Summary
The Flavivirus genus of viruses includes dengue (DENV), Zika (ZIKV), yellow fever (YFV), Japanese encephalitis (JEV), and West Nile (WNV) viruses. Infections with these species combined are prevalent in tropical and sub‐tropical areas, affecting millions of people and ranging from asymptomatic to severe forms of the disease. They therefore pose a serious threat to global public health. Several studies imply a role for T cells in the protection but also pathogenesis against the different flavivirus species. Identifying flavivirus‐specific T‐cell immune profiles and determining how pre‐exposure of one species might affect the immune response against subsequent infections from other species is important to further define the role of T cells in the immune response against infection. Understanding the immune profiles of the flavivirus‐specific T‐cell response in natural infection is important to understand the T‐cell response in the context of vaccination. In this review, we summarize the current knowledge on human immune profiles of flavivirus‐specific T‐cell reactivity, comparing natural infection with the acute form of the disease and vaccination in different flavivirus infections.
Keywords: Dengue virus, T cells, transcriptomic, yellow fever virus, Zika virus
Understanding the complexity of T‐cell responses is important in flaviviruses and requires investigation in different contexts, from natural infection to vaccination. Transcriptomic studies help with the in‐depth characterization of T‐cell responses, identifying protective immune profiles against severe disease forms or common profiles across flavivirus species. Following this concept, in this review, we summarize transcriptomic studies focused on dissecting flavivirus T‐cell‐specific immune profiles and identify common profiles across several flaviviruses and current gaps of knowledge.
Abbreviations
- C
capsid
- DENV
dengue virus
- DHF
dengue haemorrhagic fever
- E
envelope
- IFN‐γ
interferon‐γ
- JEV
Japanese encephalitis virus
- KIR
killer cell immunoglobulin‐like receptor
- NS
non‐structural
- prM
pre‐membrane
- TCR
T‐cell receptor
- WNV
West Nile virus
- YFV
yellow fever virus
- ZIKV
Zika virus
Introduction
Flaviviruses are transmitted to humans primarily by the mosquitoes Aedes aegypti, Aedes albopictus and Culex. The most prominent representative viruses such as dengue (DENV), Zika (ZIKV), yellow fever (YFV), Japanese encephalitis (JEV), and West Nile (WNV) virus1 are particularly prevalent and problematic in the large tropical and equatorial areas inhabited by hundreds of millions of people. Globally, tens of millions of infections occur worldwide each year, making the prevalence of these infections comparable to that of malaria.2
Flaviviruses are enveloped viruses with a single‐stranded positive‐sense RNA genome and share similarity in their genomic organization with a unique translated polyprotein sequence cleaved in the ten main proteins, three structural [Capsid (C), pre‐Membrane (prM) and Envelope (E)] and seven non‐structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5). Strains within the same flavivirus species are quite conserved with the exception of DENV, where four different serotypes are observed.3, 4, 5, 6, 7
The role of T‐cell responses in the context of infection with DENV has been controversial, with previous studies hypothesizing a detrimental effect (or ‘antigenic sin’)8, 9, 10 and several recent studies showing a protective effect of DENV‐specific T cells.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Although it cannot be excluded that both hypotheses are valid and depending on other factors, it is certain that the T‐cell response plays a major role in the flavivirus immune responses. Hence, it is important to define the virus‐specific T‐cell immune profiles in natural infection and compare them with those elicited in the context of vaccination.
To comprehensively assess the amount of studies performed on human T cells in the context of flavivirus infections, we queried the IEDB (http://www.IEDB.org) using the following parameters: Positive Assays Only, Organism: Dengue virus, Japanese encephalitis virus, West Nile virus, Yellow fever virus, Zika virus, No B cell assays, No MHC ligand assays, Host: Homo sapiens (human), MHC Restriction Type: Any (for both CD8 and CD4), Class I (for CD8) or Class II (for CD4). This query resulted in more than 3000 flavivirus‐derived epitopes described in 96 different studies. More specifically, 70 of these studies described flavivirus‐specific CD8 T‐cell responses and 37 studies characterized CD4 T‐cell responses. Among those, the majority were focused on DENV (reviewed by Tian et al.,21 Rivino22 and Malavige and Ogg23) followed by YFV (reviewed by Watson and Klimstra24), ZIKV (reviewed by Pardy and Richer25), WNV (reviewed by Netland and Bevan)26 and JEV.27, 28, 29
In the context of dengue, the role of T cells is still controversial. On one side, a pathogenic contribution of the T‐cell response is suggested by the antigenic sin theory, based on a cross‐reactive suboptimal T‐cell response after the first DENV primary infection that leads to an excessive pro‐inflammatory response and cytokine storm.8, 9, 10
On the other hand, many other studies have supported a protective role in the context of DENV infection. Recently, additional studies comparing the acute form of the disease have correlated the early onset of interferon‐γ (IFN‐γ)‐producing T cells with a mild form of the DENV disease as well as with increased T‐cell polyfunctionality overall, suggesting that lack of an efficient and rapid T‐cell response might be important for DENV protection and not immunopathogenesis.30, 31
A similar protective role is observed in the context of T cells in YFV, with long‐lasting memory for both CD4 and CD8 T‐cell responses.32, 33 Many studies have recently focused on dissecting the contribution of the T‐cell response in ZIKV, and particularly the role of previous DENV exposure in shaping ZIKV‐specific T‐cell responses.34, 35 Also in JEV, the T cell plays a major role, with divergent functionality between JEV‐specific CD4 and CD8 T cells and cross‐reactivity capability across different neurotropic flaviviruses.27, 28 Finally, T cells can also be detrimental in other flavivirus contexts depending on tissue location; for instance the presence of CD8+ T cells in the brain is associated with a severe form of disease, as shown in the context of WNV where reduction of antigen‐specific T cells protects from neurological complications.36
The number of studies focusing on T cells in flavivirus infections increases if the query is extended to include data derived from animal models, as some of the strongest data confirming important roles for T‐cell responses against flaviviruses come from animal models, particularly mice (reviewed by Hassert et al.,37 Wen and Shresta,38 and Diamond et al. 39) but also non‐human primates.40, 41, 42, 43 For example, in mice, it has been shown that heterologous peptides were able to contribute to protection.44, 45 A recent study in non‐human primates has characterized the T‐cell response following subsequent ZIKV and DENV infection, showing that the time elapsed between the two infections influences the virus‐specific B‐cell and T‐cell responses differently.40
It is important to note that the vast majority of these studies on T‐cell responses did not perform transcriptomic analyses of the virus‐specific T‐cell response. Performing comprehensive transcriptomic studies of these flavivirus‐specific T cells would be immensely useful to establish immune profiles associated with these different disease states as well as commonly shared immune profiles across the different flaviviruses. Following this concept, in this review, we summarize studies that have used transcriptomic analyses to investigate the T‐cell‐specific immune profiles in the context of different flavivirus infections.
Defining the quantity and quality of T‐cell responses in different settings
Dissecting the contribution of T‐cell responses in infection is complex, so it is advantageous to study the T‐cell reactivity in various different contexts such as natural infection and vaccination. Establishing the T‐cell immune profiles in natural infection allows evaluation of the T‐cell memory response in the endemic population naturally exposed to the virus. Studying the general population allows a baseline signature of the T‐cell response to be established that then enables comparison with acute infection and vaccination. (Fig. 1, top circle). This baseline signature can then be compared with T‐cell profiles in acute infection associated with different severities of disease and can provide insight if the better cross‐protection is associated with an aberrant T‐cell profile (Fig. 1, left circle). Finally, analysis of T‐cell responses induced by vaccination is of high relevance and allows evaluation as to whether different vaccine constructs and regimens induce T‐cell responses that are similar in phenotype and specificity to those induced by natural infection (Fig. 1, right circle). In the next paragraphs, we will describe the role of T cells in natural immunity, acute infection and vaccination by using DENV infection as a model and compare these findings with closely related flaviviruses.
Figure 1.
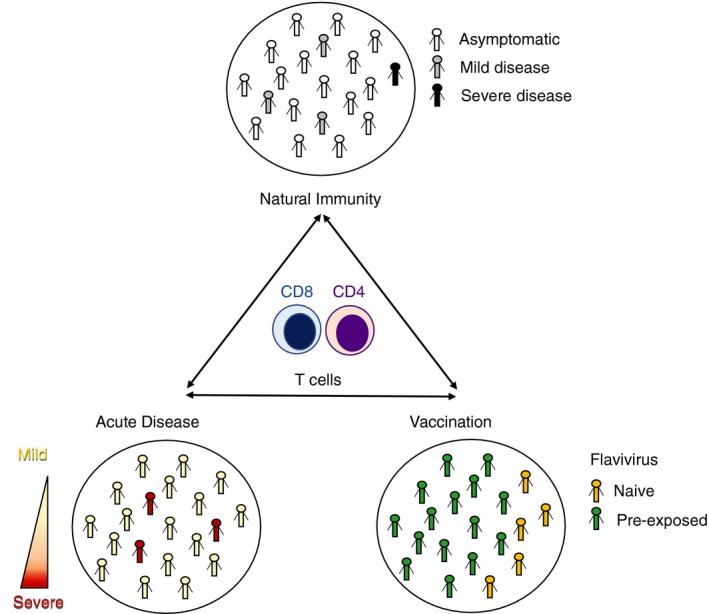
Contributing factors in dissecting flavivirus‐specific T‐cell immunity. Natural infection (top circle), Acute disease (left circle) and Vaccination (right circle).
Flavivirus T‐cell responses in natural immunity as a model to dissect flavivirus‐specific responses
The transcriptomic profiles of CD4 and CD8 T cells have been systemically investigated in DENV‐exposed individuals from endemic regions. In terms of CD4 T cells, studies from our group and others have revealed the gene expression profiles of effector memory T cells re‐expressing CD45RA (Temra cells),46, 47 which expand in individuals with multiple previous DENV infections and up‐regulate the expression of the chemokine receptor CX3CR1.48, 49 CD4 Temra cells have highly heterogeneous transcriptomic profiles and a subset of these cells is characterized by its high expression of cytotoxic molecules such as granzyme B and perforin as well as transcription factors including Runx3 and Hobit, and can be identified by surface phenotypic markers such as GPR56 and CD244.46, 47 Furthermore, it has been demonstrated that cytotoxic CD4 Temra cells have more restricted T‐cell receptor (TCR) repertoires, so may have undergone extensive clonal expansions.46, 47 Combining TCR profiling and single‐cell analysis allowed the determination of the precursors of these cytotoxic CD4 T cells. The precursor cells shared TCR clonotypes with CD4‐CTL effectors and were distinguished by high expression of the interleukin‐7 receptor.46
In terms of CD8 T cells, our group recently characterized the transcriptomic profiles of DENV‐specific memory CD8 T‐cell subsets. It was previously demonstrated that DENV‐specific CD8 T cells can be found predominantly in the two effector memory CD8 T‐cell subsets (Tem and Temra).50 DENV‐specific Tem and especially Temra cells have highly specialized gene expression programs and up‐regulated genes related to T‐cell activation, co‐stimulation, and effector functions.50 DENV‐specific CD8 T cells from both effector memory subsets responded to DENV‐specific stimuli by producing IFN‐γ in association with genes involved in activation and cytotoxicity, such as CD69, CD160, CRTAM, SLAMF7, TNFRSF9, TNF, CCL3, CCL4, and GZMB, XCL1, and XCL2.50 Additionally, DENV‐specific Temra cells express a few killer cell immunoglobulin‐like receptor (KIR) genes including KIR2DL3, which are innate immunity signatures and are expressed on natural killer cells.50 In addition, DENV‐specific CD8 T cells showed preferential usage of TCR β‐chain variable (TRBV) genes such as TRBV7‐9 and TRBV7‐8,50 pointing to a clonally expanded TCR repertoire.
Interestingly, a very similar gene expression pattern has also been found in the context of ZIKV‐specific CD8 T‐cell responses51 (Fig. 2). More specifically, ZIKV‐specific CD8 T‐cell gene profiles were more similar to the DENV‐specific CD8 profiles of Tem cells then Temra (Fig. 2). This is in line with previous findings showing that ZIKV‐specific CD8 T cells have mainly been detected in the Tem compartment.35 A set of genes sharing a similar gene expression pattern was found in DENV‐specific as well as ZIKV‐specific T cells. In both viral infections, these genes were found to be up‐regulated in the IFN‐γ + CD8+ T cells and are associated with pathways involved in the regulation of cytokine production, T‐cell activation, and the cellular response to IFN‐γ. Overall, this might suggest that flavivirus‐specific T cells share similarities in terms of quality of response whereas the magnitude and the protein immunodominance (preferential T‐cell reactivity against one or more structural or non‐structural proteins) depend on the specific flavivirus analyzed.35 Another important point when analyzing the natural immunity against flavivirus is the co‐circulation of many of these viruses and the fact that previous exposure to one flavivirus might impact the T‐cell signature to a subsequent infection with a different flavivirus.
Figure 2.
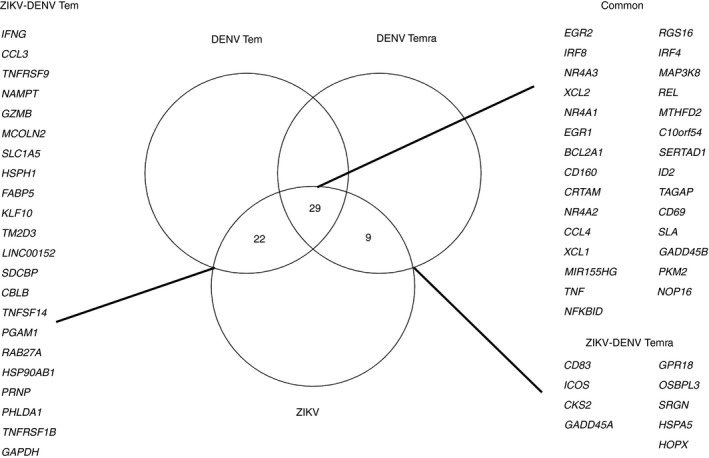
Immune profile comparison in dengue virus (DENV) and Zika virus (ZIKV) ‐specific CD8 T cells. Gene expression comparison between DENV‐specific and ZIKV‐specific CD8 T cells. Gene list has been extracted based on DEG between interferon‐γ‐positive (IFN‐γ +) and IFN‐γ − after CD8 MegaPool stimulation with log2FoldChange |2| Padj < 0·05 derived from two studies analyzing transcriptomic profiles of DENV and ZIKV‐specific T‐cell responses, respectively.50, 51 The overlap between gene lists has been performed comparing ZIKV with DENV‐Tem and DENV‐Temra, respectively. The common list represents the overlap of the three different data sets.
In the context of ZIKV, we previously showed that DENV pre‐exposure skewed the protein immunodominance of ZIKV‐specific CD8+ T cells towards non‐structural proteins both in the context of CD8 and CD4 T‐cell reactivity.35 However, when the gene immune signature was evaluated, the quality of ZIKV‐specific CD8+ T cells did not differ based on DENV pre‐exposure.51 No transcriptomic profiles of other flavivirus‐specific CD4 and CD8 T cells have been systematically investigated in the context of natural immunity and additional studies are needed to dissect the immune profiles of YF‐, JEV‐, and WNV‐specific T cells in human populations to take into account the human genetic variability and provide new insights for vaccine development and validation.
Flavivirus T‐cell responses in acute disease
Studying immune responses during acute viral infections presents several challenges: sample collection is challenged by the severity of disease status affecting both the volume of the sample available and the homogeneity of the cohort analyzed. For those reasons, many studies in the DENV context have dissected the overall peripheral blood mononuclear cell immune signature during the acute phase of the disease, trying to identify potential gene candidates able to distinguish the severe form of the diseases.52, 53, 54, 55, 56, 57, 58, 59, 60, 61 Robinson and co‐authors combined all these studies by in silico approaches and identified a 20‐gene set able to predict severe dengue disease that is significantly enriched in natural killer and natural killer T cell populations.62 Interestingly, very similar gene expression patterns are found in CX3CR1, which is under‐expressed in dengue hemorrhagic fever (DHF)/dengue shock syndrome.62 As cytotoxic CD4 Temra cells up‐regulate the expression of CX3CR1,48 this finding may support the notion that these cells provide protective immunity against severe dengue disease.
In a recent study from our group, we characterized antigen‐specific CD4 T cells during acute DENV infection and investigated whether there was any difference in CD4 T‐cell response between patients with dengue fever or DHF.63 We identified a unique interleukin‐10+ IFN‐γ + double‐positive (DP) CD4 T‐cell subset that predominated the antigen‐specific CD4 T‐cell response during acute DENV infection. Moreover, although the frequency of DENV‐specific DP cells is higher in DHF patients than in dengue fever patients, the transcriptomic profile of DP cells was similar in dengue fever and DHF patients. These findings suggest that dengue disease severity is not associated with altered phenotypic or functional attributes of this specific CD4 T‐cell subset and argue against the notion that altered CD4 T‐cell phenotype or function may be a determinant of severe dengue disease.63
In terms of CD8 T cells, it has been shown that HLA‐DR+ CD38+‐activated CD8 T cells isolated from DENV‐infected patients exhibit increased expression of genes involved in T‐cell proliferation, activation, migration, and cytotoxicity.64 These HLA‐DR+ CD38+ CD8 T cells up‐regulate the expression of multiple inhibitory receptors such as Lag‐3, Tim‐3, and CD160.64 Conversely, they down‐regulate several genes that mediate TCR signaling, including AKT3, SOS1, ITK, PLCG1, NCK2, and RASGRP1.64
In summary, both the CD4 and CD8 T‐cell responses show an immune signature of genes associated with proliferation, activation, migration, and cytotoxicity pointing to a fully functional effector capability of flavivirus‐specific T cells.64
Importance of T cells in flavivirus vaccination: the dengue example
In the absence of an effective antiviral therapy, the development of effective vaccines that are able to protect from flavivirus infections is the most desired strategy to be able to control flavivirus‐induced disease. However, although it has been possible to develop efficient vaccinations for some members of the family (e.g. YFV, JEV), multiple factors are challenging the design of protective vaccination against WNV, DENV, and ZIKV.65 Among those, the viral variability has a considerable impact, and is particularly important in the case of DENV, where the co‐circulation of multiple serotypes with only 50%–60% homology leads to heterologous infections strongly associated with increased risk of severe DENV disease.2, 4, 66, 67
Additionally, co‐circulation of multiple flaviviruses in the same geographical areas is often observed,66 making it difficult to separate the immune response specific for a single flavivirus without considering the effect that previous flavivirus exposure or the following exposure can have in shaping the immune system, as previously reported between DENV and ZIKV.35, 44, 68 This observation also has implications in vaccine development, as in order to design an efficient vaccination able to protect from a specific flavivirus infection, it should be considered that the target populations are most likely flavivirus‐exposed. Increasing progress is observed in the development of flavivirus vaccination, with YFV and JEV being the most successful targeted flaviviruses, and many promising vaccine candidates in different stages of approval for DENV, WNV, and ZIKV.65
The vaccine design for flaviviruses encompasses different strategies ranging from live attenuation, inactivation, or the development of chimeric vaccine constructs combining the structural proteins of the different flaviviruses with the YF17D vaccine strain backbone.65 The chimeric CYD‐TDV (Dengvaxia®) developed by Sanofi (Lyon, France) has recently been licensed as a vaccine against DENV. The vaccine construct contains the prM and E proteins of the four major DENV serotypes strains within a YF backbone comprised of C and NS proteins. The lack of protection observed in the DENV seronegative population in this vaccination69 raised questions about the need for DENV NS proteins in the vaccination to establish an efficient immune response despite the presence of neutralizing antibodies against prM and E proteins.7, 70, 71, 72, 73, 74 Previous studies have shown that the main reactivity of DENV‐specific T‐cell responses is against C and NS proteins, suggesting that the inclusion of proteins for which DENV‐specific T‐cell responses are detected might be required for efficient vaccine development.11, 12, 16, 75, 76, 77 Another DENV vaccine in development by Takeda (Tokyo, Japan) is TAK‐003, which contains prM and E proteins from all four DENV serotypes in a DENV2 backbone and so all DENV proteins. In a recent paper, Waickman and co‐authors have shown vaccine‐reactive memory‐precursor CD8+ T cells in TAK‐003 vaccination, with a similar CD8+ T‐cell immune profile to that shared by the DENV‐specific and ZIKV‐specific CD8+ T cells in the natural immunity context (summarized above).78 Additionally, in the same study after DENV vaccination, a specific subpopulation of activated CD8+ T cells in the memory compartment with transferrin receptor (TfR1/CD71) up‐regulation is associated with long lasting memory and increased cytotoxic capability within the vaccine‐reactive CD8+ T cells defined as HLA‐DR+ CD38+.78 In conclusion, this underlines the importance of establishing T‐cell immune signatures after vaccination and analyzing them with respect to immune profiles observed in natural infection.
Conclusion
This review summarizes the current landscape of genome‐wide transcriptomic studies of flavivirus‐specific T cells and underlines the potential of such studies that allow the characterization of antigen‐specific cells in an unbiased way. The knowledge of immune signatures and biomarkers associated with natural infection, severe disease and vaccination have the potential to identify novel immune signatures associated with protection or progression to severe disease.
Disclosures
DW has been on the scientific advisory boards on dengue vaccine evaluation for Merck and Takeda. All other authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases grants U19 AI118626 as well as National Institutes of Health contracts HHSN272200900042C and HHSN27220140045C. YT was supported through The American Association of Immunologists Intersect Fellowship Program for Computational Scientists and Immunologists.
Contributor Information
Alba Grifoni, Email: agrifoni@lji.org.
Daniela Weiskopf, Email: dweiskopf@lji.org.
References
- 1. Burke D, Monath T. Flaviviruses In: Knipe D, Howley P, eds. Field's Virology. Philadelphia: Lippincott, Williams and Wilkins, 2001: 1043–126. [Google Scholar]
- 2. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL et al The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guarner J, Hale GL. Four human diseases with significant public health impact caused by mosquito‐borne flaviviruses: West Nile, Zika, dengue and yellow fever. Semin Diagn Pathol 2019; 36:170–6. [DOI] [PubMed] [Google Scholar]
- 4. Slon Campos JL, Mongkolsapaya J, Screaton GR. The immune response against flaviviruses. Nat Immunol 2018; 19:1189–98. [DOI] [PubMed] [Google Scholar]
- 5. Sharma A, Lal SK. Zika virus: transmission, detection, control, and prevention. Front Microbiol 2017; 8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coffey LL, Mertens E, Brehin AC, Fernandez‐Garcia MD, Amara A, Despres P et al Human genetic determinants of dengue virus susceptibility. Microbes Infect 2009; 11:143–56. [DOI] [PubMed] [Google Scholar]
- 7. Collins ND, Beck AS, Widen SG, Wood TG, Higgs S, Barrett ADT. Structural and nonstructural genes contribute to the genetic diversity of RNA viruses. MBio 2018; 9: e01871‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 2011; 11:532–43. [DOI] [PubMed] [Google Scholar]
- 9. Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A et al Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003; 9:921–7. [DOI] [PubMed] [Google Scholar]
- 10. Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A et al T cell responses in dengue hemorrhagic fever: are cross‐reactive T cells suboptimal? J Immunol 2006; 176:3821–9. [DOI] [PubMed] [Google Scholar]
- 11. Grifoni A, Angelo MA, Lopez B, O'Rourke PH, Sidney J, Cerpas C et al Global assessment of dengue virus‐specific CD4+ T cell responses in dengue‐endemic areas. Front Immunol 2017; 8:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN et al Comprehensive analysis of dengue virus‐specific responses supports an HLA‐linked protective role for CD8+ T cells. Proc Natl Acad Sci USA 2013; 110:E2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiskopf D, Angelo MA, Grifoni A, O'Rourke PH, Sidney J, Paul S et al HLA‐DRB1 alleles are associated with different magnitudes of dengue virus‐specific CD4+ T‐cell responses. J Infect Dis 2016; 214:1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiskopf D, Sette A. T‐cell immunity to infection with dengue virus in humans. Front Immunol 2014; 5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rivino L. T cell immunity to dengue virus and implications for vaccine design. Expert Rev Vaccines 2016; 15:443–53. [DOI] [PubMed] [Google Scholar]
- 16. Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW et al Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013; 87:2693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rivino L, Tan AT, Chia A, Kumaran EA, Grotenbreg GM, MacAry PA et al Defining CD8+ T cell determinants during human viral infection in populations of Asian ethnicity. J Immunol 2013; 191:4010–9. [DOI] [PubMed] [Google Scholar]
- 18. Malavige GN, McGowan S, Atukorale V, Salimi M, Peelawatta M, Fernando N et al Identification of serotype‐specific T cell responses to highly conserved regions of the dengue viruses. Clin Exp Immunol 2012; 168:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeewandara C, Adikari TN, Gomes L, Fernando S, Fernando RH, Perera MK et al Functionality of dengue virus specific memory T cell responses in individuals who were hospitalized or who had mild or subclinical dengue infection. PLoS Negl Trop Dis 2015; 9:e0003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malavige GN, Jeewandara C, Alles KM, Salimi M, Gomes L, Kamaladasa A et al Suppression of virus specific immune responses by IL‐10 in acute dengue infection. PLoS Negl Trop Dis 2013; 7:e2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian Y, Grifoni A, Sette A, Weiskopf D. Human T cell response to dengue virus infection. Front Immunol 2019; 10:2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivino L. Understanding the human T cell response to dengue virus. Adv Exp Med Biol 2018; 1062:241–50. [DOI] [PubMed] [Google Scholar]
- 23. Malavige GN, Ogg GS. T cell responses in dengue viral infections. J Clin Virol 2013; 58:605–11. [DOI] [PubMed] [Google Scholar]
- 24. Watson AM, Klimstra WBT. Cell‐mediated immunity towards yellow fever virus and useful animal models. Viruses 2017; 9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pardy RD. Richer MJ. Protective to a T: the role of T cells during Zika virus infection. Cells 2019; 8: 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Netland J, Bevan MJ. CD8 and CD4 T cells in West Nile virus immunity and pathogenesis. Viruses 2013; 5:2573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turtle L, Tatullo F, Bali T, Ravi V, Soni M, Chan S et al Cellular immune responses to live attenuated Japanese Encephalitis (JE) vaccine SA14‐14‐2 in adults in a JE/dengue co‐endemic area. PLoS Negl Trop Dis 2017; 11:e0005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turtle L, Bali T, Buxton G, Chib S, Chan S, Soni M et al Human T cell responses to Japanese encephalitis virus in health and disease. J Exp Med 2016; 213:1331–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar P, Sulochana P, Nirmala G, Haridattatreya M, Satchidanandam V. Conserved amino acids 193–324 of non‐structural protein 3 are a dominant source of peptide determinants for CD4+ and CD8+ T cells in a healthy Japanese encephalitis virus‐endemic cohort. J Gen Virol 2004; 85:1131–43. [DOI] [PubMed] [Google Scholar]
- 30. Wijeratne DT, Fernando S, Gomes L, Jeewandara C, Ginneliya A, Samarasekara S et al Quantification of dengue virus specific T cell responses and correlation with viral load and clinical disease severity in acute dengue infection. PLoS Negl Trop Dis 2018; 12:e0006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wijeratne DT, Fernando S, Gomes L, Jeewandara C, Jayarathna G, Perera Y et al Association of dengue virus‐specific polyfunctional T‐cell responses with clinical disease severity in acute dengue infection. Immun Inflamm Dis 2019;7:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW et al Origin and differentiation of human memory CD8 T cells after vaccination. Nature 2017; 552:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James EA, LaFond RE, Gates TJ, Mai DT, Malhotra U, Kwok WW. Yellow fever vaccination elicits broad functional CD4+ T cell responses that recognize structural and nonstructural proteins. J Virol 2013; 87:12794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim MQ, Kumaran EAP, Tan HC, Lye DC, Leo YS, Ooi EE et al Cross‐reactivity and anti‐viral function of dengue capsid and ns3‐specific memory t cells toward Zika virus. Front Immunol 2018; 9:2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grifoni A, Pham J, Sidney J, O'Rourke PH, Paul S, Peters B et al Prior dengue virus exposure shapes T cell immunity to Zika virus in humans. J Virol 2017; 91:e01469‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graham JB, Swarts JL, Thomas S, Voss KM, Sekine A, Green R et al Immune correlates of protection from West Nile virus neuroinvasion and disease. J Infect Dis 2019; 219:1162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hassert M, Brien JD, Pinto AK. Mouse models of heterologous flavivirus immunity: a role for cross‐reactive T cells. Front Immunol 2019; 10:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wen J, Shresta S. T cell immunity to Zika and dengue viral infections. J Interferon Cytokine Res 2017; 37:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol 2003; 16:259–78. [DOI] [PubMed] [Google Scholar]
- 40. Pantoja P, Perez‐Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C et al Zika virus pathogenesis in rhesus macaques is unaffected by pre‐existing immunity to dengue virus. Nat Commun 2017; 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wertheimer AM, Uhrlaub JL, Hirsch A, Medigeshi G, Sprague J, Legasse A et al Immune response to the West Nile virus in aged non‐human primates. PLoS ONE 2010; 5:e15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cong Y, McArthur MA, Cohen M, Jahrling PB, Janosko KB, Josleyn N et al Characterization of yellow fever virus infection of human and non‐human primate antigen presenting cells and their interaction with CD4+ T cells. PLoS Negl Trop Dis 2016; 10:e0004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mladinich KM, Piaskowski SM, Rudersdorf R, Eernisse CM, Weisgrau KL, Martins MA et al Dengue virus‐specific CD4+ and CD8+ T lymphocytes target NS1, NS3 and NS5 in infected Indian rhesus macaques. Immunogenetics 2012; 64:111–21. [DOI] [PubMed] [Google Scholar]
- 44. Elong Ngono A, Chen HW, Tang WW, Joo Y, King K, Weiskopf D et al Protective role of cross‐reactive CD8 T cells against dengue virus infection. EBioMedicine 2016; 13:284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K et al Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe 2017; 21:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patil VS, Madrigal A, Schmiedel BJ, Clarke J, O'Rourke P, de Silva AD et al Precursors of human CD4+ cytotoxic T lymphocytes identified by single‐cell transcriptome analysis. Sci Immunol 2018; 3:eaan8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G et al Unique phenotypes and clonal expansions of human CD4 effector memory T cells re‐expressing CD45RA. Nat Commun 2017; 8:1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM et al Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci USA 2015; 112:E4256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tian Y, Sette A, Weiskopf D. Cytotoxic CD4 T cells: differentiation, function, and application to dengue virus infection. Front Immunol 2016; 7:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian Y, Babor M, Lane J, Seumois G, Liang S, Goonawardhana NDS et al Dengue‐specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J Clin Invest 2019; 130:1727–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grifoni A, Costa‐Ramos P, Pham J, Tian Y, Rosales SL, Seumois G et al Cutting edge: transcriptional profiling reveals multifunctional and cytotoxic antiviral responses of Zika virus‐specific CD8+ T cells. J Immunol 2018; 201:3487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoang LT, Lynn DJ, Henn M, Birren BW, Lennon NJ, Le PT et al The early whole‐blood transcriptional signature of dengue virus and features associated with progression to dengue shock syndrome in Vietnamese children and young adults. J Virol 2010; 84:12982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC et al Dengue virus infection induces expansion of a CD14+CD16+ monocyte population that stimulates plasmablast differentiation. Cell Host Microbe 2014; 16:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Loke P, Hammond SN, Leung JM, Kim CC, Batra S, Rocha C et al Gene expression patterns of dengue virus‐infected children from Nicaragua reveal a distinct signature of increased metabolism. PLoS Negl Trop Dis 2010; 4:e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Long HT, Hibberd ML, Hien TT, Dung NM, Van Ngoc T, Farrar J et al Patterns of gene transcript abundance in the blood of children with severe or uncomplicated dengue highlight differences in disease evolution and host response to dengue virus infection. J Infect Dis 2009; 199:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Popper SJ, Gordon A, Liu M, Balmaseda A, Harris E, Relman DA. Temporal dynamics of the transcriptional response to dengue virus infection in Nicaraguan children. PLoS Negl Trop Dis 2012; 6:e1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun P, Garcia J, Comach G, Vahey MT, Wang Z, Forshey BM et al Sequential waves of gene expression in patients with clinically defined dengue illnesses reveal subtle disease phases and predict disease severity. PLoS Negl Trop Dis 2013; 7:e2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van de Weg CA, van den Ham HJ, Bijl MA, Anfasa F, Zaaraoui‐Boutahar F, Dewi BE et al Time since onset of disease and individual clinical markers associate with transcriptional changes in uncomplicated dengue. PLoS Negl Trop Dis 2015; 9:e0003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Devignot S, Sapet C, Duong V, Bergon A, Rihet P, Ong S et al Genome‐wide expression profiling deciphers host responses altered during dengue shock syndrome and reveals the role of innate immunity in severe dengue. PLoS ONE 2010; 5:e11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nascimento EJ, Silva AM, Cordeiro MT, Brito CA, Gil LH, Braga‐Neto U et al Alternative complement pathway deregulation is correlated with dengue severity. PLoS ONE 2009; 4:e6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Simmons CP, Popper S, Dolocek C, Chau TN, Griffiths M, Dung NT et al Patterns of host genome‐wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis 2007; 195:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Robinson M, Sweeney TE, Barouch‐Bentov R, Sahoo MK, Kalesinskas L, Vallania F et al A 20‐Gene set predictive of progression to severe dengue. Cell Rep 2019; 26:1104–11.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tian Y, Seumois G, De‐Oliveira‐Pinto LM, Mateus J, Herrera‐de la Mata S, Kim C et al Molecular signatures of dengue virus-specific IL-10/IFN-γ co‐producing CD4 T cells and their association with dengue disease. Cell Rep 2019; 29: 4482–95.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chandele A, Sewatanon J, Gunisetty S, Singla M, Onlamoon N, Akondy RS et al Characterization of human CD8 T cell responses in dengue virus‐infected patients from India. J Virol 2016; 90:11259–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Collins MH, Metz SW. Progress and works in progress: update on flavivirus vaccine development. Clin Ther 2017; 39:1519–36. [DOI] [PubMed] [Google Scholar]
- 66. Saron WAA, Rathore APS, Ting L, Ooi EE, Low J, Abraham SN et al Flavivirus serocomplex cross‐reactive immunity is protective by activating heterologous memory CD4 T cells. Sci Adv 2018; 4:eaar4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsang TK, Ghebremariam SL, Gresh L, Gordon A, Halloran ME, Katzelnick LC et al Effects of infection history on dengue virus infection and pathogenicity. Nat Commun 2019; 10:1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K et al Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross‐reactive CD8+ T cells. Nat Microbiol 2017; 2:17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Halstead SB. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017; 35:6355–8. [DOI] [PubMed] [Google Scholar]
- 70. Guy B. Which dengue vaccine approach is the most promising, and should we be concerned about enhanced disease after vaccination? questions raised by the development and implementation of dengue vaccines: example of the Sanofi Pasteur tetravalent dengue vaccine. Cold Spring Harb Perspect Biol 2018; 10:a029462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res 2016; 2016:6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: one more step forward. Vaccine 2015; 33:7100–11. [DOI] [PubMed] [Google Scholar]
- 73. Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS et al Lack of durable cross‐neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis 2017; 23:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Patel B, Longo P, Miley MJ, Montoya M, Harris E, de Silva AM. Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl Trop Dis 2017; 11:e0005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Angelo MA, Grifoni A, O'Rourke PH, Sidney J, Paul S, Peters B et al Human CD4+ T cell responses to an attenuated tetravalent dengue vaccine parallel those induced by natural infection in magnitude, HLA restriction, and antigen specificity. J Virol 2017; 91: e02147‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rivino L, Lim MQ. CD4+ and CD8+ T‐cell immunity to dengue ‐ lessons for the study of Zika virus. Immunology 2017; 150:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sharma M, Glasner DR, Watkins H, Puerta‐Guardo H, Kassa Y, Egan MA et al Magnitude and functionality of the NS1‐specific antibody response elicited by a live‐attenuated tetravalent dengue vaccine candidate. J Infect Dis 2019:jiz081. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Waickman AT, Victor K, Li T, Hatch K, Rutvisuttinunt W, Medin C et al Dissecting the heterogeneity of DENV vaccine‐elicited cellular immunity using single‐cell RNA sequencing and metabolic profiling. Nat Commun 2019; 10:3666. [DOI] [PMC free article] [PubMed] [Google Scholar]


