Summary
As a pineal gland hormone, melatonin acts through its receptors to modulate the immune system. The immune system is composed of primary and secondary organs, and immune organs are adapted to the presence of the fetal alloantigen during pregnancy. However, it is unclear whether melatonin affects maternal immune organs during early pregnancy in sheep. In this study, the ovine thymus, lymph node, spleen and liver were sampled at day 16 of the oestrous cycle, and at days 13, 16 and 25 of pregnancy. The expression of melatonin receptor 1A (MT1), melatonin receptor 1B (MT2) and cluster of differentiation 4 (CD4) was detected by quantitative real‐time polymerase chain reaction, Western blot and immunohistochemistry experiments. Our results showed that during early pregnancy there was an upregulation of MT1 mRNA and protein in the thymus, lymph node and liver, and there was a downregulation in the spleen. The expression of MT2 mRNA and protein was increased in the thymus but decreased in the spleen and liver, and there was no significant change in the lymph node during early pregnancy. CD4 protein was upregulated in the thymus, lymph node and liver, but there were no significant changes in the spleen during early pregnancy. In conclusion, early pregnancy induces tissue‐specific expression of MT1, MT2 and CD4, which may be due to the different functions of the thymus, lymph node, spleen and liver. Further, melatonin is involved in immune regulation of the maternal thymus, lymph node, spleen and liver during early pregnancy in sheep.
Keywords: liver, lymph node, melatonin receptor, spleen, thymus
Early pregnancy induces tissue‐specific expression of melatonin receptor 1A (MT1), melatonin receptor 1B (MT2) and cluster of differentiation 4 (CD4), which may be due to the different functions of the thymus, lymph node, spleen and liver, and melatonin is involved in immune regulation of the maternal thymus, lymph node, spleen and liver during early pregnancy in sheep.
Abbreviations
- CD4
cluster of differentiation 4
- HE
haematoxylin and eosin
- HNF4α
hepatocyte nuclear factor 4α
- IFNT
interferon‐tau
- IFN‐γ
interferon‐gamma
- IL
interleukin
- ISG15
interferon‐stimulated gene 15 kDa protein
- MT1
melatonin receptor 1A
- MT2
melatonin receptor 1B
- P4
progesterone
- PGR
progesterone receptor
- PIBF
progesterone‐induced blocking factor
- qRT‐PCR
quantitative real‐time polymerase chain reaction
- TNF‐β
tumour necrosis factor‐beta
Introduction
Maternal immune tolerance exists when the fetus and placenta are not rejected by the maternal immune system during pregnancy. 1 It is through a variety of mechanisms of peripheral tolerance that the maternal immune system adapts to the presence of fetal alloantigen in sheep and cattle. 2 , 3 , 4 The immune system is composed of different immune organs, cells and tissues, and there are two groups of immune organs (primary and secondary organs). Primary organs are the bone marrow and thymus, and secondary organs refer to the lymph nodes, spleen, tonsils, Peyer's patches, appendix and liver. 5
Lymphocyte development is generally blocked in the maternal immune system during pregnancy in mice, rats and humans. 6 , 7 , 8 This developmental change occurs via thymic involution through a progesterone (P4) receptor (PGR)‐dependent paracrine mechanism. 6 The weights of lumbar and renal lymph nodes and inguinal lymph nodes increase during pregnancy in mice, 9 and the number of large pyroninophilic cells from iliac lymph nodes increases significantly during early pregnancy in rats. 10 PGR and P4‐induced blocking factor (PIBF) are also upregulated in the lymph node during early pregnancy in sheep. 11 There is a downregulation of cofilin‐1, F‐actin capping protein subunit alpha and malate dehydrogenase proteins in splenic CD4+ lymphocytes during the preimplantation period in pregnant mice, 12 and the expression of PGR and PIBF is changed in ovine spleen during early pregnancy. 13 The number of telocytes in the liver increases significantly during pregnancy in mice, which is coincident with the occurrence of two peaks of hepatic cell proliferation. 14 Pregnancy modulates both phase I and II metabolism, and alters the biological potency of the hepatocarcinogen aflatoxin B1 in mouse liver. 15
Melatonin is also known as N‐acetyl‐5‐methoxytryptamine, and it was initially thought to be produced exclusively by the pineal gland. However, it has been demonstrated that melatonin‐synthesizing enzymes exist in multiple extrapineal tissues, such as the ovary, placenta, bone marrow and bile. 16 , 17 , 18 The circadian production of melatonin from the pineal gland has chronobiotic influence on organismal activity, including sleep−wake timing, blood pressure regulation and seasonal reproduction. 19 Melatonin also has antioxidant 20 and anti‐inflammatory properties, and it functions in the protection of nuclear and mitochondrial DNA 21 and in the regulation of mitochondrial homeostasis. 18 The immunological effects of melatonin are achieved through its interactions with its receptors in animals. 22 Melatonin can downregulate a variety of proinflammatory cytokines, including interleukins (ILs) and tumour neurosis factor‐alpha (TNF‐α). 16 Melatonin plays a fundamental role in neuroimmunomodulation, which is related to lymphocytes, and it acts as a stimulant under basal or immunosuppressive conditions or as an anti‐inflammatory compound to exert pleiotropic effects on the immune system. 23
Melatonin works as a circadian rhythm modulator, an endocrine modulator, an immunomodulator, a direct free radical scavenger, an indirect antioxidant and a cytoprotective agent during pregnancy in humans, and it is necessary for normal pregnancy. 24 Free radical damage is commonplace during pregnancy, and it has negative effects on the mother, placenta and fetus. Because of its antioxidant ability, melatonin can protect the fetus, maternal tissues and placenta from oxidative damage, which is beneficial for normal reproductive physiology. 25 , 26 , 27 Melatonin treatment can increase pregnenolone synthesis in the ovary and endometrium, which promotes endometrial development and embryo implantation early in mouse pregnancy. 28 T‐cell subpopulations, IL‐17 (Th17) and regulatory T (Treg) cells, have an essential role during pregnancy, and endogenous melatonin is involved in regulating the Th17/Treg balance during pregnancy in humans. 29 It has been reported that the characteristics of the melatonin secretory rhythm are unaffected by steroids during the oestrous cycle and pregnancy in Ile‐de‐France ewes. 30 , 31 Cluster of differentiation 4 (CD4) is a glycoprotein found on the surface of CD4 cells, such as T helper cells, monocytes, macrophages and dendritic cells, and CD4 cells are an essential part of the human immune system. 32
However, it is unclear whether melatonin works on immune organs through melatonin receptors during early pregnancy in sheep. Therefore, in this study, the expression of melatonin receptor 1A (MT1), melatonin receptor 1B (MT2) and CD4 was explored in the main immune organs (thymus, lymph node, spleen and liver) from nonpregnant and early pregnant ewes, and the results may be helpful in understanding the effects of melatonin on maternal immune organs during early pregnancy in sheep.
Materials and methods
Animals and experimental design
Ewes (Small‐tail Han) approximately 18 months old were housed at the farm of Handan Boyuan Animal Husbandry in China. All experimental procedures used in animals were approved by the Hebei University of Engineering Animal Care and Use Committee, and humane animal care and handling procedures were followed throughout the experiment. Controlled internal drug‐releasing (InterAg, Hamilton, New Zealand) devices were used to improve synchrony of oestrus of ewes and ensure that the ewes were under the same circadian rhythms. The ewes were assessed for the onset of oestrus (designated as day 0 of the oestrous cycle or pregnancy) using a vasectomized ram. The ewes that mated with intact rams were randomly divided into three groups, and there was an additional group of non‐pregnant ewes that were mated with a vasectomized ram (n = 6 for each group). The effects of early pregnancy on the expression of MT1 and MT2 in the ovine thymus, lymph node, spleen and liver are mainly due to P4 and interferon‐tau (IFNT). There are significantly higher concentrations of P4 in plasma on days 12−13, and lower concentrations of P4 on days 15–16 during the ovine oestrous cycle. 33 IFNT (Protein X) and additional proteins secreted by the trophoblasts of blastocysts in the uterus were detected between days 14 and 21 in sheep. 34 Therefore, samples of the thymus, lymph node, spleen and liver were obtained from ewes at days 13, 16 and 25 of pregnancy, and day 16 of the oestrous cycle at the time of slaughter. Pregnancy was confirmed by anatomizing the uterus and observing the presence of a conceptus. Transverse pieces (0·3 cm3) of thymus, lymph node, spleen and liver were washed three times with phosphate‐buffered saline solution (PBS, pH 7·4) and then were fixed in fresh 4% (w/v) paraformaldehyde in PBS. The remaining thymus, lymph node, spleen and liver samples were frozen in liquid nitrogen for subsequent quantitative real‐time polymerase chain reaction (qRT‐PCR) and protein analysis.
RNA extraction and qRT‐PCR assays
Total RNA was isolated from thymus, lymph node, spleen and liver samples using the TRIzol method. The cDNA was synthesized with a FastQuant RT kit (Tiangen Biotech, Beijing, China), and it was analysed by qPCR with a SuperReal PreMix Plus kit (Tiangen Biotech) using a CFX96™ real‐time PCR System (BIO‐RAD, Hercules, CA, USA) according to the manufacturer's instructions. The primer sequences of MT1, MT2 and GAPDH were designed and synthesized by Shanghai Sangon Biotech (Table 1). The relative expression levels of mRNA were calculated using a reference gene (GAPDH). The relative expression value was set as 1 for the group on day 16 of the oestrous cycle, and the ratios comparing the pregnant ewes and the ewes on day 16 of the oestrous cycle were relative expression values.
Table 1.
Primers used for qRT‐PCR
| Gene | Primer | Sequence | Size (bp) | Accession numbers |
|---|---|---|---|---|
| MT1 | Forward | CCTCCATCCTCATCTTCACC | 116 | NM_001009725.1 |
| Reverse | CAGGCTCACCACAAACACAT | |||
| MT2 | Forward | ATCCCAGAGGGGTTGTTTGT | 130 | NM_001130938.1 |
| Reverse | TCCAGAGGGCAGAGACGAT | |||
| GAPDH | Forward | GGGTCATCATCTCTGCACCT | 176 | NM_001190390.1 |
| Reverse | GGTCATAAGTCCCTCCACGA |
Western blot analysis
Total proteins from thymus, lymph node, spleen and liver samples were extracted with RIPA lysis buffer (Biosharp, BL504A) and then were quantified using a bicinchoninic acid protein assay kit (Tiangen Biotech). The proteins were separated using 12% sodium dodecyl sulphate−polyacrylamide gel electrophoresis, and then were transferred to 0·22‐μm polyvinylidene fluoride membranes (Millipore, Bedford, MA). The membranes were blocked with 5% (w/v) fat‐free milk. MT1, MT2 and CD4 were detected by a mouse anti‐MT1 monoclonal antibody (Santa Cruz Biotechnology; sc‐390328, 1:1000), a rabbit anti‐MT2 polyclonal antibody (Abcam; ab203346, 1:1000; Cambridge, UK) and a mouse anti‐CD4 monoclonal antibody (Santa Cruz Biotechnology; sc‐19641, 1:1000), respectively. Secondary goat anti‐mouse IgG‐HRP (Biosharp, BL001A) and goat anti‐rabbit IgG‐HRP (Biosharp, BL003A) were diluted 1:10 000. Positive signals were detected by pro‐light HRP chemiluminescence detection reagent (Tiangen Biotech). Sample loading was monitored with a GAPDH antibody (Santa Cruz Biotechnology; sc‐20357), which was used at a dilution of 1:1000. Quantity One software (v450; Bio‐Rad Laboratories, Hercules, CA) was used to quantify the blots.
Immunohistochemistry analysis
The fixed thymus, lymph node, spleen and liver samples were embedded in paraffin, and paraffin‐embedded sections were deparaffinized in xylene and rehydrated in ethanol. The sections were stained with haematoxylin and eosin (HE). The rehydrated sections were treated to quench endogenous peroxidase activity with 3% H2O2, and non‐specific binding was reduced by incubating with 5% normal goat serum in PBS. Immunohistochemical localization of MT1 in the lymph node and liver was performed using the mouse anti‐MT1 monoclonal antibody (sc‐390328, 1:200), and localization of MT2 in the thymus and spleen was performed using the rabbit anti‐MT2 polyclonal antibody (ab203346, 1:200). Negative controls were treated with antiserum‐specific isotype instead of the MT1 antibody, and the isotype control and antibody were used at the same protein concentration. A DAB kit (Tiangen Biotech) was used to visualize the antibody binding sites in sections of the thymus, lymph node, spleen and liver. Finally, the images were captured on a light microscope (Nikon Eclipse E800, Japan) with a digital camera DP12, and the intensity of staining and density of stained cells were analysed in the captured images. The immunostaining intensity in several fields of thymus, lymph node, spleen and liver samples from different ewes (n = 4 for each group) was randomly chosen and rated by two different investigators in a blinded fashion. The expression of MT1 and MT2 in different cell types and structures was analysed by assigning immunoreactive intensity on a scale of 0 to 3, as described previously. 35 An intensity of 3+ was given to the cells with the highest staining intensity, and an intensity of 0 was assigned to cells with no immunoreactivity.
Statistical analysis
The 2−ΔΔ Ct analysis method was used to calculate relative expression values for qRT‐PCR assays, GAPDH was used as the housekeeping gene. 36 The data for the relative expression levels of MT1 and MT2 mRNA, MT1 and MT2 isoforms, and CD4 protein were analysed as a completely randomized design with at least four animals per group using the Proc Mixed models of SAS (Version 9.1; SAS Institute, Cary, NC). For the thymuses, lymph nodes, spleens and livers from different stages of gestation or pregnancy status, the model contained the random effect of ewe and fixed effects of stage of gestation, pregnancy status and the interaction of stage of gestation and pregnancy status. The comparisons among the relative expression levels of different groups were performed using the Duncan method and controlling the experiment‐wise type ± error equal to 0·05. Data are presented as least squares means. Groups were considered to be significantly different at P < 0·05.
Results
Expression levels of MT1 and MT2 in the thymus
The qRT‐PCR and Western blot analysis revealed (Fig. 1a,b) that the relative expression levels of MT1 mRNA and protein were higher in the thymuses from pregnant ewes than they were in those from the non‐pregnant ewes (P < 0·05), and the relative expression level of MT1 was higher at day 13 of pregnancy than at days 16 and 25 of pregnancy (P < 0·05). Furthermore, the relative expression levels of MT2 mRNA and protein were also higher in the thymuses from the pregnant ewes than it was in those from the non‐pregnant ewes (P < 0·05), but there was no significant difference among the pregnant ewes (P > 0·05; Fig. 1a,b). Immunohistochemistry for the MT2 protein showed that its expression was limited to epithelial reticular cells, capillaries and thymic corpuscles (Fig. 1c). The staining intensity for MT2 protein was 0, 0, 3+, 3+ and 3+ for the negative control, the thymuses from day 16 of the oestrous cycle, and thymuses from days 13, 16 and 25 of pregnancy, respectively. The staining intensity indicated the following: 0 = no staining; and 3+ = very strong staining.
Figure 1.
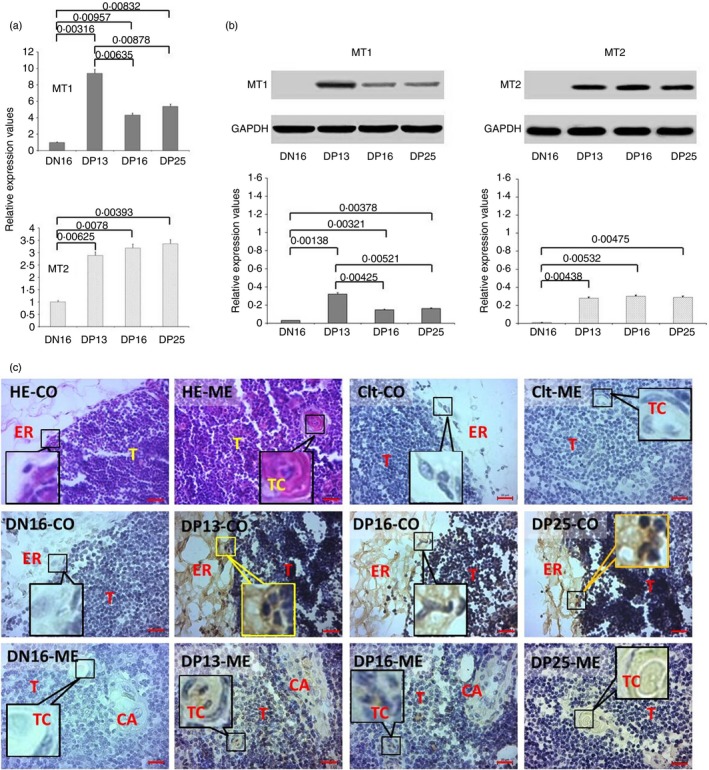
Expression of melatonin receptor 1A (MT1) and melatonin receptor 1B (MT2) in thymuses of non‐pregnant and pregnant ewes. (a) Expression values of MT1 and MT2 mRNA in the thymus. (b) Expression of MT1 and MT2 proteins in the thymus. (c) Representative immunohistochemical localization of MT2 protein in the thymuses. The thymus is divided into the cortex (CO) and the medulla (ME). Note: HE = stained by haematoxylin and eosin; Ctl = negative control; DN16 = day 16 of the oestrous cycle; DP13 = day 13 of pregnancy; DP16 = day 16 of pregnancy; DP25 = day 25 of pregnancy; T = thymocyte; ER = epithelial reticular cell; CA = capillary; TC = thymic corpuscle. Scale bar: 20 µm. P < 0·05 indicates significant difference.
Expression levels of MT1 and MT2 in the lymph node
There was an upregulation of the relative expression levels of MT1 mRNA and protein in the lymph nodes at day 25 of pregnancy (P < 0·05), but there was no significant difference among the non‐pregnant ewes and those at days 13 and 16 of pregnancy (P > 0·05; Fig. 2a,b). Furthermore, there was no significant difference in the relative expression levels of MT2 mRNA and protein among the four groups (P > 0·05; Fig. 2a,b). Immunohistochemistry for the MT1 protein revealed that its expression was limited to the subcapsular sinus and lymph sinus (Fig. 2c). The staining intensity for MT1 was 0, 1+, 1+, 1+ and 2+ for the negative control, the lymph nodes from day 16 of the oestrous cycle, and lymph nodes from days 13, 16 and 25 of pregnancy, respectively. The staining intensity indicated the following: 0 = no staining; 1+ = weak staining; 2+ = and strong staining.
Figure 2.
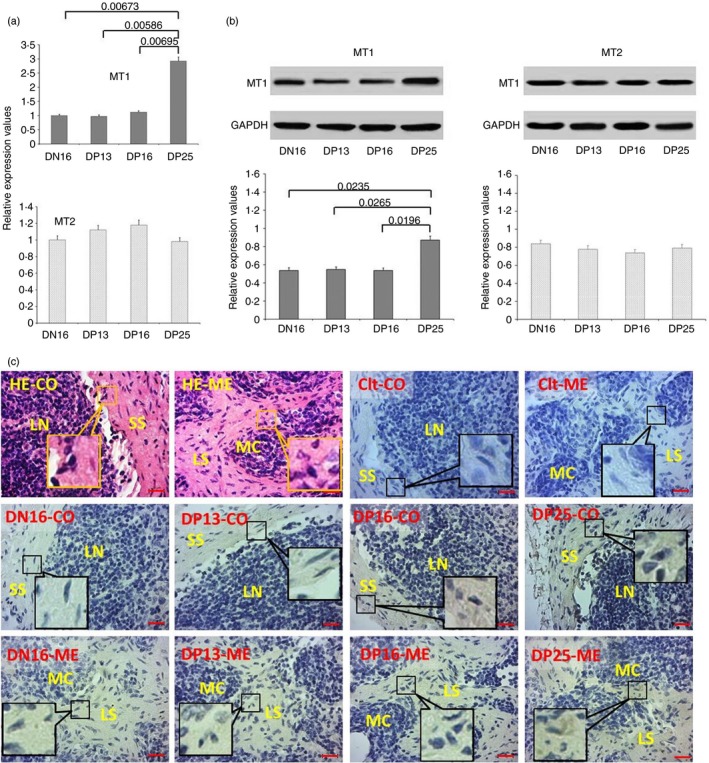
Expression of melatonin receptor 1A (MT1) and melatonin receptor 1B (MT2) in lymph nodes of non‐pregnant and pregnant ewes. (a) Expression values of MT1 and MT2 mRNA in the lymph node. (b) Expression of MT1 and MT2 proteins in the lymph node. (c) Representative immunohistochemical localization of MT1 protein in the lymph node. The lymph node is divided into an outer cortex (CO) and an inner medulla (ME). Lymph enters the convex through the subcapsular sinus (SS) and trabeculae (TRs) around the lymphoid nodules (LN) and flows into the medulla through the lymph sinus (LS) around the medullary cord (MC). Note: HE = stained by haematoxylin and eosin; Ctl = negative control; DN16 = day 16 of the oestrous cycle; DP13 = day 13 of pregnancy; DP16 = day 16 of pregnancy; DP25 = day 25 of pregnancy. Scale bar: 20 µm. P < 0·05 indicates significant difference.
Expression levels of MT1 and MT2 in the spleen
There was a downregulation of MT1 mRNA and protein in the spleens from pregnant ewes (P < 0·05), but there was no significant difference among the pregnant ewes (P > 0·05; Fig. 3a,b). The relative expression levels of MT2 mRNA and protein among the four groups were the lowest in the spleen at day 25 of pregnancy (P < 0·05), but there was no significant difference among non‐pregnant ewes or those at days 13 and 16 of pregnancy (P > 0·05; Fig. 3a,b). Furthermore, immunohistochemistry for the MT2 protein showed that its expression was limited to the capsules, trabeculae and splenic cords (Fig. 3c). The staining intensity for MT2 was 0, 2+, 2+, 2+ and 1+ for the negative control, spleens from day 16 of the oestrous cycle, and spleens from days 13, 16 and 25 of pregnancy, respectively. The staining intensity indicated the following: 0 = no staining; 1+ = weak staining; and 2+ = strong staining.
Figure 3.
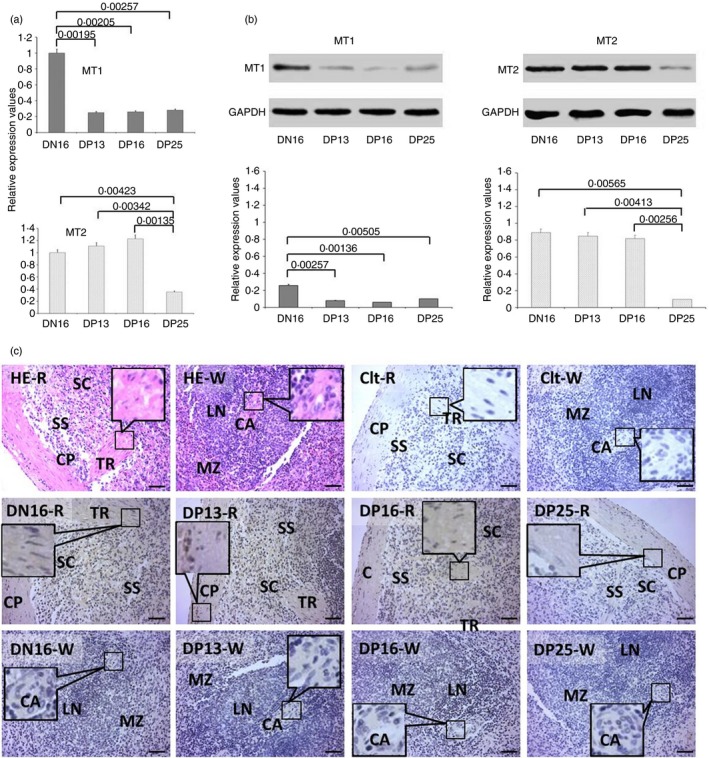
Expression of melatonin receptor 1A (MT1) and melatonin receptor 1B (MT2) in the spleens of non‐pregnant and pregnant ewes. (a) Expression values of MT1 and MT2 mRNA in the spleen. (b) Expression of MT1 and MT2 proteins in the spleen. (c) Representative immunohistochemical localization of MT2 protein in the spleen. The spleen is divided into red pulp (R) and white pulp (W), and surrounded by a thickened capsule (CP). Capsules (CPs) with several trabeculae (TRs) project into the substance of the spleen. Note: HE = stained by haematoxylin and eosin; Ctl = negative control; SS = splenic sinuses; SC = splenic cords; MZ = marginal zone; LN = lymphoid nodule; CA = central arteriole; DN16 = day 16 of the oestrous cycle; DP13 = day 13 of pregnancy; DP16 = day 16 of pregnancy; DP25 = day 25 of pregnancy. Scale bar: 50 µm. P < 0·05 indicates significant difference.
Expression levels of MT1 and MT2 in the liver
There was an upregulation of MT1 mRNA and protein in the livers at day 25 of pregnancy (P < 0·05), and the relative expression levels were higher at days 16 and 25 of pregnancy than they were at non‐pregnant ewes or those at day 13 of pregnancy (P < 0·05; Fig. 4a,b). However, the relative expression levels of MT2 mRNA and protein were higher in the livers from non‐pregnant ewes than they were in those from pregnant ewes (P < 0·05), but there was no significant difference among the pregnant ewes (P > 0·05; Fig. 4a,b). Furthermore, the immunohistochemistry for the MT1 protein showed that its expression was limited to the endothelial cells of the proper hepatic arteries, hepatic portal veins and hepatocytes (Fig. 4c). The staining intensity for MT1 was 0, 1+, 1+, 2+ and 3+ for the negative control, the livers from day 16 of the oestrous cycle, and the livers from days 13, 16 and 25 of pregnancy, respectively. The staining intensity indicated the following: 0 = no staining; 1+ = weak staining; 2+ = strong staining; and 3+ = very strong staining.
Figure 4.
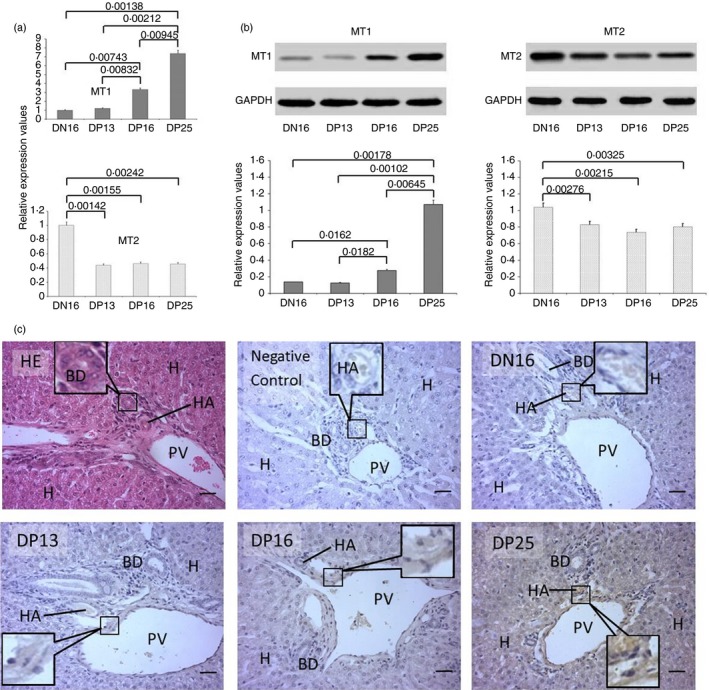
Expression of melatonin receptor 1A (MT1) and melatonin receptor 1B (MT2) in the livers of non‐pregnant and pregnant ewes. (a) Expression values of MT1 and MT2 mRNA in the liver. (b) Expression of MT1 and MT2 proteins in the liver. (c) Representative immunohistochemical localization of MT1 protein in the liver. The liver is divided into lobes, and each lobe is made up of hepatic lobules. A portal triad is a component of the hepatic lobule and consists of the proper hepatic artery (HA), hepatic portal vein (PV), small bile ductile (BD) and lymphatic vessels (LV). Note: H = hepatic cell; HE = stained by haematoxylin and eosin; DN16 = day 16 of the oestrous cycle; DP13 = day 13 of pregnancy; DP16 = day 16 of pregnancy; DP25 = day 25 of pregnancy. Scale bar: 50 µm. P < 0·05 indicates significant difference.
Expression of CD4 in the thymus, lymph node, spleen and liver
The CD4 protein was expressed in the thymus at days 13 and 16 of pregnancy (P < 0·05; Fig. 5a), but it was only expressed in the lymph node at day 25 of pregnancy (P < 0·05; Fig. 5b). In addition, CD4 protein was expressed in the spleen, but early pregnancy had no effect on the expression of CD4 protein in the spleen (P > 0·05; Fig. 5c). Furthermore, the expression level of CD4 protein was the highest in the liver at day 16 of pregnancy (P < 0·05; Fig. 5d), but there was almost no CD4 expression in the liver at day 25 of pregnancy.
Figure 5.
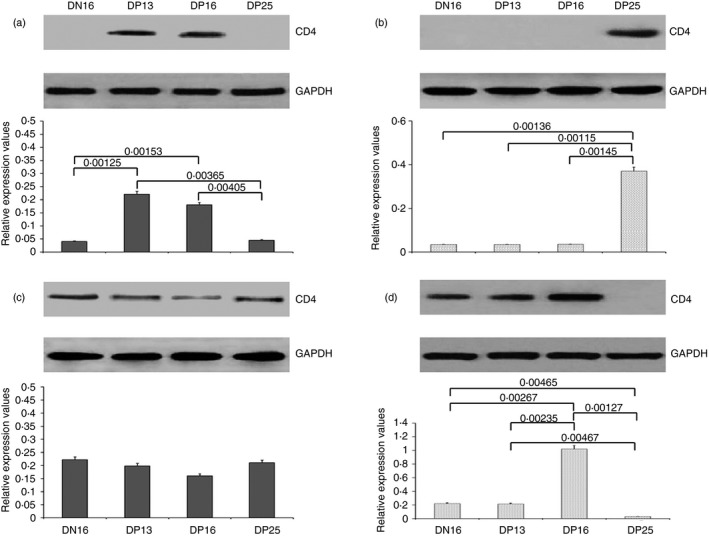
Expression of cluster of differentiation 4 (CD4) protein in the thymus, lymph node, spleen and liver. (a) Thymus; (b) lymph node; (c) spleen; (d) liver. DN16 = day 16 of the oestrous cycle; DP13 = day 13 of pregnancy; DP16 = day 16 of pregnancy; DP25 = day 25 of pregnancy. P < 0·05 indicates significant difference.
Discussion
As a primary lymphoid organ, the thymus is involved in the thymocyte differentiation into mature T lymphocytes, and T lymphocytes migrate from the thymus to constitute peripheral T‐cells, which are essential for the adaptive immune system. There is thymic involution during pregnancy, and thymus weight is negatively correlated with P4 levels in mice. 37 A significant depression of thymocyte production is obvious during pregnancy in mice, but peripheral lymphocyte numbers are not depressed. 38 It has been reported that the interferon‐stimulated gene 15 kDa protein (ISG15), PGR and PIBF are upregulated in the maternal thymus during early pregnancy in sheep. 39 Melatonin has a physiological role in regulating thymus activity during early postnatal development in rats, 40 and exogenous melatonin supplementation can partially restore age‐related thymic involution and peripheral immune dysfunctions in old mice. 41 MT1 and MT2 mRNA and protein expression levels are detected in the mouse thymus, 42 and the expression levels of MT1 and MT2 mRNA, as well as MT1 protein do not decrease in the rat thymus during physiological ageing. 43 Melatonin receptor mRNA is expressed in the rat thymus, 44 and human and rat thymuses can synthesize melatonin that has an immunomodulatory role, which occurs through intracrine, autocrine and paracrine processes. 45 Melatonin can be used as a biological‐response modifier of the immune system to improve Th2‐biased immune responses by alum adjuvants, 46 and Th2‐biased immune responses in the thymus are necessary for successful pregnancy in sheep. 47 Our results showed that the mRNA and protein levels of MT1 and MT2 were increased in the thymus during early pregnancy, while CD4 protein was only expressed at days 13 and 16 of pregnancy. Moreover, immunostaining for MT2 protein showed that its expression was limited to epithelial reticular cells, capillaries and thymic corpuscles. The numbers of CD4+ CD25+ Treg cells are higher in the thymus in normal pregnant mice than in mice that have abortions. 48 Therefore, it is suggested that melatonin exerts its effect on the thymus to regulate thymic immune functions through upregulation of MT1 and MT2 during early pregnancy in sheep.
The lymph node is an ovoid organ of the lymphatic system, and its functions include immune reaction, suppression or tolerance. 49 There is an increase in the lymph node weight and in the number of large pyroninophilic cells from the iliac lymph nodes during pregnancy in mice and rats. 9 , 10 There are peaks in the expression of interferon‐gamma (IFN‐γ), tumour necrosis factor‐beta (TNF‐β), IL‐2, IL‐4, IL‐5, IL‐6, IL‐10, ISG15 and prostaglandin synthases in the lymph node during early pregnancy, which is due to the high level of IFNT derived from the early conceptus in sheep. 50 , 51 There is a relationship between pineal melatonin synthesis, immune cell proliferation and autonomic activity in lymph nodes in rats. 52 Melatonin has immunomodulatory activity that suppresses the Th1‐dependent immune response by inhibiting the production of IFN‐γ by cells in the lymph node. 53 Our results showed that the relative expression level of MT1 was enhanced during early pregnancy, but there was not a significant change in the expression of MT2 in the lymph node; further, CD4 protein was only expressed at day 25 of pregnancy. Moreover, MT1 protein was localized in the subcapsular sinus and lymph sinus. There is an obvious expansion of Treg lymphocytes of the CD4+ CD25+ FOXP3+ phenotype in the uterine draining lymph nodes during pregnancy in mice. 54 Therefore, the increase of MT1 and CD4 may inhibit the Th1‐dependent immune response in the lymph node, which is beneficial for the establishment of pregnancy in sheep.
There is an abundant diverse population of immune cells in the spleen, and they are implicated in the regulation of innate and adaptive immunity. 55 The cofilin‐1, F‐actin capping protein subunit alpha and malate dehydrogenase proteins were strongly downregulated in splenic lymphocytes during the preimplantation period in pregnant mice. 12 There is a downregulation of the 26‐kDa isoform of PGR and the 22‐kDa variant of PIBF, 13 but ISG15 and cyclooxygenase‐2 were upregulated in the spleen during early pregnancy in sheep. 56 Melatonin receptor mRNA is expressed in the rat spleen, 44 and there is a decline in melatonin receptors (MT1/MT2) in the spleen throughout ageing in goats, which indicates that melatonin receptors play a key role in the regulation of immune function in rats and goats. 57 The age‐related decrease in MT1 and MT2 proteins in the spleen is a consequence of a generalized deterioration of immune activity during physiological ageing in mice. 58 The expression of MT1 and MT2 proteins increases under a short‐day photoperiod and decreases under the long‐day photoperiod in the spleen of the Indian palm squirrel. 59 Short‐day photoperiodic conditions and melatonin treatment increase the expression of receptor MT1 in hamsters under long‐day photoperiodic conditions. 60 In this study, the relative expression levels of MT1 and MT2 declined during early pregnancy, but early pregnancy had no effect on the expression of CD4 protein. Moreover, the MT2 protein expression was limited to the capsules, trabeculae and splenic cords. Therefore, the decline in MT1 and MT2 may be due to maternal immune tolerance during early pregnancy in sheep, which is not mediated by CD4 expression in the spleen.
As the primary haematopoietic organ, the liver contains T and B lymphocytes, Kupffer cells, liver‐adapted natural killer cells and dendritic cells, which play a key role in the removal of activated T‐cells and induction of tolerance to self‐antigens. 61 There are marked pregnancy‐dependent adjustments in maternal hepatic physiology, and the hepatic changes include genes related to cell proliferation, cytokine signalling, liver regeneration and metabolism in rats. 62 Cytochrome P450 (CYP) 2d40 is upregulated during pregnancy through enhanced transactivation by hepatocyte nuclear factor 4α (HNF4α) in mouse liver. 63 HNF4α is implicated in the regulation of the cytokine‐induced inflammatory response in the liver, 64 which indicates that the hepatic immune response is regulated by pregnancy in mice. Prenatal glucocorticoid overexposure induces liver steatosis by altering leptin expression, and melatonin can reverse the methylation of leptin and decrease liver steatosis at postnatal day 7. 65 Melatonin treatment can reduce lipid peroxidation induced by obstructive cholestasis during the last third of pregnancy in rats by stimulating the expression of components of the endogenous cellular antioxidant defence in the maternal liver. 66 The melatonin levels in hepatocytes are independent of pineal‐derived melatonin, which provides hepatocytes with a buffer against free radicals. 29 Hepatocytes play a role as ‘accessory’ cells in both the afferent and efferent arms of the cell‐mediated immune response. 67 Our results revealed that MT1 was upregulated, but MT2 was downregulated in the ovine liver during early pregnancy; further, MT1 protein was limited to the endothelial cells of the proper hepatic arteries, hepatic portal veins and hepatic cells. There is an increase in the expression of CD4 in the livers of children who have received liver transplants. 68 In this study, the expression of CD4 protein reached a peak at day 16 of pregnancy, but there was almost no expression of CD4 at day 25 of pregnancy. Therefore, it is suggested that the increase in MT1 may have protective antioxidant activity in the liver, which is helpful for successful pregnancy in sheep. Moreover, there may be enhanced immune rejection exhibited by the liver at day 16 of pregnancy, but immune rejection may disappear at day 25 of pregnancy.
In conclusion, early pregnancy induces tissue‐specific expression of MT1, MT2 and CD4 in the thymus, lymph node, spleen and liver during early pregnancy in sheep. There was an increase in the expression of MT1 in the thymus, lymph node and liver, and a decrease in the spleen. The expression of MT2 was upregulated in the thymus but downregulated in the spleen and liver, and there were no significant changes in the lymph node during early pregnancy. There was an upregulation of CD4 protein in the thymus, lymph node and liver, but there were no significant changes in the spleen during early pregnancy. Overall, the tissue‐specific expression of MT1, MT2 and CD4 may be caused by the different functions of the thymus, lymph node, spleen and liver, and melatonin is involved in the immune regulation of the maternal thymus, lymph node, spleen and liver during early pregnancy in sheep.
Disclosures
The author has no conflict of interests to disclose.
Author contribution
LY designed the project and drafted the manuscript. JCB and NL were involved in performance of the experiments. ZMZ and JCB were involved in the sampling procedures. BW was involved in supervising the project and interpretation of the data. LYZ was involved in revising the manuscript. Additionally, all authors read and approved the final manuscript.
Acknowledgements
This work was supported by the Science and Technology Project of Hebei Province, China (18236601D).
References
- 1. Williams Z. Inducing tolerance to pregnancy. N Engl J Med 2012; 367:1159–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen PJ. The immunology of early pregnancy in farm animals. Reprod Domest Anim 2011; 46:18–30. [DOI] [PubMed] [Google Scholar]
- 3. Oliveira LJ, Barreto RS, Perecin F, Mansouri‐Attia N, Pereira FT, Meirelles FV. Modulation of maternal immune system during pregnancy in the cow. Reprod Domest Anim 2012; 47:384–93. [DOI] [PubMed] [Google Scholar]
- 4. Yang L, Zhang LY, Qiao HY, Liu N, Wang YX, Li SJ. Maternal immune regulation by conceptus during early pregnancy in the bovine. Asian J Anim Vet Adv 2014; 9:610–20. [Google Scholar]
- 5. Parker GA, Papenfuss TL. Chapter 10 ‐ Immune system In: Atlas of Histology of the Juvenile Rat. New York: Academic Press, 2016: 293–347. [Google Scholar]
- 6. Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci USA 1999; 96:12021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aloe L, Micera A, Bracci‐Laudiero L, Vigneti E, Turrini P. Presence of nerve growth factor in the thymus of prenatal, postnatal and pregnant rats. Thymus 1997; 24:221–31. [PubMed] [Google Scholar]
- 8. Swami S, Tong I, Bilodeau CC, Bourjeily G. Thymic involution in pregnancy: a universal finding? Obstet Med 2012; 5:130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hetherington CM, Humber DP. The effect of pregnancy on lymph node weight in the mouse. J Immunogenet 1977; 4:271–6. [DOI] [PubMed] [Google Scholar]
- 10. Mosley JG, McLean JM, Gibbs AC. The response of iliac lymph nodes to the fetal allograft. J Anat 1975; 119:619–23. [PMC free article] [PubMed] [Google Scholar]
- 11. Yang L, Zang S, Bai Y, Yao X, Zhang L. Effect of early pregnancy on the expression of progesterone receptor and progesterone‐induced blocking factor in ovine lymph node. Theriogenology 2017; 93:78–83. [DOI] [PubMed] [Google Scholar]
- 12. Carlomagno G, Minini M, Tilotta M, Unfer V. Proteome of spleen CD4 lymphocytes in mouse preimplantation pregnancy. J Physiol Pharmacol 2014; 65:719–31. [PubMed] [Google Scholar]
- 13. Yang L, Guo R, Yao X, Yan J, Bai Y, Zhang L. Expression of progesterone receptor and progesterone‐induced blocking factor in the spleen during early pregnancy in ewes. Livest Sci 2018; 209:14–9. [Google Scholar]
- 14. Wang F, Bei Y, Zhao Y, Song Y, Xiao J, Yang C. Telocytes in pregnancy‐induced physiological liver growth. Cell Physiol Biochem 2015; 36:250–8. [DOI] [PubMed] [Google Scholar]
- 15. Sriwattanapong K, Slocum SL, Chawanthayatham S, Fedeles BI, Egner PA, Groopman JD et al Pregnancy alters Aflatoxin B1 metabolism and increases DNA damage in mouse liver. Toxicol Sci 2017; 160:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci 2000; 917:376–86. [DOI] [PubMed] [Google Scholar]
- 17. Reiter RJ, Tan DX, Korkmaz A, Rosales‐Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update 2014; 20:293–307. [DOI] [PubMed] [Google Scholar]
- 18. Acuña‐Castroviejo D, Escames G, Venegas C, Díaz‐Casado ME, Lima‐Cabello E, López LC et al Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 2014; 71:2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Altun A, Ugur‐Altun B. Melatonin: therapeutic and clinical utilization. Int J Clin Pract 2007; 61:835–45. [DOI] [PubMed] [Google Scholar]
- 20. Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005; 27:119–30. [DOI] [PubMed] [Google Scholar]
- 21. Reiter RJ, Acuña‐Castroviejo D, Tan DX, Free Burkhardt S. radical‐mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci 2001; 939:200–15. [PubMed] [Google Scholar]
- 22. Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci 2005; 26:412–9. [DOI] [PubMed] [Google Scholar]
- 23. Carrillo‐Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández‐Montesinos R, Guerrero JM et al The modulatory role of melatonin on immune responsiveness. Curr Opin Investig Drugs 2006; 7:423–31. [PubMed] [Google Scholar]
- 24. Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan DX et al Melatonin and pregnancy in the human. Reprod Toxicol 2008; 25:291–303. [DOI] [PubMed] [Google Scholar]
- 25. Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM. Melatonin and reproduction revisited. Biol Reprod 2009; 81:445–56. [DOI] [PubMed] [Google Scholar]
- 26. Reiter RJ, Rosales‐Corral SA, Manchester LC, Liu X, Tan DX. Melatonin in the biliary tract and liver: health implications. Curr Pharm Des 2014; 20:4788–801. [DOI] [PubMed] [Google Scholar]
- 27. Carlomagno G, Minini M, Tilotta M, Unfer V. From implantation to birth: insight into molecular melatonin functions. Int J Mol Sci 2018; 19:E2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guan S, Xie L, Ma T, Lv D, Jing W, Tian X et al Effects of melatonin on early pregnancy in mouse: involving the regulation of StAR, Cyp11a1, and Ihh expression. Int J Mol Sci 2017; 18:E1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glebezdina NS, Olina AA, Nekrasova IV, Kuklina EM. Role of endogenous melatonin in the regulation of Th17/Treg balance during pregnancy. Bull Exp Biol Med 2018; 164:462–5. [DOI] [PubMed] [Google Scholar]
- 30. Zarazaga LA, Malpaux B, Chemineau P. Characteristics of the plasma melatonin rhythm are not modified by steroids during the estrous cycle in Ile‐de‐France ewes. J Pineal Res 1996; 21:114–20. [DOI] [PubMed] [Google Scholar]
- 31. Zarazaga LA, Malpaux B, Chemineau P. The characteristics of the melatonin secretory rhythm are not modified by the stage of pregnancy in ewes. Reprod Nutr Dev 1997; 37:105–12. [DOI] [PubMed] [Google Scholar]
- 32. Brady RL, Dodson EJ, Dodson GG, Lange G, Davis SJ, Williams AF et al Crystal structure of domains 3 and 4 of rat CD4: relation to the NH2‐terminal domains. Science 1993; 260:979–83. [DOI] [PubMed] [Google Scholar]
- 33. Mcnatty KP, Revefeim KJ, Young A. Peripheral plasma progesterone concentrations in sheep during the oestrous cycle. J Endocrinol 1973; 58:219–25. [DOI] [PubMed] [Google Scholar]
- 34. Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J Reprod Fertil 1982; 65:141–50. [DOI] [PubMed] [Google Scholar]
- 35. Kandil D, Leiman G, Allegretta M, Trotman W, Pantanowitz L, Goulart R et al Glypican‐3 immunocytochemistry in liver fine‐needle aspirates: a novel stain to assist in the differentiation of benign and malignant liver lesions. Cancer 2007; 111:316–22. [DOI] [PubMed] [Google Scholar]
- 36. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101–8. [DOI] [PubMed] [Google Scholar]
- 37. Chambers SP, Clarke AG. Measurement of thymus weight, lumbar node weight and progesterone levels in syngeneically pregnant, allogeneically pregnant, and pseudopregnant mice. J Reprod Fertil 1979; 55:309–15. [DOI] [PubMed] [Google Scholar]
- 38. Shinomiya N, Tsuru S, Tsugita M, Katsura Y, Takemura T, Rokutanda M et al Thymic depletion in pregnancy: kinetics of thymocytes and immunologic capacities of the hosts. J Clin Lab Immunol 1991; 34:11–22. [PubMed] [Google Scholar]
- 39. Zhang L, Xue J, Wang Q, Lv W, Mi H, Liu Y et al Changes in expression of ISG15, progesterone receptor and progesterone‐induced blocking factor in ovine thymus during early pregnancy. Theriogenology 2018; 121:153–9. [DOI] [PubMed] [Google Scholar]
- 40. Martin‐Cacao A, Lopez‐Gonzalez MA, Reiter RJ, Calvo JR, Guerrero JM. Binding of 2‐[125I]melatonin by rat thymus membranes during postnatal development. Immunol Lett 1993; 36:59–63. [DOI] [PubMed] [Google Scholar]
- 41. Tian YM, Zhang GY, Dai YR. Melatonin rejuvenates degenerated thymus and redresses peripheral immune functions in aged mice. Immunol Lett 2003; 88:101–4. [DOI] [PubMed] [Google Scholar]
- 42. Carrillo‐Vico A, García‐Pergañeda A, Naji L, Calvo JR, Romero MP, Guerrero JM. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci 2003; 60:2272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sánchez‐Hidalgo M, Guerrero Montávez JM, Carrascosa‐Salmoral MP, Naranjo Gutierrez MC, Lardone PJ, de la Lastra Romero CA. Decreased MT1 and MT2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging. J Pineal Res 2009; 46:29–35. [DOI] [PubMed] [Google Scholar]
- 44. Pozo D, Delgado M, Fernandez‐Santos JM, Calvo JR, Gomariz RP, Martin‐Lacave I et al Expression of the Mel1a‐melatonin receptor mRNA in T and B subsets of lymphocytes from rat thymus and spleen. FASEB J 1997; 11:466–73. [DOI] [PubMed] [Google Scholar]
- 45. Naranjo MC, Guerrero JM, Rubio A, Lardone PJ, Carrillo‐Vico A, Carrascosa‐Salmoral MP et al Melatonin biosynthesis in the thymus of humans and rats. Cell Mol Life Sci 2007; 64:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Regodón S, Martín‐Palomino P, Fernández‐Montesinos R, Herrera JL, Carrascosa‐Salmoral MP, Píriz S et al The use of melatonin as a vaccine agent. Vaccine 2005; 23:5321–7. [DOI] [PubMed] [Google Scholar]
- 47. Zhang L, Zhao Z, Mi H, Liu B, Wang B, Yang L. Modulation of helper T cytokines in thymus during early pregnancy in ewes. Animals 2019; 9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T et al Abnormal T‐cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy‐induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 2005; 166:811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buettner M, Bode U. Lymph node dissection–understanding the immunological function of lymph nodes. Clin Exp Immunol 2012; 169:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang L, Wang P, Mi H, Lv W, Liu B, Du J et al Comparison of Th1 and Th2 cytokines production in ovine lymph nodes during early pregnancy. Theriogenology 2019; 123:177–84. [DOI] [PubMed] [Google Scholar]
- 51. Yang L, Wang Q, Liu Y, Zhang L, Lv W, Liu B. Expression profiles of interferon‐stimulated gene 15 and prostaglandin synthases in the ovine lymph nodes during early pregnancy. Mol Reprod Dev 2019; 86:100–8. [DOI] [PubMed] [Google Scholar]
- 52. Cardinali DP, Brusco LI, García Bonacho M, Esquifìno AI. Effect of melatonin on 24‐hour rhythms of ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats. Neuroendocrinology 1998; 67:349–62. [DOI] [PubMed] [Google Scholar]
- 53. Majewska M, Zajac K, Zemelka M, Szczepanik M. Influence of melatonin and its precursor L‐tryptophan on Th1 dependent contact hypersensitivity. J Physiol Pharmacol 2007; 58:125–32. [PubMed] [Google Scholar]
- 54. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5:266–71. [DOI] [PubMed] [Google Scholar]
- 55. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 2005; 5:606–16. [DOI] [PubMed] [Google Scholar]
- 56. Yang L, Liu Y, Lv W, Wang P, Wang B, Xue J et al Expression of interferon‐stimulated gene 15‐kDa protein, cyclooxygenase (COX) 1, COX‐2, aldo‐keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim Sci J 2018; 89:1540–8. [DOI] [PubMed] [Google Scholar]
- 57. Singh SS, Laskar P, Acharjee S. Age‐ and sex‐dependent effect of exogenous melatonin on expression pattern of melatonin receptor (MT1 and MT2) proteins in spleen of mice. Biol Rhythm Res 2015; 46:403–15. [Google Scholar]
- 58. Singh AK, Haldar C. Age dependent nitro‐oxidative load and melatonin receptor expression in the spleen and immunity of goat Capra hircus. Exp Gerontol 2014; 60:72–8. [DOI] [PubMed] [Google Scholar]
- 59. Ahmad R, Haldar C. Photoperiodic regulation of MT1 and MT2 melatonin receptor expression in spleen and thymus of a tropical rodent Funambulus pennanti during reproductively active and inactive phases. Chronobiol Int 2010; 27:446–62. [DOI] [PubMed] [Google Scholar]
- 60. Vishwas DK, Haldar C. Photoperiodic induced melatonin regulates immunity and expression pattern of melatonin receptor MT1 in spleen and bone marrow mononuclear cells of male golden hamster. J Photochem Photobiol B 2013; 128:107–14. [DOI] [PubMed] [Google Scholar]
- 61. Parker GA, Picut CA. Immune functioning in non lymphoid organs: the liver. Toxicol Pathol 2012; 40:237–47. [DOI] [PubMed] [Google Scholar]
- 62. Bustamante JJ, Copple BL, Soares MJ, Dai G. Gene profiling of maternal hepatic adaptations to pregnancy. Liver Int 2010; 30:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ning M, Koh KH, Pan X, Jeong H. Hepatocyte nuclear factor (HNF) 4α transactivation of cytochrome P450 (Cyp) 2d40 promoter is enhanced during pregnancy in mice. Biochem Pharmacol 2015; 94:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Z, Bishop EP, Burke PA. Expression profile analysis of the inflammatory response regulated by hepatocyte nuclear factor 4α. BMC Genom 2011; 12:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tiao MM, Huang LT, Chen CJ, Sheen JM, Tain YL, Chen CC et al Melatonin in the regulation of liver steatosis following prenatal glucocorticoid exposure. Biomed Res Int 2014; 2014:942172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Perez MJ, Castano B, Gonzalez‐Buitrago JM, Marin JJ. Multiple protective effects of melatonin against maternal cholestasis‐induced oxidative stress and apoptosis in the rat fetal liver‐placenta‐maternal liver trio. J Pineal Res 2007; 43:130–9. [DOI] [PubMed] [Google Scholar]
- 67. Volpes R, van den Oord JJ, Desmet VJ. Can hepatocytes serve as ‘activated’ immunomodulating cells in the immune response? J Hepatol 1992; 16:228–40. [DOI] [PubMed] [Google Scholar]
- 68. Senaldi G, Mieli‐Vergani G, Portmann B, Mowat AP, Vergani D. CD4 expression on liver sinusoidal cells: enhancement in liver transplant rejection. Hepatology 1991; 13:1265. [PubMed] [Google Scholar]


