Summary
The transcription factor Foxp3 controls the differentiation and function of regulatory T‐cells (Treg). Studies in the past decades identified numerous Foxp3‐interacting protein partners. However, it is still not clear how Foxp3 produces the Treg‐type transcriptomic landscape through cooperating with its partners. Here I show the current understanding of how Foxp3 transcription factor complexes regulate the differentiation, maintenance and functional maturation of Treg. Importantly, T‐cell receptor (TCR) signalling plays central roles in Treg differentiation and Foxp3‐mediated gene regulation. Differentiating Treg will have recognized their cognate antigens and received TCR signals before initiating Foxp3 transcription, which is triggered by TCR‐induced transcription factors including NFAT, AP‐1 and NF‐κB. Once expressed, Foxp3 seizes TCR signal‐induced transcriptional and epigenetic mechanisms through interacting with AML1/Runx1 and NFAT. Thus, Foxp3 modifies gene expression dynamics of TCR‐induced genes, which constitute cardinal mechanisms for Treg‐mediated immune suppression. Next, I discuss the following key topics, proposing new mechanistic models for Foxp3‐mediated gene regulation: (i) how Foxp3 transcription is induced and maintained by the Foxp3‐inducing enhanceosome and the Foxp3 autoregulatory transcription factor complex; (ii) molecular mechanisms for effector Treg differentiation (i.e. Treg maturation); (iii) how Foxp3 activates or represses its target genes through recruiting coactivators and corepressors; (iv) the ‘decision‐making’ Foxp3‐containing transcription factor complex for Th17 and Treg differentiation; and (v) the roles of post‐translational modification in Foxp3 regulation. Thus, this article provides cutting‐edge understanding of molecular biology of Foxp3 and Treg, integrating findings by biochemical and genomic studies.
Keywords: AML1/Runx1, Foxp3, protein–protein interaction, regulatory T‐cells, TCR signalling, transcription factor
Foxp3 controls the differentiation and function of Treg. However, it is still not clear how Foxp3 establishes the transcriptional programme of Treg through cooperating with its partners. Here I highlight the central roles of TCR‐induced transcription factors and Runx1 in Treg, showing models of how Foxp3 overrides Runx1 to modulate TCR‐induced transcription factor complexes in Treg, which cardinal feature is antigen‐reactivity and the resultant active state.

Abbreviations
- AML1
acute myeloid leukaemia‐1
- AP‐1
activator protein‐1
- APC
antigen‐presenting cell
- CBP
CREB‐binding protein
- ChIP
chromatin immunoprecipitation
- CNS
conserved non‐coding sequences
- eTreg
effector Treg
- H3K27ac
histone H3 lysine 27 acetylation
- H3K27me3
histone H3 lysine 27 trimethylation
- HAT
histone acetyltransferase
- IL
interleukin
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X‐linked
- iTreg
induced Treg
- PI(3)K
phosphoinositide 3‐kinase
- SP
single positive
- TCR
T‐cell receptor
- TNFRSF
TNF receptor superfamily
- Tocky
timer of cell kinetics and activity
- TRAF
TNF receptor‐associated factor
- Treg
regulatory T‐cells
- TSDR
Treg‐specific demethylation region
Introduction
Upon recognising antigens, T‐cells receive T‐cell receptor (TCR) signals, thereby get activated and differentiate into effector T‐cells, and thus induce immune responses. In inflammatory environments, in which antigen‐presenting cells (APCs) are matured,1 T‐cells effectively receive CD28 signals from its ligands CD80/CD86 on APCs when TCRs engage with peptide‐MHC complex. These two signals induce T‐cell activation and proliferation.2, 3 Then, activated T‐cells produce interleukin (IL)‐2 and CD25 (IL‐2 receptor α chain), which forms the high‐affinity IL‐2 receptor with β and γ chains. Activated T‐cells efficiently receive IL‐2 signals, which promote their own survival and differentiation. Thus, a positive feedback loop for T‐cell activation is established.4, 5 T‐cell activation also induces molecules for negative feedback regulation in any T‐cells, including CTLA‐4, which binds to and inhibits CD80/CD86 on APCs and thereby blocks CD28 signalling, inducing T‐cell quiescence.6
However, upon recognizing antigens, a minor subset of CD4+ T‐cells do not induce immune responses but suppress the activities of other T‐cells. These cells are designated as regulatory T‐cells (Treg), or natural Treg.7, 8 Treg specifically express the transcription factor Foxp3, which controls their differentiation and function.9, 10, 11 Notably, Treg do not produce IL‐2 by TCR stimulation,12 and the majority of them have an activated memory phenotype (CD25+CD45RBlow).13, 14 Foxp3+ Treg constitute 10−15% of CD4+ T‐cells in the periphery and 5−6% of CD4‐single positive (SP) cells in the thymus of healthy mice.15, 16
Although the mechanism of Treg‐mediate suppression is still debated, Foxp3 represses Il2 transcription, upregulates the expression of CD25 and other Treg markers, and confers suppressive activities to CD4+ T‐cells.9 Mutations of the Foxp3 gene impair Treg function and lead to autoimmune diseases in mice (Scurfy disease17, 18) and humans [immune dysregulation, polyendocrinopathy, enteropathy, X‐linked (IPEX) syndrome19]. The depletion of Foxp3+ Treg can induce autoinflammation20, 21 and can enhance anti‐cancer immunity.22 On the other hand, cell transfer of ex vivo isolated Treg or Foxp3‐transduced T‐cells can suppress the activities of autoinflammatory T‐cells.23 Collectively, these indicate that the regulation of Foxp3 expression is critically important for controlling immune responses.
The unique gene expression dynamics of TCR‐induced genes constitute key mechanisms for the suppressive activities of Treg. Treg highly express CD25 and do not produce IL‐2 and, thus, they can absorb IL‐2 proteins in microenvironments and prevent effector T‐cells from proliferating and surviving.24 In addition, Treg highly express CTLA‐4 and, thus, when inflammation occurs, Treg can immediately prevent other T‐cells from receiving CD28 signalling.25
Foxp3 has a DNA‐binding domain (forkhead domain), and binds DNA as homodimer or heterodimer with Foxp1.26 Foxp3 interacts with key transcription factors for T‐cell function, including NFAT,27 NF‐κB,28 and AML1/Runx1 (hereafter Runx1).29 The Foxp3 transcription factor complex further associates with transcriptional repressors and activators to regulate transcriptional activities of its target genes in a context‐dependent manner. Studies in the past 16 years identified more than 300 proteins as Foxp3‐interacting partners, although many of these interactions still need further investigations.
In this article, I will show the up‐to‐date understanding of how Foxp3 regulates the differentiation, maintenance and functional maturation of Treg through interacting with other transcription factors. To this end, I will firstly discuss the key evidence for the current understanding of Treg, highlighting the importance of Foxp3 expression dynamics.
From Treg classification to Foxp3 expression dynamics
Treg are heterogeneous. The identification of functional Treg subsets is key to understand Treg function. Treg are often classified into thymic and peripheral Treg, depending on whether they become Treg in the thymus or the periphery, respectively.30 In addition, both Treg subsets can become activated and show enhanced suppressive activities in vivo as effector Treg (eTreg).
Thymic Treg and peripheral Treg
Thymic Treg (tTreg) are defined as the Treg that differentiate in the thymus.30 In addition, Foxp3−CD4+ T‐cells (designated as conventional T‐cells) can become Treg in the periphery (peripheral Treg, pTreg), especially in the intestinal system31 and the placenta.32 Food antigens and commensal bacteria induce Foxp3 expression in T‐cells in the small intestine and the colon, respectively, generating pTreg.33, 34 Intriguingly, recent thymic emigrant CD4+ T‐cells have higher capacities to initiate Foxp3 expression in the periphery.35 The majority of natural Treg are believed to be tTreg,36 although quantitative data are not yet available.
While Helios (Ikzf2) and neuropilin‐1 (Nrp1) are often used as markers for tTreg, their expression can be changed depending on experimental conditions.36 The majority of Treg in the colon express ROR‐γt (also known as a Th17 transcription factor). ROR‐γt+ Treg are considered to be pTreg, as they do not express Helios and are induced by gut microbiota, while Helios+ Treg are regarded as tTreg. However, it is debated how specific these markers are, and a recent single cell study showed that conventional T‐cells can generate both Helios+ and ROR‐γt+ Treg in the colon.37
By definition, tTreg must have TCRs that recognize self‐antigens presented in the thymus, because Treg differentiation requires the recognition of cognate antigens.38, 39 On the other hand, the TCR repertoire of pTreg may be enriched with those for commensal and food antigens.40, 41 However, investigations are hampered by the lack of methods to specifically identify tTreg and pTreg.42
Understanding eTreg and Treg maturation by Foxp3 expression dynamics
Regardless of in which organ Foxp3 expression is initiated, functional Treg have the common characteristics: high Foxp3 expression. Foxp3 expression is primarily controlled by transcriptional and epigenetic regulation. A recent technological advancement to analyse temporal dynamics of Foxp3 transcription is providing new insights into Treg differentiation and function.43 Notably, highly sustained Foxp3 transcription over time induces the differentiation of eTreg, which is also known as Treg maturation.44 On the other hand, once Foxp3 expression is completely lost, Treg can become memory‐phenotype T‐cells during homeostasis,45 or fully function as effector T‐cells that promote inflammation (also known as ‘ex‐Treg’).46 Interestingly, Nrp1 expression is high in resting Treg but is substantially reduced in eTreg with persistent Foxp3 transcription.44 In a skin allergy model, both eTreg differentiation and de novo Foxp3 expression in effector T‐cells are promoted.44 This suggests that new Foxp3 induction and Treg maturation use a common mechanism, which most likely involves TCR‐induced transcription factors.
Foxp3 expression can be induced just by TCR stimulation in most of CD4+ T‐cells in humans, and by the combination of TGF‐β, IL‐2, and TCR stimulation in mice (induced Treg, iTreg).47 Because this in vitro induced Foxp3 expression is not ‘stable’ over time, the induction of Foxp3 has been dismissed as ‘transient’. However, ‘transient’ Foxp3 expression has cell‐intrinsic roles to suppress TCR‐induced activation,48, 49, 50 and may have large impacts on T‐cell regulation, considering that any acute inflammation is also ‘transient’. In humans, notably, the majority of Foxp3+ T‐cells are low Foxp3 expressors that produce pro‐inflammatory cytokines and are not suppressive in vitro, whereas Foxp3high CD25highCD45RO+ CD4+ T‐cells are suppressive and designated as eTreg.51, 52
The difference in Foxp3 dynamics between humans and mice can be explained by epigenetic regulation. Importantly, Foxp3 promoter is TCR‐responsive.53 CpG islands in the human Foxp3 promoter are demethylated in naïve T‐cells,54 whereas those in mouse Foxp3 promoter are only partially demethylated in naïve T‐cells and fully demethylated by TGF‐β signals.53 These can explain why human T‐cells can express Foxp3 just by TCR signals, while mouse T‐cells generally require TCR and TGF‐β signals for efficient induction of Foxp3. On the other hand, the demethylation of CpG islands in Treg‐specific demethylation region (TSDR) is associated with ‘stable’ Foxp3 expression. TSDR is methylated in conventional T‐cells and iTreg cells in mice and humans, and human Foxp3low T‐cells, while it is fully demethylated in mouse Treg and human Foxp3highCD25high CD45RO+ eTreg.52, 55
Collectively, Treg function is dependent of Foxp3 expression level in mice and humans. Both mouse and human Treg have an activated memory phenotype.
TCR signalling downstream is continuously active in Treg due to infrequent‐but‐regular antigen recognition
This section shows that Treg have a constitutive active status, which is induced by TCR signals independently from Foxp3, and that Foxp3 exploits TCR‐induced mechanisms to produce Treg phenotype and function. To understand these, it is important to analyse temporal dynamics of TCR signalling and Treg differentiation in vivo.
A recent breakthrough was made through the new technology Timer of Cell Kinetics and Activity (Tocky). Tocky uses as a reporter gene Fluorescent Timer protein that spontaneously and irreversibly changes its emission spectrum from blue to red within 4 hr on average.56 Using the TCR downstream gene Nr4a3, Nr4a3‐Tocky allows analysis of real‐time dynamics of TCR signal downstream activities at the single cell level. In addition, Foxp3‐Tocky enables analysis of temporal dynamics of Foxp3 transcription, and thereby reveals temporal orders of Treg differentiation events in the thymus and the periphery.56
Importantly, when Foxp3 is expressed in differentiating or mature Treg, TCR signal‐induced transcription factors will have been already active. Thus, once expressed, Foxp3 can immediately make a complex with these transcription factors during Treg differentiation and homeostasis. This is a non‐obvious fact but can be safely concluded from the following three lines of evidence.
Firstly, TCR signals always come first before Foxp3 expression. Foxp3 is a TCR signal downstream gene as discussed above. This means that, when Treg differentiate, they will have been already activated by TCR signals.56, 57 Treg differentiation is triggered and controlled by TCR signals in the thymus and the periphery. When differentiating CD4‐SP cells receive strong TCR signals, they upregulate CD25 expression, becoming Treg precursors. If these precursor cells continue to receive persistent TCR signals across time, they initiate Foxp3 transcription and become Treg.56 In the periphery, CD4+ conventional T‐cells can differentiate into pTreg by recognizing food and microbial antigens as discussed above. Furthermore, new Foxp3 transcription can be initiated in effector T‐cells upon antigen recognition during inflammation.44
Secondly, Treg receive infrequent‐yet‐regular TCR signals in the periphery. Moran et al. developed Nr4a1‐GFP reporter mice and showed that Treg have higher GFP, suggesting that Treg received more TCR signals, although their temporal dynamics were not known.58 Nr4a3‐Tocky analysis showed that the majority of Treg spontaneously recognized their cognate antigen and received TCR signals approximately once a few days.56 Thus, Treg regularly recognize their cognate antigens and receive TCR signals. Because T‐cells specific to tissue antigens or microbiota can differentiate into Treg (either as tTreg or as pTreg), it is not surprising that Treg regularly encounter with their cognate antigens while circulating the body. This also indicates that the relative ‘short‐life’ of pTreg may be due to the removal of their cognate antigens from the system,50 especially when the antigens are exogenous.
Thirdly, genes that are stimulated downstream of TCR signalling are actively transcribed in Tregs. Our multidimensional transcriptome analysis showed that Treg were as activated as tissue‐infiltrating effector T‐cells and memory‐phenotype T‐cells at the transcriptome level.59 Furthermore, upon the deletion of TCRα in the periphery, Treg downregulate Treg markers60 and lose the transcriptional signature of activated T‐cells.59
Summarizing, Foxp3 is induced in T‐cells that have received strong and persistent TCR signals, and Foxp3 expression is maintained in a unique type of activated T‐cells, which regularly encounter with their cognate antigens. In other words, as we previously proposed, whether in tTreg or pTreg, Foxp3 is induced and maintained as a negative feedback on cognate antigen‐induced activation processes, and that Treg are activated T‐cells in which negative regulatory mechanisms are actively operating.50 Accordingly, transcription factors downstream of TCR signalling are always available to Foxp3 protein in Treg.
In the following sections, I highlight the following key Foxp3‐interacting transcription factors as central players for Treg differentiation and function: (i) TCR signal‐induced transcription factors (e.g. NFAT); (ii) Runx and cofactors; (iii) Foxp3 autoregulatory transcription factor complex; (iv) transcription factors for eTreg maturation; and (v) Foxp3‐containing transcription factor complex for fate bifurcation (e.g. Foxp3/ROR‐γt/Hif1a; Table 1).
Table 1.
Key Foxp3 transcription factor complexes
| Classification | Key Foxp3‐interacting partners | Possible involvements | Role |
|---|---|---|---|
| TCR signal‐induced transcription factors | NFAT, NF‐kB (c‐Rel, RelA) | AP‐1 (Fos/Jun), p300 | Modulation of T‐cell activation programme |
| Runx proteins and their cofactors | Runx1, CBF‐β | Runx3, Est1, p300 | Formation of Treg differentiation |
| Foxp3 autoregulatory complex | Runx1, Stat5, Smad3 | Activation and maintenance of Foxp3 transcription | |
| Transcription factors for eTreg maturation | Myb, IRF4, Blimp1, JunB, RelA | Promote Treg maturation (eTreg differentiation) | |
| Intermediate complex for fate bifurcation | Runx1, ROR‐γt Hif1a, Deltex | RORa | Fate bifurcation between Treg and Th17 |
| Transcriptional repressor and activator | Eos (Ikzf4) | Aiolos (Ikzf3), Ezh2, p300, Tip60 | Transcriptional regulation |
Foxp3 interacts with TCR signalling downstream transcription factors
Foxp3 interacts with TCR‐induced transcription factors, which are activated through signalling cascades initiated by TCR and CD28 costimulatory signals. TCR‐MHC engagement activates proximal signalling molecules including PLCγ1 and PKC, which subsequently activate calcium signalling and NF‐κB and Ras/MAPK pathways.61 Calcium influx activates and translocates NFAT into the nucleus.62 TCR and CD28 costimulatory signals cooperatively activate MAPK and NF‐κB pathway, and efficiently activate AP‐1 transcription factors63 and NF‐κB.64 Notably, NFAT and AP‐1 make a complex, and bind to and activate the IL‐2 promoter.62
NFAT and AP‐1
The AP‐1 transcription factors are formed by heterodimerization of bZIP proteins, including Jun (e.g. c‐Jun, JunB and JunD), Fos (e.g. c‐Fos) and ATF (e.g. ATF2, BATF).65 Particularly, Fos and Jun regulate TCR signal downstream genes.65 Foxp3 is proposed to bind c‐Jun and inhibit AP‐1 activities,66 although the evidence is limited.
Foxp3 binds NFAT and inhibits its transcriptional activities.27, 28 Wu et al. analysed the crystal structure of NFATc2 (NFAT1), the forkhead domain of FOXP2 (which is considerably similar to that of FOXP3) and a 19 base‐pair‐DNA sequence from the IL2 promoter (namely, ARRE2), and characterized the NFATc2:FOXP2:DNA complex. Interestingly, when making a complex with NFATc2, the forkhead domain bound the DNA sequence that are occupied by Fos and Jun when they bind NFAT, suggesting that Foxp3 competitively inhibit the NFATc2:Fos:Jun complex by binding NFATc2 and replacing Fos and Jun.27 However, this view is challenged, as another Foxp member, Foxp1, is constitutively expressed by CD4+ T‐cells,67 which means that Foxp1 may constitutively make a complex with NFAT in the absence of Foxp3.
Functional significance of the Foxp3−NFAT interaction is still not fully clear. Wu et al. showed that Foxp3 mutants that did not bind NFATc2 were defective in mediating Il2 repression, the upregulaton of Treg‐associated surface proteins (e.g. CD25) and suppressive activities,27 suggesting that the Foxp3−NFATc2 complex regulates transcriptional activities of a wide range of genes. The Foxp3‐binding domain of NFATc2 is conserved with other NFAT isoforms (i.e. NFAT2, NFAT3 and NFAT4).27 However, it is not known whether different NFAT isoforms play different roles in regulating Foxp3 function.
NF‐κB
Immunoprecipitation experiments showed that Foxp3 physically interacted with RelA (p65), an NF‐κB subunit,28, 68, 69 although another study failed to confirm this.70 Camperio et al. 68 showed that Foxp3 and RelA cooperatively activated the transcription of the promoter of Il2ra (CD25) by a reporter assay, while Foxp3 rather repressed NF‐κB reporters in other studies.70, 71 Loizou et al.71 showed that Foxp3 interacted with the DNA‐binding domain of another NF‐κB component, c‐Rel, and repressed NF‐κB transcriptional activities, although the evidence is limited to overexpression and reporter assays. These conflicting results suggest that the interaction between Foxp3 and NF‐κB may be indirect through NFAT, which can make a complex with RelA72 and c‐Rel,73 or through Runx1, which interacts with the NF‐κB subunit p50.74
Collectively, Foxp3 participates in TCR‐induced transcription factor complexes and modulates their activities. This is considered to be critically important to modulate the gene expression dynamics of TCR‐induced genes in Treg, which constitute cardinal mechanisms for Treg‐mediated immune suppression.
Foxp3 seizes Runx transcription factor complex for shaping the Treg‐type transcriptome
Foxp3 interacts with Runx1 and modulates its transcriptional regulation in Treg. Thus, this section briefly discusses the roles of Runx1 in non‐regulatory T‐cells, and thereafter shows the Foxp3−Runx1 complex‐mediated regulation of Treg differentiation and function.
Roles of Runx1 in CD4+ T‐cells
Runx1 directly bind to specific DNA sequences through its N‐terminal DNA‐binding domain (Runt domain) by making a complex with the cofactor CBF‐β (Fig. 1). As its original name (acute myeloid leukaemia‐1) implies, Runx1 is a frequent target of chromosomal translocation and fusion protein formation in acute leukaemia.75 In normal cells, Runx1 mediates DNA‐sequence‐specific transcriptional regulation and plays key roles in multiple stages of haematopoietic cell development, from haematopoietic stem cells to peripheral T‐cells.76 Importantly, Runx1 controls the differentiation and homeostasis of CD4+ T‐cells, although underlying mechanisms are not clear. Deletion of Runx1 in thymic T‐cells results in the blockade of CD4‐SP maturation, and CD4+ T‐cells are reduced in the periphery.77 On the other hand, transgenic expression of Bcl2 in CD4+ T‐cells restores the number of CD4+ T‐cells in the Runx1‐deficient background,78 suggesting that Runx1 inhibits the activities of pro‐apoptotic Bcl‐2 family proteins.
Figure 1.
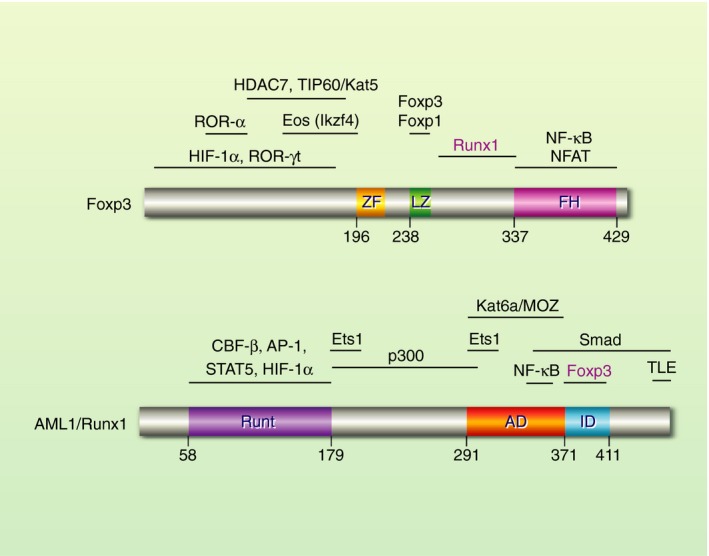
Structure of Foxp3 and AML1/Runx1 proteins. Foxp3 binds DNA through the forkhead (FH) domain, which directly interacts with NFAT. Foxp3 makes a homodimer or heterodimer with Foxp1 through its leucine zipper (LZ) domain. Foxp3 directly interacts with AML1/Runx1 through the inter‐domain region between FH and LZ domains,29 which is distinct from the interacting domains for other key partners including NFAT,27 Foxp3/Foxp1,26 HDAC7 and TIP60/Kat5.154 AML1/Runx1 binds DNA through the Runt domain by making a complex with CBF‐β. AML1/Runx1 interacts with Foxp3 through its inhibition domain (ID).29 AML1/Runx1 can associate with key transcription factors for T‐cell activities including Stat5, Smads, AP‐1, Ets1 and NF‐κB. In addition, AML1/Runx1 can recruit and interact with major transcriptional activators and repressors including p300, Kat6a/Myst3/Moz and TEL.75, 155, 156 AD, transactivation domain; FH, forkhead; LZ, leucine zipper; ZF, zinc finger.
The Foxp3−Runx1 interaction
Foxp3 physically interacts with Runx1 and thereby modifies Runx1‐mediated gene regulation. The Runx1‐binding region of Foxp3 is the inter‐domain region between the forkhead domain and the leucine zipper motif, which does not have other protein interaction or any known function29 (Fig. 1). Ono et al. showed that the Foxp3 mutants that did not bind Runx1 lacked the abilities to produce Treg function and phenotype, including Il2 repression, Treg marker expression (including CD25 and GITR), and suppressive activities.29 Treg‐specific deletion of Runx1 or CBF‐β induces the proliferation of CD4+ T‐cells and the development of autoimmune gastritis, indicating that Runx1 is required for suppressive function of Treg.79
In the absence of Foxp3, Runx1 binds Il2 enhancers in CD4+ T‐cells and is poised to activate Il2 transcription.29 Although Runx1 is dispensable for initiating Il2 transcription,80 upon receiving TCR signals, Runx1 enhances Il2 transcription in non‐Treg, and the amounts of Runx1 protein are correlated with Il2 transcriptional activities.29, 80 Retroviral gene transduction using murine conventional T‐cells showed that Runx1 binds to the Il2 promoter and enhances IL‐2 production, which is suppressed by Foxp3.29 Importantly, IL‐2 production is markedly increased in Cbfb‐deficient Treg,79 indicating that Foxp3 cannot repress Il2 in the absence of the Runx1−CBF‐β complex. Thus, it is likely that Runx1 is required for the optimal expression dynamics of IL‐2, orchestrating NFAT, AP‐1 and NF‐κB to control temporal dynamics of Il2 transcriptional activities. However, once Foxp3 is expressed, the Foxp3−Runx1 complex functions as a repressor for Il2 transcription.
The transcription factor Ets1 interacts and cooperates with Runx1, promoting the binding of Runx1 to DNA,81 activating transcription of their target genes82 (Fig. 1). Ets1 −/− mice show B‐cell activation, and have reduced numbers of T‐cells, which are also highly activated. This phenotype can be rescued by adoptive transfer of wild‐type Treg, indicating the functional impairment of Ets1 −/−Treg.83 Interestingly, Kwon et al. showed chromatin immunoprecipitation (ChIP) peak data that Ets1 was excluded from Foxp3‐binding sites specifically in Foxp3‐repressed genes.69
Runx‐guided control of active enhancers and Treg transcriptome
Samstein et al. showed that Foxp3‐binding sites were significantly enriched with Runx, Ets and forkhead motif sequences.84 Importantly, nucleosome‐free genomic regions are largely overlapped between Treg and activated conventional T‐cells, and are enriched with AP‐1 motif.84 These regions are likely to be opened and maintained by the cooperation of NFAT and AP‐1, because they lose chromatin accessibility in Treg‐specific knockout of calcineurin B (Cnb1), which is required for the nuclear translocation of NFAT upon calcium influx.84 Interestingly, another ChIP‐seq analysis of H3K4me2 showed that primed enhancers in activated CD4+ T‐cells were enriched with the motifs for Runx1, Ets1, IRF4, NF‐κB/Rel and AP‐1.85 These studies highlight the central role of Runx1 in regulating enhancers in conventional T‐cells and Treg.
Importantly, Treg transcriptomes are largely similar to memory‐phenotype T‐cell transcriptomes, and their shared feature is TCR‐induced T‐cell activation.59 As demonstrated by multidimensional transcriptome analysis, Runx1 enhances transcription of some of the TCR‐induced genes in memory‐phenotype T‐cells, which are mostly repressed by Foxp3. On the other hand, Foxp3 largely sustains the expression of Runx1‐independent TCR‐induced genes.59 Thus, Runx1 enhances transcription of genes downstream of TCR signalling, while Foxp3 binds Runx1 and modifies the transcriptional regulation of their common target genes.
Accordingly, here I propose a model for Runx1‐guided control of transcriptional programme for Treg differentiation (Fig. 2). In the absence of Foxp3, Runx1 binds to a wide range of primed or poised enhancers of the genes for T‐cell differentiation and response (Fig. 2a). Upon antigen recognition, TCR‐induced transcription factors (including NFAT, AP‐1 and NF‐κB) are recruited to Runx1‐bound genes, and their cooperation controls gene expression dynamics of TCR‐induced genes (including IL‐2 and CD25; Fig. 2b). However, once Foxp3 expression is induced, Foxp3 binds to the primed and active enhancers through interacting with Runx1 and NFAT (Fig. 2c). Subsequently, Foxp3 will further recruit cofactors, modifying the composition of pre‐existing Runx1‐containing complex. When acting as a transcriptional repressor complex, Foxp3 may recruit corepressors (Fig. 2c). On the other hand, when acting as a transcriptional activator, Foxp3 recruits p300 and other coactivators, stabilizing TCR‐induced enhanceosomes, and thereby maintains constitutively active transcription of its target genes (Fig. 2d).
Figure 2.

A model for Runx1‐guided control of Treg‐type transcriptome. AML1/Runx1 has different roles in Foxp3− T‐cells (conventional T‐cells) (a, b) and Foxp3+ Treg (c, d). (a) When T‐cells are matured, the AML1/Runx1‐CBF‐β complex may bind primed and poised enhancers, which are transcriptionally inactive, and AML1/Runx1 is poised to execute its function for T‐cell differentiation and response. Primed enhancers are marked by H3K4me1 but not by H3K27me3. Poised enhancers are similar to active enhancers in p300 binding and nucleosome depletion, but are marked by H3K27me3 in addition to H3K4me1. Histones at the promoter are marked by H3K4me3, which is the feature of active promoter. In poised enhancer, AML1/Runx1 presumably interacts with p300, while PRC2 and HDACs actively suppress p300‐mediated histone acetylation and prevent transcriptional activation. (b) Upon cognate antigen recognition, TCR signals activate its downstream transcription factors, including NF‐κB, NFAT and AP‐1, which are recruited to promoters and enhancers of AML1/Runx1‐bound genes. This will form a p300‐containing enhanceosome, which acetylates histones together with other HATs such as Kat2b and TIP60, increasing H3K27Ac, and enhances RNA polymerase II (Pol II)‐mediated transcription at the transcription start site (TSS). (c) In Treg, Foxp3 is highly expressed and TCR signals are regularly conveyed. When Foxp3 expression is induced, whether in the thymus or in the periphery, T‐cells have received strong TCR signals. Some genes such as Il2 have repressed enhancers, to which the Foxp3‐AML1/Runx1 complex binds. Foxp3 and AML1/Runx1 may further recruit transcriptional corepressors such as NCoR1/NCoR2 (through HDAC3) and TLE, respectively (Fig. 1). (d) Treg highly express activation‐induced proteins including CD25 and coinhibitory molecules, which transcriptional activities are sustained with the help of Foxp3 and infrequent‐but‐regular TCR signals. Foxp3 is considered to stabilize TCR signal‐induced enhanceosomes by interacting with AML1/Runx1 and TCR signal downstream transcription factors.
Foxp3‐inducing transcription factors and Foxp3 autoregulatory transcriptional circuit
When Foxp3 transcription is newly induced in differentiating thymic CD4‐SP and peripheral non‐Treg CD4+ T‐cells, transcription factors downstream of TCR signalling and IL‐2 signalling play key roles. However, once Foxp3 transcription is sustained and becomes stabilized, it is regulated by the Foxp3 autoregulatory transcription factor complexes that are bound to the key regulatory regions of the Foxp3 gene. This section discusses these two layers of mechanisms for regulating Foxp3 expression.
Transcription factors for the induction of Foxp3 expression
There are two significantly conserved sequences between species in the first intron of the Foxp3 gene, and these are designated as the conserved non‐coding sequences (CNS) 1−2. In addition, the sequence just downstream of the first coding exon is designated as CNS3.86 The genetic deletion of each CNS showed that CNS1 and CNS3 control the induction of Foxp3 expression in the periphery and the thymus, respectively.86
Foxp3 transcription is initiated after thymic CD4‐SP T‐cells receive strong and persistent TCR signals and upregulate the expression of CD25 and GITR.56 In addition, Foxp3 transcription is controlled by CD28, IL‐2 and TGF‐β signals in vivo.87 Importantly, the genetic deletion of either c‐Rel or RelA (p65) markedly reduces both Foxp3+ Treg and CD25+GITRhighFoxp3− Treg precursors in the thymus,88, 89 indicating that NF‐κB promotes the differentiation of Treg precursors (Fig. 3a). ChIP polymerase chain reaction experiments showed that c‐Rel bound to the Foxp3 promoter90 and CNS3.86, 90 NF‐κB activities are further enhanced by TNF receptor superfamily (TNFRSF) molecules (including TNFR2, OX40 and GITR) through TNF receptor‐associated factors (TRAFs) and promote thymic Treg differentiation.91
Figure 3.

A model for dynamic regulation of Foxp3 expression in Treg precursors and differentiated Treg. (a) The signalling and transcriptional landscape of Treg precursors. Before Foxp3 transcription is initiated, Treg precursors receive signals from TCR, CD28 and TNFRSF. These signals induce transcription factors required for the initiation of Foxp3, including NFAT, c‐Rel and AP‐1, which bind the promoter and CNS3 of the Foxp3 gene in thymic T‐cells. Here prolonged CD28 signals can also inhibit Foxp3 transcription through activating Akt and mTOR and thereby inactivating Foxo proteins, which have roles in activating Foxp3 transcription. TNFRSF molecules such as GITR, OX40 and TNFR2 are expressed in both mature Treg and differentiating Treg including Foxp3‐ Treg precursors, and activate the NF‐κB pathway. (b) Maintenance of Foxp3 transcription through Foxp3 autoregulatory transcriptional circuit in mature Treg. Once Foxp3 protein is translated, it will join the regulation of Foxp3 transcription, establishing Foxp3 autoregulatory transcriptional loop. CNS2 plays key roles in the maintenance of Foxp3 expression, and is bound by both AML1/Runx1 and Foxp3 in mature Treg. Thus, it is considered that Foxp3 interacts with AML1/Runx1 in CNS2, which triggers DNA demethylation of the region, which is also known as TSDR. In addition, IL‐2 signalling may consolidate Foxp3 transcription through STAT5 binding to CNS2.
CD28 signalling enhances TCR‐triggered NF‐κB activities, and is required for thymic Foxp3 expression.92 However, CD28 signals can also inhibit Foxp3 transcription by activating the cascade of signalling proteins, including phosphoinositide 3‐kinase [PI(3)K], Akt and mTOR.93, 94 Given that Foxo1/Foxo3 promotes Treg differentiation,95, 96 the inhibitory effect of CD28 is by activating PI(3)K and Akt, which phosphorylates and inhibits Foxo1/Foxo3 by nuclear exclusion.
In addition to NF‐κB (c‐Rel), the Foxp3 promoter is bound by TCR‐induced transcription factors, including NFAT, AP‐1,97 and Nr4a proteins98 (Fig. 3a). Among these factors, Nr4a may play non‐redundant roles in mediating TCR‐induced Foxp3 expression, as Nr4a1 −/− Nr4a3 −/− double‐knockout mice have markedly reduced Foxp3+ T‐cells.98 In addition, CNS1 enhances TCR‐ and TGF‐β‐induced Foxp3 expression.86 In fact, NFAT can activate the Foxp3 promoter and also binds to CNS1.99 CNS1 is also bound by Smad3, which activates Foxp3 transcription in a TGF‐β‐ and TCR‐dependent manner.99
The enhancer regions of the Foxp3 gene also participate in the initiation of Foxp3 transcription. The CNS0 enhancer, which is 8·5 kb upstream of the transcription start site, controls the initiation of Foxp3 transcription in the thymus and the periphery. CNS0 is bound by the histone methyltransferase MLL4, which promotes Foxp3 transcription by increasing the histone modification H3K27me1 that is commonly found in primed and active enhancers.100 CNS0 is also bound by the genome organizer Satb1, which promotes Foxp3 expression in the thymus.101 In addition, the CNS1 enhancer promotes Treg differentiation in the periphery, and is bound by NFAT and Smad399 (Fig. 3a). The key enhancers (CNS0‐3) can interact with the Foxp3 promoter by forming chromatin loops.100, 102 TCR‐induced transcription factors may thus interact with transcription factors in the distal enhancers to form a Foxp3‐inducing enhanceosome.
In addition, the transcription factor Bach2 indirectly promotes Foxp3 expression in thymic and peripheral T‐cells by repressing effector T‐cell genes.103, 104 However, given that effector T‐cell genes can be highly expressed by eTreg44 and other differentiated Treg subsets (e.g. Th1‐Treg, Th2‐Treg),50 the repression of effector T‐cell genes per se does not explain the promotion of Treg differentiation. Further studies are expected to elucidate new mechanisms for Foxp3 induction through Bach2.
Foxp3 autoregulatory transcription factor complex for the maintenance of Foxp3 expression
The TSDR is a part of CNS2. CNS2‐deficient Treg cannot maintain Foxp3 expression after cell divisions,105 indicating that CNS2 is required for the maintenance of Foxp3 expression. Importantly, CNS2 is bound by Foxp3,86 Runx1‐CBF‐β,86 Ets‐1,106 and Stat5105 (Fig. 3b). The binding of Stat5 to CNS2 is compatible with the finding that IL‐2 signalling is dispensable for Foxp3 induction but is required for maintaining Foxp3 expression.107, 108
Runx1 can directly activate Foxp3 transcription, as the deletion of Runx1 or CBF‐β reduces Foxp3 protein expression levels in peripheral Treg.79, 109 Bruno et al. 110 showed that Runx proteins bind the Foxp3 promoter and activate Foxp3 transcription and, thus, proposed a feedforward regulation in which Runx proteins control Foxp3 expression and subsequently make transcription factor complexes with Foxp3 to control target genes.
Using Foxp3‐Tocky, we recently showed that the demethylation of CNS2 occurs after Foxp3 transcription was initiated and sustained in CD4‐SP cells for some time, most probably for a day.56 Thus, it is likely that Foxp3 binds to CNS2 and coordinates its DNA demethylation process. Intriguingly, sustained Foxp3 transcription requires Foxp3 protein. Using Foxp3 mutant (scurfy) carrying Foxp3‐Tocky, we recently showed that Foxp3 protein‐deficient T‐cells with Foxp3 transcription (‘want‐to‐be Treg’) could not sustain Foxp3 transcription, and that each Foxp3 transcription was short and prematurely terminated.44 Thus, Foxp3 enhances its own transcription by forming the Foxp3 autoregulatory transcription circuit, which likely involves the Foxp3−Runx1 interaction, as both Foxp3 and Runx1 bind CNS286 (Fig. 3c).
Summarizing, TCR‐induced transcription factors form ‘Foxp3‐inducing enhanceosome’ across CNS0‐1‐3, integrating TCR and costimulatory signalling pathways to initiate Foxp3 transcription. However, once Foxp3 transcription is sustained over time, the Foxp3 autoregulatory transcriptional circuit is established and maintains Foxp3 transcription through consolidating epigenetic modifications in CNS2.
Foxp3 transcription factor complex for Treg maturation
Functionally mature Treg can be found in the CD44high CD62Llow Treg fraction, which is activated at the transcriptome level in a TCR‐dependent manner.59 eTreg highly express CD25, coinhibitory molecules including CTLA‐4, Tigit, ICOS and Klrg1,111 and migrate to lymph nodes and infiltrate inflamed tissues in which they suppress immune responses.112 CD44high CD62Llow Treg are more dependent on TCR signals than resting Treg, as shown by the induced deletion of Tcra.113 TCR signal dynamics for inducing eTreg differentiation must be different to infrequent‐yet‐regular TCR signals (see above) from self‐antigens in non‐inflammatory conditions.56
Treg maturation is considered to be promoted by cognate antigen signalling together with CD28, TNFRSF and cytokine signals, which are generally enhanced in inflammatory environments. Transcription factors for Treg maturation include Myb,114 IRF4,70, 115 Blimp1,115 JunB,116 CARMA1,117 and the NF‐κB subunit RelA.118 TCR signalling by high‐affinity antigens induces the expression of IRF4, which binds and cooperates with AP‐1 transcription factors.119 Notably, IRF4 activates Prdm1 (Blimp‐1) transcription.120 Interestingly, IRF4 may physically interact with Foxp3,70 although the evidence is limited. CARMA1 is required for TCR signal‐induced NF‐κB activation.121 Although the mechanism of Myb activation in T‐cells is less clear, Myb expression is induced by IL‐2 signalling and PI(3)K,122 which activities are induced by TCR and CD28 signals.
Another cardinal hallmark of eTreg is their temporally sustained Foxp3 transcriptional activities,44 which maintains Foxp3 protein levels for inducing Tregs' suppressive activities while inhibiting their own differentiation into effector T‐cells. Human studies also indicate that the high FOXP3 protein expression is key for the suppressive activities of Treg, as shown by the analysis of FOXP3high CD45RO+ (CD45RA−) CD4+ eTreg in autoimmunity52 and in cancer.51, 123 Foxp3 proteins may participate in TCR‐induced transcription factor complex in eTreg. Further studies are required.
Collectively all the findings above indicate that TCR signalling triggers and promotes Treg maturation, and that NF‐κB activating signals (e.g. TNFRSF signals) can have synergistic effects. These signals also induce persistent Foxp3 transcription and thereby promote eTreg differentiation.
Mechanisms for controlling transcriptional activities of Foxp3 transcription factor complex
Early studies characterized Foxp3 as a transcriptional repressor that inhibits NFAT‐mediated Il2 transcription.124 However, Foxp3 can also activate transcription of TCR/CD28‐induced genes, particularly Il2ra (CD25) and other surface proteins.9, 29 It is still unclear how Foxp3 switches between activator and repressor, but a clue was obtained by the most common IPEX mutation, A384T.
The A384T mutation disrupts the interaction between Foxp3 and the HAT TIP60 (Kat5), suggesting that the Foxp3−TIP60 interaction is required for Treg suppression.125 In addition, pharmacological inhibition of p300 impairs the suppressive function of Treg while less affecting effector T‐cell responses.126 While it is widely believed that HATs mediate their effects on Treg through acetylating and increasing the stability of Foxp3 protein127, 128 (see below), p300 is a potent transcriptional coactivator129 and may directly support the Treg‐type transcriptional regulation.
The HATs p300 and CREB‐binding protein (CBP) are potent transcriptional coactivators and may play central roles in activating transcription of Foxp3 target genes.129 CBP/p300 can bind and assemble NF‐κB, AP‐1 and other major transcription factors to form the enhanceosome complex that associates with RNA polymerase II and enhance transcription.129 Liu et al. 126 showed that pharmacological inhibition or conditional deletion of p300 impaired Treg's suppressive function. Importantly, CBP and p300 interact with Runx1 and activate Runx1‐mediated transcription as coactivators.130 Thus, it is plausible that Foxp3, Runx1 and CBP/p300 cooperate to activate transcriptional activities of Foxp3 target genes. However, the presence of p300 in an enhancer is not sufficient for its activity, as p300 can occupy both active and poised enhancers, the latter of which is distinguished by the absence of histone H3 lysine 27 acetylation (H3K27ac), enrichment of histone H3 lysine 27 trimethylation (H3K27me3), and are linked to transcriptionally inactive genes131 (Fig. 2). Although the upstream signal of p300 in Treg is not known, the MAPK/ERK pathway is known to activate p300 activity,132 which suggests that TCR signals play key roles.
When Foxp3 functions as a repressor, Foxp3 may recruit transcriptional corepressors. Eos (Ikzf4) and HDAC3 contribute to Il2 repression, and both bind the proline‐rich N‐terminal domain of Foxp3 protein (Fig. 1). Eos and HDAC3 can recruit the transcriptional corepressors CtBp1133 and NCoR1/NCoR2,134 respectively, although roles of these corepressors in the Foxp3 transcription factor complex are yet to be elucidated. The histone methyltransferase Ezh2 also has significant roles in the maintenance of Treg transcriptome.135 Ezh2 is a functional subunit of the Polycomb Repressive Complex 2 (PRC2), which mediates heritable transcriptional silencing.136 Arvey et al.137 showed that Ezh2 protein co‐immunoprecipitated with Foxp3 in activated Treg, and that histones in Foxp3‐bound genomic sites were more marked with H3K27me3, suggesting the recruitment of Ezh2 to Foxp3‐bound regions. However, PRC2 preferentially binds unmethylated CpG islands, which have high CG content, unique DNA conformations in transcriptionally inactive genes.138 Therefore, it is not clear whether the association of Foxp3−Ezh2 is the cause or the consequence of transcriptional repression. Further studies are required to elucidate mechanisms for the roles of PRC2 in Foxp3‐mediated gene regulation.
Summarizing, Foxp3 functions as a transcriptional activator by recruiting transcriptional coactivators including CBP/p300, which can interact and cooperate with Runx1. When functioning as a repressor, Foxp3 can recruit corepressors. Further studies are required to understand the coordination between Foxp3, coactivators, corepressors, and epigenetic factors.
Roles of bivalent Foxp3‐containing transcription factor complexes in bifurcation of Treg and Teff differentiation
It is a common regulation that two lineage‐specific transcription factors compete with each other for the lineage choice. Notably, mechanisms for the differentiation of Th17 and Treg are closely related. Th17 cells produce Th17‐cytokines (e.g. IL‐17 and IL‐21) and play key roles in autoimmune inflammation. Importantly, when stimulated by TCR ligation and TGF‐β, CD4+ T‐cells can express not only Foxp3 but also ROR‐γt.139 Subsequently, IL‐2 promotes Treg differentiation by enhancing Foxp3 expression while repressing ROR‐γt expression, whereas IL‐6 promotes Th17 differentiation and inhibits Treg differentiation.140 In these differentiating T‐cells, Foxp3 interacts with Runx1 and ROR‐γt.141 Thus, Runx1 provides a platform for the competitive interaction between Foxp3 and ROR‐γt.
Hif1a plays important roles for the choice between Th17 and Treg differentiation. Hypoxia stabilizes Hif1a, which enhances Th17 differentiation by making a complex with ROR‐γt and p300. Hif1a binds and promotes the degradation of Foxp3 protein.142 On the other hand, the ubiquitin E3 ligase Deltex1 promotes the degradation of Hif1a and thereby stabilises Foxp3 protein.143
The bivalent Foxp3‐containing transcription factor may be further controlled by TCR signals. Molinero et al. showed that low concentrations of anti‐CD3 antibody (~0·5 µg/ml) together with IL‐2 and TGF‐β promoted Treg differentiation, while high concentrations (~5 µg/ml) rather suppressed Foxp3 expression and induced Th17 differentiation.144 Ashouri et al.145 used Nur77‐GFP reporter in an arthritis‐prone background and showed that Nur77‐GFPhigh cells are enriched with arthritogenic Th17 cells. These results suggest that TCR‐induced transcription factors may control the Th17−Treg bivalent transcription factor complex.
Collectively, Foxp3 and Th17‐inducing factors make a transcriptional complex, which works as a decision‐making complex for the choice of Treg and Th17 differentiation.
Roles of Foxp3 post‐translational modifications in the activities of Foxp3 transcription factor complex
Post‐translational modifications (PTMs) regulate Foxp3 protein stability and thereby control Foxp3 transcription factor complex. Major PTMs for Foxp3 include ubiquitination, phosphorylation and acetylation.
Foxp3 is degraded by the ubiquitin‐proteasome‐system (UPS). The molecular chaperon Hsp70 interacts with Foxp3 and recruits the E3 ubiquitin ligase Stub1, which promotes polyubiquitination and proteasome‐dependent degradation of Foxp3.146 On the other hand, the deubiquitinase USP7 can interact with Foxp3 protein, removing ubiquitin and thereby increasing the stability of Foxp3 protein.147 Intriguingly, the pro‐inflammatory cytokine IL‐6 upregulates Stub1 and decreases USP7 expression, and thus induces Foxp3 degradation.146, 147 Importantly, IL‐6 represses Foxp3 transcription as well in a Stat3‐dependent manner.148 In addition, Morawski et al. showed that Cdk2 phosphorylated and enhanced the stability of Foxp3,149 although the evidence is largely from the analysis of a Foxp3 mutant.
The other types of phosphorylation and acetylation can stabilize Foxp3 protein. Nemo‐like kinase (NLK) phosphorylates Foxp3, and prevents Stub1 from interacting with and ubiquitinating Foxp3.150 Fleskens et al.150 showed that TCR and costimulatory signals induce Foxp3 phosphorylation through the activation of NLK in a TGF‐β‐activated kinase 1 (TAK1)‐dependent manner. TAK1 is a part of the MAPK cascade151 and, also, activates IκB kinase (IKK) and NF‐κB.152 Thus, TCR and other NF‐κB‐inducing signals phosphorylate and stabilize Foxp3 protein, which may promote TCR‐induced Foxp3 expression (see above). Meanwhile, van Loosdregt et al.153 showed that the HAT p300 interacts with and acetylates Foxp3, and thereby prevents proteasome‐dependent degradation of Foxp3. This suggests that p300‐containing Foxp3 transcription factor complex can stably regulate transcription of its target genes.
Summarizing, PTMs of Foxp3 regulate the expression level of Foxp3 protein, which may have significant impacts in the fate of each Foxp3+ cell. This may be particularly relevant for differentiating Treg, Foxp3+ non‐suppressive T‐cells in humans, and proliferating Treg, in which Foxp3 protein expression level can determine their fate. In addition, Foxp3 PTMs may play roles in context‐dependent gene regulation by changing the stability of each Foxp3 transcription factor complex.
Perspectives
Outstanding questions include: (i) how Foxp3 transcription factor complex switches between transcriptional repressor and activator; (ii) how signals from cytokines and antigen recognition can change the compositions and activities of Foxp3 transcription factor complex; (iii) whether and how Foxp3 transcription factor complex regulates epigenetic modifications. The current challenge is to obtain mechanistic understanding by integrating molecular, immunological and genomic data, including epigenomic and single cell data. Bioinformatics analysis is becoming more and more important. Tocky is useful for analysing the temporal aspects of these cellular and molecular processes. These new technologies will make breakthroughs in the understanding of the molecular mechanisms of Foxp3‐mediated immune suppression, which may well lead to the development of next‐generation immunotherapy for cancer and autoimmunity in the future. Learning from the history of Treg biology and immunotherapy,43 it is needed to obtain both mechanistic understanding of molecular and cellular mechanisms and systemic view on the entire T‐cell system and the immune system.
Disclosures
The author has no conflicts of interest to declare.
Acknowledgement
MO is supported by an MRC research grant (MR/S000208/1).
References
- 1. Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol 2006; 6:476–83. [DOI] [PubMed] [Google Scholar]
- 2. Park S‐G, Schulze‐Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M et al The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF‐κB and activate T cells. Nat Immunol 2009; 10:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rincón M, Flavell RA. AP‐1 transcriptional activity requires both T‐cell receptor‐mediated and co‐stimulatory signals in primary T lymphocytes. EMBO J 1994; 13:4370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimizu A, Kondo S, Sabe H, Ishida N, Honjo T. Structure and function of the interleukin 2 receptor: affinity conversion model. Immunol Rev 1986; 92:103–20. [DOI] [PubMed] [Google Scholar]
- 5. Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A et al Competing feedback loops shape IL‐2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci USA 2010; 107:3058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM et al Trans‐endocytosis of CD80 and CD86: a molecular basis for the cell‐extrinsic function of CTLA‐4. Science 2011; 332:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775–87. [DOI] [PubMed] [Google Scholar]
- 8. Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol 2016; 16:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 10. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 11. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337–42. [DOI] [PubMed] [Google Scholar]
- 12. Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998; 188:287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. [PubMed] [Google Scholar]
- 14. Powrie F, Mason D. OX‐22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX‐22low subset. J Exp Med 1990; 172:1701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the Forkhead transcription factor Foxp3. Immunity 2005; 22:329–41. [DOI] [PubMed] [Google Scholar]
- 16. Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid‐induced TNF receptor family‐related protein(high), Foxp3‐expressing CD25+ and CD25‐ regulatory T cells. J Immunol 2006; 176:4748–56. [DOI] [PubMed] [Google Scholar]
- 17. Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci USA 1991; 88:5528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Godfrey VL, Wilkinson JE, Russell LB. X‐linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 1991; 138:1379–87. [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L et al The immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27:20–1. [DOI] [PubMed] [Google Scholar]
- 20. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G et al Selective depletion of Foxp3+ regulatory T cells induces a scurfy‐like disease. J Exp Med 2007; 204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 2006; 8:191. [DOI] [PubMed] [Google Scholar]
- 22. Teng MWL, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T‐cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Can Res 2010; 70:7800–9. [DOI] [PubMed] [Google Scholar]
- 23. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003; 170:3939–43. [DOI] [PubMed] [Google Scholar]
- 24. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation‐mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007; 8:1353. [DOI] [PubMed] [Google Scholar]
- 25. Walker LSK, Sansom DM. The emerging role of CTLA4 as a cell‐extrinsic regulator of T cell responses. Nat Rev Immunol 2011; 11:852–63. [DOI] [PubMed] [Google Scholar]
- 26. Song X, Li B, Xiao Y, Chen C, Wang Q, Liu Y et al Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep 2012; 1:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC et al FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 2006; 126:375–87. [DOI] [PubMed] [Google Scholar]
- 28. Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF‐kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA 2005; 102:5138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T et al Foxp3 controls regulatory T‐cell function by interacting with AML1/Runx1. Nature 2007; 446:685–9. [DOI] [PubMed] [Google Scholar]
- 30. Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S et al Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 2013; 14:307–8. [DOI] [PubMed] [Google Scholar]
- 31. Whibley N, Tucci A, Powrie F. Regulatory T cell adaptation in the intestine and skin. Nat Immunol 2019; 20:386–96. [DOI] [PubMed] [Google Scholar]
- 32. Samstein Robert M, Josefowicz Steven Z, Arvey A, Treuting Piper M, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal‐fetal conflict. Cell 2012; 150:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim KS, Hong S‐W, Han D, Yi J, Jung J, Yang B‐G et al Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016; 351:858–63. [DOI] [PubMed] [Google Scholar]
- 34. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H et al Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232–6. [DOI] [PubMed] [Google Scholar]
- 35. Paiva RS, Lino AC, Bergman M‐L, Caramalho Í, Sousa AE, Zelenay S et al Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc Natl Acad Sci USA 2013; 110:6494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 2014; 259:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pratama A, Schnell A, Mathis D, Benoist C. Developmental and cellular age direct conversion of CD4+ T cells into RORγ+ or Helios+ colon Treg cells. J Exp Med 2019; 217:e20190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA et al Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self‐peptide. Nat Immunol 2001; 2:301–6. [DOI] [PubMed] [Google Scholar]
- 39. Weissler KA, Caton AJ. The role of T‐cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3(+) regulatory T cells. Immunol Rev 2014; 259:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C‐W, Santacruz N et al Peripheral education of the immune system by colonic commensal microbiota. Nature 2011; 478:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nutsch K, Chai Jiani N, Ai Teresa L, Russler‐Germain E, Feehley T, Nagler Cathryn R et al Rapid and efficient generation of regulatory T cells to commensal antigens in the periphery. Cell Rep 2016; 17:206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yadav M, Bluestone J, Stephan S. Peripherally induced Tregs – role in immune homeostasis and autoimmunity. Front Immunol 2013; 4:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bending D, Ono M. From stability to dynamics: understanding molecular mechanisms of regulatory T cells through Foxp3 transcriptional dynamics. Clin Exp Immunol 2018; 197:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bending D, Paduraru A, Ducker CB, Prieto Martin P, Crompton T, Ono M. A temporally dynamic Foxp3 autoregulatory transcriptional circuit controls the effector Treg programme. EMBO J 2018; 37:e99013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H et al Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 2012; 36:262–75. [DOI] [PubMed] [Google Scholar]
- 46. Bailey‐Bucktrout SL, Martinez‐Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H et al Self‐antigen‐driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013; 39:949–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N et al Conversion of peripheral CD4+CD25‐ naive T cells to CD4+CD25+ regulatory T cells by TGF‐β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith KA, Popmihajlov Z. The quantal theory of immunity and the interleukin‐2‐dependent negative feedback regulation of the immune response. Immunol Rev 2008; 224:124–40. [DOI] [PubMed] [Google Scholar]
- 49. McMurchy AN, Gillies J, Gizzi MC, Riba M, Garcia‐Manteiga JM, Cittaro D et al A novel function for FOXP3 in humans: intrinsic regulation of conventional T cells. Blood 2013; 121:1265–75. [DOI] [PubMed] [Google Scholar]
- 50. Ono M, Tanaka RJ. Controversies concerning thymus‐derived regulatory T cells: fundamental issues and a new perspective. Immunol Cell Biol 2016; 94:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fujii H, Arakawa A, Kitoh A, Miyara M, Kato M, Kore‐eda S et al Perturbations of both nonregulatory and regulatory FOXP3+ T cells in patients with malignant melanoma. Br J Dermatol 2011; 164:1052–60. [DOI] [PubMed] [Google Scholar]
- 52. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A et al Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30:899–911. [DOI] [PubMed] [Google Scholar]
- 53. Kim H‐P, Leonard WJ. CREB/ATF‐dependent T cell receptor‐induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 2007; 204:1543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minskaia E, Saraiva BC, Soares MMV, Azevedo RI, Ribeiro RM, Kumar SD et al Molecular markers distinguishing T cell subtypes with TSDR strand‐bias methylation. Front Immunol 2018; 9:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J et al Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bending D, Martin PP, Paduraru A, Ducker C, Marzaganov E, Laviron M et al A timer for analyzing temporally dynamic changes in transcription during differentiation in vivo. J Cell Biol 2018; 217: 2931–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA et al Foxp3‐dependent programme of regulatory T‐cell differentiation. Nature 2007; 445:771–5. [DOI] [PubMed] [Google Scholar]
- 58. Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J et al T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011; 208:1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradley A, Hashimoto T, Ono M. Elucidating T cell activation‐dependent mechanisms for bifurcation of regulatory and effector T cell differentiation by multidimensional and single‐cell analysis. Front Immunol 2018; 9:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J et al Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 2014; 41:722–36. [DOI] [PubMed] [Google Scholar]
- 61. Smith‐Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009; 27:591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Crabtree GR, Olson EN. NFAT Signaling: choreographing the social lives of cells. Cell 2002; 109:S67–79. [DOI] [PubMed] [Google Scholar]
- 63. Rincon M, Flavell RA. AP‐1 transcriptional activity requires both T‐cell receptor‐mediated and co‐stimulatory signals in primary T lymphocytes. EMBO J 1994; 13:4370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paul S, Schaefer BC. A new look at T cell receptor signaling to nuclear factor‐κB. Trends Immunol 2013; 34:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shaulian E, Karin M. AP‐1 as a regulator of cell life and death. Nat Cell Biol 2002; 4:E131–6. [DOI] [PubMed] [Google Scholar]
- 66. Lee SM, Gao B, Fang D. FoxP3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA‐binding activity of AP‐1. Blood 2008; 111:3599–606. [DOI] [PubMed] [Google Scholar]
- 67. Konopacki C, Pritykin Y, Rubtsov Y, Leslie CS, Rudensky AY. Transcription factor Foxp1 regulates Foxp3 chromatin binding and coordinates regulatory T cell function. Nat Immunol 2019; 20:232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Camperio C, Caristi S, Fanelli G, Soligo M, Del Porto P, Piccolella E. Forkhead transcription factor FOXP3 upregulates CD25 expression through cooperation with RelA/NF‐kappaB. PLoS ONE 2012; 7:e48303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kwon H‐K, Chen H‐M, Mathis D, Benoist C. Different molecular complexes that mediate transcriptional induction and repression by FoxP3. Nat Immunol 2017; 18:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu T‐T et al Regulatory T‐cell suppressor program co‐opts transcription factor IRF4 to control TH2 responses. Nature 2009; 458:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loizou L, Andersen KG, Betz AG. Foxp3 interacts with c‐Rel to mediate NF‐kappaB repression. PLoS ONE 2011; 6:e18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu Q, Chen Y, Auger‐Messier M, Molkentin JD. Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 2012; 110:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pham LV, Tamayo AT, Yoshimura LC, Lin‐Lee YC, Ford RJ. Constitutive NF‐kappaB and NFAT activation in aggressive B‐cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood 2005; 106:3940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luo MC, Zhou SY, Feng DY, Xiao J, Li WY, Xu CD et al Runt‐related Transcription Factor 1 (RUNX1) binds to p50 in macrophages and enhances TLR4‐triggered inflammation and septic shock. J Biol Chem 2016; 291:22011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koh CP, Wang CQ, Ng CEL, Ito Y, Araki M, Tergaonkar V et al RUNX1 meets MLL: epigenetic regulation of hematopoiesis by two leukemia genes. Leukemia 2013; 27:1793–802. [DOI] [PubMed] [Google Scholar]
- 76. Voon DC‐C, Hor YT, Ito Y. The RUNX complex: reaching beyond haematopoiesis into immunity. Immunology 2015; 146:523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med 2007; 204:1945–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wong WF, Kohu K, Nakamura A, Ebina M, Kikuchi T, Tazawa R et al Runx1 deficiency in CD4+ T cells causes fatal autoimmune inflammatory lung disease due to spontaneous hyperactivation of cells. J Immunol 2012; 188:5408–20. [DOI] [PubMed] [Google Scholar]
- 79. Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H et al Indispensable role of the Runx1‐Cbfbeta transcription complex for in vivo‐suppressive function of FoxP3+ regulatory T cells. Immunity 2009; 31:609–20. [DOI] [PubMed] [Google Scholar]
- 80. Wong WF, Kurokawa M, Satake M, Kohu K. Down‐regulation of Runx1 expression by TCR signal involves an autoregulatory mechanism and contributes to IL‐2 production. J Biol Chem 2011; 286:11110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gu TL, Goetz TL, Graves BJ, Speck NA. Auto‐inhibition and partner proteins, core‐binding factor beta (CBFbeta) and Ets‐1, modulate DNA binding by CBFalpha2 (AML1). Mol Cell Biol 2000; 20:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim WY, Sieweke M, Ogawa E, Wee HJ, Englmeier U, Graf T et al Mutual activation of Ets‐1 and AML1 DNA binding by direct interaction of their autoinhibitory domains. EMBO J 1999; 18:1609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mouly E, Chemin K, Nguyen HV, Chopin M, Mesnard L, Leite‐de‐Moraes M et al The Ets‐1 transcription factor controls the development and function of natural regulatory T cells. J Exp Med 2010; 207:2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R et al Foxp3 exploits a pre‐existent enhancer landscape for regulatory T cell lineage specification. Cell 2012; 151:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Allison KA, Sajti E, Collier JG, Gosselin D, Troutman TD, Stone EL et al Affinity and dose of TCR engagement yield proportional enhancer and gene activity in CD4+ T cells. eLife 2016; 5:e10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non‐coding DNA elements in the Foxp3 gene in regulatory T‐cell fate. Nature 2010; 463:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A et al B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000; 12:431–40. [DOI] [PubMed] [Google Scholar]
- 88. Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N et al c‐Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 2009; 206:3001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Oh H, Grinberg‐Bleyer Y, Liao W, Maloney D, Wang P, Wu Z et al An NF‐κB transcription‐factor‐dependent lineage‐specific transcriptional program promotes regulatory T cell identity and function. Immunity 2017; 47:450–65.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Long M, Park S‐G, Strickland I, Hayden MS, Ghosh S. Nuclear factor‐κB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 2009; 31:921–31. [DOI] [PubMed] [Google Scholar]
- 91. Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL et al Costimulation via the tumor‐necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol 2014; 15:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 2005; 6:152–62. [DOI] [PubMed] [Google Scholar]
- 93. Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL‐2‐Independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol 2006; 176:2730–8. [DOI] [PubMed] [Google Scholar]
- 94. Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M et al T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA 2008; 105:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kerdiles YM, Stone EL, Beisner DL, McGargill MA, Ch'en IL, Stockmann C et al Foxo transcription factors control regulatory T cell development and function. Immunity 2010; 33:890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ouyang W, Beckett O, Ma Q, Paik J‐H, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 2010; 11:618–27. [DOI] [PubMed] [Google Scholar]
- 97. Mantel P‐Y, Ouaked N, Rückert B, Karagiannidis C, Welz R, Blaser K et al Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol 2006; 176:3593–602. [DOI] [PubMed] [Google Scholar]
- 98. Sekiya T, Kondo T, Shichita T, Morita R, Ichinose H, Yoshimura A. Suppression of Th2 and Tfh immune reactions by Nr4a receptors in mature T reg cells. J Exp Med 2015; 212:1623–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 2008; 9:194–202. [DOI] [PubMed] [Google Scholar]
- 100. Placek K, Hu G, Cui K, Zhang D, Ding Y, Lee JE et al MLL4 prepares the enhancer landscape for Foxp3 induction via chromatin looping. Nat Immunol 2017; 18:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kakugawa K, Kojo S, Tanaka H, Seo W, Endo TA, Kitagawa Y et al Essential roles of SATB1 in specifying T lymphocyte subsets. Cell Rep 2017; 19:1176–88. [DOI] [PubMed] [Google Scholar]
- 102. Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis‐element in protecting regulatory T cell identity. Cell 2014; 158:734–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M et al BACH2 represses effector programs to stabilize Treg‐mediated immune homeostasis. Nature 2013; 498:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim EH, Gasper DJ, Lee SH, Plisch EH, Svaren J, Suresh M. Bach2 regulates homeostasis of Foxp3+ regulatory T cells and protects against fatal lung disease in mice. J Immunol 2014; 192:985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 2014; 158:749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Polansky JK, Schreiber L, Thelemann C, Ludwig L, Kruger M, Baumgrass R et al Methylation matters: binding of Ets‐1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med 2010; 88:1029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3‐expressing regulatory T cells. Nat Immunol 2005; 6:1142–51. [DOI] [PubMed] [Google Scholar]
- 108. Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL‐2 controls the stability of Foxp3 expression in TGF‐β‐induced Foxp3+ T cells in vivo . J Immunol 2011; 186:6329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx‐CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol 2009; 10:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bruno L, Mazzarella L, Hoogenkamp M, Hertweck A, Cobb BS, Sauer S et al Runx proteins regulate Foxp3 expression. J Exp Med 2009; 206:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol 2013; 34:74–80. [DOI] [PubMed] [Google Scholar]
- 112. Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D et al Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009; 30:458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol 2014; 15:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dias S, D'Amico A, Cretney E, Liao Y, Tellier J, Bruggeman C et al Effector regulatory T cell differentiation and immune homeostasis depend on the transcription factor myb. Immunity 2017; 46:78–91. [DOI] [PubMed] [Google Scholar]
- 115. Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M et al The transcription factors Blimp‐1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 2011; 12:304–11. [DOI] [PubMed] [Google Scholar]
- 116. Koizumi S‐i, Sasaki D, Hsieh T‐H, Taira N, Arakaki N, Yamasaki S et al JunB regulates homeostasis and suppressive functions of effector regulatory T cells. Nat Commun 2018; 9:5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Di Pilato M, Kim EY, Cadilha BL, Prussmann JN, Nasrallah MN, Seruggia D et al Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy. Nature 2019; 570:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vasanthakumar A, Liao Y, Teh P, Pascutti MF, Oja AE, Garnham AL et al The TNF receptor superfamily‐NF‐kappaB axis is critical to maintain effector regulatory T cells in lymphoid and non‐lymphoid tissues. Cell Rep 2017; 20:2906–20. [DOI] [PubMed] [Google Scholar]
- 119. Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM et al BATF‐JUN is critical for IRF4‐mediated transcription in T cells. Nature 2012; 490:543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S et al The transcription factor IRF4 is essential for TCR affinity‐mediated metabolic programming and clonal expansion of T cells. Nat Immunol 2013; 14:1155. [DOI] [PubMed] [Google Scholar]
- 121. Wang D, You Y, Case SM, McAllister‐Lucas LM, Wang L, DiStefano PS et al A requirement for CARMA1 in TCR‐induced NF‐κB activation. Nat Immunol 2002; 3:830. [DOI] [PubMed] [Google Scholar]
- 122. Lauder A, Castellanos A, Weston K. c‐myb Transcription is activated by protein kinase B (PKB) following interleukin 2 stimulation of T cells and is required for PKB‐mediated protection from apoptosis. Mol Cell Biol 2001; 21:5797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fujii H, Josse J, Tanioka M, Miyachi Y, Husson F, Ono M. Regulatory T cells in melanoma revisited by a computational clustering of FOXP3+ T Cell subpopulations. J Immunol 2016; 196:2885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem 2001; 276:37672–9. [DOI] [PubMed] [Google Scholar]
- 125. Bin Dhuban K, d'Hennezel E, Nagai Y, Xiao Y, Shao S, Istomine R et al Suppression by human FOXP3+ regulatory T cells requires FOXP3‐TIP60 interactions. Sci Immunol 2017; 2:eaai9297. [DOI] [PubMed] [Google Scholar]
- 126. Liu Y, Wang L, Predina J, Han R, Beier UH, Wang L‐CS et al Inhibition of p300 impairs Foxp3+ T regulatory cell function and promotes antitumor immunity. Nat Med 2013; 19:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. van Loosdregt J, Coffer PJ. Post‐translational modification networks regulating FOXP3 function. Trends Immunol 2014; 35:368–78. [DOI] [PubMed] [Google Scholar]
- 128. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol 2017; 17:703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vo N, Goodman RH. CREB‐binding protein and p300 in transcriptional regulation. J Biol Chem 2001; 276:13505–8. [DOI] [PubMed] [Google Scholar]
- 130. Aikawa Y, Nguyen LA, Isono K, Takakura N, Tagata Y, Schmitz ML et al Roles of HIPK1 and HIPK2 in AML1‐ and p300‐dependent transcription, hematopoiesis and blood vessel formation. EMBO J 2006; 25:3955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rada‐Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 2011; 470:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gusterson R, Brar B, Faulkes D, Giordano A, Chrivia J, Latchman D. The transcriptional co‐activators CBP and p300 are activated via phenylephrine through the p42/p44 MAPK cascade. J Biol Chem 2002; 277:2517–24. [DOI] [PubMed] [Google Scholar]
- 133. Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF et al Eos mediates Foxp3‐dependent gene silencing in CD4+ regulatory T cells. Science 2009; 325:1142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang L, Liu Y, Han R, Beier UH, Bhatti TR, Akimova T et al FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest 2015; 125:1111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D et al The chromatin‐modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity 2015; 42:227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Grossniklaus U, Paro R. Transcriptional silencing by Polycomb‐Group proteins. Cold Spring Harb Perspect Biol 2014; 6:a019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation‐induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol 2014; 15:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Laugesen A, Hojfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell 2019; 74:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD et al TGF‐β‐induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008; 453:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235–8. [DOI] [PubMed] [Google Scholar]
- 141. Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17–producing T cells. Nat Immunol 2008; 9:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y et al Control of T(H)17/T(reg) balance by hypoxia‐inducible factor 1. Cell 2011; 146:772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hsiao HW, Hsu TS, Liu WH, Hsieh WC, Chou TF, Wu YJ et al Deltex1 antagonizes HIF‐1alpha and sustains the stability of regulatory T cells in vivo. Nat Commun 2015; 6:6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Molinero LL, Miller ML, Evaristo C, Alegre M‐L. High TCR stimuli prevent induced regulatory T cell differentiation in a NF‐κB‐dependent manner. J Immunol 2011; 186:4609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ashouri JF, Hsu L‐Y, Yu S, Rychkov D, Chen Y, Cheng DA et al Reporters of TCR signaling identify arthritogenic T cells in murine and human autoimmune arthritis. Proc Natl Acad Sci USA 2019; 116:18517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chen Z, Barbi J, Bu S, Yang H‐Y, Li Z, Gao Y et al The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 2013; 39:272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. van Loosdregt J, Fleskens V, Fu J, Brenkman Arjan B, Bekker Cornelis PJ, Pals Cornelieke EGM et al Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg‐Cell‐Suppressive capacity. Immunity 2013; 39:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z et al Interleukin‐2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007; 26:371–81. [DOI] [PubMed] [Google Scholar]
- 149. Morawski PA, Mehra P, Chen C, Bhatti T, Wells AD. Foxp3 protein stability is regulated by cyclin‐dependent Kinase 2. J Biol Chem 2013; 288:24494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fleskens V, Minutti CM, Wu X, Wei P, Pals CEGM, McCrae J et al Nemo‐like kinase drives Foxp3 stability and is critical for maintenance of immune tolerance by regulatory T cells. Cell Rep 2019; 26:3600–12.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kishimoto K, Matsumoto K, Ninomiya‐Tsuji J. TAK1 mitogen‐activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem 2000; 275:7359–64. [DOI] [PubMed] [Google Scholar]
- 152. Wang C, Deng L, Hong M, Akkaraju GR, Inoue J‐i, Chen ZJ. TAK1 is a ubiquitin‐dependent kinase of MKK and IKK. Nature 2001; 412:346–51. [DOI] [PubMed] [Google Scholar]
- 153. van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YYJ, Beekman JM, van Beekum O et al Regulation of Treg functionality by acetylation‐mediated Foxp3 protein stabilization. Blood 2010; 115:965. [DOI] [PubMed] [Google Scholar]
- 154. Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R et al FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA 2007; 104:4571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kitabayashi I, Yokoyama A, Shimizu K, Ohki M. Interaction and functional cooperation of the leukemia‐associated factors AML1 and p300 in myeloid cell differentiation. EMBO J 1998; 17:2994–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chuang LS, Ito K, Ito Y. RUNX family: regulation and diversification of roles through interacting proteins. Int J Cancer 2013; 132:1260–71. [DOI] [PubMed] [Google Scholar]


