Summary
The immune response is governed by a highly complex set of interactions among cells and mediators. T cells may be rendered dysfunctional by the presence of high levels of antigen in the absence of co‐stimulation while myeloid cells may be programmed towards an immunosuppressive state that promotes cancer growth and metastasis while deterring tumor immunity. In addition, inhibitory programs driven by immune checkpoint regulators dampen anti‐tumor immunity. The ideal cancer immunotherapy treatment will improve both cross‐priming in the tumor microenvironment and relieve suppression by the inhibitory checkpoints. Recently, blockade of programmed cell death 1 (PD‐1) and cytotoxic T lymphocyte antigen 4 (CTLA‐4) has elicited impressive results, but not in all patients, so additional targets are under investigation. V‐set immunoglobulin domain suppressor of T cell activation (VISTA) is a novel immunoregulatory receptor that is broadly expressed on cells of the myeloid and lymphoid lineages, and is frequently implicated as a poor prognostic indicator in multiple cancers. Importantly, antibody targeting of VISTA uniquely engages both innate and adaptive immunity. This, combined with the expression of VISTA and its non‐redundant activities compared to other immune checkpoint regulators, qualifies VISTA to be a promising target for improving cancer immunotherapy.
Keywords: cancer, immune checkpoint, VISTA
VISTA is an immunoregulatory protein with an important nonredundant role in maintaining immune homeostasis. VISTA is expressed in numerous human cancers and correlates with poor clinical responses. Antibody‐mediated VISTA blockade has shown promise in multiple tumor models, synergizing with the blockade of other inhibitory checkpoints.
Introduction: what is VISTA?
V‐set immunoglobulin domain suppressor of T cell activation (VISTA) is an immunoregulatory receptor expressed on most cell subsets of the hematopoietic lineage 1. Myeloid cells present the highest levels of VISTA expression, with a remarkably high level on microglia and neutrophils 2. This is followed by other myeloid subsets, including monocytes, macrophages, basophils and dendritic cells. Unlike other checkpoints, which are induced at different stages after activation, VISTA is constitutively expressed on these subsets at steady state. This broad and unique pattern of expression implies an important homeostatic role in regulating immune system responses and distinguishes VISTA among other immunoregulatory receptors. On lymphocytes, VISTA is most highly expressed on naive CD4+ T cells as well as forkhead box protein 3 (FoxP3)+ T regulatory cells (Tregs). It exhibits lower expression levels on CD8+ T cells, natural killer (NK) cell subsets and thymocytes 1, 3. While VISTA is not expressed on B cells, it is significantly expressed on plasma cells (Lines and ElTanbouly, unpublished observations). Of note, we and others have detected reduced VISTA expression under inflammatory conditions 2, 3, 4.
Structure
The human VISTA protein is 279 amino acids (aa) long, which includes an extracellular domain of 162 amino acids, a 21 aa transmembrane domain, and a 96 aa cytoplasmic domain. There are no immunoreceptor tyrosine‐based signaling motifs in the cytoplasmic domain. However, there are potential sites for protein kinase C and casein kinase 2 phosphorylation sites 5, 6, 7. Phylogenetic and protein sequence analysis revealed similarities between VISTA and B7 family members which contain a conserved immunoglobulin (Ig)V‐like fold. There are multiple notable differences that distinguish VISTA from these proteins. On the genetic level, VISTA is present on chromosome 10 (10q22.1) at a distant site from all clustered B7 family members. Secondly, VISTA is positioned within an intron of the Cdh23 gene in all genomes. Of importance, VISTA is the most conserved B7 member showing 76% identity between mouse and human, its cytoplasmic tail sharing up to 91% identity, as well as the highest sequence identity between mouse and zebrafish genomes. Based on IgV domain analysis, the closest homolog to VISTA within the B7 family is PD‐L1, which shares 22% sequence identity. Very recently, the structure of human VISTA extracellular domain (ECD) has been elucidated, highlighting structural features that distinguish VISTA as well as fine‐epitope mapping for residues important for antibody and ligand binding 6. Multiple differences exist in the extracellular domain of VISTA compared to the canonical B7 IgV fold. The classical B7 family fold contains nine beta strands, while VISTA contains 10 beta strands. In place of the longer beta strand C′, the VISTA ECD contains an extra helix sequence (FQDL) in its positively charged patch. Thirdly, VISTA contains a C‐C′ unstructured loop of 21 residues that is unique from all B7 homologs. Of importance, VISTA contains two extra disulphide bonds absent in all other B7 family proteins. VISTA contains a single IgV‐like domain, while all the other B7 family members include both IgV and IgC domains. Multiple studies have inferred a role for VISTA as either a ligand, or receptor, or both. The B7 family includes proteins that function as ligands or receptors. B7‐1 (CD80), B7‐2 (CD86), B7‐DC [programmed cell death ligand 2 (PD‐L2)], B7‐H1 (PD‐L1) and B7‐H3 (CD276) contain 2 IgV domains and exhibit ligand activity. In contrast, CD28, cytotoxic T lymphocyte antigen 4 CTLA‐4), inducible co‐stimulator (ICOS) and programmed cell death 1 (PD‐1) have single IgV domains and primarily function as receptors. VISTA is also composed of a single IgV domain and therefore, based on structural insights, we predict that VISTA probably functions primarily as a receptor. This has been corroborated by a number of studies that elucidated the receptor role for VISTA using multiple systems 5, 8, 9, 10, 11. The second evidence for VISTA function is the ability to impart opposing outcomes of immune responses using agonist and antagonist antibodies.
VISTA‐deficiency enhances immunity
Our group and others have demonstrated that the loss of VISTA enhances tumor responses and precipitates autoimmunity. VISTA−/− mice have enhanced CD44high CD62Llow memory‐phenotype T cells and when crossed on an autoimmune transgenic myelin oligodendrocyte glycoprotein (MOG)‐specific background 12, VISTA−/− mice had a dramatic exacerbation of disease incidence and lethality in a T cell‐driven transgenic model of experimental autoimmune encephalomyelitis (EAE) 11. Another group reported that older VISTA−/− mice develop glomerulonephritis and lupus‐like symptoms 13. Interbreeding VISTA‐deficient mice with Sle1.Sle3 mice significantly enhanced lupus nephritis development 14, and this was also evident with VISTA blockade 15. Similar results were recently reported in the Faslpr lupus model where VISTA‐deficiency enhanced disease while VISTA agonists suppressed both cutaneous and systemic lupus 16. In addition, VISTA‐deficiency was shown to exacerbate allergic inflammation and experimental asthma, suggesting a role in the regulation of type II immunity 10, 17. VISTA−/− mice were also more susceptible to concanavalin A (ConA)‐induced lethality 8. This presents evidence that VISTA plays a broad role in fine‐tuning and establishment of a normal response, thereby restraining autoimmunity and excessive damage. The role of VISTA as an inhibitory receptor on T cells was first confirmed by Chen and colleagues, who showed that targeting VISTA with a novel class of agonistic antibodies can completely prevent acute graft‐versus‐host disease (GVHD) induction 5. We have expanded upon these data and showed that selective targeting of donor alloreactive T cells is sufficient for the anti‐VISTA prophylactic effect in GVHD 3. Of relevance, a recent study found that VISTA was expressed by myeloid cells of the corneal stroma and that genetic deficiency or blockade of VISTA significantly reduced corneal allograft survival 18. We have observed similar results where VISTA‐deficiency or blockade also markedly reduced skin graft tolerance and survival and superseded the immunosuppressive effects of CD40L blockade (ElTanbouly and LeMercier, unpublished observations). Flies et al. and others have shown that VISTA−/− CD4+ T cells show an enhanced effector response, and expression of VISTA can restrain T cell differentiation 8, 9, 19.
Gene regulation
Although the expression patterns of VISTA have been evaluated in various studies, the identity of the regulatory networks that constitutively maintain VISTA expression in leukocytes remains far from elucidated. Previous work revealed that the transcription factors p53 and HIF‐1α up‐regulate VISTA expression 13, 20. In the tumor microenvironment, VISTA was shown to be induced by hypoxia‐inducible factor 1‐alpha (HIF‐1α) under hypoxic conditions and, in vitro, VISTA appears to exert a greater impact when cells are cultured in hypoxic chambers 20. Therefore, regulation of VISTA expression within the tumor microenvironment may govern the quality of the inflammatory infiltrate.
Our analysis of the Encyclopaedia of DNA Elements (ENCODE) database 21 for putative transcription factor (TF) binding to the VISTA promoters revealed multiple candidates. These include Fos, transcription factor JunD and nuclear factor kappa B (NF‐κB) 3. As these TFs are up‐regulated under inflammatory conditions, we predict that they are potential repressors of VISTA expression. In line with this, deacetylation of H3K27 upstream of the Vsir gene in response to lipopolysaccharide (LPS) has also been reported, suggesting an additional layer of regulation upon inflammation that can reduce VISTA expression 2. The genomic locus containing Vsir is unique among other immunoregulatory molecules. The entire Vsir gene is a ‘nested gene’ 22 located within the negative strand of an intron within the CDH23 gene 7. It is unclear how CDH23 and VISTA expression are related; however, VISTA‐specific gene regulation seems highly likely because, similar to most nested genes, VISTA has its own promoter region. Currently, no enhancers are known to regulate VISTA expression, although some conserved putative enhancers have been suggested in CD14+ myeloid cells 23. Post‐transcriptional regulation of VISTA seems to be an important regulatory mechanism, as both microRNA (miR)‐125a and miR‐125b can bind VISTA mRNA and promote its degradation 24, 25.
VISTA function in non‐hematopoietic cells in cancer
Several single nucleotide polymorphisms (SNPs) have been identified in the VISTA locus. The most well‐annotated is rs3747869, which results in a D (aspartic acid) to E (glutamic acid) missense variant and occurs in 5–10% of the study population 26, 27, 28. This variant has been associated with an increased leukocyte count in carriers by multiple independent studies 26, 27, 28. Interestingly, another annotated SNP in the VISTA locus, rs748113, that occurs in the 3′ untranslated region (UTR), is associated with a decrease in lymphocyte count in carriers 26. Besides naturally occurring variants, several mutations have been observed in the VISTA gene in a range of cancer types, albeit at very low frequencies. The most commonly observed mutation (six samples in TCGA and ICGC combined) is an R (arginine)/C (cysteine) substitution at position 86, which is located in the Ig‐like V‐type domain and probably results in loss of function 29, 30. Interestingly, the next most frequent mutation (five samples in TCGA and ICGC combined) that may result in loss of function occurs at the next position, position 87, where R is substituted for W (tryptophan). Both mutations are observed mainly in stomach and bladder cancer 29, 30. Copy number variants (CNVs) seem to be equally infrequent tumors, with only 26/> 32 000 patients displaying copy number alterations in the COSMIC database 31. Most tumors (22 of 26) display VISTA amplifications, suggesting that VISTA gains are more advantageous to tumors than VISTA losses. Of note, two studies have reported copy number losses in four of 538 healthy individuals, suggesting that VISTA is a non‐essential protein 32, 33. However, as discussed below, VISTA expression on tumor or immune cells may have a large impact on tumor growth and anti‐tumor immunity.
VISTA expression in on non‐hematopoietic cells in cancer
Initial work on VISTA showed that MCA105 fibrosarcoma cells that over‐expressed VISTA grew with accelerated kinetics 1. Similarly, mice inoculated with ovarian cancer cell lines HM‐1 or ID8 over‐expressing VISTA showed impaired survival compared to control cell lines 34. In both studies, growth differences were not observed in fully immune‐incompetent mice, indicating that VISTA expressed on tumor cells is able to impair anti‐tumor immunity. Interestingly, although initial studies showed minimal expression of VISTA on human tumor cells and suggested a predominant myeloid expression in the tumor 35, a number of recent studies have established that while some tumor types such as melanoma are associated with immune‐restricted VISTA expression 36, tumor cells can show significant expression on CD45‐negative tumor cells. This has been demonstrated in gastric cancer 37, ovarian cancer 38, colorectal cancer 39 and primary oral squamous cell mesothelioma 40. Interestingly, in non‐small‐cell lung cancer (NSCLC) and hepatocellular carcinoma (HCC), tumor cell expression appears to be associated with improved objective survival (OS) 41, 42. Currently, it is not clear if and how VISTA expression induced in CD45‐negative cells might be supportive for anti‐tumor immunity. VISTA has been implicated in the uptake of dead cells 13 It is possible, therefore, that non‐hematopoietic VISTA expression may enhance uptake of tumor cell‐derived antigen to cross‐priming dendritic cells. As discussed above, the promoter of VISTA is predicted to bind inflammatory TFs 3. Therefore, another possibility is that the non‐hematopoietic expression of VISTA may be a tumor biomarker for the engagement of beneficial inflammatory programs that override the negative impacts of VISTA expression.
VISTA expression in tumor‐infiltrating immune cells
This positive association of tumor cell VISTA expression is in contrast with the impact of high VISTA expression in immune cells on OS. In oral squamous cell carcinoma the poorest OS was associated with high VISTA expression, together with low levels of CD8+ T cell infiltration 40. In colon cancer, analysis of the The Cancer Genome Atlas (TGCA) database showed association of high VISTA expression with poor OS 20. While expression of VISTA (or PD‐1 and CTLA‐4) in cutaneous melanoma by analysis of the TGCA database showed a positive association with survival, immunohistochemistry staining (IHC) demonstrated that high VISTA expression on immune cells was associated with poor survival 36. In ovarian cancer, while VISTA expression was not predictive for OS, VISTA expression increased with progression, and was generally increased in advanced disease 38. Furthermore, VISTA expression may contribute to the acquired resistance to anti‐PD‐1 or anti‐CTLA‐4 immunotherapy. In melanoma, the density of VISTA‐positive immune cells is increased in patients after progression of disease in the face of anti‐PD‐1 treatment 43. Similarly, VISTA expression is up‐regulated after ipilimumab (anti‐CTLA‐4) therapy in prostate cancer 44. In addition, VISTA expression seems to correlate with PD‐1/PD‐L1 expression; for example, in colon cancer, NSCLC and gastric cancer 20, 37, 41. Overall, these data on the expression of VISTA in human cancer are highly supportive that targeting of VISTA as part of immunotherapeutic strategies may be of great value.
The promise of VISTA in cancer immunotherapy
Immunotherapy has demonstrated extreme therapeutic efficacy, but only in a minority of patients. The frequency of tumor regression from anti‐PD‐L1/PD‐1 antibody monotherapy ranges from 10 to 40%, depending on the indication 45 . Responses to other monotherapeutic indications such as CTLA‐4 blockade or interleukin (IL)‐2 are even lower in most cancers 46, 47. Several factors can determine whether a response occurs. However, clinical studies have elucidated distinct phenotypes that can predict the response to immunotherapy. Histological sections of tumor tissue collected from patients prior to receiving anti‐PDL1/PD‐1 reveals three distinct immune profiles that directly correlate with response and therapeutic efficacy 48, 49, 50.
The first profile is the immune‐inflamed tumor (Fig. 1a). This is defined by the presence of T cells (CD4+ and CD8+) T cells in the tumor parenchyma, as well as myeloid cells. Of note, the immune cells are positioned in proximity to the tumor cells 51, 52, 53, 54, 55, 56, 57, 58. These environments usually express staining for checkpoint molecules such as PD‐L1, CTLA‐4 and VISTA 47, 52, 54, 58, 59, 60. These tumors also have elevated levels of proinflammatory cytokines, indicative of an active (but probably insufficient) immune response 51, 53, 54, 56. As such, clinical responses to PD‐1/PD‐L1 blockade mainly occur in patients with an inflamed‐tumor profile. Another profile is the immune‐excluded tumor 61, 62, 63, 64 (Fig. 1b). This tumor phenotype also exhibits abundant immune cells. However, the immune cells do not penetrate the parenchyma of the tumor, but are rather excluded in the stroma that surrounds the tumor hotbeds 48, 54, 63, 65. Blockade of the PD‐1/PD‐L1 signaling axis results in activation and proliferation of stroma‐associated T cells but not infiltration. Intuitively, clinical responses are uncommon and are probably impeded by the lack of T cell migration into the tumor nests. Indeed, there is evidence that transforming growth factor (TGF)‐β may be an important constituent of this rate‐limiting step 64, 66, 67.
Figure 1.
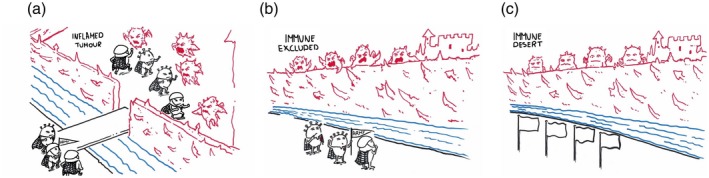
The tumor immune response profiles. The tumor microenvironment (TME) from patients prior to ICB exhibit three different immune profiles that correlate with therapeutic efficacy. The first profile is the immune‐inflamed tumor (a). This tumor profile is associated with the infiltration of T cells and myeloid cells within the tumor parenchyma, with immune cell expression of inflammatory cytokines and checkpoint molecules. The second profile is that of the immune‐excluded TME (b). These tumors have immune cells, but these cells are excluded from the tumor hotbeds. The last profile is the immune‐desert tumor (c). This is characterized by the scarcity of tumor‐infiltrating leukocytes and the lack of an immune response. Figure illustration reproduced courtesy of Roche Communications department 105.
Our group and others have shown that VISTA‐deficiency or blockade can dramatically modulate the TME towards a more proinflammatory myeloid phenotype that favors the infiltration of tumor‐reactive T cells. These studies have shown success in multiple tumor models, including tumors that mimic human immune‐excluded tumors 68, 69. Of particular interest, a recent paper showed that VISTA‐deficient myeloid cells, including monocytic tumor myeloid‐derived suppressor cells (MDSC), were defective in migration 70. Furthermore, tumors grown in VISTA deficient mice were strikingly devoid of macrophages 70 . Although a direct impact of anti‐VISTA on migration has not yet been demonstrated, it is possible that modulation of myeloid cell migration and conversion of tumors from the immune‐excluded to immune‐inflamed tumor is part of the mechanism of action. Of equal relevance, VISTA is highly expressed on human immune‐excluded tumors, such as ovarian and pancreatic cancers 34, 71, 72, and is up‐regulated by checkpoint blockade on tumors such as prostate cancer 44. This evidence suggests that VISTA is a promising therapeutic target in combination with other checkpoint immunotherapies, especially in the context of immune‐excluded ICT‐resistant tumors.
The immune‐desert tumor represents the third profile (Fig. 1c). This phenotype is characterized by a scarcity of tumor‐reactive T cells or other immune cells either in the tumor parenchyma or stroma 48, 49, 52, 54, 73. In these patients, the generation of tumor‐reactive T cells is the rate‐limiting step and prerequisite for successful immunotherapy. There are two indications that predict a promising role for VISTA blockade in the immunotherapy of these cancer settings. First, VISTA is highly expressed on myeloid cells, including antigen‐presenting cells [APCs; e.g. macrophages, dendritic cells (DCs)]. Indeed, previous work has shown that VISTA on the APCs suppresses T cell proliferation and cytokine production 1, 8. In consequence, VISTA blockade enhanced the activation status of tumor‐associated DCs resulting in a greater production of inflammatory cytokines and up‐regulation of co‐stimulatory and antigen‐presenting molecules by these cells 69. Therefore, VISTA is expected to enhance the antigen‐presenting function of myeloid cells in the tumor and draining lymph nodes, thereby enhancing the potential for tumor‐reactive T cell priming and effector function. Le Mercier et al. found that prophylactic treatment with the VISTA blocking clone (13F3) of anti‐VISTA delayed growth of B16‐OVA and B16BL6 melanoma, MB49 bladder cancer and the inducible BRAF/PTEN tumor models in a monotherapy approach 69. In combination with vaccine 69 or PD‐L1 blockade 74, 13F3 treatment could also trigger rejection of CT26 colon cancer and/or B16BL6. Therefore, there is evidence suggesting that VISTA blockade can activate the myeloid compartment within the tumour microenvironment (TME) to enhance T cell activation and infiltration and, consequently, unleash anti‐tumor T cell immunity. An immune desert tumor type where anti‐VISTA is predicted to augment immunotherapeutic responses is pancreatic cancer. The TME in pancreatic ductal adenocarcinoma (PDA) is characterized by extensive stromal and fibrotic tissue, with low vascular permeability. This tissue architecture promotes T cell exclusion and, indeed, the most abundant immune cells in PDA are CD68+ macrophages. The most successful immunotherapy for PDA so far has utilized activation of the CD40 pathway to redirect macrophages and other APCs towards an activated more immune‐promoting phenotype 75. VISTA blockade has repeatedly shown a similar impact on redirecting the TME in other preclinical models, and has the advantage (more than CD40 agonists) in multi‐lineage targeting to induce broad‐spectrum immune activation of both T cells and myeloid cells. Of equal importance, VISTA expression was higher on macrophages localized within the pancreatic stroma of PDAC patients compared to its expression in melanoma 71.
The most critical changes of anti‐VISTA treatment on the TME are summarized in Fig. 2. Aside from the identity of the tumor microenvironment, one promising aspect of VISTA targeted therapy is that the suppressive activity of VISTA is non‐redundant with the PD‐1/PD‐L1 pathway. There are several lines of evidence that support this. First, the magnitude of T cell response was synergistically enhanced in the absence of VISTA and PD‐1 76. More importantly, blockade of VISTA and PD‐1/PD‐L1 more profoundly enhances tumor immunity than targeting either pathway 68, 76, 77. This has sparked interest in VISTA as a potential target for cancer immunotherapy. In VISTA‐deficient mice, B16‐OVA melanoma and CT26 colon cancer tumors grow with reduced kinetics 11, 70, and VISTA‐deficient mice also show reduced susceptibility to GL261 glioma, particularly when undergoing radiation therapy 8. Cells mediating VISTA suppression may be of both the T cell and myeloid lineages 8, 11, 78, 79, 80. Myeloid‐derived suppressor cells (MDSC) from an LP‐BM5 virus model showed a significant dependence on VISTA for suppressive function 81. In colorectal cancer patients and the CT26 model, we showed that VISTA is an important MDSC checkpoint that is essential for optimal suppression of T cell responses 20. Indeed, VISTA‐deficiency or blockade reduced MDSC suppression of T cells in both in‐vitro and in‐vivo settings. In human prostate cancer, Sharma and colleagues showed that VISTA and PD‐L1 are expressed on independent subsets of macrophages, which further suggests potential combination therapy. Importantly, VISTA combination with PD‐1 blockade engendered strong immunity to tumors resistant to either checkpoint as monotherapy 68, 77, 82. A subtle yet critical aspect for the success of immunotherapy is the timing of checkpoint blockade. A recent study presented compelling evidence that blocking PD‐1 during early priming or under settings of suboptimal priming of T cells abolished therapeutic outcomes by enhancing T cell dysfunction 83. Moreover, blocking PD‐1 after sufficient priming or concurrent with vaccination can enhance tumor immunity. This is supported by elegant work by Chang and colleagues, elucidating the mechanism by which PD‐1 blockade enhances T cell immunity 84. By coupling T cell receptor (TCR) and transcriptional sequencing, they tracked T cell clones at a single‐cell resolution prior to and after anti‐PD‐1 therapy, conclusively demonstrating that the therapeutic impact of PD‐1 blockade was not derived from reinvigorating exhausted TILs, but rather restricting the exhaustion of new distinct novel clonotypes that are nascently primed. We have evidence that VISTA is an important checkpoint on naive T cells that impedes the earlies stages of T cell activation and transition from quiescence to priming 3. This presents the intriguing speculation that targeting VISTA prior to or concurrent with PD‐1 blockade may augment T cell responses through removing the restraints on both priming and exhaustion.
Figure 2.
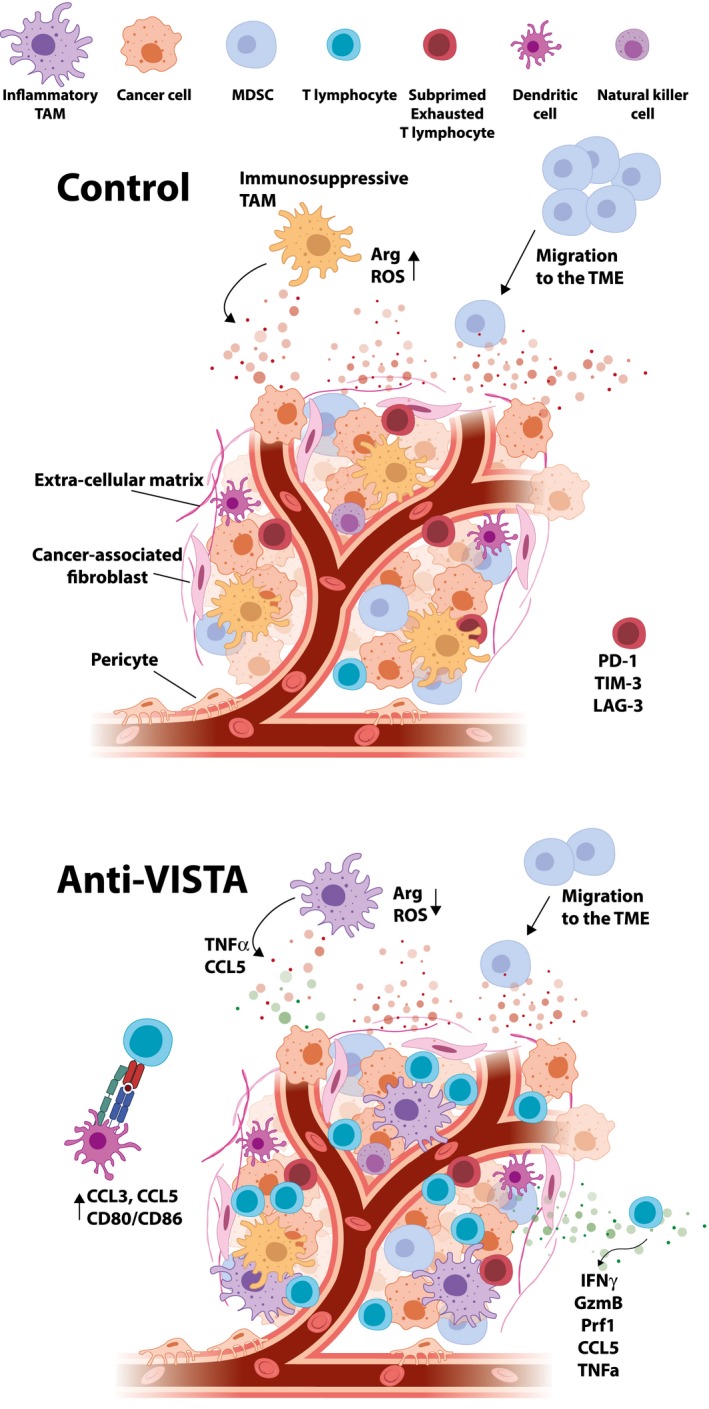
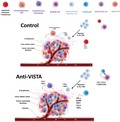
The impact of V‐domain Ig suppressor of T cell activation (VISTA) blockade on the tumor microenvironment profile. Anti‐VISTA treatment improves T cell priming and infiltration while impeding myeloid infiltration. Additionally, anti‐VISTA appears to reprogram myeloid cells, reducing expression of suppressive mediators such as arginase 1 and reactive oxygen species (ROS), and improving expression of co‐stimulatory molecules. This can result in improved T cell effector function and anti‐tumor immunity.
The VISTA ligand mystery
A critical rate‐limiting step in the development of successful anti‐VISTA targeting agents is the chronic knowledge‐gap concerning VISTA ligands. Multiple published studies have highlighted potential VISTA ligands. The first group suggested that VISTA mediates a homotypical interaction important for its activities 13. These interactions have not yet been supported by other independent studies. More recently, a study identified VSIG3 (or IgSF11) as a VISTA–ligand and that this interaction contributes to inhibition of T cell responses 85. As VSIG3 expression is undetectable in the hematopoietic compartment, the in‐vivo physiological relevance of this interaction remains to be validated. Alan Korman and colleagues presented the exciting finding of a pH‐dependent interaction between VISTA and P‐selectin glycoprotein ligand‐1 (PSGL‐1) 77. The investigators highlight a unique property of the extracellular domain of VISTA, which is enriched in histidine residues. The side chain of histidine becomes deprotonated at acidic pH conditions, such as those found within the TME, and this post‐translational modification permits binding to PSGL‐1 77. The importance of this post‐translational modification in dictating VISTA ligand interactions cannot be understated, given that VISTA multimers (including VISTA–Fc) only bind leukocytes under acidic conditions. Indeed, one group reported binding of VISTA multimer binding to a T cell clone under physiological pH 86, but neither our group nor Johnston et al. were able to reproduce these findings. This finding is also intriguing from the perspective of elucidating PSGL‐1 biology. It is well established that naive T cells express significant levels of PSGL‐1, but lack the post‐translational modifications (e.g. fucosylation and sialylation) necessary for it to bind selectins 87, 88. Therefore, selectins can only bind PSGL‐1 on activated T cells, which express the fucosyl‐ and sialyl‐transferases that modify PSGL‐1 to become a true selectin ligand. On naive T cells, underglycosylated PSGL‐1 is a receptor for the chemokine CCL21 and competes with CCR7 in binding CCL19, suggesting an important role for regulation of naive T cell trafficking 89, 90. Moreover, the binding of VISTA requires tyrosine sulfation of PSGL‐1 77.
The second promising aspect of the VISTA : PSGL‐1 potential relationship is the very similar pattern of expression of these two molecules on leukocytes. By viewing the Immgen database 91, we observed that both VISTA and PSGL‐1 have their highest expression on microglia. While the role of PSGL‐1 is not clearly established in microglia, VISTA plays a critical regulatory role as a checkpoint of microglia activation and restricts their proinflammatory polarization 2. Both VISTA and PSGL‐1 are also highly expressed on granulocytes, macrophages and endothelial cells and play important functions in their biology 78, 92, 93. From the perspective of immune cell function, there are interesting parallels between VISTA and PSGL‐1 on the outcome of immune responses. For example, VISTA deficiency or targeting, and PSGL‐1‐Ig can all ameliorate mouse models of arthritis 94, 95. VISTA‐ or PSG‐L1‐targeting antibodies can also inhibit GVHD induction by inducing T cell death 3, 5, 96. While the study by Johnston et al., demonstrating direct interactions between VISTA and PSGL‐1, warrant interest, there are several points to consider for future investigation. The investigators did not demonstrate any in‐vivo interactions between VISTA and PSGL‐1, therefore the physiological relevance of this binding remains a mere speculation. Secondly, we and others have reported several VISTA‐specific phenotypes in vitro and in vivo where the pH conditions were not altered. From a translational standpoint, the authors introduced novel pH‐dependent anti‐VISTA antibodies, but showed neither a therapeutic advantage of these clones over the ‘conventional’ anti‐VISTA antagonists nor a reduction in adverse events. Based on recent findings of PD‐L1 cis‐interactions with CD80 on myeloid cells and its profound functions in regulating immunity 97, 98, and the very similar of PSGL1 and VISTA expression on the same leukocyte populations, one can reasonably hypothesize that these two proteins may be interacting (in cis) on the same cell‐forming heterodimers and may both be required for co‐signalling in some contexts. Utilization of available VISTA‐deficient and PSGL‐1‐deficient mice could readily address these questions.
However, there are multiple reasons for enthusiasm about this discovery. First, the roles of PSGL‐1 and VISTA in regulating T cell activities and tumor immunity is gaining traction 99, 100. Secondly, the pH and ionic environment being a factor that regulates immune receptor interactions is a novel and valuable concept. This is particularly important in tumors where the ionic environment has been clearly implicated in influencing the fate of T cell immunity 101, 102. More importantly, this study presents an innovative strategy for engineering pH‐selective antibodies, whereby the ligand is only targeted at the sites of pH dysregulation (e.g. tumor). Indeed, Korman and colleagues showed that targeting VISTA via this strategy had a similar outcome on anti‐tumor responses and synergized with PD‐1 blockade to drive tumor regression of MC38 colorectal adenocarcinoma.
VISTA targeting agents under development
Given the significant therapeutic activity of VISTA targeting agents in multiple preclinical tumor models, VISTA‐specific antagonists have moved forward into the clinic. One of the first agents to move into the clinic is a small molecule, CA‐170, developed by Aurigene and licensed by Curis. CA‐170 is an oral PD‐L1, PD‐L2 and VISTA checkpoint antagonist that has been shown to interfere with the suppression of T cell activation and cytokine production by VISTA 103. Clinical development (NCT02812875) of CA‐170 is ongoing with evaluation of potentially pharmacologically active doses in VISTA‐expressing tumors. In addition to this Phase I trial, a Phase II study is ongoing in India conducted by Aurigene.
Monoclonal antibodies that can block the suppressive effects of VISTA are also in commercial and therapeutic development. In January 2016, Janssen Research & Development launched a Phase I trial of anti‐VISTA antagonist (JNJ‐61610588) to assess safety in treating participants with advanced cancer. JNJ‐61610588 is a picomolar IgG1 anti‐VISTA monoclonal antibody (mAb) for which extensive insights into the therapeutic mechanism were gained, and has been proved to have biological activities in treated patients. In March 2018, Janssen terminated this study due to financial concerns, and all drug products and data were returned to ImmuNext, Inc. for continued clinical development. In addition, Hummingbird Biosciences has reported the development of a nanomolar anti‐VISTA antibody that would be ready for a Phase Ia/b clinical trial in 2020 68. No additional information is available.
There are currently no anti‐PSGL1 antibodies in the clinic, but the potential of targeting this pathway is highly recognized by investigators and investors alike. Verseau Therapeutics launched this fall with a primary focus on the development of lead PSGL‐1 antibody (VTX‐0811), which they advocate could reprogram tumor‐associated macrophages (TAMs) to a more proinflammatory anti‐tumor phenotype 104.
Conclusion
VISTA is a novel immunoregulatory protein with broad expression on both the lymphocyte and myeloid compartments at steady state, which underlies a unique and important homeostatic role in the immune system. The advantage of targeting VISTA by antibodies that suppress or enhance immunity represents a unique facet for the therapeutic normalization of immune responses in multiple diseases. In addition, the intriguing genetics and biology of VISTA warrants interest and efforts from multiple disciplines to resolve the exact molecular role of this molecule in immune homeostasis.
Disclosure
R. J. N. is co‐founder of ImmuNext involved in commercial development of anti‐VISTA antibodies. The other authors have no additional financial interests.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immune checkpoint inhibition: from molecules to clinical application. Clinical and Experimental Immunology 2020, 200: 105‐107.
TIGIT as an emerging immune checkpoint. Clinical and Experimental Immunology 2020, 200: 108‐119.
Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clinical and Experimental Immunology 2020, 200: 131‐140.
Mechanisms of checkpoint inhibition‐induced adverse events. Clinical and Experimental Immunology 2020, 200: 141‐154.
Role of inflammasome activation in tumor immunity triggered by immune checkpoint blockers. Clinical and Experimental Immunology 2020, 200: 155‐162.
Contributor Information
R. J. Noelle, Email: RJN@dartmouth.edu.
J. L. Lines, Email: JLL@dartmouth.edu.
References
- 1. Wang L, Rubinstein R, Lines JL et al VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 2011; 208:577–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borggrewe M, Grit C, Den Dunnen WFA et al VISTA expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia 2018; 66:2645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ElTanbouly MA, Zhao Y, Nowak EC et al VISTA is a checkpoint regulator for naive T cell quiescence and peripheral tolerance. Science 2020;367 10.1126/science.aay0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharaj P, Chahar HS, Alozie OK et al Characterization of programmed death‐1 homologue‐1 (PD‐1H) expression and function in normal and HIV infected individuals. PLOS ONE 2014; 9:e109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death‐1 homolog prevents graft‐versus‐host disease in mouse models. J Immunol 2011; 187:1537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta N, Maddineni S, Mathews II, Sperberg AP, Huang P‐S, Cochran JR. Structure and functional binding epitope of V‐domain Ig suppressor of T‐cell activation (VISTA). Cell Rep 2019; 28:2509–16.e5. [DOI] [PubMed] [Google Scholar]
- 7. Nowak EC, Lines JL, Varn FS et al Immunoregulatory functions of VISTA. Immunol Rev 2017; 276:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flies DB, Han X, Higuchi T et al Coinhibitory receptor PD‐1H preferentially suppresses CD4(+) T cell‐mediated immunity. J Clin Invest 2014; 124:1966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flies DB, Higuchi T, Chen L. Mechanistic assessment of PD‐1H coinhibitory receptor‐induced T cell tolerance to allogeneic antigens. J Immunol 2015; 194:5294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Li X, Hu L et al A crucial role of the PD‐1H coinhibitory receptor in suppressing experimental asthma. Cell Mol Immunol 2018; 15:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Le Mercier I, Putra J et al Disruption of the immune‐checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci USA 2014; 111:14846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein‐specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med 2003; 197:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoon KW, Byun S, Kwon E et al Control of signaling‐mediated clearance of apoptotic cells by the tumor suppressor p53. Science 2015; 349:1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceeraz S, Sergent PA, Plummer SF et al VISTA deficiency accelerates the development of fatal murine lupus nephritis. Arthritis Rheumatol 2017; 69:814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sergent PA, Plummer SF, Pettus J et al Blocking the VISTA pathway enhances disease progression in (NZB × NZW) F1 female mice. Lupus 2018; 27:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han X, Vesely MD, Yang W et al PD‐1H (VISTA)‐mediated suppression of autoimmunity in systemic and cutaneous lupus erythematosus. Sci Transl Med 2019;11:eaax1159. [DOI] [PubMed] [Google Scholar]
- 17. Ohno T, Zhang C, Kondo Y et al The immune checkpoint molecule VISTA regulates allergen‐specific Th2‐mediated immune responses. Int Immunol 2018; 30:3–11. [DOI] [PubMed] [Google Scholar]
- 18. Kunishige T, Taniguchi H, Ohno T, Azuma M, Hori J. VISTA is crucial for corneal allograft survival and maintenance of immune privilege. Invest Ophthalmol Vis Sci 2019; 60:4958–65. [DOI] [PubMed] [Google Scholar]
- 19. Hid Cadena R, Reitsema RD, Huitema MG et al Decreased expression of negative immune checkpoint VISTA by CD4+ T cells facilitates T helper 1, T helper 17, and T follicular helper lineage differentiation in GCA. Front Immunol 2019; 10:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng J, Li J, Sarde A et al Hypoxia‐induced VISTA promotes the suppressive function of myeloid‐derived suppressor cells in the tumor microenvironment. Cancer Immunol Res 2019; 7:1079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Consortium EP . An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar A. An overview of nested genes in eukaryotic genomes. Eukaryot Cell 2009; 8:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao T, He B, Liu S, Zhu H, Tan K, Qian J. EnhancerAtlas: a resource for enhancer annotation and analysis in 105 human cell/tissue types. Bioinformatics 2016; 32:3543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Battista M, Musto A, Navarra A, Minopoli G, Russo T, Parisi S. miR‐125b regulates the early steps of ESC differentiation through dies1 in a TGF‐independent manner. Int J Mol Sci 2013; 14:13482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parisi S, Battista M, Musto A, Navarra A, Tarantino C, Russo T. A regulatory loop involving Dies1 and miR‐125a controls BMP4 signaling in mouse embryonic stem cells. FASEB J 2012; 26:3957–68. [DOI] [PubMed] [Google Scholar]
- 26. Astle WJ, Elding H, Jiang T et al The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 2016; 167:1415–29.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanai M, Akiyama M, Takahashi A et al Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet 2018; 50:390–400. [DOI] [PubMed] [Google Scholar]
- 28. Kichaev G, Bhatia G, Loh PR et al Leveraging polygenic functional enrichment to improve GWAS power. Am J Hum Genet 2019; 104:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simonyan V, Chumakov K, Dingerdissen H et al High‐performance integrated virtual environment (HIVE): a robust infrastructure for next‐generation ence data analysis. Database (Oxf) 2016; 2016:baw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simonyan V, Mazumder R. High‐performance integrated virtual environment (HIVE) tools and applications for big data analysis. Genes (Basel) 2014; 5:957–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tate JG, Bamford S, Jubb HC et al COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res 2019; 47:D941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jakobsson M, Scholz SW, Scheet P et al Genotype, haplotype and copy‐number variation in worldwide human populations. Nature 2008; 451:998–1003. [DOI] [PubMed] [Google Scholar]
- 33. Wong KK, deLeeuw RJ, Dosanjh NS et al A comprehensive analysis of common copy‐number variations in the human genome. Am J Hum Genet 2007; 80:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mulati K, Hamanishi J, Matsumura N et al VISTA expressed in tumour cells regulates T cell function. Br J Cancer 2019; 120:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad‐spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2014; 2:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuklinski LF, Yan S, Li Z et al VISTA expression on tumor‐infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease‐specific survival. Cancer Immunol Immunother 2018; 67:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boger C, Behrens HM, Kruger S, Rocken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD‐L1/PD‐1: a future perspective for a combined gastric cancer therapy? Oncoimmunology 2017; 6:e1293215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liao H, Zhu H, Liu S, Wang H. Expression of V‐domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett 2018; 16:3465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie S, Huang J, Qiao Q et al Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother 2018; 67:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu L, Deng WW, Huang CF et al Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother 2017; 66:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villarroel‐Espindola F, Yu X, Datar I et al Spatially resolved and quantitative analysis of VISTA/PD‐1H as a novel immunotherapy target in human non‐small cell lung cancer. Clin Cancer Res 2018; 24:1562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang M, Pang HJ, Zhao W et al VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer 2018; 18:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kakavand H, Jackett LA, Menzies AM et al Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti‐PD‐1 therapy in metastatic melanoma patients. Mod Pathol 2017; 30:1666–76. [DOI] [PubMed] [Google Scholar]
- 44. Gao J, Ward JF, Pettaway CA et al VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017; 23:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zou W, Wolchok JD, Chen L. PD‐L1 (B7–H1) and PD‐1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hodi FS, O’Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg SA. IL‐2: the first effective immunotherapy for human cancer. J Immunol 2014; 192:5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res 2016; 22:1865–74. [DOI] [PubMed] [Google Scholar]
- 49. Kim JM, Chen DS. Immune escape to PD‐L1/PD‐1 blockade: seven steps to success (or failure). Ann Oncol 2016; 27:1492–504. [DOI] [PubMed] [Google Scholar]
- 50. Larkin J, Chiarion‐Sileni V, Gonzalez R et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fehrenbacher L, Spira A, Ballinger M et al Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016; 387:1837–46. [DOI] [PubMed] [Google Scholar]
- 52. Gajewski TF, Woo SR, Zha Y et al Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013; 25:268–76. [DOI] [PubMed] [Google Scholar]
- 53. Harlin H, Meng Y, Peterson AC et al Chemokine expression in melanoma metastases associated with CD8+ T‐cell recruitment. Cancer Res 2009; 69:3077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McDermott DF, Sosman JA, Sznol M et al Atezolizumab, an anti‐programmed death‐ligand 1 antibody, in metastatic renal cell carcinoma: long‐term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016; 34:833–42. [DOI] [PubMed] [Google Scholar]
- 56. Rosenberg JE, Hoffman‐Censits J, Powles T et al Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spranger S, Spaapen RM, Zha Y et al Up‐regulation of PD‐L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tumeh PC, Harview CL, Yearley JH et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Powles T, Eder JP, Fine GD et al MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558–62. [DOI] [PubMed] [Google Scholar]
- 60. Taube JM, Klein A, Brahmer JR et al Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin Cancer Res 2014; 20:5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity 2013; 39:1–10. [DOI] [PubMed] [Google Scholar]
- 62. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017; 541:321–30. [DOI] [PubMed] [Google Scholar]
- 63. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348:74–80. [DOI] [PubMed] [Google Scholar]
- 64. Vanpouille‐Box C, Formenti SC. Dual transforming growth factor‐beta and programmed death‐1 blockade: a strategy for immune‐excluded tumors? Trends Immunol 2018; 39:435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salmon H, Franciszkiewicz K, Damotte D et al Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012; 122:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mariathasan S, Turley SJ, Nickles D et al TGFbeta attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature 2018; 554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tauriello DVF, Palomo‐Ponce S, Stork D et al TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018; 554:538–43. [DOI] [PubMed] [Google Scholar]
- 68. Ingram PJ, Thakkar D, Boyd‐Kirkup JD. Abstract 587: HMBD002, a novel neutralizing antibody targeting a specific epitope on the co‐inhibitory immune checkpoint receptor VISTA, displays potent anti‐tumor effects in pre‐clinical models. Cancer Res 2017; 77(Suppl 13):587. [Google Scholar]
- 69. Le Mercier I, Chen W, Lines JL et al VISTA regulates the development of protective antitumor immunity. Cancer Res 2014; 74:1933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Broughton T, El Tanbouly MA, Schaafsma E et al Defining the signature of VISTA on myeloid cell chemokine responsiveness. Front Immunol 2019; 10:2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blando J, Sharma A, Higa MG et al Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci USA 2019; 116:1692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu J, Xie X, Xuan C et al High‐density infiltration of V‐domain immunoglobulin suppressor of T‐cell activation up‐regulated immune cells in human pancreatic cancer. Pancreas 2018; 47:725–31. [DOI] [PubMed] [Google Scholar]
- 73. Kather JN, Suarez‐Carmona M, Charoentong P et al Topography of cancer‐associated immune cells in human solid tumors. eLife 2018; 7:e36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu J, Yuan Y, Chen W et al Immune‐checkpoint proteins VISTA and PD‐1 nonredundantly regulate murine T‐cell responses. Proc Natl Acad Sci USA 2015; 112:6682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Beatty GL, Chiorean EG, Fishman MP et al CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu J, Yuan Y, Chen W et al Immune‐checkpoint proteins VISTA and PD‐1 nonredundantly regulate murine T‐cell responses. Proc Natl Acad Sci USA 2015; 112:6682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Johnston RJ, Su LJ, Pinckney J et al VISTA is an acidic pH‐selective ligand for PSGL‐1. Nature 2019; 574:565–70. [DOI] [PubMed] [Google Scholar]
- 78. El Tanbouly MA, Croteau W, Noelle RJ, Lines JL. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin Immunol 2019; 42:101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang L, Jia B, Claxton DF et al VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. Oncoimmunology 2018; 7:e1469594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yamanouchi S, Kuwahara K, Sakata A et al A T cell activation antigen, Ly6C, induced on CD4+ Th1 cells mediates an inhibitory signal for secretion of IL‐2 and proliferation in peripheral immune responses. Eur J Immunol 1998; 28:696–707. [DOI] [PubMed] [Google Scholar]
- 81. Green KA, Wang L, Noelle RJ, Green WR. Selective involvement of the checkpoint regulator VISTA in suppression of B‐cell, but not T‐cell, responsiveness by monocytic myeloid‐derived suppressor cells from mice infected with an immunodeficiency‐causing retrovirus. J Virol 2015; 89:9693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boyd‐Kirkup JD, Thakkar D, Paszkiewicz K, Ingram PJ. Abstract 1729: Integrative immune profiling of syngeneic tumor models provides predictive immune signatures for treatment response with HMBD002, a novel anti‐VISTA neutralizing antibody. Cancer Research 2018; 78(Suppl 13):1729. [Google Scholar]
- 83. Verma V, Shrimali RK, Ahmad S et al PD‐1 blockade in subprimed CD8 cells induces dysfunctional PD‐1(+)CD38(hi) cells and anti‐PD‐1 resistance. Nat Immunol 2019; 20:1231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yost KE, Satpathy AT, Wells DK et al Clonal replacement of tumor‐specific T cells following PD‐1 blockade. Nat Med 2019; 25:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang J, Wu G, Manick B et al VSIG‐3 as a ligand of VISTA inhibits human T‐cell function. Immunology 2019; 156:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Prodeus A, Abdul‐Wahid A, Sparkes A et al VISTA.COMP ‐ an engineered checkpoint receptor agonist that potently suppresses T cell‐mediated immune responses . JCI Insight 2017; 2:e94308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Abadier M, Ley K. P‐selectin glycoprotein ligand‐1 in T cells. Curr Opin Hematol 2017; 24:265–73. [DOI] [PubMed] [Google Scholar]
- 88. Ley K, Kansas GS. Selectins in T‐cell recruitment to non‐lymphoid tissues and sites of inflammation. Nat Rev Immunol 2004; 4:325–35. [DOI] [PubMed] [Google Scholar]
- 89. Veerman KM, Williams MJ, Uchimura K et al Interaction of the selectin ligand PSGL‐1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol 2007; 8:532–9. [DOI] [PubMed] [Google Scholar]
- 90. Veldkamp CT, Kiermaier E, Gabel‐Eissens SJ et al Solution Structure of CCL19 and Identification of Overlapping CCR7 and PSGL‐1 Binding Sites. Biochemistry 2015; 54:4163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Heng TS, Painter MW, Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 2008; 9:1091–4. [DOI] [PubMed] [Google Scholar]
- 92. Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL‐1 function in immunity and steady state homeostasis. Immunol Rev 2009; 230:75–96. [DOI] [PubMed] [Google Scholar]
- 93. Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL. P‐selectin glycoprotein ligand‐1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood 1996; 88:3010–21. [PubMed] [Google Scholar]
- 94. Ceeraz S, Eszterhas SK, Sergent PA et al VISTA deficiency attenuates antibody‐induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res Ther 2017; 19:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sumariwalla PF, Malfait AM, Feldmann M. P‐selectin glycoprotein ligand 1 therapy ameliorates established collagen‐induced arthritis in DBA/1 mice partly through the suppression of tumour necrosis factor. Clin Exp Immunol 2004; 136:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang CC, Lu YF, Wen SN et al A novel apoptosis‐inducing anti‐PSGL‐1 antibody for T cell‐mediated diseases. Eur J Immunol 2005; 35:2239–49. [DOI] [PubMed] [Google Scholar]
- 97. Sugiura D, Maruhashi T, Okazaki IM et al Restriction of PD‐1 function by cis‐PD‐L1/CD80 interactions is required for optimal T cell responses. Science 2019; 364:558–66. [DOI] [PubMed] [Google Scholar]
- 98. Zhao Y, Lee CK, Lin CH et al PD‐L1:CD80 Cis‐heterodimer triggers the co‐stimulatory receptor CD28 while repressing the inhibitory PD‐1 and CTLA‐4 pathways. Immunity 2019; 51:1059–73.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tinoco R, Carrette F, Barraza ML et al PSGL‐1 Is an immune checkpoint regulator that promotes T cell exhaustion. Immunity 2016; 44:1190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tinoco R, Otero DC, Takahashi AA, Bradley LM. PSGL‐1: a new player in the immune checkpoint landscape. Trends Immunol 2017; 38:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eil R, Vodnala SK, Clever D et al Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016; 537:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vodnala SK, Eil R, Kishton RJ et al T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019; 363:eaau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Musielak B, Kocik J, Skalniak L et al CA‐170 – a potent small‐molecule PD‐L1 inhibitor or not? Molecules. 2019;24:1420–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wong S.Verseau unveils tumor macrophage reprogramming strategy [online article]. 2019. Available at: https://www.biocentury.com/bc-innovations/emerging-company-profile/2019-10-21/verseau-debuts-50m-therapies-make-tumor- (accessed 1 October 2019).
- 105. Roche . Beyond the immunity cycle [online article]. [Available at: https://www.roche.com/research_and_development/what_we_are_working_on/oncology/cancer-immunotherapy/beyond-the-immunity-cycle.htm (accessed 11 November 2019).


