Abstract
Background
Cartilage degeneration during osteoarthritis (OA) most adversely affects the quality of life by hindering the movement. The present study investigated the role of verbascoside in the protection of cartilage degeneration induced by osteoarthritis.
Material/Methods
The enzyme-linked immunosorbent (ELISA) and western blot assays were used for determination of inflammatory cytokine secretion in serum and cartilage tissues, respectively.
Results
Treatment of the OA rats with verbascoside inhibited overproduction of interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β in serum as well as cartilage tissues. The expression of P2X7R and matrix metalloproteinase (MMP)-13 was much higher in the rats induced with OA. However, administration of verbascoside reversed the OA-induced upregulation of P2X7R and MMP-13 expression in the cartilage tissues. The OA-mediated increase in substance P (SP) and prostaglandin E2 (PGE2) expression was also reduced in the cartilage tissues by the verbascoside treatment. Western blot assay revealed that verbascoside treatment markedly decreased the activation of IκBα and NF-κB p65 in the OA rats.
Conclusions
Thus, verbascoside inhibited inflammatory cytokine secretion in the OA rats by targeting P2X7R expression, production of matrix metalloproteinase, PGE2 and downregulation of NF-κB signaling pathway. Therefore, verbascoside may be used as potent agent for osteoarthritis treatment.
MeSH Keywords: Cytokines, Matrix Metalloproteinase 10, Osteoarthritis
Background
Osteoarthritis (OA) is detected most commonly in the people belonging to over 45 years of age group and is characterized by the degradation of cartilage at joints, inflammation, and osteophyte formation [1,2]. Studies for understanding the mechanism of osteoarthritis and development of treatment are being conducted continuously [3–7]. There is degradation of proteoglycan, breakdown of collagen network and finally erosion of the cartilage which is associated with the joint stiffness, pain and loss of movement [5–11]. The proteolysis and decomposition of different components of extra cellular matrix are catalyzed by the overproduction of inflammatory cytokines, interleukins, matrix metalloproteinases (MMPs) [11,12]. MMPs are the endopeptidases which contain zinc and depend on the calcium ions for their activity [6]. Therefore, suppression of MMPs is believed to play a vital role in OA treatment. The interleukin (IL)-1β acts as the main inducer of osteoarthritis by leading to chondrocyte metabolic imbalance through upregulation of catabolic enzymes [13,14]. The overproduction of catabolic enzymes interferes with the normal physiological functioning of the chondrocytes [13–15]. During OA chondrocytes secrete cytokines such as interleukin (IL)-1β and IL-6, prostaglandins (PGs) and tumor necrosis factor (TNF)-α in much higher level compared to normal chondrocytes [16]. The cytokines secreted by the synovial membrane penetrate and erode the cartilage tissues leading to OA development [17]. The prostaglandin E2 (PGE2), a member of PG family also catalyzes erosion of cartilage by promoting MMP production [18]. The tissue inflammation and pain in OA patients is regulated the expression of a neuropeptide known as substance P (SP) [19]. The expression of P2X7R has been found markedly higher in the macrophages and injured spinal cord tissues [20,21]. It has been found that P2X7R is an important target for rheumatoid arthritis treatment [22,23]. Although clinicians are making enormous efforts to develop an effective treatment strategy for OA, but no successful results have been obtained so far. Therefore, present study was designed to investigate the protective role verbascoside against OA in the rat model. The study found that verbascoside prevented OA induced cartilage tissue damage through inhibition of cytokine production.
Material and Methods
Animals and ethics committee approval
The 60 male Wistar rats weighing 290–395 g (10–13 weeks old) were obtained from the Animal Center, Henan Province, laboratory, Zhengzhou, China. The rats were placed in wire-bottomed cages individually in the animal center under a constant temperature of 25±2°C and 50–60% humidity. The rats were provided access to tap water plus standard pellet diet and exposed to 12-hour light/dark cycles. The Ethics Committee of The Xi’an Hospital, Weiyang, Shaanxi Province, China approved the study. The experiments were conducted in accordance with the guidelines from National Institute of Health, China.
Osteoarthritis induction and treatment strategy
Osteoarthritis was induced in the rats by injecting 5 mg/kg solution of monosodium iodoacetate (MIA) in normal saline after anaesthetization with sodium 5% sorbitol through intra-articular route. The Wistar rats were divided into 6 groups containing 10 rats in each group; OA group, normal control, 5 mg/kg, 10 mg/kg, 15 mg/kg and 20 mg/kg verbascoside treatment groups. Among the 6 groups OA was established in 5 groups including OA group, 5 mg/kg, 10 mg/kg, 15 mg/kg, and 20 mg/kg treatment groups. The treatment groups were administered 5, 10, 15, and 20 mg/kg doses of verbascoside after 2 days of MIA injection by intraperitoneal route alternately for 2 weeks. The rats in the normal control and OA groups were administered equal volumes of normal saline alone.
Determination of cytokine levels in rat serum
The rats were sacrificed on day 28 of MIA injection after sodium sorbitol anaesthetization for determination of cytokine levels in serum. In brief, the blood samples of the rats were collected from the carotid artery and subjected to centrifugation for 25 minutes at 4000×g. The isolated supernatant was stored for measurement of IL-1β, IL-6, and TNF-α content at −80°C. The commercially available enzyme-linked immunosorbent assay (ELISA) kits were used for measurement of IL-1β, IL-6, and TNF-α in the rat serum in accordance with the manufacturer instructions.
Western blot analysis
The articular cartilage of the Wistar rats at the knee joint was excised carefully after sacrificing under sodium sorbitol anesthesia. The excised cartilage was cut into thin sections and then homogenized in the RIPA buffer [sodium chloride (150 Mm), NP-40 (1%), sodium deoxycholate (0.5%), SDS (0.2%), Tris-hydrochloride (50 mM at pH 8.0), EDTA (10 mM) and PMSF (1 mM; Sigma, Chemical, St. Louis, MO, USA) for 45 minutes at 4°C. The lysate was freed of the debris by centrifugation for 10 minutes at 12 000×g at 4°C. The concentration of proteins in the supernatant was measured by BCA protein assay kit (Beyotime Institute of Biotechnology, Nanjing, China) according to manual protocol. The protein samples (20–30 μg) were resolved by electrophoresis on 8–10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and subsequently electroblotted onto polyvinylidene fluoride (PVDF) membranes. The membrane blocking was achieved by incubation with 3% non-fat milk at 37°C for 2.5 hours. The incubation of membranes with primary antibodies was carried out for overnight at 4°C. The antibodies used were: anti-IL-6 (ab6672), anti-TNF-α (ab9635), anti-P2X7R (ab48871), anti-MMP-13 (ab39012), anti-SP (ab67006), anti-PGE2 (ab2318), anti-IL-1β (ab9787), anti-p-NF-κBp65 (ab86299), anti-NF-κBp65 (ab16502), anti-IκBα (sc-371), anti-p-IκBα (ab59195) and anti-GAPDH (G9545; all from Abcam, Cambridge, UK). Then incubation was carried out at room temperature with HRP-conjugated secondary antibodies for 2 hours. The enhanced chemiluminescence detection system was employed for visualization of the bands and Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was used for densitometry analysis.
Statistical analysis
The data expressed are the mean of ±standard deviation (SD) of triplicate experiments. The statistical analysis of the data was performed by one-way analysis of variance (ANOVA) and Bonferroni tests. The SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Differences were taken significant statistically at P<0.05.
Results
Inhibitory effect of verbascoside on serum cytokine levels in OA-rats
In the serum of OA-rats the levels of inflammatory cytokines after verbascoside treatment were measured using ELISA assays (Figure 1). It was found that OA-induced secretion of IL-6, TNF-α and IL-1β in the serum of rats markedly suppressed than those of normal group. However, the OA-induced levels of IL-6, TNF-α, and IL-1β were reduced by verbascoside treatment significantly (P<0.05). Among the 5, 10, 15, and 20 mg/kg treatment groups, the OA-mediated secretion of IL-6, TNF-α, and IL-1β levels were completely inhibited on treatment with 20 mg/kg doses of verbascoside.
Figure 1.
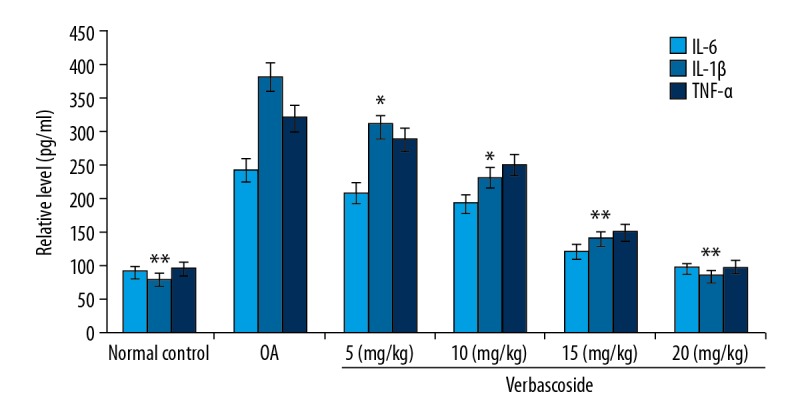
Effect of verbascoside on cytokine levels in osteoarthritis (OA)-rat serum. The OA-rats were treated with 5, 10, 15, and 20 mg/kg doses of verbascoside and then IL-6, TNF-α, and IL-1β levels were measured by enzyme-linked immunosorbent assay (ELISA). * P<0.05 and ** P<0.02 versus OA-group.
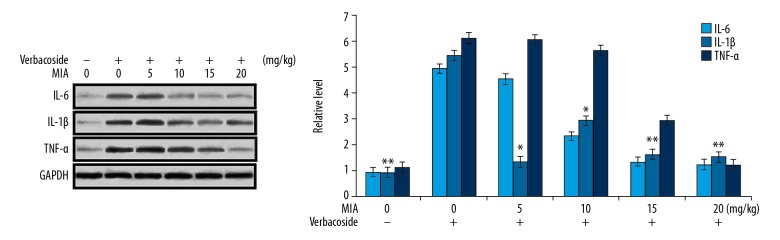
Verbascoside ameliorated OA-induced cytokines in the rat cartilage of knee tissues
The suppression of IL-6, TNF-α, and IL-1β levels by verbascoside in the knee cartilage of OA-rats was determined by western blot assay (Figure 2). The data showed that the levels of cytokines were markedly higher in the OA-rats than those of normal control rats. On the other hand, verbascoside treatment of the OA-rats ameliorated the increase of IL-6, TNF-α, and IL-1β levels. Although the reduction of OA-induced cytokines was observed in all the 4 treatment groups, but the effect was maximum in 20 mg/kg treatment group.
Figure 2.
Effect of verbascoside on cytokine level in osteoarthritis (OA)-rat knee cartilage. Treatment of the OA-rats with 5, 10, 15, and 20 mg/kg doses of verbascoside was followed by analysis of IL-6, TNF-α, and IL-1β levels in knee cartilage by western blotting. * P<0.05 and ** P<0.02 versus OA-group.
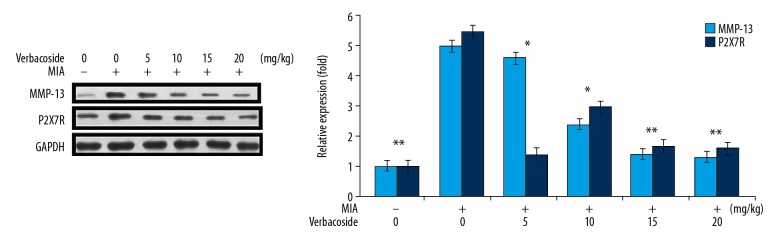
Inhibition of P2X7R and MMP-13 expression by verbascoside in OA-rats
The western blot showed a markedly higher expression of P2X7R and MMP-13 in the cartilage tissues of the OA-rats (Figure 3). However, treatment with 5, 10, 15, and 20 mg/kg doses of verbascoside reversed the OA-induced upregulation of P2X7R and MMP-13 expression. Again, the downregulatory effect of verbascoside on OA-mediated P2X7R and MMP-13 expressions was maximum at 20 mg/kg doses.
Figure 3.
Effect of verbascoside on P2X7R and MMP-13 expression in osteoarthritis (OA)-rats. The OA-rats were treated with 5, 10, 15, and 20 mg/kg doses of verbascoside and then western blotting was used for assessment of P2X7R and MMP-13 expression in knee cartilage tissues. * P<0.05 and ** P<0.02 versus OA-group.
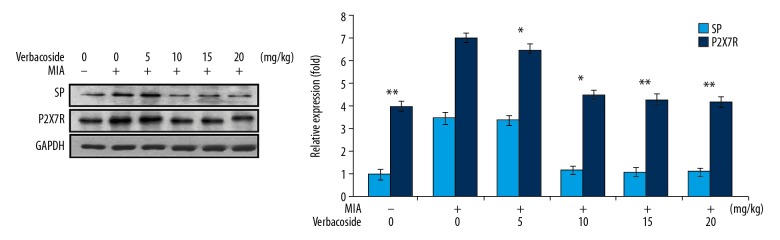
Inhibition of SP and PGE2 expression by verbascoside in OA-rats
It was found that expression of SP and PGE2 was markedly higher in the cartilage tissues of the OA-rats (Figure 4). Treatment with 5, 10, 15, and 20 mg/kg doses of verbascoside exhibited inhibitory effect on OA-induced SP and PGE2 upregulation.
Figure 4.
Effect of verbascosideon substance P (SP) and prostaglandin E2 (PGE2) expression in osteoarthritis (OA)-rats. The OA-rats were treated with 5, 10, 15, and 20 mg/kg doses of verbascoside and then western blotting was used for assessment of substance P (SP) and prostaglandin E2 (PGE2) expression in knee cartilage tissues. * P<0.05 and ** P<0.02 versus OA-group.
Inhibition of NF-κB signaling pathway by verbascoside in OA-rats
The expression of p-IκBα and p-NF-κB p65 in the OA-rats was much higher in comparison to the normal control rats (Figure 5). Western blot assay showed that verbascoside treatment at 5, 10, 15 and 20 mg/kg doses markedly decreased the expression of p-IκBα and p-NF-κB p65. The OA-induced increase of p-IκBα and p-NF-κB p65 expression was decreased almost completely by verbascoside at 20 mg/kg doses.
Figure 5.
Effect of verbascoside on p-IκBα and p-NF-κB p65 in osteoarthritis (OA)-rats. The changes in expression of p-IκBα and p-NF-κB p65 by 5, 10, 15, and 20 mg/kg doses of verbascoside was assessed in OA rats by western blot assay. * P<0.05 and ** P<0.02 versus OA-group.
Discussion
In the present study preventive role of verbascoside against osteoarthritis was investigated by measuring the changes in cytokines, P2X7R, MMP, PGE2 and NF-κB. The study demonstrated that verbascoside downregulated levels of cytokines, P2X7R, MMP and PGE2 through inhibition of NF-κB signaling pathway in OA-rats.
Inflammation of joints is usually followed by the articular cartilage decomposition, subchondral bone degradation and hyperplasia of the synovial tissues in OA patients [24]. The OA induced joint inflammation is accompanied by neuronal activation which leads to acute pain and involves the chondrocytes [16]. During OA, the chondrocytes produce inflammatory cytokines in higher levels in comparison to those of the normal cases [17]. Studies have found overproduction of TNF-α, nitric oxide, and IL-8/6/α in the chondrocytes of OA patients [17]. In consistence with the earlier reports the presents study found markedly higher levels of IL-6, TNF-α, and IL-1β in the serum of OA rats. The article cartilage tissues were also found to secrete higher levels of these inflammatory cytokines in the OA rats. However, verbascoside exhibited inhibitory effect on OA induced overproduction of IL-6, TNF-α, and IL-1β. Treatment of the rats with verbascoside reversed the up-regulatory effect of OA on IL-6, TNF-α, and IL-1β inflammatory cytokines. These findings suggested anti-osteoarthritic potential of verbascoside in the rat model. It is reported that production of inflammatory cytokines in the mouse mast cells is promoted by P2X7R activation [25]. The levels of IL6/-1β and TNF-α was also markedly increased in the immune cells of rats by P2X7R activation [26]. Moreover, TNF-α blocking or silencing in different animal models has been found to inhibit inflammation [27–29]. The MMP-13 is secreted only in the cartilage of OA patients and is therefore considered to be indicator for the cartilage degradation [30–33]. There is chondrocyte apoptosis and decomposition of cartilage in the patients following MMP activation [32,34]. In the present study expression of P2X7R and MMP-13 in articular cartilage was markedly higher OA rats compared to the control group. However, verbascoside treatment inhibited the OA induced overexpression of P2X7R and MMP-13 in the cartilage tissues. This suggested that verbascoside inhibited inflammatory cytokine production in OA rats by downregulation of P2X7R expression.
The PGE2 catalyzes degradation of the cartilage in OA patients by promoting the generation of matrix metalloproteinase and aggrecanases [18]. In the present study the production of PGE2 was markedly higher in cartilage tissues of OA tissues. Treatment with verbascoside caused a marked reduction in the level of PGE2 in the cartilage tissues of rats with OA. It has been found that overproduction of IL-1β in the chondrocytes leads to activation of NF-κB pathway [35–37]. The NF-κB activation is also triggered by P2X7R which then leads to the development of various inflammatory diseases including osteoarthritis [38]. In the present study IκBα and NF-κB p65 phosphorylation was markedly promoted by OA induction in the rats. Treatment with verbascoside inhibited phosphorylation of IκBα and NF-κB p65 in the OA rat cartilage tissues.
Conclusions
In summary, the present study showed that verbascoside suppressed inflammatory cytokine secretion in the OA rats by targeting the expression of P2X7R, production of MMP, PGE2, and downregulation of NF-κB signaling pathway. Thus, verbascoside may be used as chemotherapeutic candidate for the treatment of osteoarthritis.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Lajeunesse D, Martel Pelletier J, Fernandes JC, et al. Treatment with licofelone prevents abnormal subchondral bone cell metabolism in experimental dog osteoarthritis. Ann Rheum Dis. 2004;63:78–83. doi: 10.1136/ard.2002.003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: Rational approach to treating the individual. Best Pract Res Clin Rheumatol. 2006;20:721–40. doi: 10.1016/j.berh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Gongming G, Huimin D, Chao Z, Weimin F. Effects of hesperidin on H2O2-treated chondrocytes and cartilage in a rat osteoarthritis model. Med Sci Monit. 2018;24:9177–86. doi: 10.12659/MSM.913726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong R, Yueju L, Bing Z, et al. A novel design of a plate for posterolateral tibial plateau fractures based on computed tomography mapping of the proximal tibiofibular joint. Med Sci Monit. 2018;24:9300–6. doi: 10.12659/MSM.911738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H, Sung-Hyoun C, Bo-Kyung S, Byung-Jun C. Effect of resistance exercise on serum osteoprotegerin levels and insulin resistance in middle-aged women with metabolic syndrome. Med Sci Monit. 2018;24:9385–91. doi: 10.12659/MSM.911548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhiwang Z, Lin W, Kaijun H, Yu L. Characteristics of plantar loads during walking in patients with knee osteoarthritis. Med Sci Monit. 2017;23:5714–19. doi: 10.12659/MSM.905136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z, Xiaofei L, Heng H, et al. Cross-coupling effects of silencing of cyclooxygenase-2 (COX-2)/aggrecanase-1 and over-expressed insulin-like growth factor 1 (IGF-1) in an osteoarthritis animal model. Med Sci Monit. 2017;23:5302–10. doi: 10.12659/MSM.907150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobasheri A. Osteoarthritis year 2012 in review: Biomarkers. Osteoarthritis Cartilage. 2012;20:1451–64. doi: 10.1016/j.joca.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Daans M, Lories RJ, Luyten FP. Dynamic activation of bone morphogenetic protein signaling in collagen induced arthritis supports their role in joint homeostasis and disease. Arthritis Res Ther. 2008;10:R115. doi: 10.1186/ar2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeser RF. Molecular mechanisms of cartilage destruction in osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8:303–6. [PubMed] [Google Scholar]
- 12.Loeser RF. Molecular mechanisms of cartilage destruction: Mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–60. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldring MB. Osteoarthritis and cartilage: The role of cytokines. Curr Rheumatol Rep. 2000;2:459–65. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 14.Largo R, Alvarez-Soria MA, Díez-Ortego I, et al. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11:290–98. doi: 10.1016/s1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 15.Fan Z, Bau B, Yang H, et al. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum. 2005;52:136–43. doi: 10.1002/art.20725. [DOI] [PubMed] [Google Scholar]
- 16.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier JP, Fernandes JC, Jovanovic DV, et al. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: Role of cyclooxygenase-2 and inducible nitric oxide synthase. J Rheumatol. 2001;28:2509–19. [PubMed] [Google Scholar]
- 18.Bar-Or D, Rael LT, Thomas GW, Brody EN. Inflammatory pathways in knee osteoarthritis: Potential targets for treatment. Curr Rheumatol Rev. 2015;11:50–58. doi: 10.2174/1573397111666150522094131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam FF, Ng ES. Substance P and glutamate receptor antagonists improve the anti-arthritic actions of dexamethasone in rats. Br J Pharmacol. 2010;159:958–69. doi: 10.1111/j.1476-5381.2009.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dray A, Read SJ. Arthritis and pain. Future targets to control osteoarthritis pain. Arthritis Res Ther. 2007;9:212. doi: 10.1186/ar2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang K, Zhuang Y, Yan M, et al. Effects of riluzole on P2X7R expression in the spinal cord in rat model of neuropathic pain. Neurosci Lett. 2016;618:127–33. doi: 10.1016/j.neulet.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 22.McInnes IB, Cruwys S, Bowers K, Braddock M. Targeting the P2X7 receptor in rheumatoid arthritis: Biological rationale for P2X7 antagonism. Clin Exp Rheumatol. 2014;32:878–82. [PubMed] [Google Scholar]
- 23.Portales-Cervantes L, Niño-Moreno P, Doníz-Padilla L, et al. Expression and function of the P2X(7) purinergic receptor in patients with systemic lupus erythematosus and rheumatoid arthritis. Hum Immunol. 2010;71:818–25. doi: 10.1016/j.humimm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Konttinen YT, Sillat T, Barreto G, et al. Osteoarthritis as an autoinflammatory disease caused by chondrocyte-mediated inflammatory responses. Arthritis Rheum. 2012;64:613–16. doi: 10.1002/art.33451. [DOI] [PubMed] [Google Scholar]
- 25.Lister MF, Sharkey J, Sawatzky DA, et al. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourine AV, Poputnikov DM, Zhernosek N, et al. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005;146:139–45. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catal F, Mete E, Tayman C, et al. A human monoclonal anti-TNF alpha antibody (adalimumab) reduces airway inflammation and ameliorates lung histology in a murine model of acute asthma. Allergol Immunopathol (Madr) 2015;43:14–18. doi: 10.1016/j.aller.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Johnsen-Soriano S, Sancho-Tello M, Arnal E, et al. Comparison of the acute effects of anti-TNF-alpha drugs on a uveitis experimental model. Ocul Immunol Inflamm. 2010;18:208–15. doi: 10.3109/09273940903521964. [DOI] [PubMed] [Google Scholar]
- 29.Grounds MD, Davies M, Torrisi J, et al. Silencing TNFalpha activity by using Remicade or Enbrel blocks inflammation in whole muscle grafts: An in vivo bioassay to assess the efficacy of anti-cytokine drugs in mice. Cell Tissue Res. 2005;320:509–15. doi: 10.1007/s00441-005-1102-z. [DOI] [PubMed] [Google Scholar]
- 30.Li NG, Shi ZH, Tang YP, et al. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem. 2011;18:977–1001. doi: 10.2174/092986711794940905. [DOI] [PubMed] [Google Scholar]
- 31.Malemud CJ, Islam N, Haqqi TM. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs. 2003;174:34–48. doi: 10.1159/000070573. [DOI] [PubMed] [Google Scholar]
- 32.Schlomann U, Wildeboer D, Webster A, et al. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002;277:48210–19. doi: 10.1074/jbc.M203355200. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berg WB. Osteoarthritis year 2010 in review: Patho mechanisms. Osteoarthritis Cartilage. 2011;19:338–41. doi: 10.1016/j.joca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Chubinskaya S, Kuettner KE, Cole AA. Expression of matrix metalloproteinases in normal and damaged articular cartilage from human knee and ankle joints. Lab Invest. 1999;79:1669–77. [PubMed] [Google Scholar]
- 35.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappa B-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YJ, Tsai KS, Chan DC, et al. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J Orthop Res. 2014;32:573–80. doi: 10.1002/jor.22577. [DOI] [PubMed] [Google Scholar]
- 37.Ding Q, Zhong H, Qi Y, et al. Anti-arthritic effects of crocin in interleukin-1β-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm Res. 2013;62:17–25. doi: 10.1007/s00011-012-0546-3. [DOI] [PubMed] [Google Scholar]
- 38.He C, Chen X, Zhao C, et al. Eleutheroside E ameliorates arthritis severity in collagen-induced arthritis mice model by suppressing inflammatory cytokine release. Inflammation. 2014;37:1533–43. doi: 10.1007/s10753-014-9880-7. [DOI] [PubMed] [Google Scholar]