Abstract
Background
Evidence about COVID-19 on cardiac injury is inconsistent.
Objectives
We aimed to summarize available data on severity differences in acute cardiac injury and acute cardiac injury with mortality during the COVID-19 outbreak.
Methods
We performed a systematic literature search across Pubmed, Embase and pre-print from December 1, 2019 to March 27, 2020, to identify all observational studies that reported cardiac specific biomarkers (troponin, creatine kinase–MB fraction, myoglobin, or NT-proBNP) during COVID-19 infection. We extracted data on patient demographics, infection severity, comorbidity history, and biomarkers during COVID-19 infection. Where possible, data were pooled for meta-analysis with standard (SMD) or weighted (WMD) mean difference and corresponding 95% confidence intervals (CI).
Results
We included 4189 confirmed COVID-19 infected patients from 28 studies. More severe COVID-19 infection is associated with higher mean troponin (SMD 0.53, 95% CI 0.30 to 0.75, p < 0.001), with a similar trend for creatine kinase–MB, myoglobin, and NT-proBNP. Acute cardiac injury was more frequent in those with severe, compared to milder, disease (risk ratio 5.99, 3.04 to 11.80; p < 0.001). Meta regression suggested that cardiac injury biomarker differences of severity are related to history of hypertension (p = 0.030). Also COVID19-related cardiac injury is associated with higher mortality (summary risk ratio 3.85, 2.13 to 6.96; p < 0.001). hsTnI and NT-proBNP levels increased during the course of hospitalization only in non-survivors.
Conclusion
The severity of COVID-19 is associated with acute cardiac injury, and acute cardiac injury is associated with death. Cardiac injury biomarkers mainly increase in non-survivors. This highlights the need to effectively monitor heart health to prevent myocarditis in patients infected with COVID-19.
Abbreviations and acronyms: ACE2, angiotensin converting enzyme 2; CI, confidence intervals; CK-MB, creatine kinase–MB; COVID-19, coronavirus disease 2019; hsTnI, hypersensitive troponin I; NT-proBNP, N-terminal pro–B-type natriuretic peptide; RR, risk ratio; SARS-CoV, severe acute respiratory syndrome coronavirus; SMD, standard mean difference; WMD, weighted mean difference
Keywords: Coronavirus, COVID-19, Cardiac injury, Mortality
Graphical abstract
Introduction
Up to Mar 29, 2020, there were 665,361 (82,342 in China and 583,019 outside of China) confirmed cases of novel coronavirus (COVID-2019) infection reported worldwide, causing 30,864 deaths (http://www.nhc.gov.cn). The target of COVID-2019, angiotensin converting enzyme 2(ACE2), is expressed in the heart, esophagus, kidney, bladder and ileum as well as the alveoli.1 Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) has many similarities to COVID-2019 and has been shown in an animal model to mediate myocardial inflammation and damage through down-regulation of myocardial ACE2. Direct effects of SARS-CoV on the myocardium may have been responsible for adverse cardiac outcomes in patients with SARS2 and the myocardium may be directly affected by COVID-2019.1 Recently, two case reports from China3 and Italy4 have found that COVID-2019 could cause fulminant myocarditis, even without symptoms and signs of interstitial pneumonia. But whether acute cardiac injury is common and whether it is associated with death is still unclear. We thus undertook a systemic search of the literature for evidence of cardiac injury among individuals infected with COVID-2019.
Methods
Search strategy and selection criteria
The systematic review is reported following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.5 No language restrictions were imposed on the search. We searched the electronic databases Pubmed, and Embase from Dec 12,019 to Mar 272,020 using the keywords “coronavirus”, “pneumonia”, “nCoV”, “HCoV”, “SARS-CoV-2”, “COVID*”, “NCP*”, alone and in combinations as well as medRxiv (https://www.medrxiv.org), SSRN (https://www.ssrn.com) and the reference lists of all studies identified. Eligible studies were those that reported cardiac specific biomarkers (troponin, creatine kinase–MB fraction, myoglobin, or NT-proBNP) or acute cardiac injury. Any studies reporting cardiac specific biomarkers or acute cardiac injury according to severity of COVID-19, or mortality according to cardiac injury, were included. Studies of the same hospital with period within range of other studies, or with data that could not be reliably extracted were excluded.
Data collection and extraction
TH and JL scrutinized the titles and abstracts of the 2961 reports identified and excluded clearly irrelevant studies. Two authors (TH and JL) then did independent review of the full reports for the remaining 553 studies and extracted data independently from the 28 with relevant information (eFig. 1 in Supplementary material). Any disagreements were resolved by a third author (YC). When estimates were only presented graphically, we used the software g3data (version 1.51, www.frantz.fi/software/g3data.php) to extract estimates.
Data analysis
For analysis of cardiac injury biomarkers, first standard mean differences (SMD) were calculated for all biomarkers using mean (SD) values and group size, and pooled using random effects models. Then, separate meta-analysis was carried out, with SMD using for troponin (troponin I and T, or high sensitivity troponin I and T), and weighted mean differences (WMD) for other biomarkers. Summary relative risks (RRs) with 95% CIs were estimated for the association between acute cardiac injury and death. The degree of heterogeneity was assessed using the I2-index. An I2 statistic was considered to reflect low likelihood (0%–25%), moderate likelihood (26%–75%), and high likelihood (76%–100%) of differences beyond chance, as was a p value, from a Q test of heterogeneity, of less than or equal to 0.05.6 If the results were homogeneous (I2 < 50% and p > 0.05), fixed effects models were utilized, whereas if these results were heterogeneous (I2 ≥ 50% or p ≤ 0.05), then random effect models were used. If only median and interquartile range (Q25, Q75) were reported, then we assumed the median was equal to the mean and that the standard deviation (SD) was (Q75-Q25)/2. We also conducted meta-regression to assess whether severity differences in cardiac injury biomarkers were modified by patient characteristics: age, sex, smoking, diabetes, hypertension, history of cardiovascular diseases, coronary heart disease, cerebrovascular diseases, chronic obstructive pulmonary diseases, chronic kidney disease, and severity definitions of COVID-19. In this meta-regression analysis cardiac injury biomarkers are selected with priority troponin>CK-MB > myogloblin>NT-proBNP for each study, for example if one study reported troponin and CK-MB, we used troponin as the outcome and used the standard mean difference model to combine the results. Evidence of publication bias was examined using Egger's regression test for funnel asymmetry, in addition to visual inspection of the funnel plots. Combined means of hsTroponin I and NT-proBNP were calculated as the sum, over studies, of the mean value of the biomarker and n is the number of participants. Data were summarised using inverse variance weighted meta-analysis and a 2-sided p value of less than or equal to 0.05 was deemed significant. Statistical analysis was performed with Stata, version 15.1.
Results
Literature search and study characteristics
There were 28 reports of 4189 patients included in 22 studies that provided data describing effects on myocardial injury.7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29. Nine reported the association of acute cardiac injury with death13 , 17 , 20 , 21 , 24 , 28 , 30., 31., 32. and 4 reported dynamic changes of cardiac biomarkers during hospitalization20 , 32., 33., 34.. Three included duplicate information from the same participants35., 36., 37. and were not excluded. Among the included studies reporting effect of infection severity on cardiac injury, two studies compared values between those admitted to intensive care unit (ICU) and those not,8 , 12 another 10 compared values between non-survivors with survivors, and the remainder (10 studies) compared severe versus non-severe cases. Fourteen studies reported data from patients in Wuhan, where the epidemic first emerged. Those with more severe disease were somewhat older, fewer women than men, and had higher prevalence of coexisting disorders (Table 1 ).
Table 1.
Characteristics of included studies comparing more severe and less severe cases.
| Author | No. | Period | Age (yr) | Female | Concomitant chronic diseases |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | Hypertension | Cardiovascular | Cerebral | |||||||||||
| Wuhan | ||||||||||||||
| Huang et al8 | 41 | 2019/12/16–2020/01/02 | 49 | 49 | 15 | 32 | 8 | 25 | 15 | 14 | 23 | 11 | ||
| Wang et al12 | 138 | 2020/01/01–2020/01/28 | 66 | 51 | 39 | 48 | 22 | 6 | 58 | 22 | 25 | 11 | 17 | 1 |
| Zhou et al14 | 34 | 2020/02/05–2020/02/13 | 67 | 63 | 38 | 54 | ||||||||
| Yang et al13 | 52 | 2019/12/24–2020/01/26 | 65 | 52 | 34 | 30 | 22 | 10 | 9 | 10 | ||||
| He et al17 | 54 | 2020/02/3–2020/02/24 | 70 | 66.5 | 38.5 | 35.7 | 19.2 | 10.7 | ||||||
| Peng et al19 | 112 | 2020/1/20–2020/2/15 | 57.5 | 62 | 43.75 | 54.17 | 25 | 19.79 | 62.5 | 85.42 | 62.5 | 54.17 | ||
| Zhou et al20 | 191 | 2019/12/29–2020/01/31 | 69 | 52 | 30 | 41 | 31 | 14 | 48 | 23 | 24 | 1 | ||
| Fu et al21 | 200 | 2020/1/1–2020/1/30 | 52.9 | 50 | 76.5 | 66.9 | 64.7 | 47.5 | ||||||
| Ruan et al23 | 150 | NA | 67 | 50 | 28 | 35 | 18 | 16 | 43 | 28 | 19 | 0 | 10 | 6 |
| Han et al26 | 47 | 2020/2/1–2020/2/18 | 65.08 | 64.74 | 29.16 | 60.87 | 16.67 | 13.04 | 41.67 | 34.78 | 16.67 | 4.35 | ||
| Chen et al24 | 274 | 2020/1/13–2020/02/12 | 68 | 51 | 27 | 45 | 21 | 14 | 48 | 24 | 14 | 4 | 4 | 0 |
| Chen et al25 | 150 | 2020/1/1–2020/02/29 | 68.5 | 57.1 | 25 | 47.7 | 20.8 | 11.9 | 58.3 | 27.8 | 25 | 2.4 | ||
| Zhang et al28 | 48 | 2019/12/25–2020/02/15 | 78.65 | 66.16 | 29.4 | 32.3 | 29.4 | 16.1 | 70.6 | 64.5 | 23.5 | 29 | 35.3 | 16.1 |
| Hu et al29 | 323 | 2020/1/8–2020/02/20 | 70 | 58 | 41.3 | 50.4 | 30.2 | 10.8 | 42.9 | 30 | 3.2 | 1.9 | ||
| Outside Wuhan | ||||||||||||||
| Ji et al9 | 49 | 2020/01/20–2020/02/16 | 57 | 38 | 33 | 38 | ||||||||
| Cai et al7 | 298 | 2020/01/11–2020/02/09 | 64 | 40 | 57 | 46 | ||||||||
| Lu et al11 | 265 | up to 2020/02/7 | 27 | 6 | 46 | 17 | 18 | 4 | 5 | 0.4 | ||||
| Hui et al15 | 41 | 2020/01/21–2020/02/03 | 0 | 57 | ||||||||||
| Wang et al16 | 242 | 2020/01/17–2020/02/20 | 57 | 41 | 45.9 | 52.5 | 10.8 | 5.4 | 37.8 | 10.7 | 13.5 | 2 | 5.4 | 2 |
| Wan et al18 | 135 | 2020/01/23–2020/02/8 | 56 | 44 | 47.5 | 45.3 | 22.5 | 3.1 | 10 | 9.4 | 15 | 1 | ||
| Gong et al22 | 189 | 2020/1/20–2020/3/2 | 63.5 | 45 | 42.9 | 55.3 | ||||||||
| Ma et al27 | 84 | 2020/1/21–2020/03/2 | 58 | 46.5 | 40 | 43.8 | 35 | 4.7 | 20 | 12.5 | 10 | 4.7 | 10 | 3.1 |
Data are mean or %. Under each item, the first column is for more severe group, the second column is for less severe group.
The association of severity with cardiac injury
Overall, cardiac injury biomarkers were higher in severe compared to less severe cases (SMD 0.69, 95% confidence interval [CI] 0.48 to 0.89, p < 0.001) (Fig. 1). Mean troponin was higher in severe, compared to less severe cases (SMD 0.53, 95% CI 0.30 to 0.75, p < 0.001) as was mean creatine kinase–MB (WMD 1.16, 95% CI 0.73 to 1.59 U/L, p < 0.001) and NT-proBNP (WMD 430.2, 95% CI 109.6 to 750.8; p = 0.009) but not myoglobin (WMD 75.4, 95% CI -0.67 to 151.4 ng/mL; p = 0.052) (eFig. 2, eFig. 3, eFig. 4). Acute cardiac injury, defined as a troponin above upper limit, except in three studies which combined with ECG or echocardiographic abnormalities,8 , 12 , 20 was more frequent in those with severe, compared to mild, disease (summary risk ratio 5.99, 3.04 to 11.80; p < 0.001) (eFig. 5). A higher incidence of arrhythmia in severe cases was also reported in one study (44.4% vs. 6.9%, p < 0.001).8
Fig. 1.
Forest plots of cardiac biomarkers change between more severe and less cases of COVID-19. SMD, standard mean difference.
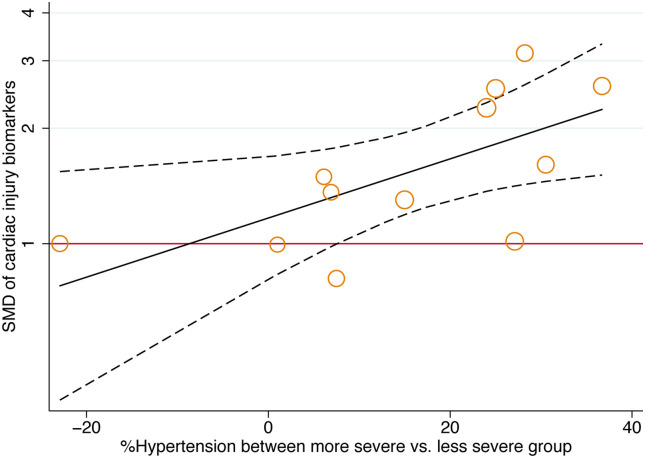
Meta regression demonstrated that standard mean differences in cardiac injury biomarkers between more and less severe cases in a study were positively associated with the prevalence of hypertension (Fig. 2 ) but not increasing age, male sex, smoking, other comorbidities and severity definitions of COVID-19 (Table 2 ).
Fig. 2.
Meta-regression of standard mean difference for difference of hypertension history on cardiac injury biomarker (more severe vs. less severe, p = 0.030).
Table 2.
Meta-regression results for baseline characteristics difference on cardiac injury biomarkers of more severe vs. less severe of COVID19.
| Between severity | No. study | Coef (exp) | 95% CI | p Value | |
|---|---|---|---|---|---|
| Age (per year older) | 16 | 1.02 | 0.99 | 1.05 | 0.220 |
| Female | 15 | 1.00 | 0.97 | 1.03 | 0.979 |
| Smoking | 5 | 1.05 | 0.97 | 1.14 | 0.160 |
| Diabetes | 12 | 1.01 | 0.99 | 1.04 | 0.324 |
| Hypertension | 12 | 1.02 | 1.00 | 1.03 | 0.030 |
| Cardiovascular diseasea | 13 | 1.02 | 0.98 | 1.06 | 0.337 |
| Coronary heart disease | 7 | 1.01 | 0.97 | 1.06 | 0.460 |
| Cerebrovascular disease | 7 | 1.01 | 0.93 | 1.11 | 0.748 |
| COPD | 8 | 1.14 | 1.00 | 1.29 | 0.052 |
| Chronic kidney disease | 6 | 0.99 | 0.94 | 1.04 | 0.562 |
| Severity definitions | 16 | 1.36 | 0.88 | 2.12 | 0.154 |
Coef = regression coefficients; CIs = confidence intervals. Severity definitions indicate groups are devided by surviors/non-surviors or severe/less severe. Cardiac injury biomarkers are selected in sequence of troponin>CK-MB > myogloblin>NT-proBNP, which indicate that if one study report troponin and CK-MB, we will use troponin as the outcome and use standard mean difference model to pool the data.
Seven of which report data on coronary heart disease were also included.
The association of COVID-19 related cardiac injury with death
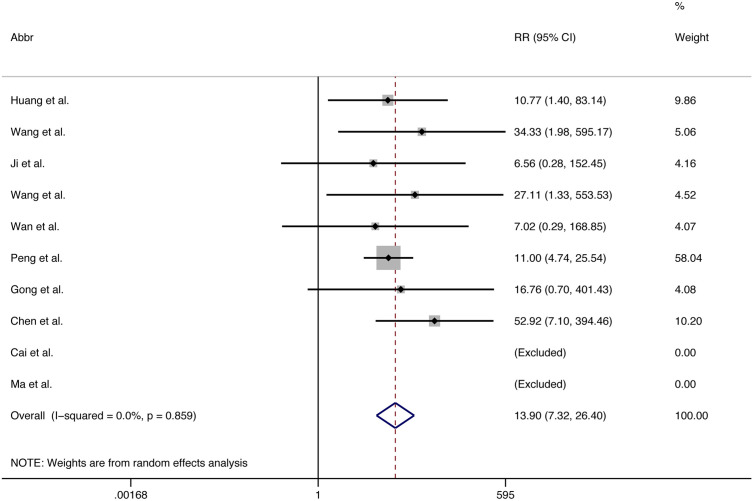
Death was more frequent in those with acute cardiac injury compared to those without (summary risk ratio 3.85, 2.13 to 6.96; p < 0.001) (Fig. 3 ). Death was also more frequent in those with more severe COVID-19 compared to those with less severe (summary risk ratio 13.90, 7.32 to 26.40; p < 0.001) (Fig. 4 ).
Fig. 3.
Forest plots showing risk ratio (RR) for death according to acute cardiac injury (yes vs. no).
Fig. 4.
Forest plots showing risk ratio (RR) for death according to severity of COVID-19 (more severe vs. less).
Dynamic changes of troponin and NT-proBNP during hospitalization
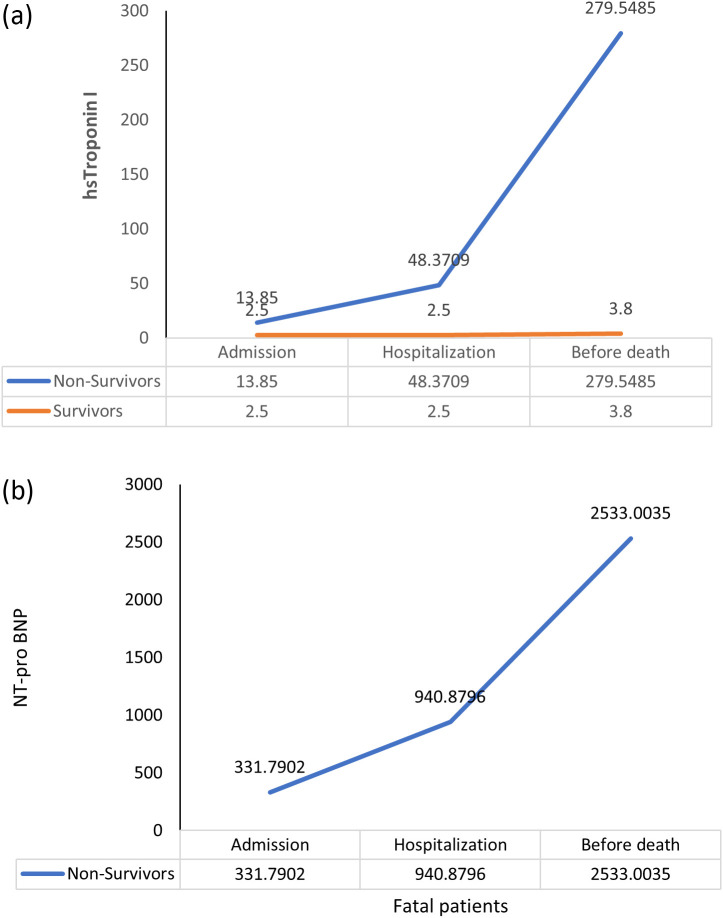
Three studies, in each case, showed dynamic escalation of hsTnI20 , 33 , 34 and NT-proBNP32., 33., 34. levels for survivors and non-survivors. Both pooled hsTnI and NT-proBNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such dynamic changes of hsTnI levels were evident in survivors, NT-proBNP in survivors was only available in one small study32 (Fig. 5 ).
Fig. 5.
Combined time series change of hs-Troponin I (a) and NT-proBNP (b).
Discussion
In this systematic review and meta-analysis, we found that there is an increased risk of acute cardiac injury associated with more severe COVID-2019 infection and this acute cardiac injury is associated with death. Patients with a history of hypertension seem to suffer more from cardiac damage.
Our results are in line with a previous meta-analysis of COVID-19 on cardiac troponin I, which found troponin I significantly increased in COVID-19 patients with severe disease compared to those with milder infection.38 Increases in troponin I, CK-MB and NT-proBNP are indicators of possible cardiac damage during COVID infection, and three published case reports have found fulminant myocarditis3 , 4 and cardiac tamponade39 after COVID infection. Assessments of the dynamic change in hsTnI and NT-proBNP show that cardiac injury biomarkers rise above normal by the midpoint of hospitalization and spike immediately before death, which seems to be most seen in severe cases. This pattern of elevation suggests that cardiac damage may already exist before multiple organ dysfunction syndrome.
In a large report containing 44,672 confirmed cases from China, approximately 81% of COVID-19 infections were mild cases which did not require hospitalization, with case-fatality rates of 49% in critical cases and 0% in mild ones.40 In our analysis more severe infection with COVID-19 is associated with 14 times higher mortality risk compared to mild infection, and acute cardiac injury mostly indicated by abnormal cardiac biomarker levels, is associated with 4 times higher mortality risk. In one of the included studies abnormal troponin I was associated with an 80 times higher risk of in-hospital death, although the sample size was small.20 It is possible that direct cardiac damage or cardiac shock partially explain death.
Based on these results, we are in support of previous suggestions that longitudinal measurement of cardiac damage biomarkers is needed during hospitalization stay for SARS-CoV-2 infection, which may help to identify a subset of patients with cardiac injury, and thereby predict the progression of COVID-19 towards an unfavorable outcome.38 Moreover, studies shall also be planned to determine whether or not cardiac supportive measures, such as echocardiographic testing and heart failure treatments such as mechanical ventilation, inotropic agents, and vasopressors, are beneficial in patients with significant elevation of cardiac injury biomarkers.
Limitations
Our meta-analysis has several potential limitations. Firstly, there was obvious heterogeneity among studies regarding definitions of severity of COVID-19, acute cardiac injury and biomarkers to detect cardiac injury although no significant publication bias were observed (eFig. 6). Secondly, this meta-analysis was conducted for studies that failed to describe all relevant characteristics of individual patients and it was hard to adjust for potentially confounding factors such as age, gender and use of treatments, such as renin angiotensin aldosterone system inhibitors. Finally, all included studies were retrospective and there are risk of bias in the data collected.
Conclusions
The severity of COVID-19 is associated with acute cardiac injury, and the latter is associated with death. Patients with a history of hypertension seem to suffer more from this kind cardiac damage. Future studies are needed identify whether cardiac supportive measures and heart failure treatments are beneficial in severe patients infected with COVID-19.
The following are the supplementary data related to this article.
Literature search and selection process.
Forest plots of creatine kinase–MB change between more severe and less cases of COVID-19.
Forest plots of myoglobin change between more severe and less cases of COVID-19.
Forest plots of NT-proBNP change between more severe and less cases of COVID-19.
Forest plots showing risk ratio (RR) for acute cardiac injury according to severity of COVID-19 (more severe vs. less).
Egger's funnel plot showing publication bias regarding severity of COVID-19 on cardiac injury biomarkers (p = 0.805). SMD, standard mean difference.
Funding
None of the authors have any funding with regard to this publication.
Author contributions
YC and BN designed the study. JL and TH identified and acquired reports of trials and extracted data. JL and TH performed all data analyses, checked for statistical inconsistency, and interpreted data. MW, CA, and CY contributed to data interpretation. LJ drafted the letter and all other authors critically reviewed the letter. All authors approved the final version of manuscript. YC is the guarantor of this work.
Declaration of competing interest
BN is supported by an Australian National Health and Medical Research Council Principal Research Fellowship; holds a research grant for this study from Janssen; and has held research grants for other largescale cardiovascular outcome trials from Roche, Servier, and Merck Schering Plough; and his institution has received consultancy, honoraria, or travel support for contributions he has made to advisory boards or the continuing medical education programmes of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier. MW is a consultant for Amgen, Inc., and Kirin. CA holds a NHMRC Senior Principal Research Fellowship and has received fees from Boehringer Ingelheim and Amgen for participating in advisory panels, from Takeda China and Boehringer Ingelheim for speaking at seminars, and a research grant from Takeda China paid to his institution. The other authors have no disclosures.
References
- 1.Xin Zou KC, Jiawei Zou, Peiyi Han, Jie Hao, Zeguang Han. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Frontiers of Medicine. 2020:0-. [DOI] [PMC free article] [PubMed]
- 2.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European Journal of Clinical Investigation. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [published online ahead of print, 2020 Mar 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [published online ahead of print, 2020 Mar 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 6.Rothman K.J., Greenland S., Lash T.L. 2008. Modern epidemiology. [Google Scholar]
- 7.Cai Q., Huang D., Ou P., Yu H., Zhu Z. 2019-nCoV pneumonia in a normal work infectious diseases hospital besides Hubei Province, China. 2020. https://ssrn.com/abstract=3542163 SSRN pre-print. [DOI] [PubMed]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji D., Zhang D., Chen Z., Xu Z., Zhao P. Clinical characteristics predicting progression of COVID-19. 2020. https://ssrn.com/abstract=3539674 SSRN pre-print.
- 10.Liu J., Qin X., Qiu S., Yuan Y., Zong Y. Clinical characteristics and treatment of patients infected with COVID-19 in Shishou, China. 2020. https://ssrn.com/abstract=3541147 SSRN pre-print.
- 11.Lu H., Ai J., Shen Y., Li Y., Li T. medRxiv preprint. 2020. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. [DOI] [Google Scholar]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020. [DOI] [PMC free article] [PubMed]
- 13.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [published online ahead of print, 2020 Feb 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of moycardial injury in servere and very severe patients with 2019 novel coronavirus disease. 2020. https://ssrn.com/abstract=3539668 SSRN pre-print. [DOI] [PMC free article] [PubMed]
- 15.Hui H., Zhang Y., Yang X., Wang X., He B. 2020. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. medRxiv preprint. [DOI] [Google Scholar]
- 16.Wang G., Wu C., Zhang Q., Wu F., Yu B. Epidemiological and clinical features of corona virus disease 2019 (COVID-19) in Changsha, China. 2020. https://ssrn.com/abstract=3548770 SSRN pre-print.
- 17.He X.W., Lai J.S., Cheng J. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 18.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020:1–10. doi: 10.1002/jmv.25783. [published online ahead of print, 2020 Mar 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Y.D., Meng K., Guan H.Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L., Fei J., Xiang H.-X. 2020. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv. 2020.2003.2013.20035329. [Google Scholar]
- 22.Gong J., Ou J., Qiu X., Jie Y., Chen Y. SSRN pre-print. 2020. Multicenter development and validation of a novel risk nomogram for early prediction of severe 2019-novel coronavirus pneumonia.https://ssrn.com/abstract=3552819 [Google Scholar]
- 23.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 26.Han Y., Zhang H., Mu S. medRxiv. 2020. Lactate dehydrogenase, a risk factor of severe COVID-19 patients. 2020.2003.2024.20040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma K.-L., Liu Z.-H., C-f Cao. medRxiv; 2020. COVID-19 myocarditis and severity factors: an adult cohort study. 2020.2003.2019.20034124. [Google Scholar]
- 28.Zhang F., Yang D., Li J. medRxiv. 2020. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: a single center retrospective cohort study. 2020.2003.2021.20040121. [Google Scholar]
- 29.Hu L., Chen S., Fu Y. medRxiv. 2020. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. 2020.2003.2025.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S., Qin M., Shen B. China; JAMA Cardiol: 2020. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Li J., Liu D. medRxiv. 2020. Clinical features and outcomes of 2019 novel coronavirus-infected patients with cardiac injury. 2020.2003.2011.20030957. [Google Scholar]
- 32.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. e201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J., Fan H., Zhang L. medRxiv. 2020. Retrospective analysis of clinical features in 101 death cases with COVID-19. 2020.2003.2009.20033068. [Google Scholar]
- 34.Zhang D., Li Y., Wang Z., Hui Y., Tong X. SSRN pre-print. 2020. Clinical characteristics of 77 novel coronavirus 2019 infected patients with respiratory failure in the terminal stage in Wuhan.https://ssrn.com/abstract=3551325 [Google Scholar]
- 35.Wu C., Hu X., Song J., Du C., Xu J. medRxiv preprint. 2020. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) [DOI] [Google Scholar]
- 36.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C., Chen X., Cai Y. China; JAMA Intern Med: 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [published online ahead of print, 2020 Mar 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua A., O’Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa253. [published online ahead of print, 2020 Mar 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature search and selection process.
Forest plots of creatine kinase–MB change between more severe and less cases of COVID-19.
Forest plots of myoglobin change between more severe and less cases of COVID-19.
Forest plots of NT-proBNP change between more severe and less cases of COVID-19.
Forest plots showing risk ratio (RR) for acute cardiac injury according to severity of COVID-19 (more severe vs. less).
Egger's funnel plot showing publication bias regarding severity of COVID-19 on cardiac injury biomarkers (p = 0.805). SMD, standard mean difference.