J., Liu R., Savoldo B., Ferrone S., Dotti G. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35(2):221–237. doi: 10.2139/ssrn.3280238. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapoval A.I., Ni J., Lau J.S. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 23.Leitner J., Klauser C., Pickl W.F., Stockl J., Majdic O., Bardet A.F., Kreil D.P., Dong C., Yamazaki T., Zlabinger G., Pfistershammer K., Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009;39(7):1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loo D., Alderson R.F., Chen F.Z., Huang L., Zhang W., Gorlatov S., Burke S., Ciccarone V., Li H., Yang Y., Son T., Chen Y., Easton A.N., Li J.C., Rillema J.R., Licea M., Fieger C., Liang T.W., Mather J.P., Koenig S., Stewart S.J., Johnson S., Bonvini E., Moore P.A. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer Res. 2012;18(14):3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed M., Cheng M., Zhao Q., Goldgur Y., Cheal S.M., Guo H.F., Larson S.M., Cheung N.K. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J. Biol. Chem. 2015;290(50):30018–30029. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer K., Kushner B.H., Modak S., Pandit-Taskar N., Smith-Jones P., Zanzonico P., Humm J.L., Xu H., Wolden S.L., Souweidane M.M., Larson S.M., Cheung N.K. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neuro-Oncol. 2010;97(3):409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nehama D., Di Ianni N., Musio S., Du H., Patane M., Pollo B., Finocchiaro G., Park J.J.H., Dunn D.E., Edwards D.S., Damrauer J.S., Hudson H., Floyd S.R., Ferrone S., Savoldo B., Pellegatta S., Dotti G. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine. 2019 doi: 10.1016/j.ebiom.2019.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghorashian Sara, Kramer Anne Marijn, Onuoha Shimobi. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 2019;25(9):1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 29.Slaney C.Y., Wang P., Darcy P.K., Kershaw M.H. CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov. 2018;8(8):924–934. doi: 10.1158/2159-8290.CD-18-0297. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M., Wu B., Brandl C., Johnson J., Wolf A., Chow A., Doshi S. Blinatumomab, a bispecific T-cell engager (BiTE((R))) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin. Pharmacokinet. 2016;55(10):1271–1288. doi: 10.1007/s40262-016-0405-4. [DOI] [PubMed] [Google Scholar]
- 31.Choi B.D., Yu X., Castano A.P., Bouffard A.A., Schmidts A., Larson R.C., Bailey S.R., Boroughs A.C., Frigault M.J., Leick M.B., Scarfo I., Cetrulo C.L., Demehri S., Nahed B.V., Cahill D.P., Wakimoto H., Curry W.T., Carter B.S., Maus M.V. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019;37(9):1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 32.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., Qayed M., De Moerloose B., Hiramatsu H., Schlis K., Davis K.L., Martin P.L., Nemecek E.R., Yanik G.A., Peters C., Baruchel A., Boissel N., Mechinaud F., Balduzzi A., Krueger J., June C.H., Levine B.L., Wood P., Taran T., Leung M., Mueller K.T., Zhang Y., Sen K., Lebwohl D., Pulsipher M.A., Grupp S.A. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K.H., Brawley V.S., Byrd T.T., Krebs S., Gottschalk S., Wels W.S., Baker M.L., Dotti G., Mamonkin M., Brenner M.K., Orange J.S., Ahmed N. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126(8):3036–3052. doi: 10.1172/JCI131246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegde M., Corder A., Chow K.K.H., Mukherjee M., Ashoori A., Kew Y., Zhang Y.J., Baskin D.S., Merchant F.A., Brawley V.S., Byrd T.T., Krebs S., Wu M.F., Liu H., Heslop H.E., Gottachalk S., Yvon E., Ahmed N. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther. 2013;21(11):2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burvenich I.J.G., Parakh S., Lee F.T., Guo N., Liu Z., Gan H.K., Rigopoulos A., O'Keefe G.J., Gong S.J., Goh Y.W., Tochon-Danguy H., Scott F.E., Kotsuma M., Hirotani K., Senaldi G., Scott A.M. Molecular imaging of T cell co-regulator factor B7-H3 with (89)Zr-DS-5573a. Theranostics. 2018;8(15):4199–4209. doi: 10.7150/thno.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson K.E., Bachawal S.V., Abou-Elkacem L., Jensen K., Machtaler S., Tian L., Willmann J.K. Spectroscopic photoacoustic molecular imaging of breast cancer using a B7-H3-targeted ICG contrast agent. Theranostics. 2017;7(6):1463–1476. doi: 10.7150/thno.18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bam R., Laffey M., Nottberg K., Lown P.S., Hackel B.J., Wilson K.E. Affibody-indocyanine green based contrast agent for photoacoustic and fluorescence molecular imaging of B7-H3 expression in breast cancer. Bioconjug. Chem. 2019;30(6):1677–1689. doi: 10.1021/acs.bioconjchem.9b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]