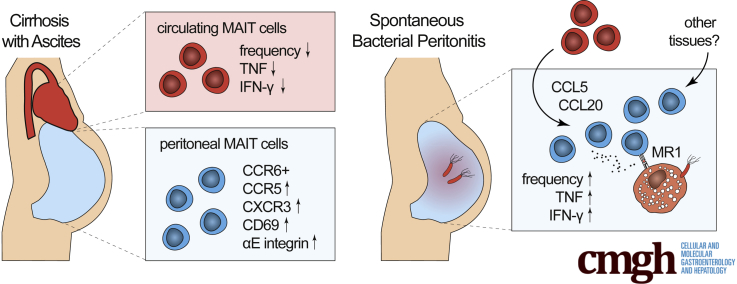
Abstract
Background & Aims
Mucosal-associated invariant T (MAIT) cells are depleted from blood in patients with advanced liver disease and show features of immune dysfunction. Because circulating MAIT cells differ from organ-resident MAIT cells, we aimed to investigate the frequency, phenotype, and function of peritoneal MAIT cells from patients with cirrhosis and spontaneous bacterial peritonitis (SBP).
Methods
MAIT cells in blood and ascitic fluid from patients with cirrhosis were characterized using flow cytometry. Healthy individuals and noncirrhotic patients undergoing peritoneal dialysis served as controls. MAIT cell migration was studied in transwell assays. Cytokine release in response to infected ascitic fluid and bacterial products was assessed in vitro.
Results
Peritoneal CD3+ CD161hi Vα7.2+ T cells had an inflammatory, tissue retention phenotype, expressing the alpha E integrin, the chemokine receptors CCR5 and CXCR3, and the activation marker CD69 at higher levels than their circulating equivalents. Seventy-seven percent bound to MR1 tetramers loaded with the pyrimidine intermediate 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil. The ratio of peritoneal to blood MAIT cell frequency increased from 1.3 in the absence of SBP to 2.6 at diagnosis and decreased by day 3. MAIT cells migrated toward infected ascitic fluid containing CCL5 and CCL20 and released cytokines in an MR1-restricted fashion. Whereas the depleted circulating MAIT cell pool displayed features of immune exhaustion, peritoneal MAIT cells remained competent producers of inflammatory cytokines in response to bacterial products. Peritoneal MAIT activation correlated with systemic inflammation, suggesting a possible link between peritoneal and systemic immunity.
Conclusions
Peritoneal MAIT cells phenotypically and functionally differ from circulating MAIT cells in decompensated cirrhosis and redistribute to the peritoneum during SBP.
Keywords: Liver Cirrhosis, Mucosal-Associated Invariant T Cells, Bacterial Infections, Spontaneous Bacterial Peritonitis
Abbreviations used in this paper: AF, ascitic fluid; CAPD, continuous ambulant peritoneal dialysis; cMAIT, circulating mucosal-associated invariant T; IFN, interferon; IL, interleukin; MAIT, mucosal-associated invariant T; MELD, Model for End-Stage Liver Disease; 5-OP-RU, 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil; pMAIT, peritoneal mucosal-associated invariant T; SBP, spontaneous bacterial peritonitis; TCR, T-cell receptor; TNF, tumor necrosis factor
Graphical abstract
Summary.
Circulating mucosal-associated invariant T (MAIT) cells are depleted and dysfunctional in advanced liver disease. Here we show that MAIT cell function is compartmentalized in decompensated cirrhosis, where peritoneal MAIT cells are immunocompetent, migratory cells that accumulate during peritonitis and contribute to inflammation.
Bacterial infections are frequent life-threatening complications in patients with advanced liver disease, particularly in the presence of decompensated cirrhosis with ascites.1, 2, 3 Spontaneous bacterial peritonitis (SBP) results from the translocation of viable bacteria from the gut across the gastrointestinal barrier into mesenteric lymph nodes or the portal vein, followed by seeding into ascitic fluid (AF) with low antimicrobial capacity.4 In patients with advanced cirrhosis, a failure of immunologic control occurs on multiple levels.5, 6, 7 Particularly, innate immune responses of intestinal, circulating, and peritoneal myeloid cells are believed to critically contribute to pathologic bacterial translocation, the susceptibility, and the outcome of SBP.8, 9, 10, 11 In addition, evidence suggests impaired adaptive immune responses in patients with advanced cirrhosis by conventional and unconventional T cells.6,12,13
Mucosal-associated invariant T (MAIT) cells are unconventional T cells that act as a bridge between the adaptive and the innate arm of the immune system.14 They were initially characterized to be preferentially located in the gut lamina propria,15 but they account for 1%–10% of total circulating T cells in the blood and comprise the major proportion of innate-like lymphocytes in human liver.16 Activation of their unique T-cell receptor (TCR), with the invariant alpha chain Vα7.2Jα33 in humans, relies on the presentation of microbial vitamin B metabolites bound to the MHC class I-like molecule MR1 on antigen-presenting cells.17,18 In the absence of antigen presentation, MAIT cells can also be activated in a TCR-independent manner via interleukins (ILs) 12 and 18.19
Because MAIT cells have the ability to relocate to sites of infection and to release inflammatory cytokines and cytotoxic granules on activation,20 we hypothesized a critical role of innate immune cells in maintaining peritoneal immunity in the context of decompensated cirrhosis and during SBP occurrence. Recent studies reported a functional exhaustion of circulating MAIT (cMAIT) cells in patients with alcoholic liver disease as a result of repetitive pathologic bacterial translocation.13 Performing and interpreting such studies is challenging because the cMAIT cell pool is depleted in patients with liver disease, and conflicting data exist whether MAIT cells accumulate in the diseased liver.13,21, 22, 23 Because the depleted cMAIT cell pool may differ from organ-resident MAIT cells, the aim of this study was to investigate the phenotype and function of human MAIT cells in the peritoneal cavity in decompensated cirrhosis and during the course of SBP.
Results
Patients’ Characteristics
One hundred two patients with decompensated cirrhosis and ascites were eligible for the study. Two patients were excluded because of secondary peritonitis. Among the remaining 100 patients, 16 had SBP at study inclusion, and 4 developed SBP during follow-up. Among the 20 patients who developed SBP, 3 patients had recurrent SBP during the study period. The majority of patients were male (84%), with a median age of the total population of 61 years (Table 1). Alcoholic liver disease was the main cause of cirrhosis. As expected, patients with SBP had features of more advanced liver disease and signs of peritoneal and systemic inflammation (Table 1). Among patients with SBP, 50% were culture positive in AF, and 15% had concomitant bacteremia. Microbial culture results, concomitant medication, and primary prophylaxis are given in Tables 2 and 3.
Table 1.
Patients’ Baseline Characteristics
| Total patients (N = 100) | Decompensated cirrhosis without SBP (N = 80) | Decompensated cirrhosis with SBP (N = 20) | P value | |
|---|---|---|---|---|
| Age, y, median (range) | 61 (36–81) | 63 (36–81) | 57 (38–73) | .18 |
| Male sex, N (%) | 84 (84) | 69 (86.3) | 15 (75.0) | .30 |
| Alcoholic liver disease, N (%) | 84 (84) | 65 (81.3) | 19 (95) | .18 |
| Previous SBP | 11 (11.0) | 3 (3.8) | 5 (25) | .008 |
| AF analysis, median (interquartiles) | ||||
| White blood cells, μL-1 | 160 (110–380) | 135 (90–190) | 2695 (1208–9350) | <.0001 |
| Neutrophils, μL-1 | 20 (10–70) | 20 (10–30) | 1885 (713–7243) | <.0001 |
| Protein, g/L | 13.3 (8.4–18.9) | 12.6 (8.4–18.2) | 15.6 (8.0–23.1) | .56 |
| Albumin, g/L | 7.2 (5.0–11.4) | 7.0 (5.0–10.9) | 9.5 (5.0–14.4) | .28 |
| Laboratory data, median (interquartiles) | ||||
| Total bilirubin, μmol/L | 39 (20–72.4) | 37 (20–72) | 50.0 (21.5–219.4) | .09 |
| Serum albumin, g/L | 28.0 (23.4–32.0) | 28.0 (24.3–31.0) | 30.0 (20.0–36.0) | .65 |
| Serum sodium, mmol/L | 135 (130–138) | 135 (130–138) | 135 (130–139) | .55 |
| Serum protein, g/L | 60.3 (57.0–70.3) | 64.0 (59.0–71.0) | 59.0 (53.0–65.3) | .048 |
| Creatinine, μmol/L | 112.0 (68.5–158.5) | 107 (67–148) | 120.5 (105.0–181.0) | .09 |
| Urea, mmol/L | 9.9 (6.9–16.5) | 9.4 (6.3–18.0) | 10.3 (8.5–17.4) | .53 |
| International normalized ratio | 1.4 (1.3–1.8) | 1.4 (1.3–1.7) | 1.7 (1.3–2.4) | .06 |
| White blood cell count, 109/L | 6.6 (4.4–10.0) | 6.1 (4.2–9.1) | 9.3 (6.4–21.7) | .001 |
| Platelet count, 109/L | 120.5 (76.0–179.8) | 122 (76–180) | 120.5 (74.5–176.0) | .73 |
| C-reactive protein, mg/L | 27.7 (10.8–57.7) | 23.8 (8.5–43.5) | 63.4 (47.5–104.2) | <.0001 |
| Clinical scores, median (interquartiles) | ||||
| Child-Pugh score | 10 (8–12) | 10 (8–12) | 10 (8–13) | .43 |
| MELD score | 17 (13–22) | 17 (13–21) | 21 (14–28) | .18 |
NOTE. Characteristics of patients included in the study. Data are given as median with interquartiles or frequency with percentages. P values are based on Mann–Whitney test for continuous data or Fisher exact test for discrete data.
Table 2.
Microorganisms Isolated From AF and Blood Cultures From Patients With SBP
| AF culture results N (%) | Blood culture results N (%) | |
|---|---|---|
| Positive culture results | 10 (50) | 3 (15) |
| E coli | 4 (20) | 2 (10) |
| Streptococcus pneumoniae | 1 (5) | 1 (5) |
| Listeria monocytogenes | 1 (5) | — |
| Klebsiella pneumoniae | 1 (5) | — |
| Enterobacter cloacae | 1 (5) | — |
| Enterococcus faecalis | 1 (5) | — |
| Enterococcus faecalis and Candida albicans | 1 (5) | — |
| Negative culture results | 10 (50) | 17 (85) |
Table 3.
Clinical Data on Previous and Current Treatment of Patients With SBP (n = 20)
| Characteristics | N (%) |
|---|---|
| Previous antibiotic exposurea | 13 (65) |
| Nonselective β-blockersa | 5 (25) |
| Proton pump inhibitorsa | 18 (90) |
| On rifaximin prophylaxis at SBP | 7 (35) |
| Empirical treatment of SBP | |
| Piperacillin/tazobactam ± linezolid | 11 (55) |
| Meropenem + linezolid | 8 (40) |
| Failure of empirical therapy | 4 (20) |
Within the last 4 weeks.
CD3+ CD161hi Vα7.2 TCR-Positive Cells in the Peritoneal Cavity Resemble Organ-Resident MAIT Cells
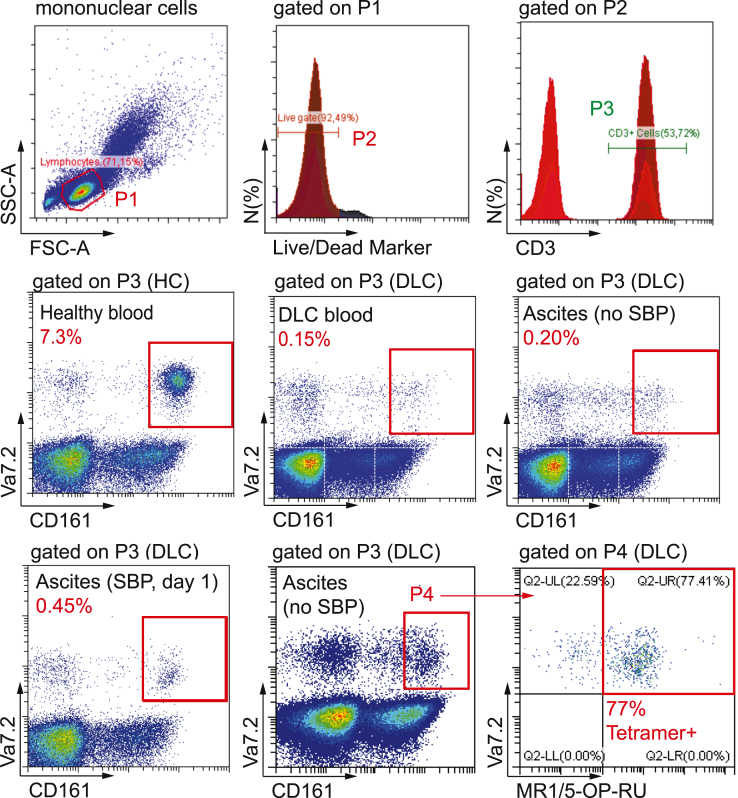
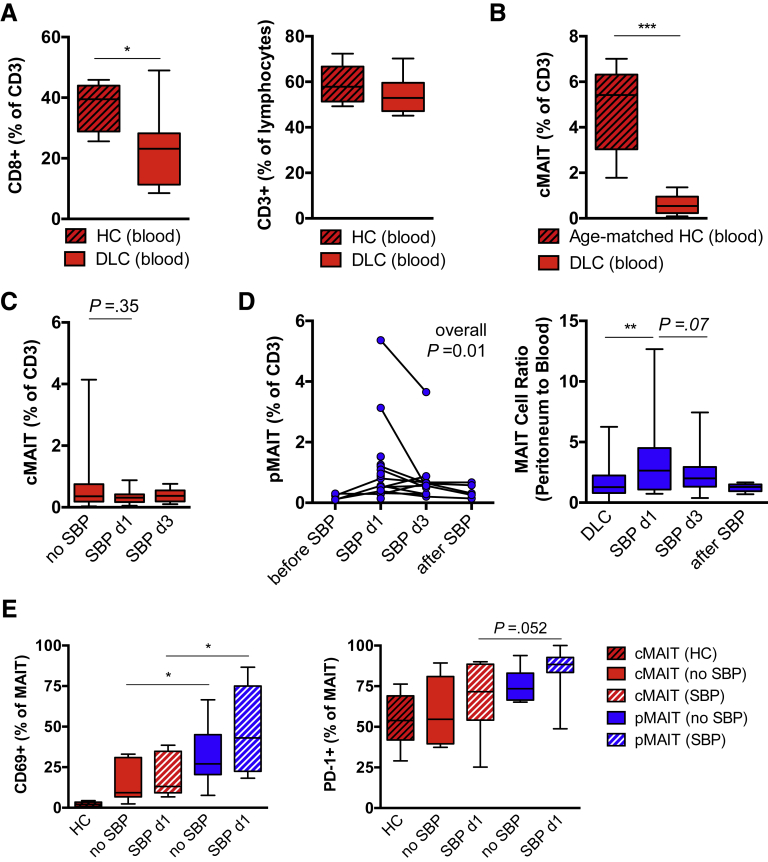
We identified circulating and peritoneal MAIT cells as viable CD3+ CD161hi Vα7.2 TCR-positive cells16,24 and confirmed antigen specificity in a subset of patients by using tetramer staining of MR1 loaded with the pyrimidine intermediate 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU) (Figure 1). Consistent with previous data,13 we observed a depletion of circulating CD3+ CD161hi Vα7.2+ cells in patients with decompensated cirrhosis (median 0.4% of CD3+ T cells) compared with healthy controls (median 4.7% of T cells; P < .0001) (Figure 2A). In the peritoneal compartment, the median frequency of CD3+ CD161hi Vα7.2+ cells in AF from patients with decompensated cirrhosis (0.5% of T cells; range, 0.1%–5.8%) was lower than in the peritoneal fluid of patients with end-stage renal disease undergoing continuous ambulant peritoneal dialysis (CAPD) (3.6%; range, 0.9%–14.1%; P < .0001) but higher than in paired blood samples from patients with cirrhosis (0.4%; range, 0.03%–4.1%; P < .001) (Figure 2A).
Figure 1.
Gating strategy. Representative flow cytometry plots from healthy controls (HC), patients with decompensated liver cirrhosis (DLC), and patients with SBP. MAIT cells were identified as viable CD3+ lymphocytes simultaneously expressing CD161 at high levels (CD161hi) and the invariant TCR Vα7.2. Representative tetramer staining of MR1 loaded with the pyrimidine intermediate 5-OP-RU is shown (bottom right panel). FSC, forward scatter; SSC, side scatter.
Figure 2.
Peritoneal CD3+ CD161hi Vα7.2+ cells resemble organ-resident MAIT cells in decompensated cirrhosis. (A) Percentage of Vα7.2+CD161hi circulating and peritoneal cells among CD3+ T cells in healthy controls (HC) (n = 19), patients with decompensated liver cirrhosis (DLC) in the absence of SBP (n = 75), and in controls without cirrhosis undergoing CAPD (n = 13). (B) Percentage of MR1/5-OP-RU tetramer positive Vα7.2+CD161hi circulating and peritoneal cells from patients (n = 9). (C) CD8/CD4 T-cell composition of Vα7.2+CD161hi, CD161hi MR1/5-OP-RU+, and MR1/5-OP-RU+ among peritoneal CD3+ T cells from patients with decompensated cirrhosis (n = 6). (D) Median fluorescent intensity (MFI) of intranuclear RORγt, Tbet, and GATA-3 in peritoneal CD3+ Vα7.2+ CD161hi cells and CD3+ Vα7.2– CD161– T cells as determined by flow cytometry (n = 8). (E) Surface expression of CCR6 (n = 8), beta 7 integrin (n = 12), and CD69 (n = 10) in peritoneal CD3+ Vα7.2+ CD161hi cells and peritoneal CD3+ Vα7.2– CD161– T cells from patients with cirrhosis in the absence of SBP. *P < .05, **P < .01, ***P < .001 in Wilcoxon signed-rank test (paired samples) and Mann-Whitney U test (unpaired samples).
To verify that CD3+ CD161hi Vα7.2+ cells were MAIT cells, we performed MR1/5-OP-RU tetramer staining in a subset of samples (n = 9). The median frequency of MR1/5-OP-RU positive CD3+ CD161hi Vα7.2+ cells was 77% (range, 61%–97%) in the peritoneum and 73% (range, 28%–98%) in blood from patients with cirrhosis (Figure 2B). The majority of peritoneal MAIT (pMAIT) cells were CD8+ positive or CD4 and CD8 double negative, irrespective of staining strategy (Figure 2C). When compared with peritoneal conventional CD161– Vα7.2– T cells, peritoneal CD161hi Vα7.2+ cells expressed higher levels of the transcription factor RORγT (Figure 2D), alongside high levels of CCR6, beta 7 integrin, and CD69 (Figure 2E), which is consistent with the phenotype of organ-resident MAIT cells.25 Because of these phenotypic markers, from here on we refer to CD3+ CD161hi Vα7.2 TCR-positive cells as MAIT cells in the article, acknowledging a possible, but limited contamination with CD161hi Vα7.2 TCR-positive conventional T cells, which are nonreactive to the vitamin B2 precursor derivative 5-OP-RU.
Peritoneal Redistribution of MAIT Cells During Early SBP
We equally observed a depletion of CD8+CD3+ T cells in blood from patients with decompensated cirrhosis compared with healthy controls and no significant changes in percentage of total CD3+ cells (Figure 3A). Because MAIT cells have been shown to decrease with age,26,27 we compared age-matched samples (5 controls vs 10 patients) and still observed a striking depletion of cMAIT cells from blood in patients with cirrhosis (median 5.4% vs 0.5%; P = .0007; Figure 3B).
Figure 3.
Peritoneal MAIT cell frequency is regulated during SBP. (A) Percentage of CD8 expressing circulating CD3+T cells and CD3+ T cells of lymphocytes from patients with decompensated liver cirrhosis (DLC) and from healthy controls (HC) are shown (n = 7–13). (B) Percentage of Vα7.2+CD161hi cMAIT cells among CD3+ T cells in age-matched patients with DLC in the absence of SBP (n = 10) and in HC (n = 5). (C) Frequency of circulating Vα7.2+ CD161hi MAIT (cMAIT) cells of CD3+ T cells in DLC and during the course of SBP on days 1 (d1) and 3 (d3). (D) Frequency of peritoneal CD3+ Vα7.2+ CD161hi MAIT (pMAIT) cells in patients with serial samples in the course of SBP (before SBP: n = 4; on day 1: n = 18; on day 3: n = 13; after SBP: n = 5). Connected samples are indicated (left panel), and ratio of pMAIT cell to cMAIT cell frequency in patients without SBP (n = 75) and patients during SBP are shown (right panel). (E) Frequency of CD69 and PD-1 positive CD3+ Vα7.2+ CD161hi cMAIT and CD3+ Vα7.2+ CD161hi pMAIT cells from HC and patients with DLC in the absence and presence of SBP. Box plots from 6–13 representative individuals are shown.*P < .05, **P < .01, ***P < .001 in Wilcoxon signed-rank test (paired samples) and Mann-Whitney U test (unpaired samples). P values from Mann-Whitney U test (unpaired samples) and Wilcoxon signed-rank test (paired samples) are shown. Overall P in (D) indicates result from Kruskal-Wallis test.
The frequency of MAIT cells in blood did not significantly change in the presence of SBP (Figure 3C). However, we observed significant changes in pMAIT cell frequency among T cells during SBP, with highest concentrations at day 1 and resolution with treatment (Figure 3D). This was confirmed when the AF to blood ratio was considered as a marker of compartmental MAIT cell distribution in the total patient cohort (median ratio 1.3 in the absence of SBP vs 2.6 at day 1 of SBP; P = .0098; Figure 3D). Peritoneal MAIT cells showed increased expression of the activation marker CD69 as compared with cMAIT cells in absence and presence of SBP, whereas the regulation marker programmed cell death protein 1 did not significantly differ (Figure 3E).
Peritoneal MAIT Cells Have an Inflammatory Migratory Phenotype
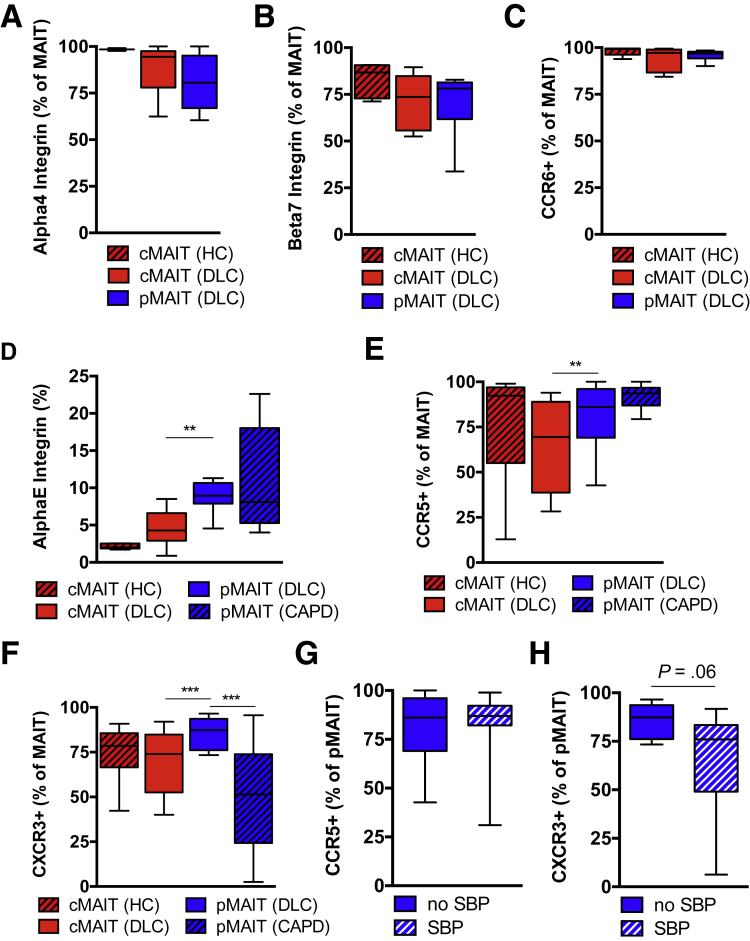
To investigate whether pMAIT cell redistribution during SBP is the result of selective recruitment to the peritoneum, we compared the expression of chemokine receptors, tissue retention markers, and gut homing markers between pMAIT cells from patients with decompensated cirrhosis and cMAIT cells. The vast majority of pMAIT cells expressed the alpha 4 integrin and the beta 7 integrin similar to cMAIT cells (Figure 4A and B). Consistent with the phenotype of MAIT cells,16 pMAIT cells expressed high levels of CCR6 similar to cMAIT cells from controls and patients with cirrhosis (Figure 4C).
Figure 4.
Integrin and chemokine receptor surface expression differ between blood and peritoneal MAIT cells. Surface expression of (A) alpha 4 integrin (n = 3–6), (B) beta 7 integrin (n = 6–12), and (C) CCR6 (n = 7) on circulating CD3+ Vα7.2+ CD161hi MAIT (cMAIT) and peritoneal CD3+ Vα7.2+ CD161hi MAIT (pMAIT) cells from healthy controls (HC) and patients with decompensated liver cirrhosis (DLC) in absence of SBP are shown. Surface expression of (D) alpha E integrin (n = 5–12), (E) CCR5 (n = 9–12), and (F) CXCR3 (n = 11–12) on CD3+ Vα7.2+ CD161hi cMAIT and CD3+ Vα7.2+ CD161hi pMAIT cells from HC, patients with DLC in absence of SBP, and in noncirrhotic patients undergoing CAPD. Surface expression of (G) CCR5 (n = 10–12) and (H) CXCR3 (n = 8–12) on CD3+ Vα7.2+ CD161hi pMAIT cells from patients with DLC in absence or presence of SBP. **P < .01, ***P < .001 in Wilcoxon signed-rank test (paired samples) and Mann-Whitney U test (unpaired samples). P value from Mann-Whitney U test.
The surface expression of the alpha E integrin (tissue retention marker CD103) was increased in pMAIT cells as compared with cMAIT cells (Figure 4D). pMAIT cells from patients with decompensated cirrhosis expressed higher levels of the chemokine receptors CXCR3 and CCR5 than their circulating counterparts, consistent with a more inflammatory migratory phenotype (Figure 4E and F). There were no significant differences in surface expression levels of CCR5 and CXCR3 in the absence or presence of SBP, although CXCR3 levels on pMAIT cells tended to be numerically lower during SBP (Figure 4G and H), consistent with CXCR3 being internalized after ligand binding and migration.28,29
Circulating MAIT Cells Preferentially Migrate Toward Infected AF
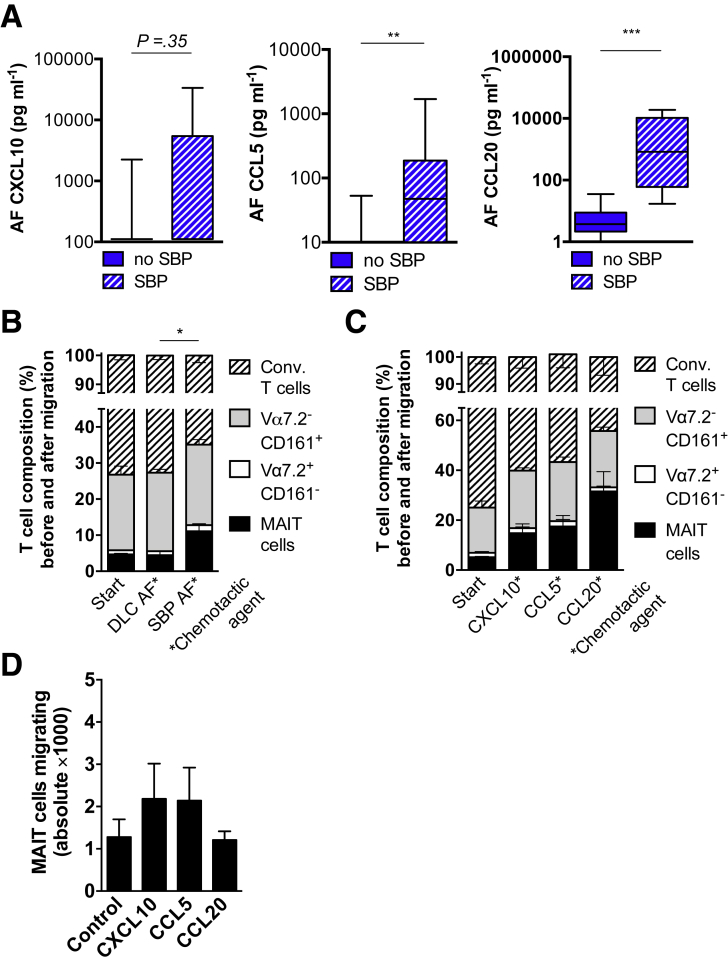
To investigate the role of chemokines in the recruitment of MAIT cells to the peritoneal cavity during SBP, we measured the ligands of CCR6, CCR5, and CXCR3 in a subset of AF samples from 22 patients with and without SBP (Table 4). We detected significantly increased levels of CCL20 (CCR6 ligand) and CCL5 (CCR5 ligand) in AF during SBP but no significant differences in CXCL10 (CXCR3 ligand) (Figure 5A).
Table 4.
Baseline Characteristics for Patients Included in Cytokine and Chemokine Analysis
| Decompensated cirrhosis without SBP (N = 12) | Decompensated cirrhosis with SBP (N = 10) | P value | |
|---|---|---|---|
| Age, y, median (range) | 63 (36–81) | 55 (38–68) | .73 |
| Male sex, N (%) | 9 (75.0) | 7 (70.0) | 1.00 |
| Neutrophils, μL-1 (IQR) | 20 (10–20) | 4850 (1435–2714) | <.0001 |
| Total bilirubin, μmol/L (IQR) | 24 (13–69) | 113 (31–366) | .04 |
| Creatinine, μmol/L (IQR) | 107 (53–150) | 94 (47–130) | .67 |
| International normalized ratio (IQR) | 1.5 (1.3–2.3) | 1.9 (1.7–3.2) | .08 |
| C-reactive protein, mg/L (IQR) | 5.7 (3.4–39.8) | 51.2 (28.1–86.2) | .01 |
| MELD score (IQR) | 16 (11–23) | 23 (12–35) | .23 |
| Culture-positive AF, N (%) | 0 (0.0) | 8 (80.0) | <.0001 |
NOTE. Characteristics of patients included for chemokine analysis. Data are given as median with range or interquartiles (IQR) or frequency with percentages. P values are based on Mann–Whitney test for continuous data or Fisher exact test for discrete data.
Figure 5.
MAIT cells preferentially migrate toward infected AF. Concentrations of (A) CXCL10, CCL5, and CCL20 measured by ELISA in AF from patients with and without SBP (n = 10 vs 12). (B and C) Composition of T-cell subsets stratified for expression of Vα7.2 and CD161 before (Start) and after migration in a transwell migration assay. Mononuclear cells from healthy controls were activated and seeded on upper chamber of 3-μm pore transwell (B) with filtered AF from patients with decompensated liver cirrhosis (DLC) without SBP or from patients with SBP or (C) with indicated chemokines at 150 ng/mL in the bottom well and were allowed to migrate for 4 hours at 37°C. MAIT cells were defined as CD3+ Vα7.2+ CD161hi; conventional (conv.) T cells were defined as CD3+ Vα7.2– CD161–. (D) Absolute numbers of CD3+ CD161hi Vα7.2 T cells were quantified using flow cytometry. Mean and standard error of the mean from 4–7 experiments are shown. *P < .05, **P < .01, ***P < .001 in Wilcoxon signed-rank test (paired samples) and Mann-Whitney U test (unpaired samples). P value from Mann-Whitney U test.
To investigate whether MAIT cells preferentially migrate over conventional T-cell subsets toward infected AF, we analyzed the T-cell composition before and after migration by using transwell migration chambers. To have sufficient numbers of MAIT cells for such functional assays and to avoid the assessment of recently migrated cells with chemokine receptor internalization,30 we used mononuclear cells from healthy individuals for migration experiments. Mononuclear cells, which were activated with Escherichia coli supernatant overnight, were put in the upper chamber and migrated along a gradient of chemokines or filtered AF in the bottom chamber. We observed that a higher percentage of MAIT cells migrated toward infected AF from patients with SBP (final MAIT cell fraction, 11.2% of CD3 T cells) as compared with patients without SBP (final MAIT cell fraction, 3.1%; P = .02) (Figure 5B). Consistent with the analysis of chemokine receptor expression, MAIT cells preferentially migrated over conventional T cells against gradients of the recombinant chemokines CCXL10, CCL5, and CCL20 (Figure 5C).
Although CCL20 was the strongest chemotactic agent to preferentially recruit MAIT cells, CXCL10 and CCL5 showed the strongest chemotactic abilities in terms of absolute T-cell recruitment at concentrations of 150 ng/mL (Figure 5D).
Peritoneal MAIT Cells Largely Remain Functional and Are Potent Producers of Inflammatory Cytokines
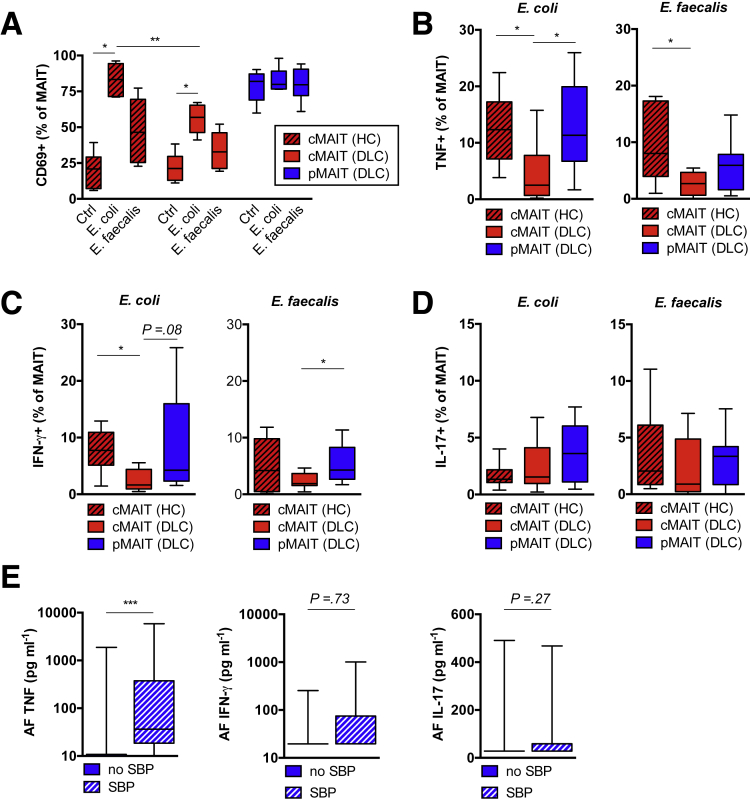
Having shown a preferential migration of MAIT cells toward infected AF (Figure 5B) alongside an enrichment of pMAIT cells in the early course of SBP (Figure 3D), we investigated whether this redistribution during SBP was associated with markers of early activation by assessing CD69 expression. Consistent with previous observations,13 CD69 expression on cMAIT cells from patients with decompensated cirrhosis was increased as compared with controls (Figure 3E).
Next, we investigated whether filtered bacterial culture supernatants from riboflavin-positive (E coli) and riboflavin-negative bacteria (Enterococcus faecalis) were sufficient to activate MAIT cells in the presence of antigen-presenting cells. In this model, E coli potently activated cMAIT cells from healthy controls, as indicated by CD69 expression, whereas cMAIT cell activation in patients with decompensated cirrhosis was significantly reduced compared with healthy controls (56.9% vs 83.3%; P = .002) (Figure 6A). Owing to high baseline expression, we could not use the CD69 read-out for pMAIT cell activation in this model. Therefore, we assessed cytokine production after stimulation with bacterial products. Consistent with the data on CD69 expression, we observed a significantly reduced number of responding cMAIT cells in patients with cirrhosis as compared with controls, when assessed for intracellular tumor necrosis factor (TNF) and interferon (IFN)-γ production (Figure 6B and C), whereas no changes in IL-17 production were observed in response to filtered E coli supernatant (Figure 6D). In contrast, pMAIT cells from patients with cirrhosis did not show impaired cytokine release under these conditions and remained potent producers of TNF and IFN-γ (Figure 6B and C). Quantification of inflammatory cytokines in the AF in the presence and absence of SBP revealed an elevated concentration of TNF during SBP, whereas IFN-γ and IL-17 in AF were not significantly regulated (Figure 6E).
Figure 6.
Peritoneal MAIT cells from patients with decompensated cirrhosis release inflammatory cytokines. (A) Frequency of CD69 expressing circulating CD3+ Vα7.2+ CD161hi MAIT (cMAIT) cells from patients with decompensated liver disease (DLC) and healthy controls (HC) and peritoneal CD3+ Vα7.2+ CD161hi MAIT (pMAIT) cells from patients with DLC after overnight stimulation with sterile filtered supernatants of bacterial cultures from riboflavin-producing E coli or riboflavin non-producing Enterococcus faecalis. Unstimulated cells (bacterial culture broth) are shown as control (Ctrl) (n = 6). Percentage of MAIT cells with intracellular expression of (B) TNF, (C) IFN-γ, and (D) IL-17 after ex vivo stimulation with sterile filtered bacterial supernatants for 6 hours as determined by flow cytometry. Results from 6–8 independent experiments are shown. Concentrations of (E) TNF (n = 17–28), IFN-γ (n = 10–12), and IL-17 (n = 10–12) measured by ELISA in AF in presence and absence of SBP. *P < .05, **P < .01, ***P < .001 in Wilcoxon signed-rank test (paired samples) and Mann-Whitney U test (unpaired samples). P values from Mann-Whitney U test.
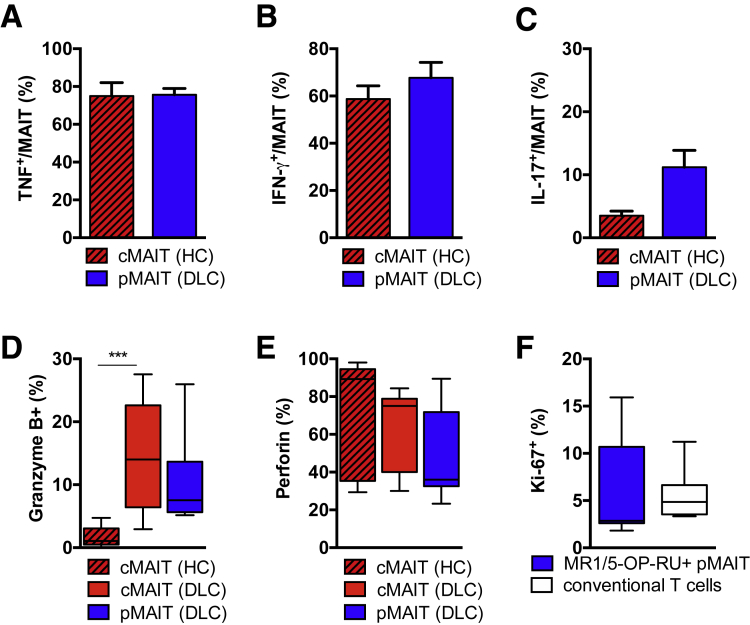
Bypassing the T-cell membrane receptor complex using phorbol 12-myristate 13-acetate and ionomycin, we observed no differences between pMAIT cells from patients with cirrhosis and healthy cMAIT cells, indicating a similar maximum release capacity for IFN-γ and TNF (Figure 7A–C). In addition, there was no evidence for reduced expression of cytotoxic granules, granzyme B and perforin, between pMAIT cells and healthy cMAIT cells (Figure 7D and E). Using combined Ki-67 and MR1/5-OP-RU tetramer staining, we did not observe a preferential proliferation of MAIT cells as compared with conventional T cells in the peritoneal cavity in the absence of SBP (Figure 7F).
Figure 7.
Peritoneal MAIT cells from patients with decompensated cirrhosis have unaltered cytokine release capacity. Percentage of (A) TNF, (B) IFN-γ, and (C) IL-17 producing circulating CD3+ CD161hi Vα7.2+ MAIT (cMAIT) cells from healthy controls (HC) in comparison with peritoneal CD3+ CD161hi Vα7.2+ MAIT (pMAIT) cells from patients with decompensated cirrhosis (DLC) after ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. Mean and standard error of the mean from 3–9 independent experiments are shown. Percentage of (D) granzyme B (n = 7–9) and (E) perforin (n = 7–9) producing CD3+ CD161hi Vα7.2 cMAIT cells from HC, CD3+ CD161hi Vα7.2+ cMAIT, and CD3+ CD161hi Vα7.2+ pMAIT cells from patients with DLC. (F) Expression of Ki-67 in MR1/5-OP-RU-tetramer positive CD3+ CD161hi Vα7.2+ pMAIT cells and peritoneal conventional T cells from patients with DLC. ***P < .001 in Mann-Whitney U test.
Infected Ascitic Fluid Activates MAIT Cells in an MR1-Restricted Fashion
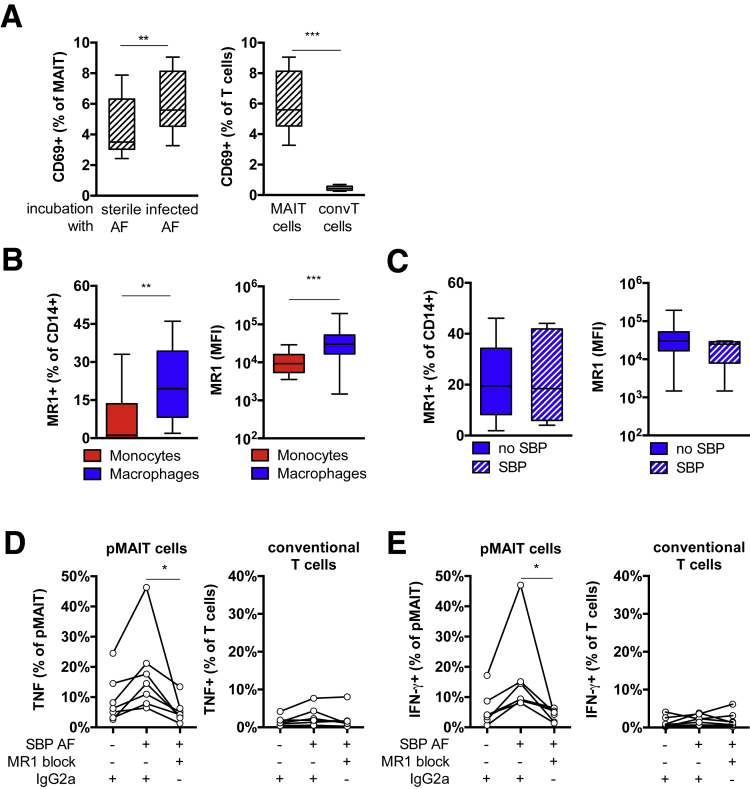
To investigate whether AF from patients with SBP was sufficient to activate immunocompetent MAIT cells ex vivo, we incubated healthy peripheral blood mononuclear cells with bacteria-free, filtered AF from patients with and without SBP for 24 hours and assessed CD69 expression. In this model, we observed a higher increase in CD69 expression after treatment of healthy cMAIT cells with infected AF from patients with SBP as compared with patients without SBP (Figure 8A). To investigate whether pMAIT cell activation was MR1-dependent, we first demonstrated surface expression of MR1 on circulating CD14+ monocytes and CD14+ peritoneal macrophages from patients with decompensated cirrhosis by flow cytometry. The number of MR1-expressing cells and the fluorescence intensity were higher in peritoneal macrophages as compared with circulating monocytes (Figure 8B), but no differences in MR1 expression on macrophages in the presence and absence of SBP were observed (Figure 8C). To determine whether pMAIT cell activation was MR1-dependent, we used MR1 blocking antibodies before treating with filtered infected AF from patients with SBP. Incubating with MR1 blocking antibodies significantly reduced the number of IFN-γ and TNF producing pMAIT cells in response to infected AF, whereas peritoneal conventional T cells did not respond (Figure 8D and E), indicating that pMAIT cell activation in the course of SBP depends on antigen presentation by the MR1 major histocompatibility complex.
Figure 8.
MAIT cells can be activated by infected AF in MR1-restricted fashion. (A) Mononuclear cells from healthy controls were incubated with filtered AF from patients with decompensated cirrhosis in absence or presence of SBP for 24 hours, and T-cell activation was analyzed. Increase in CD69 surface expression after treatment with infected AF from patients with SBP is shown in CD3+ Vα7.2+ CD161hi MAIT cells (n = 9) (left panel) but not on conventional (conv) CD3+ Vα7.2– CD161– T cells (n = 6–9) (right panel). (B) Median fluorescence intensity (MFI) and percentage MR1-expressing CD14+ monocytes and CD14+ peritoneal macrophages from patients with decompensated cirrhosis in absence of SBP (n = 17). (C) Median fluorescence intensity (MFI) and percentage of MR1-expressing CD14+ peritoneal macrophages in absence or presence of SBP (n = 5–17). Percentage of (D) TNF and (E) IFN-γ expressing pMAIT cells after incubation of mononuclear cells with infected AF from patients with SBP in presence of MR1-blocking antibodies or isotype-matched controls (IgG2a) (n = 7). *P < .05, **P < .01, ***P < .001 in Wilcoxon signed-rank test (paired samples) and Mann-Whitney U test (unpaired samples).
Peritoneal MAIT Cell Activation Status Correlates With Disease Severity
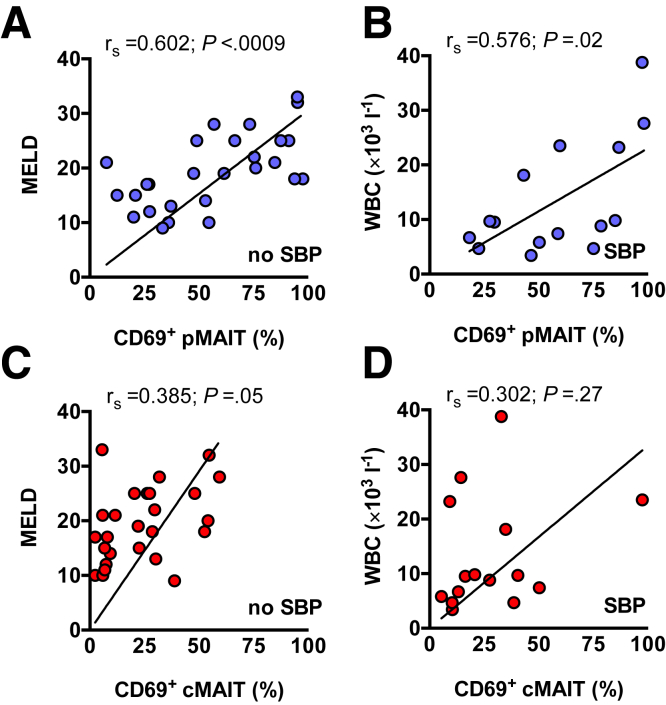
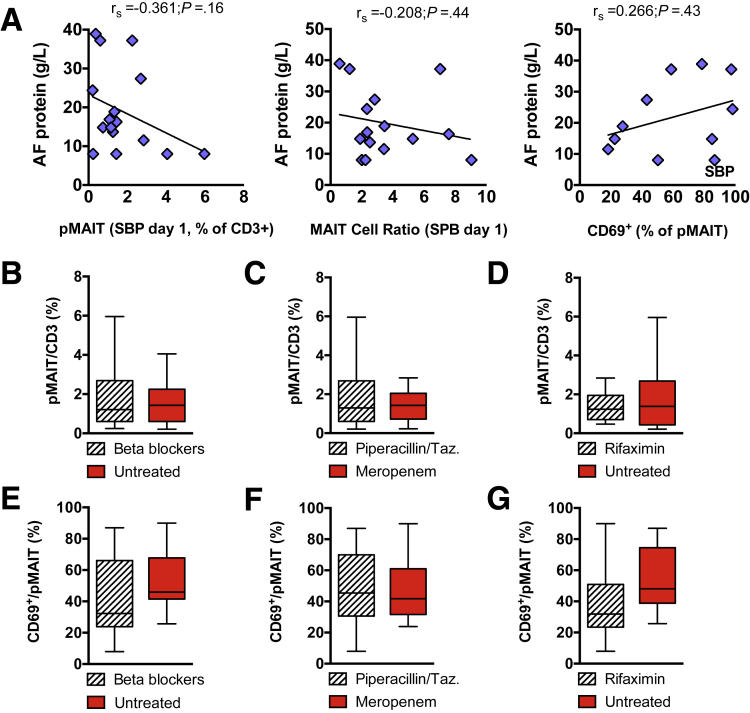
Following these observations, we correlated clinical data with MAIT cell function, specifically with immune activation indicated by CD69. In the absence of SBP, the frequency of CD69-expressing pMAIT cells correlated with the Model for End-Stage Liver Disease (MELD) score (P = .0009) (Figure 9A). In the presence of SBP, the frequency of CD69-expressing pMAIT cells significantly correlated with the white blood cell count as a marker of systemic inflammation (P = .02) (Figure 9B). In contrast to pMAIT cell activation, the activational status of cMAIT cells did not significantly correlate with these surrogate markers of disease severity (Figure 9C and D). We did not observe any correlation of pMAIT cell frequency or activation status with AF protein concentration or concomitant medication (Figure 10).
Figure 9.
Peritoneal MAIT cell activation status correlates with liver function and systemic inflammation. Dot plots, linear regression, and nonparametric Spearman’s rho (rs) with P values are indicated to demonstrate correlation of CD69-expressing pMAIT or cMAIT cells with (A and C) MELD score in patients with decompensated liver cirrhosis (DLC) without SBP, (B and D) white blood cell count (WBC) in patients with SBP. Correlation between variables was assessed using Spearman's method, and linear regression lines were only used as a graphical representation to suggest the direction of the association.
Figure 10.
Peritoneal MAIT cell frequency and activation status with AF protein concentration and co-medication. (A) Dot plots, linear regression, and nonparametric Spearman’s rho (rs) with P values are indicated to demonstrate correlation of peritoneal CD3+ CD161hi Vα7.2+ MAIT (pMAIT) cell frequency, CD3+ CD161hi Vα7.2+ MAIT cell ratio, and CD69-expressing pMAIT cells with concentrations of AF protein in patients with decompensated liver cirrhosis (DLC) with SBP. Correlation between variables was assessed using Spearman's method, and linear regression lines were used as a graphical representation to suggest the direction of the association. (B–D) pMAIT cell frequency and (E–G) CD69 expression stratified for nonselective beta blocker use, empiric antibiotic treatment for SBP, and concomittant rifaximin use in patients with SBP. Taz., tazobactam.
Conclusions
In this study we examine the frequency, phenotype, and function of MAIT cells of the peritoneal cavity from patients with decompensated cirrhosis in the absence or presence of SBP. We report a redistribution of MAIT cells, owing to a preferential migration of functional MAIT cells toward infected in contrast to noninfected AF. In contrast to cMAIT cells, which are depleted in end-stage liver disease and show signs of functional exhaustion,13,21,31 pMAIT cells from patients with decompensated cirrhosis respond to bacterial products comparable with healthy MAIT cells and remain potent producers of TNF and IFN-γ in a MR1-restricted fashion.
In the absence of SBP, the circulating as well as the resident peritoneal MAIT cell pool is significantly reduced in patients with cirrhosis. The processes by which MAIT cells are depleted could not be linked to increased apoptosis and require further investigations.13 In agreement with previous observations in blood,31 pMAIT cells from patients with cirrhosis showed significant proliferation as indicated by Ki-67 staining, but this did not differ from conventional T cells. Consistent with their role in fighting bacterial infections by linking innate and adaptive immune responses,32, 33, 34 we observed a relative enrichment of MAIT cells in AF from patients at the day of diagnosis of SBP, which resolved after antibiotic treatment by day 3. Comparative kinetics of MAIT cell frequencies in blood and AF during SBP, integrin and chemokine receptor repertoire, and ex vivo migration assays imply the redistribution of MAIT cells to sites of inflammation, as observed in CAPD-associated peritonitis35 and in hepatic fibrosis.31 Elevated concentration of CCL5 and CCL20 in the peritoneal cavity during SBP, alongside the high expression CCR5, CCR6, and CXCR3 on pMAIT cells in patients with cirrhosis, suggest recruitment and/or retention of pMAIT cells. The increased expression of the mucosal homing receptors alpha E beta 7 along with the high expression alpha 4 beta 7 integrin on pMAIT cells is consistent with a gut-homing and tissue retention phenotype.36
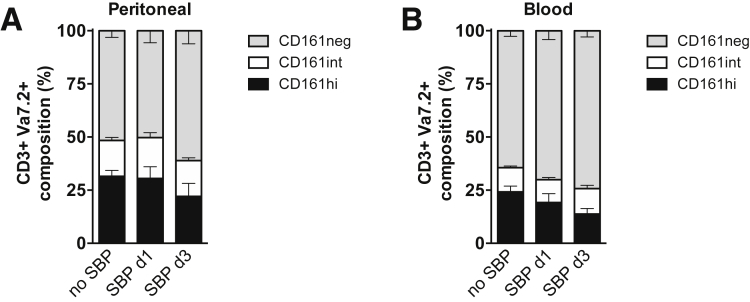
Together, our data indicate that MAIT cells from blood and AF differ in their ability to migrate to tissues, and pMAIT cells are configured to respond to proinflammatory chemotactic signals sensed by CCR5, CXCR3, and CCR6. Although the origin of pMAIT cells during SBP remains unknown, our data suggest the early recruitment from blood by chemotactic signals from infected ascites, followed by MR1-dependent activation and only short-term retention in the peritoneal cavity. We can only speculate whether other MAIT cell-rich tissues, such as liver and gut epithelium, contribute to the direct recruitment to the peritoneal cavity, and whether their subsequent reduction is a result of redistribution to other tissues or increased cell death. Because we observed a higher percentage of CD161-negative cells among CD3+ Vα7.2+ T cells at day 3 of SBP (Figure 11), down-regulation of CD161 may contribute to the relative depletion of CD3+ Vα7.2+ CD161hi T cells, which has been demonstrated in co-culture models with E coli in vitro.37
Figure 11.
CD161 expression on CD3+ Vα7.2+ T cells during SBP. CD161 expression of (A) peritoneal and (B) blood CD3+ Vα7.2+ T cells in patients with decompensated cirrhosis in absence of SBP, at day 1 and at day 3 of SBP. CD161 surface expression was stratified according to high (CD161hi), intermediate (CD161int), and absent (CD161neg) expression. Mean and standard error of the mean are shown.
We observed differences in the expression patterns of activation and immunomodulatory markers of peritoneal in contrast to circulating MAIT cells in cirrhosis. Such marked differences have been reported for colonic versus blood MAIT cells in healthy individuals,38 suggesting organ-specific activation and functionality. Stimulation with bacterial supernatants ex vivo confirmed previous studies on the dysfunctional nature of cMAIT cells as they responded with lower production of the proinflammatory cytokines TNF and IFN-γ in response to bacterial stimulation, which is consistent with previous observations in chronic liver disease.13,22 On the contrary, peritoneal cells from patients with advanced liver disease remained potent producers of IFN-γ and TNF after stimulation with bacterial supernatants or phorbol myristate acetate/ionomycin. Having shown that MAIT cells differ in function and phenotype when compared with tissue resident MAIT cells39 has important implications. First, the qualitative and quantitative assessment of the depleted cMAIT cell pool in cirrhosis is challenging and might underestimate compartmental and organ-resident MAIT cell function in cirrhosis. Second, pMAIT cells remain potent inflammatory cells with preserved antibacterial effector functions capable of fueling inflammatory complications even in end-stage liver disease.
In addition to increased pMAIT cell activation in the course of SBP, we show a direct activation of MAIT cells by bacterial products from sterile filtered infected AF in an MR1-restricted fashion, which suggests a direct role of microbial vitamin B–derived compounds in this process.17 In this capacity, they mirror liver-infiltrating peribiliary MAIT cells, whose activation is MR1-dependent but largely independent of IL-12 and IL-18.40 We propose that pMAIT cells constitute a crucial part of peritoneal immune surveillance to provide protection against spontaneous bacterial infections from the gut.4 Because of the enrichment of MAIT cells in gut and liver and their capacity to identify gut-derived bacteria and their products,39 the peritoneal migration, activation, and retention of MAIT cells may provide an important link between gut, liver, and the peritoneum. It is tempting to speculate that pMAIT cell activation and MAIT cell-derived inflammation might even drive extraperitoneal hepatic and systemic inflammation in decompensated cirrhosis. This hypothesis is supported by our observation of pMAIT cell activation correlating with higher MELD score and elevated white blood cell count, which are both surrogates of poor outcome in decompensated cirrhosis.41
There are certain limitations present in our investigation. For the majority of experiments performed, MAIT cells were defined as viable CD3+ CD161hi Vα7.2+ T cells, and TCR specificity by MR1 tetramers loaded with 5-OP-RU was only available for validation experiments. The analysis of the composition of the pMAIT cell subsets revealed the presence of CD161hi Vα7.2+ T cells nonreactive to the MR1 tetramer, suggesting that they contain conventional T cells expressing the Vα7.2 chain. Detailed MAIT cell analysis revealed a contamination with about 10% of CD8– CD4+ CD161hi Vα7.2+ MR1 tetramer-negative T cells. However, recent reports have shown only minor differences in transcription factor profiles of CD161hi Vα7.2+ T cells and MR1 tetramer+ MAIT cells.42 Future studies will be needed to investigate differences in the development and function of defined CD161hi Vα7.2+ T-cell subsets and their regulation during the onset and resolution of SBP. To reduce such confounding factors, paired investigations of CD3+ CD161hi Vα7.2+ T cells in the blood and in the peritoneum were performed, noncirrhotic controls were implemented, and differences in functional activation status were confirmed by blocking MR1.
Taken together, our findings provide evidence that pMAIT cells are functionally competent innate immune cells in decompensated cirrhosis, which accumulate in the early course of SBP. In contrast to their circulating exhausted counterparts, they retain effector functions and are preferentially recruited over conventional T cells to the peritoneal cavity in the context of SBP. We therefore propose a role of pMAIT cells for immune surveillance protecting from spontaneous gut-derived infections, thereby linking peritoneal immune activation with extraperitoneal inflammatory complications.
Methods
Patient Cohort and Healthy Donors
Patients with decompensated cirrhosis who underwent diagnostic ascites tap or therapeutic paracentesis at our hospital between April 2017 and December 2019 were eligible for this study. Exclusion criteria were evidence of secondary peritonitis, tuberculous peritonitis, peritoneal carcinomatosis, and human immunodeficiency virus coinfection. Patients were stratified for the presence of SBP by using current diagnostic criteria.43 Spontaneous fungal peritonitis was also considered SBP if secondary peritonitis was excluded. During the course of SBP, AF samples were collected as clinically indicated and recommended by guidelines.43 Thirteen patients with end-stage renal disease who underwent peritoneal dialysis catheter implantation or undergo continuous ambulatory peritoneal dialysis (CAPD) and 19 healthy blood donors were included as noncirrhotic controls. The study was approved by the Internal Review Board of the Jena University Hospital (Ethics committee of the Jena University Hospital, no 3683-02/3). Patients and donors gave written informed consent before inclusion. All authors had access to the study data and reviewed and approved the final manuscript.
Isolation of Mononuclear Cells
Up to 500 mL AF and 8 mL EDTA blood were collected from each patient. AF cells were enriched by centrifugation, and mononuclear cells were isolated from AF and corresponding blood by using Lympholyte-H separation media (Cedarlane, Burlington, Ontario, Canada) and washed in phosphate-buffered saline (Thermo Fisher Scientific, Waltham, MA). In selected experiments, T cells were enriched by magnetic cell separation using positive selection of CD3+ cells or negative selection of CD14– (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) for downstream analysis.
Cell Culture
Mononuclear cells were cultured in 96-well plates (3 × 105/well) using RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10% pooled heat-inactivated fetal bovine serum (PAN-Biotech GmbH, Aidenbach, Germany), 100 IU/mL penicillin/streptomycin, and 2.2 mmol/L L-glutamine (Thermo Fisher Scientific) at 37°C and 5% CO2. To assess the intracellular cytokine repertoire, cells were treated with 50 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin (Invitrogen, Carlsbad, CA) for 6 hours and brefeldin A (BD Biosciences, San Jose, CA) for 4 hours. To activate MAIT cells by bacterial products, cells were treated with sterile filtered bacterial supernatants at a final concentration of 20% in supplemented media. We used supernatants from 2 strains of riboflavin-producing bacteria, ie, E coli (ATCC 25922) and E coli (SO1), and 1 strain that has been shown not to induce MAIT cell activation,32 ie, Enterococcus faecalis (ATCC 29212). Bacteria were grown in 25 mL Luria Broth (Gibco/Thermo Fisher Scientific) at 37°C agitated at 160 rpm for 4–6 hours. The broth was sterile filtered through a 0.45-μm filter twice. In a further approach, mononuclear cells were incubated with sterile filtered AF from patients with and without SBP at a final concentration of 10%. In some experiments, mononuclear cells were incubated by using 10 μg/mL LEAF anti-MR1 blocking antibodies (clone 26.5) or the IgG2a isotype (Biolegend, San Diego, CA) for 1 hour before stimulation.
Immunoassay
Cytokines and chemokines were quantified in AF from patients with SBP and in the absence of SBP. TNF was determined by using human TNF-α ELISA Kit (Invitrogen), CCL20 was determined by using human CCL20/MIP-3 alpha Quantikine ELISA Kit (R & D Systems, Minneapolis, MN), IL-17, IFN-γ, CXCL10, and CCL5 (RANTES) were determined by using a customized human multi-analyte ELISArray kit (Qiagen, Hilden, Germany), all according to the manufacturer’s manual.
Cell Migration Assays
To assess the migratory properties, cMAIT cells were activated by using incubation with filtered E coli bacterial supernatants overnight, washed, and resuspended in RPMI 1640 medium supplemented with 0.1% bovine serum albumin (Thermo Fisher Scientific, Waltham, MA). Cells were seeded in the upper chamber of 24-well transwell plate inserts with a pore size of 3 μm at a density of 3 × 106 cells per well (Corning/Greiner, Frickenhausen, Germany). Mononuclear cells were allowed to migrate against a gradient of 150 ng/mL recombinant chemokines CXCL10, CXCL12, CCL5, or CCL20 (PeproTech, Hamburg, Germany) or against diluted AF from patients with or without SBP (33% final concentration in RPMI 1640 supplemented with 0.1% bovine serum albumin) for 4 hours at 37°C. Cells having migrated into the bottom chamber were collected, washed in phosphate-buffered saline, stained, and counted using flow cytometry.
Flow Cytometry
For the analysis of expression of surface antigens on leukocytes, cells were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) and incubated with fluorochrome-conjugated primary antibodies (Table 5) at optimal dilutions at 4°C in phosphate-buffered saline containing 2% fetal calf serum and 2 mmol/L EDTA. In some experiments, subsequent intracellular staining was performed by using the Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences) for cytokines and cytotoxic granules or the Transcription factor buffer set (BD Biosciences, San Jose, CA) for transcription factor expression analysis. Single color staining, isotype-matched controls, and fluorescence minus one controls were performed. MR1/5-OP-RU tetramers18 were obtained from the NIH tetramer core facility at Emory University (Atlanta, GA). The MR1 tetramer technology was developed jointly by Drs James McCluskey, Jamie Rossjohn, and David Fairlie, and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne.
Table 5.
List of Fluorochrome-Conjugated Primary Antibodies
| Antibody | Isotype | Company | Clone | Fluorochromes |
|---|---|---|---|---|
| CD4 | Mouse IgG1, κ | BD Pharmingen | RPA-T4 | APC |
| CD4 | Mouse IgG1, κ | Miltenyi | M-T466 | FITC |
| CD8 | Human IgG1 | Miltenyi | REA734 | APC |
| CD8 | Mouse IgG1, κ | BD Pharmingem | HIT8a | PE |
| CD8 | Mouse IgG1, κ | Biolegend | HIT8a | BV510 |
| CD3 | Mouse IgG2a, κ | Biolegend | OKT3 | BV421 |
| CD14 | Human IgG1 | Miltenyi | REA599 | PE |
| CD14 | Mouse IgG1, κ | Biolegend | HCD14 | BV421 |
| CD49d (beta 7 integrin) | Mouse IgG1, κ | BD Pharmingen | 9F10 | APC |
| CD69 | Mouse IgG1, κ | BD Pharmingen | N50 | APC |
| CD103 (alpha E integrin) | Mouse IgG1, κ | Biolegend | Ber-ACT8 | APC |
| CD161 | Mouse IgG1, κ | Biolegend | HP-3G10 | PE |
| CD161 | Mouse IgG1, κ | Biolegend | HP-3G10 | APC |
| CD183 (CXCR3) | Mouse IgG1, κ | BD Pharmingen | 1C6/CXCR3 | APC |
| CD195 (CCR5) | Mouse IgG2a | BD Pharmingen | 2D7/CCR5 | APC |
| CD196 (CCR6) | Mouse IgG1, κ | BD Pharmingen | 11A9 | APC |
| CD197 (CCR7) | Mouse IgG2a | Biolegend | G043H7 | APC |
| CD279 (PD-1) | Mouse IgG1, κ | Biolegend | EH12.2H7 | APC |
| GATA-3 | Human IgG1 | Miltenyi | REA174 | APC |
| Granzyme B | Mouse IgG1, κ | Biolegend | QA16A02 | APC |
| IFN-γ | Mouse IgG1, κ | Biolegend | 4S.B3 | APC |
| IL-17A | Mouse IgG1, κ | Biolegend | BL168 | APC |
| Ki-67 | Human IgG1 | Miltenyi | REA183 | APC |
| TNF alpha | Mouse IgG1, κ | Biolegend | MAb11 | APC |
| MR1 | Mouse IgG2a, κ | Biolegend | 26.5 | APC |
| MR1/6-FP | Tetramer | NIH tetramer core facility | APC/FITC | |
| MR1/5-OP-RU | Tetramer | NIH tetramer core facility | APC/FITC | |
| Perforin | Mouse IgG1 | Biolegend | B-D48 | APC |
| RORγ(t) | Human IgG1 | Miltenyi | REA278 | APC |
| T-bet | Human IgG1 | Miltenyi | REA102 | APC |
| TCR Vα7.2 | Mouse IgG1, κ | Biolegend | 3C10 | FITC |
| TCR Vα7.2 | Mouse IgG1, κ | Biolegend | 3C10 | APC |
Statistical Analysis
Data are expressed as median with interquartiles and visualized by using box plots unless otherwise indicated. Differences between groups were analyzed by the Fisher exact test, Wilcoxon signed-rank test for paired samples, or the Mann-Whitney U test for unpaired samples as appropriate. The Spearman rank correlation test was used to assess the nonparametric correlation between variables. Statistical analysis was performed by using SPSS versions 16 and 21 (IBM, Armonk, NY) and Prism 6 (GraphPad, La Jolla, CA). Owing to the exploratory nature of the study, we applied a two-sided significance level of P <.05 for tests without correction for multiple testing. All authors had access to the study data and had reviewed and approved the final manuscript. Systematic randomization and blinding were not performed.
Acknowledgments
The authors thank Kathrin Schulze and Lisa Jasef (Institute for Infectious Diseases and Infection Control, Jena University Hospital) for their excellent technical assistance.
Footnotes
Author contributions O.I.-O. performed the majority of the experiments and wrote the manuscript. S.S., N.K., and T.B. performed experiments on patient samples. P.A.R. and M.Bu. included patients and controls. S.Q. provided and analyzed clinical data. A.S. and M.Ba. assisted with the experimental design and the writing of the manuscript. O.I.-O. and T.B. performed statistical analyses. T.B. conceived of and supervised the study and provided guidance with experimental design and writing of the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This work was funded by grant DFGBR 4182/3-1 from the German Research Foundation. T.B. and M.Ba. were supported by grant BMBF01 E0 1002/1502 from the German Federal Ministry of Education and Research.
References
- 1.Caly W.R., Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 2.Borzio M., Salerno F., Piantoni L., Cazzaniga M., Angeli P., Bissoli F., Boccia S., Colloredo-Mels G., Corigliano P., Fornaciari G., Marenco G., Pistarà R., Salvagnini M., Sangiovanni A. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 3.Arvaniti V., D’Amico G., Fede G., Manousou P., Tsochatzis E., Pleguezuelo M., Burroughs A.K. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. 1256.e1–e5. [DOI] [PubMed] [Google Scholar]
- 4.Wiest R., Lawson M., Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Bonnel A.R., Bunchorntavakul C., Reddy K.R. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Bruns T., Zimmermann H.W., Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appenrodt B., Grünhage F., Gentemann M.G., Thyssen L., Sauerbruch T., Lammert F. Nucleotide-binding oligomerization domain containing 2 (NOD2) variants are genetic risk factors for death and spontaneous bacterial peritonitis in liver cirrhosis. Hepatology. 2010;51:1327–1333. doi: 10.1002/hep.23440. [DOI] [PubMed] [Google Scholar]
- 9.Nischalke H.D., Berger C., Aldenhoff K., Thyssen L., Gentemann M., Grünhage F., Lammert F., Nattermann J., Sauerbruch T., Spengler U., Appenrodt B. Toll-like receptor (TLR) 2 promoter and intron 2 polymorphisms are associated with increased risk for spontaneous bacterial peritonitis in liver cirrhosis. J Hepatol. 2011;55:1010–1016. doi: 10.1016/j.jhep.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Bruns T., Reuken P.A., Stengel S., Gerber L., Appenrodt B., Schade J.H., Lammert F., Zeuzem S., Stallmach A. The prognostic significance of bacterial DNA in patients with decompensated cirrhosis and suspected infection. Liver Int. 2016;36:1133–1142. doi: 10.1111/liv.13095. [DOI] [PubMed] [Google Scholar]
- 11.Mai M., Stengel S., Al-Herwi E., Peter J., Schmidt C., Rubio I., Stallmach A., Bruns T. Genetic variants of TRAF6 modulate peritoneal immunity and the risk of spontaneous bacterial peritonitis in cirrhosis: a combined prospective-retrospective study. Sci Rep. 2017;7:4914. doi: 10.1038/s41598-017-04895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peter J., Frey O., Stallmach A., Bruns T. Attenuated antigen-specific T cell responses in cirrhosis are accompanied by elevated serum interleukin-10 levels and down-regulation of HLA-DR on monocytes. BMC Gastroenterol. 2013;13:37. doi: 10.1186/1471-230X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riva A., Patel V., Kurioka A., Jeffery H.C., Wright G., Tarff S., Shawcross D., Ryan J.M., Evans A., Azarian S., Bajaj J.S., Fagan A., Patel V., Mehta K., Lopez C., Simonova M., Katzarov K., Hadzhiolova T., Pavlova S., Wendon J.A., Oo Y.H., Klenerman P., Williams R., Chokshi S. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67:918–930. doi: 10.1136/gutjnl-2017-314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey D.I., Uldrich A.P., McCluskey J., Rossjohn J., Moody D.B. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 15.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 16.Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 17.Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., Williamson N.A., Purcell A.W., Dudek N.L., McConville M.J., O'Hair R.A., Khairallah G.N., Godfrey D.I., Fairlie D.P., Rossjohn J., McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 18.Corbett A.J., Eckle S.B.G., Birkinshaw R.W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H., Williamson N.A., Strugnell R.A., Van Sinderen D., Mak J.Y., Fairlie D.P., Kjer-Nielsen L., Rossjohn J., McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 19.Ussher J.E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T.H., Klenerman P., Willberg C.B. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurioka A., Ussher J.E., Cosgrove C., Clough C., Fergusson J.R., Smith K., Kang Y.H., Walker L.J., Hansen T.H., Willberg C.B., Klenerman P. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottcher K., Rombouts K., Saffioti F., Roccarina D., Rosselli M., Hall A., Luong T., Tsochatzis E.A., Thorburn D., Pinzani M. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. 2018;68:172–186. doi: 10.1002/hep.29782. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Huang B., Jiang X., Chen W., Zhang J., Wei Y., Chen Y., Lian M., Bian Z., Miao Q., Peng Y., Fang J., Wang Q., Tang R., Gershwin M.E., Ma X. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front Immunol. 2018;9:1994. doi: 10.3389/fimmu.2018.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billerbeck E., Kang Y.-H., Walker L., Lockstone H., Grafmueller S., Fleming V., Flint J., Willberg C.B., Bengsch B., Seigel B., Ramamurthy N., Zitzmann N., Barnes E.J., Thevanayagam J., Bhagwanani A., Leslie A., Oo Y.H., Kollnberger S., Bowness P., Drognitz O., Adams D.H., Blum H.E., Thimme R., Klenerman P. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A. 2010;107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin E., Treiner E., Duban L., Guerri L., Laud H., Toly C. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:0525–0536. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundström P., Ahlmanner F., Akéus P., Sundquist M., Alsén S., Yrlid U., Börjesson L., Sjöling Å., Gustavsson B., Wong S.B., Quiding-Järbrink M. Human mucosa-associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN-γ. J Immunol. 2015;195:3472–3481. doi: 10.4049/jimmunol.1500258. [DOI] [PubMed] [Google Scholar]
- 26.Novak J., Dobrovolny J., Novakova L., Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol. 2014;80:271–275. doi: 10.1111/sji.12193. [DOI] [PubMed] [Google Scholar]
- 27.Lee O.J., Cho Y.N., Kee S.J., Kim M.J., Jin H.M., Lee S.J., Park K.J., Kim T.J., Lee S.S., Kwon Y.S., Kim N., Shin M.G., Shin J.H., Suh S.P., Ryang D.W., Park Y.W. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014;49:47–54. doi: 10.1016/j.exger.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Dagan-Berger M., Feniger-Barish R., Avniel S., Wald H., Galun E., Grabovsky V., Alon R., Nagler A., Ben-Baruch A., Peled A. Role of CXCR3 carboxyl terminus and third intracellular loop in receptor-mediated migration, adhesion and internalization in response to CXCL11. Blood. 2006;107:3821–3831. doi: 10.1182/blood-2004-01-0214. [DOI] [PubMed] [Google Scholar]
- 29.Meiser A., Mueller A., Wise E.L., McDonagh E.M., Petit S.J., Saran N., Clark P.C., Williams T.J., Pease J.E. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J Immunol. 2008;180:6713–6724. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neel N.F., Schutyser E., Sai J., Fan G.-H., Richmond A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16:637–658. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegde P., Weiss E., Paradis V., Wan J., Mabire M., Sukriti S., Rautou P.E., Albuquerque M., Picq O., Gupta A.C., Ferrere G., Gilgenkrantz H., Kiaf B., Toubal A., Beaudoin L., Lettéron P., Moreau R., Lehuen A., Lotersztajn S. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun. 2018;9:2146. doi: 10.1038/s41467-018-04450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bourhis L., Martin E., Peguillet I., Guihot A., Froux N., Coré M., Lévy E., Dusseaux M., Meyssonnier V., Premel V., Ngo C., Riteau B., Duban L., Robert D., Huang S., Rottman M., Soudais C., Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11 doi: 10.1038/ni.1890. 701–U66. [DOI] [PubMed] [Google Scholar]
- 33.Gold M.C., Cerri S., Smyk-Pearson S., Cansler M.E., Vogt T.M., Delepine J., Winata E., Swarbrick G.M., Chua W.J., Yu Y.Y., Lantz O., Cook M.S., Null M.D., Jacoby D.B., Harriff M.J., Lewinsohn D.A., Hansen T.H., Lewinsohn D.M. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ussher J.E., Klenerman P., Willberg C.B. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol. 2014;5:450. doi: 10.3389/fimmu.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liuzzi A.R., Kift-Morgan A., Lopez-Anton M., Friberg I.M., Zhang J., Brook A.C., Roberts G.W., Donovan K.L., Colmont C.S., Toleman M.A., Bowen T., Johnson D.W., Topley N., Moser B., Fraser D.J., Eberl M. Unconventional human T cells accumulate at the site of infection in response to microbial ligands and induce local tissue remodeling. J Immunol. 2016;197:2195–2207. doi: 10.4049/jimmunol.1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorfu G., Rivera-Nieves J., Ley K. Role of β7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leeansyah E., Ganesh A., Quigley M.F., Sönnerborg A., Andersson J., Hunt P.W., Somsouk M., Deeks S.G., Martin J.N., Moll M., Shacklett B.L., Sandberg J.K. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121:1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmaler M., Colone A., Spagnuolo J., Zimmermann M., Lepore M., Kalinichenko A., Bhatia S., Cottier F., Rutishauser T., Pavelka N., Egli A., Azzali E., Pieroni M., Costantino G., Hruz P., Sauer U., Mori L., De Libero G. Modulation of bacterial metabolism by the microenvironment controls MAIT cell stimulation. Mucosal Immunol. 2018;11:1060–1070. doi: 10.1038/s41385-018-0020-9. [DOI] [PubMed] [Google Scholar]
- 39.Kurioka A., Walker L.J., Klenerman P., Willberg C.B. MAIT cells: new guardians of the liver. Clin Transl Immunol. 2016;5:e98. doi: 10.1038/cti.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery H.C., van Wilgenburg B., Kurioka A., Parekh K., Stirling K., Roberts S., Dutton E.E., Hunter S., Geh D., Braitch M.K., Rajanayagam J., Iqbal T., Pinkney T., Brown R., Withers D.R., Adams D.H., Klenerman P., Oo Y.H. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol. 2016;64:1118–1127. doi: 10.1016/j.jhep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreau R., Jalan R., Gines P., Pavesi M., Angeli P., Cordoba J., Durand F., Gustot T., Saliba F., Domenicali M., Gerbes A., Wendon J., Alessandria C., Laleman W., Zeuzem S., Trebicka J., Bernardi M., Arroyo V. CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1–e9. [DOI] [PubMed] [Google Scholar]
- 42.Kurioka A., Jahun A.S., Hannaway R.F., Walker L.J., Fergusson J.R., Sverremark-Ekström E., Corbett A.J., Ussher J.E., Willberg C.B., Klenerman P. Shared and distinct phenotypes and functions of human CD161++ Vα7.2+ T cell subsets. Front Immunol. 2017;8:1031. doi: 10.3389/fimmu.2017.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Association for the Study of the Liver EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]