We vaccinated the Diversity Outbred (DO) population of mice with BCG, the only vaccine currently used to protect against tuberculosis, and then challenged them with M. tuberculosis by aerosol. We found that the BCG-vaccinated DO mouse population exhibited a wide range of outcomes, in which outcomes in individual mice ranged from minimal respiratory or systemic disease to fulminant disease and death. The breadth of these outcomes appears similar to the range seen in people, indicating that DO mice may serve as an improved small-animal model to study tuberculosis infection and immunity. Moreover, sophisticated tools are available for the use of these mice to map genes contributing to control of vaccination. Thus, the present studies provided an important new tool in the fight against tuberculosis.
KEYWORDS: Mycobacterium tuberculosis, animal models, Diversity Outbred, immunization, tuberculosis vaccines, vaccines
ABSTRACT
Many studies of Mycobacterium tuberculosis infection and immunity have used mouse models. However, outcomes of vaccination and challenge with M. tuberculosis in inbred mouse strains do not reflect the full range of outcomes seen in people. Previous studies indicated that the novel Diversity Outbred (DO) mouse population exhibited a spectrum of outcomes after primary aerosol infection with M. tuberculosis. Here, we demonstrate the value of this novel mouse population for studies of vaccination against M. tuberculosis aerosol challenge. Using the only currently licensed tuberculosis vaccine, we found that the DO population readily controlled systemic Mycobacterium bovis BCG bacterial burdens and that BCG vaccination significantly improved survival across the DO population upon challenge with M. tuberculosis. Many individual DO mice that were vaccinated with BCG and then challenged with M. tuberculosis exhibited low bacterial burdens, low or even no systemic dissemination, little weight loss, and only minor lung pathology. In contrast, some BCG-vaccinated DO mice progressed quickly to fulminant disease upon M. tuberculosis challenge. Across the population, most of these disease parameters were at most modestly correlated with each other and were often discordant. This result suggests the need for a multiparameter metric to better characterize “disease” and “protection,” with closer similarity to the complex case definitions used in people. Taken together, these results demonstrate that DO mice provide a novel small-animal model of vaccination against tuberculosis that better reflects the wide spectrum of outcomes seen in people.
IMPORTANCE We vaccinated the Diversity Outbred (DO) population of mice with BCG, the only vaccine currently used to protect against tuberculosis, and then challenged them with M. tuberculosis by aerosol. We found that the BCG-vaccinated DO mouse population exhibited a wide range of outcomes, in which outcomes in individual mice ranged from minimal respiratory or systemic disease to fulminant disease and death. The breadth of these outcomes appears similar to the range seen in people, indicating that DO mice may serve as an improved small-animal model to study tuberculosis infection and immunity. Moreover, sophisticated tools are available for the use of these mice to map genes contributing to control of vaccination. Thus, the present studies provided an important new tool in the fight against tuberculosis.
INTRODUCTION
People exposed to Mycobacterium tuberculosis experience many outcomes. About 5% to 10% of those repeatedly exposed, such as health care workers in areas where tuberculosis (TB) is endemic, resist or possibly clear M. tuberculosis infection (1). The majority of those who become infected do not have symptoms for many years, a state generally termed latency but that probably includes a range of underlying disease activities. Active tuberculosis is also characterized by a wide spectrum of disease manifestations, ranging from localized pulmonary disease to disseminated infection. The underlying causes of the variability in mammalian responses to M. tuberculosis have been topics of intense study. Among these is host genetics, and human genetic loci have been linked to host susceptibility to M. tuberculosis infection and disease (2–4).
A variety of animal models are currently used to study immunity to M. tuberculosis, with variable success in recapitulating the wide range of tuberculosis disease seen in humans. Mice are the most commonly used models because they are immunologically similar to humans, genetically tractable, and economical. Studies in inbred mice demonstrated the importance of CD4+ T cells, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) among other immunological mediators that are clearly relevant to tuberculosis in humans (5–10). However, M. tuberculosis infection of the most commonly used inbred mouse strains, such as C57BL/6J (B6) or BALB/c, does not result in all aspects of human tuberculosis or outcomes after vaccination. In particular, traditional caseating granulomas are not usually formed in lungs of mice like those seen in humans, nonhuman primates (NHPs), and guinea pigs (11). Studies using inbred C3HeB/FeJ mice overcome at least one of the limitations of the use of B6 or BALB/c, in that these mice develop necrotizing lung granulomas (12). The use of these mice was instrumental in describing multigenic control of tuberculosis (13, 14). Nonetheless, within groups of inbred mice given similar low doses of M. tuberculosis (typically, 50 to 100 bacteria by aerosol), all have similar bacterial burdens, and all untreated animals eventually succumb to disease. Additionally, due to their origin and inbreeding, these common inbred mouse strains have a restricted gene pool. Even outbred Swiss Webster mice have limited genetic diversity and have relatively uniform responses to M. tuberculosis infection (15, 16).
In contrast, Diversity Outbred (DO) mice are a new population whose level of genetic diversity is on par with that of humans and nonhuman primates (NHPs) (17). The eight founding inbred strains that comprised the initial breeding pool for DO mice included traditional laboratory strains such as B6 and A/J, as well as three wild-caught mouse strains (CAST/EiJ, PWK/PhJ, and WSB/EiJ) that represent different murine genetic clades found throughout the world. Extensive interbreeding of these eight strains has now resulted in an outbred population of unique individuals representing infinite variety. As a result, divergent or rare phenotypes are more likely to be detected in this population than in conventional inbred mice (17).
Studies examining primary aerosol M. tuberculosis infection in DO mice demonstrated a wide heterogeneity of disease presentations (18–20), according to which DO mice could be categorized based on survival and weight outcomes into “supersusceptible,” susceptible, and resistant groups. Disease in the supersusceptible animals was marked by large necrotic and/or diffuse lung lesions dominated by neutrophils, while lung lesions in resistant mice had small lymphocytic foci resembling lesions seen in inbred B6 and BALB/c mice. Therefore, tuberculosis studies using DO mice may overcome some of the limitations of existing inbred mouse strains. Further, sophisticated genetic tools are available to perform mapping of quantitative trait loci (QTL), using a panel of >140,000 single nucleotide polymorphisms (SNPs) derived from the founder strains; thus, findings can be linked to founder allele effects (21). Inbred Collaborative Cross (CC) mice, developed in parallel to DO mice, represent a complementary resource that may aid in testing hypotheses generated in DO studies (22).
Animal models, including mice, guinea pigs, and NHPs, have been widely used to test new drugs and vaccines against M. tuberculosis. M. bovis BCG is the only currently licensed tuberculosis vaccine and has been administered to over 3 billion people worldwide (23). While this vaccine prevents disseminated tuberculosis in children, BCG’s efficacy against pulmonary TB in adults is highly variable for a variety of reasons, including BCG and M. tuberculosis strain variation, environmental factors, and underlying genetic causes (24, 25). Despite limitations, however, BCG remains the “gold standard” for vaccine protection against which all novel vaccine candidates are compared. In experimental models of tuberculosis, “protection” is typically defined as the reduction of M. tuberculosis CFU levels in organs of vaccinated mice compared to naive controls, usually assessed at 4 weeks after a low-dose aerosol M. tuberculosis challenge. For example, we recently demonstrated that the degrees of BCG-induced protection differed among the eight inbred DO founder strains and CC lines (26). At the population level, survival rates can be compared between vaccinated and nonvaccinated groups. However, defining protection using single measures such as bacterial burdens or survival may not be sufficient in genetically heterogeneous populations such as humans, where multicomponent case definitions are usually needed for clinical studies.
Outbred small-animal models that exhibit a human-like range of host immune responses induced by vaccination are likely to be useful not only for studying immunity to M. tuberculosis generally but also for identifying genes that control vaccination outcomes. Here, we used DO mice to establish a novel model to evaluate vaccine-induced protection against M. tuberculosis. Using BCG as a prototype, we found that the use of DO mice represents a robust approach with which to evaluate tuberculosis vaccines. Most importantly, we expect that the heterogeneous outcomes seen in DO mice after vaccination and M. tuberculosis challenge will enable studies of genetic control of vaccination, studies of novel vaccines, and development of immune correlates of vaccine-induced protection against tuberculosis.
RESULTS
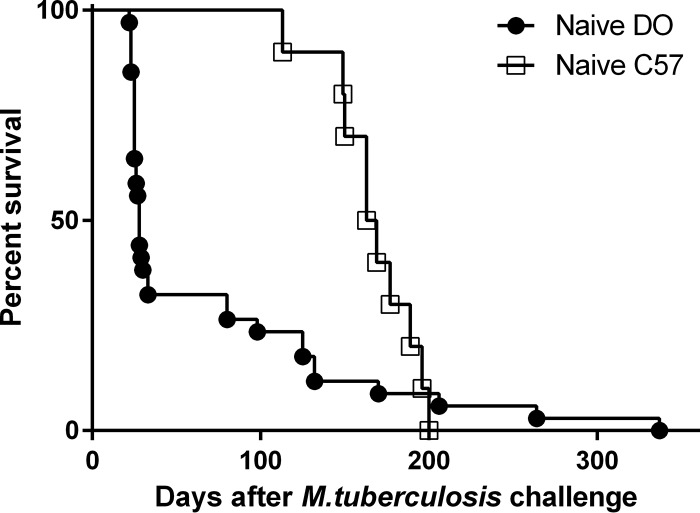
To assess the suitability of DO mice for vaccination studies, we first examined long-term survival of these mice after primary M. tuberculosis infection. B6 and DO male mice were infected by aerosol with a targeted dose of ∼150 CFU of M. tuberculosis and followed for survival. Previous studies that evaluated short-term survival in female DO mice found a supersusceptible population of mice that succumbed to M. tuberculosis infection within 35 days (19). Similarly to those results, we observed a supersusceptible population of DO mice (Fig. 1); at this relatively high challenge dose, ∼70% were supersusceptible. This was a higher proportion than previously observed with a lower (∼100 CFU) dose (19). About day 80, a second group of DO mice started to succumb to infection; in contrast, B6 mice survived until at least day 113. Among the supersusceptible DO mice that succumbed to infection prior to day 35, the mean time to death (TTD) was 26 days (± 3 days), while the remaining DO mice had a mean TTD of 160 days (± 81 days); for all of the B6 mice, the mean TTD was 167 days (± 26 days). The overall survival rates of vaccinated mice were significantly different between the B6 and DO mice (Mantel-Cox test, P < 0.0001). An examination of fixed lung sections from BCG-vaccinated DO mice by a board-certified veterinary pathologist revealed that the lungs of most of the supersusceptible mice that were subjected to necropsy exhibited necrosuppurative pneumonia marked by heavy neutrophilia. In contrast, the lungs of the animals that succumbed later, at day 80 or beyond, generally exhibited diffuse histiocytic and lymphocytic pneumonia dominated by the presence of macrophages, often with coalescing lung lesions. These phenotypes are consistent with those previously described for the supersusceptible and resistant DO groups with primary M. tuberculosis infection (19).
FIG 1.
A subpopulation of Diversity Outbred mice is highly susceptible to aerogenic M. tuberculosis challenge. Groups of 10 male B6 and 35 male DO mice were infected by aerosol with ∼150 CFU of M. tuberculosis. Bacterial uptake was determined by euthanizing one group of five B6 mice 4 h after infection and determining total lung M. tuberculosis burdens. Survival curves determined for B6 and DO mice were significantly different (Wilcoxon test, P = 0.0007).
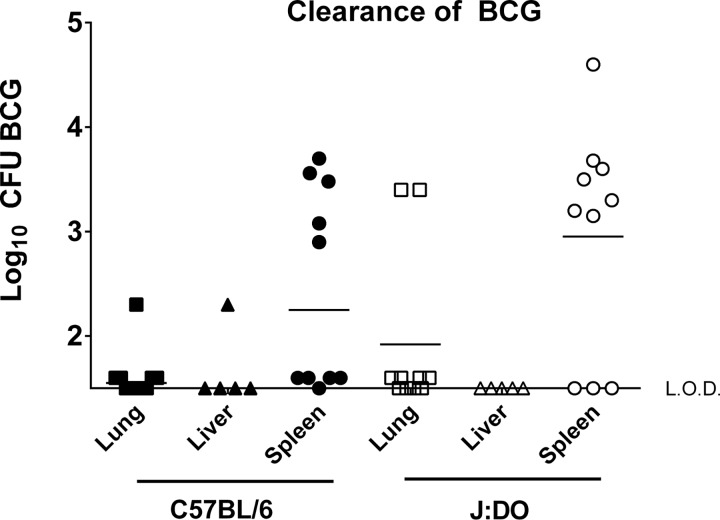
Because nearly 75% of the DO mice were supersusceptible to M. tuberculosis infection, we tested whether some DO mice were immunodeficient by vaccinating mice with the live attenuated M. bovis BCG vaccine strain. We vaccinated male DO mice with 105 CFU of BCG by the subcutaneous route. We followed five B6 and five male DO mice for survival after BCG vaccination, and, similarly to the B6 mice, all of the DO mice survived for 4 months (data not shown). Further, to assess systemic control of BCG, we euthanized five BCG-vaccinated DO and B6 mice 4 weeks after vaccination and determined bacterial burdens in lungs, livers, and spleens. BCG growth was controlled in all organs of each DO mouse to a degree equivalent to that seen with the inbred B6 mice (Fig. 2). These results demonstrated that DO mice control and survive BCG vaccination and thus could be challenged with M. tuberculosis to determine protection outcomes.
FIG 2.
B6 and DO mice vaccinated subcutaneously with M. bovis BCG clear bacteria from organs at similar rates. Groups of five B6 and DO male mice were vaccinated subcutaneously with 105 BCG. Animals were euthanized 4 weeks after vaccination, and organs were extracted, homogenized, and plated to enumerate BCG CFU. This experiment was repeated twice, and results combined from the two experiments are shown. Dots represent BCG CFU in individual animals, and lines represent median CFU per group. Pairwise comparisons between B6 and DO mice for each organ did not detect significant differences (P > 0.05 by Student's t test for all combinations). L.O.D., limit of detection.
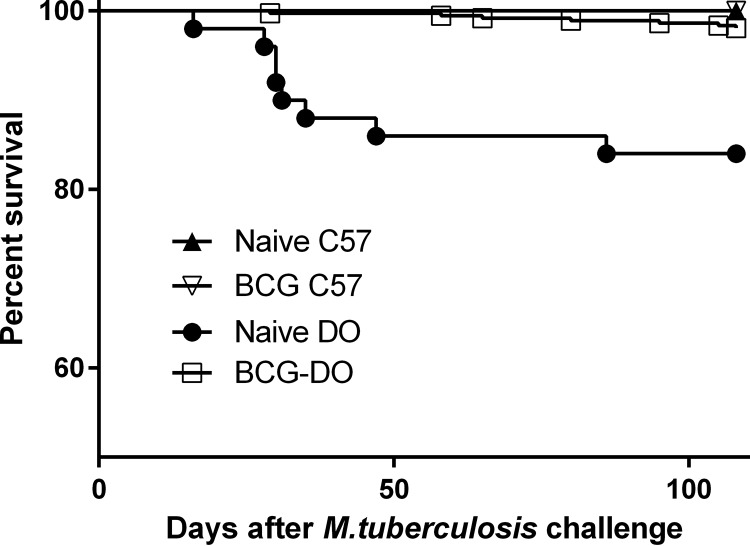
To test the ability of BCG to protect DO mice against M. tuberculosis, we vaccinated groups of female DO mice subcutaneously with phosphate-buffered saline (PBS) or 105 BCG bacteria and then challenged them by aerosol 8 weeks later. For these experiments, we targeted a dose of 50 CFU; across five experiments, the average dose was ∼45 CFU of M. tuberculosis (range, 16 to 65 CFU). This lower dose ensured that all of the mice would be infected but that a higher proportion of naive DO mice would survive primary M. tuberculosis challenge. For comparison, a group of inbred B6 mice were BCG vaccinated and infected with M. tuberculosis in parallel. We followed mice for 14 weeks after challenge, a time point chosen to allow extended observations of survival as well as to reveal declines in BCG-induced protection, such as that seen in B6 mice as reported previously (27). All of the naive and BCG-vaccinated B6 mice survived through 14 weeks after infection (Fig. 3). The naive DO mice began to succumb to infection by day 30 after challenge, with a mean TTD of 38 days (± 18 days) for all naive animals that died within 14 weeks after challenge. However, BCG vaccination decreased the overall proportion of DO mice that succumbed to infection within 14 weeks from 16% in the naive group to 2% in the BCG-vaccinated group (P < 0.001). The mean TTD for BCG-vaccinated DO mice that did succumb within 14 weeks was 63 days (± 21 days), significantly longer than that seen with the naive DO mice (P < 0.0001), due primarily to extension of survival of the supersusceptible group. Thus, BCG vaccination extended the survival time of most of the members of the supersusceptible subpopulation of the DO mice.
FIG 3.
BCG vaccination enhances survival of M. tuberculosis-challenged DO mice. Groups of female B6 and DO mice were vaccinated subcutaneously with 105 BCG, and control mice were sham vaccinated with PBS. Mice were then challenged by aerosol with ∼45 CFU M. tuberculosis. Survival was followed for 115 days, at which point surviving mice were euthanized. Data represent combined results from five independent experiments similar in design, where the total numbers of animals for each group were 35 naive B6 mice, 60 naive DO mice, 30 BCG-vaccinated B6 mice, and 240 BCG-vaccinated DO mice. The survival of naive DO mice was significantly different from that of all other groups (Wilcoxon test, P < 0.0001).
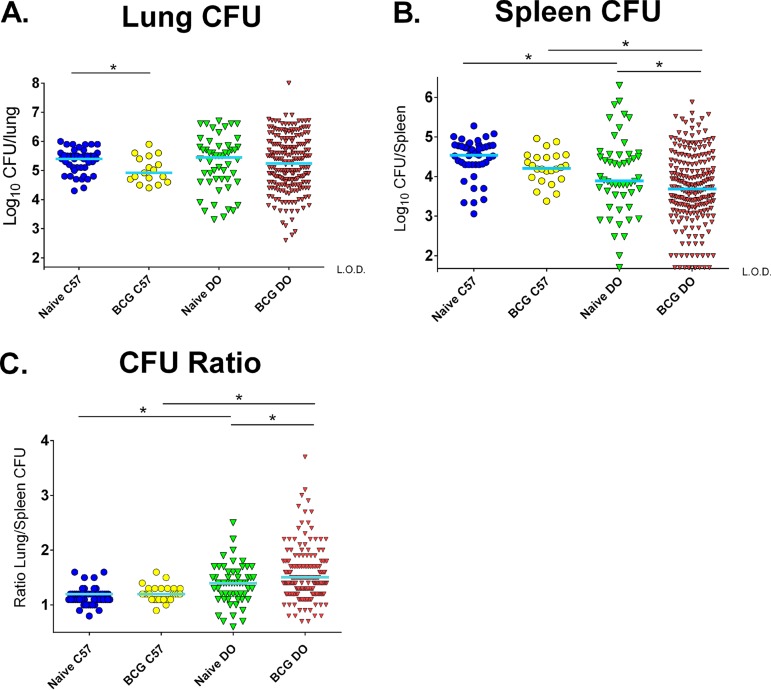
We euthanized all of the remaining surviving mice at 14 weeks after M. tuberculosis challenge and enumerated M. tuberculosis burdens in the lungs and spleens. Of note, data from the nine naive and three BCG-vaccinated mice that were euthanized prior to 14 weeks and that were available for sampling are included in the applicable figures. Consistent with previous findings of declining effects of BCG vaccination in B6 mice by this time point (27), BCG-vaccinated B6 mice showed a modest and yet significant reduction in lung CFU compared to naive mice (P < 0.05; Fig. 4A). M. tuberculosis burdens in the lungs of challenged naive DO exhibited a wide 4-log range, and median lung CFU counts were similar to those in naive B6 mice (P > 0.05). The DO mice that were vaccinated and challenged exhibited an even larger, 6-log distribution of lung M. tuberculosis burdens that were not significantly reduced compared to those seen with the naive DO mice at this late time point (P = 0.77). Therefore, the BCG-vaccinated DO mice exhibited heterogenous lung bacterial burdens following aerogenic M. tuberculosis infection.
FIG 4.
Lungs and spleens of naive and BCG-vaccinated DO mice challenged with M. tuberculosis exhibit a wide range of bacterial burdens. Groups of female B6 and DO mice were vaccinated subcutaneously with 105 BCG, and control mice were sham vaccinated with PBS. Mice were challenged by aerosol with ∼50 CFU M. tuberculosis. Surviving animals were euthanized 14 weeks after infection, and M. tuberculosis organ burdens were enumerated by plating for CFU. Animals that became moribund before 14 weeks were euthanized when humane endpoints were reached, and the M. tuberculosis burdens in their organs were enumerated when possible. (A and B) Dots represent the M. tuberculosis CFU in individual animals, and lines represent the median CFU per group for (A) lungs and (B) spleens. Data represent combined results from five independent experiments similar in design, where the total numbers of animals for each group were 35 naive B6 mice, 60 naive DO mice, 30 BCG-vaccinated B6 mice, and 240 BCG-vaccinated DO mice. The limit of detection (L.O.D.) for spleen samples in panel B was 50 CFU. (C) For each individual animal, lung CFU data were divided by spleen CFU data to derive the ratio of lung/spleen M. tuberculosis burdens. *, differences between the groups indicated by the lines were significant (unpaired t test, P < 0.05).
In addition to lung M. tuberculosis burdens, we measured splenic M. tuberculosis burdens. M. tuberculosis CFU counts were modestly reduced in the spleens of BCG-vaccinated B6 mice compared to naive B6 mice, although the reduction was not statistically significant at this time point (Fig. 4B). Similarly to the pattern of CFU levels in lungs, the naive DO mice exhibited a wide range of M. tuberculosis CFU levels in spleens that were significantly lower than the burdens in naive mice (P = 0.02), ranging from below the limit of detection to over 6 logs. Interestingly, about 4% of the BCG-vaccinated DO mice had levels of M. tuberculosis in spleens below the limit of detection (50 CFU), suggesting either that the bacteria did not disseminate from the lungs or that the bacteria disseminated and were then cleared.
Typically, in B6 mice challenged by aerosol with M. tuberculosis, BCG vaccination decreases M. tuberculosis burdens proportionally in lungs and spleens. To compare the effects of BCG on the relative distributions of M. tuberculosis in DO mice, we calculated the ratios of lung CFU to spleen CFU for individual mice and compared these ratios for the populations of naive and BCG-vaccinated B6 and DO mice. The ratios determined for the naive B6 mice and BCG-vaccinated B6 mice were relatively homogeneous and similar (∼1.2 ± 0.1; Fig. 4C). In contrast, the both naive and BCG-vaccinated DO mice exhibited a wider range in CFU distribution; some mice had a predominantly pulmonary infection, with lung/spleen CFU ratios of up to 2.5, while others had more extrapulmonary bacteria, with lung/spleen ratios as low as 0.6. Compared to the naive DO mice, the BCG-vaccinated DO mice exhibited a significant shift toward a pulmonary disease (P < 0.05).
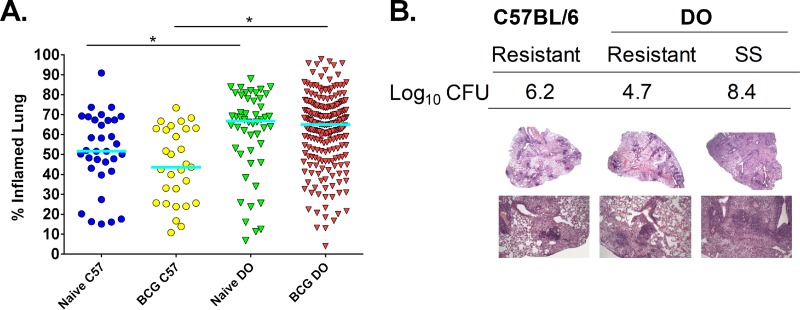
For each mouse that was challenged with M. tuberculosis, a portion of lung and spleen were preserved for histopathological analyses performed using staining with hematoxylin and eosin. The total area of lung tissue with disease involvement was measured by densitometry, and the data are presented as percent inflamed lung tissue per total lung (Fig. 5A) (28). Despite a wide range of outcomes, the median percentages of tissue involvement were roughly equivalent between the naive and BCG-vaccinated B6 mice, as well as between the naive and BCG-vaccinated DO mice. Interestingly, as a group, both the naive and BCG-vaccinated DO mice had more lung tissue damage than their B6 counterparts (P < 0.001). This indicates that DO mice are more likely to exhibit lung pathology than the relatively resistant B6 mice, despite having similar mean lung bacterial burdens (Fig. 4A).
FIG 5.
Naive and BCG-vaccinated DO mice challenged with M. tuberculosis exhibit a range of lung inflammation levels and unique lung pathologies. Groups of female B6 and DO mice were vaccinated subcutaneously with 105 M. bovis BCG, and control mice were sham vaccinated with PBS. Mice were challenged by aerosol with ∼45 CFU M. tuberculosis. Animals were euthanized 14 weeks after infection, and lung sections were isolated and prepared for slides with H&E staining. Animals that became moribund before 14 weeks were euthanized, and their lung samples were similarly isolated. H&E-stained slides were scanned, and images were analyzed to assess the percentage of lung tissue with disease involvement compared to the total lung area. (A) Dots represent the percentage of each lung that was inflamed and/or had disease involvement in individual animals, and lines represent the median for the group. Data represent the combined results from five independent experiments similar in design, where the total numbers of animals for each group were 35 naive B6 mice, 60 naive DO mice, 30 BCG-vaccinated B6 mice, and 240 BCG-vaccinated DO mice. (B) Representative images of lung sections prepared for H&E staining that demonstrate various disease pathologies. Lung section images from a BCG-vaccinated B6 mouse with typical disease presentation (resistant), a “resistant” BCG-vaccinated DO mouse that had high lung M. tuberculosis burdens, and a “supersusceptible” mouse with low M. tuberculosis CFU are shown, alongside the log10 lung M. tuberculosis CFU. Images in the top panel used ×2 magnification, and images in the bottom panel used ×10 magnification. *, differences between the groups indicated by the lines were significant (unpaired t test, P < 0.05).
Lung sections were also examined in more detail by a board-certified veterinary pathologist. Representative tissue section images illustrating the range of pathologies observed are shown (Fig. 5B), including images from a BCG-vaccinated “resistant” B6 mouse (assumed to be protected) and from a “resistant” DO mouse that had 4.7 log10 M. tuberculosis in the lungs (Fig. 5B). Lung lesions in the BCG-vaccinated “resistant” B6 and BCG-vaccinated “resistant” DO mice were similar and characterized by their small size; these lesions were dominated by dense lymphoid aggregates and nonnecrotic macrophages. However, the pathologies in lungs from the BCG-vaccinated DO mice were varied. For example, a BCG-vaccinated DO mouse that had high lung M. tuberculosis burden and was still supersusceptible (as defined by rapid development of morbidity) even in the face of BCG vaccination had lung tissue with diffuse macrophage-rich pneumonia and sparse lymphoid aggregates (Fig. 5B). Similar lung pathology was described previously in supersusceptible DO mice as defined by primary M. tuberculosis infection (19).
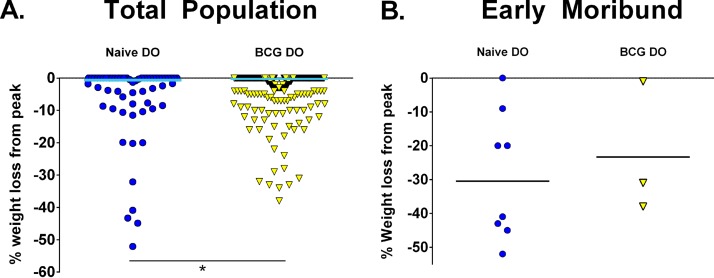
Weight loss is associated with advanced tuberculosis disease in humans, and weight loss was a marker of susceptibility of DO mice following primary M. tuberculosis infection that also correlated with lung bacterial burdens (19). We examined changes in weight in both naive and BCG-vaccinated DO mice, shown here as the percentage of weight loss of each animal compared to peak body weight. At the M. tuberculosis challenge dose of ∼45 CFU, relatively few naive or BCG-vaccinated DO mice lost weight over the course of 14 weeks of M. tuberculosis infection, but BCG vaccination further protected DO mice from weight loss (unpaired t test, P < 0.05; Fig. 6A). Only a few mice in either group succumbed to infection before 14 weeks, but there was no obvious relationship between weight loss and morbidity nor any difference between naive and BCG-vaccinated DO mice in this subset of mice with early morbidity (Fig. 6B); of note, several mice that died never lost weight. We extracted organs from some obviously moribund mice to determine bacterial burdens and to examine lung pathology, and indeed, all of the animals had high M. tuberculosis burdens and lung disease consistent with tuberculosis (data not shown). Thus, weight loss differentiated between naive and BCG-vaccinated DO mouse populations but was not a strong marker of morbidity.
FIG 6.
BCG vaccination reduces weight loss in DO mice compared to naive mice, but weight loss does not predict morbidity. Groups of female B6 and DO mice were vaccinated subcutaneously with 105 M. bovis BCG, and control mice were sham vaccinated with PBS. Mice were aerogenically challenged with ∼45 CFU M. tuberculosis. Mice were weighed a week prior to aerosol challenge and weekly after challenge until the end of the experiment at 14 weeks after challenge. Mice that showed signs of distress were weighed daily. The percentage of weight loss from peak was calculated using the final weight (or final weight before euthanasia) divided by the peak body weight, and that value was subtracted from 100. (A) Data represent combined results from five independent experiments similar in design, where the total numbers of animals for each group are 35 naive B6 mice, 60 naive DO mice, 30 BCG-vaccinated B6 mice, and 240 BCG-vaccinated DO mice. (B) The percentage of weight loss from peak for all mice that succumbed to M. tuberculosis challenge before 14 weeks is shown; these results include data from eight naive DO mice and three BCG-vaccinated DO mice. *, differences between the groups indicated by the lines were significant (unpaired t test, P < 0.05).
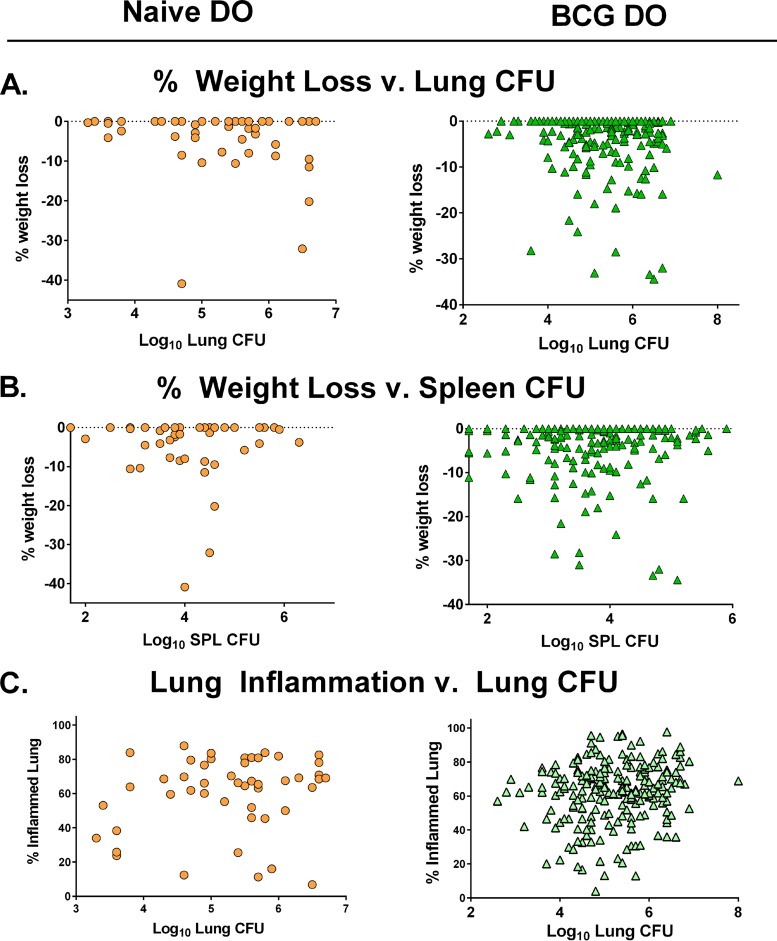
Finally, to evaluate the utility of the use of single versus multiple parameters in defining vaccine-induced protection in DO mice, we assessed correlations between all traits that were measured. We performed pairwise analyses for each mouse, comparing the percentages of weight loss to either lung CFU or spleen CFU at 14 weeks after challenge. We observed a spectrum of weight loss/lung CFU combinations in both groups, such that some individual animals (particularly the BCG-vaccinated DO mice) had high lung CFU burdens and yet lost no weight; conversely, at least one animal with low lung CFU had extreme weight loss. We found no correlation between percent weight loss and lung CFU in naive DO mice but found a modest correlation between weight loss and lung CFU in BCG-vaccinated DO mice (Fig. 7A; see also Table 1). There was no correlation between weight loss and splenic M. tuberculosis burden in either naive or BCG-vaccinated DO mice (Fig. 7B; see also Table 1). Many animals with high spleen M. tuberculosis burdens did not lose weight, while some animals with moderate bacterial burdens lost up to 30% of their body weight.
FIG 7.
Individual disease measurements from DO mice provide nonoverlapping information about effects of BCG vaccination on M. tuberculosis disease. The relationship between individual disease outcomes in terms of lung CFU, spleen CFU, lung histopathology, and weight loss was determined using pairwise analyses of the combined data described above. Spearman’s rank correlation was performed on each pairwise set of data. (A) Comparisons between lung CFU and percent weight loss for naive and BCG-vaccinated DO mice. (B) Comparisons between spleen CFU and percent weight loss for naive and BCG-vaccinated DO mice. (C) Comparisons between lung CFU and percent inflamed lung for naive and BCG-vaccinated DO mice.
TABLE 1.
Individual disease measurements had weak or no correlationsa
| Mouse group |
Lung CFU versus weight loss |
Spleen CFU versus weight loss |
Lung CFU versus lung inflammation |
|---|---|---|---|
| Naive DO | −0.15 (P = 0.29) | −0.009 (P = 0.94) | 0.17 (P = 0.26) |
| BCG-DO | −0.19 (*P = 0.0043) | −0.73 (P = 0.27) | 0.15 (*P = 0.023) |
Values shown represent Spearman’s rank (Spearman r) correlations and P values corresponding to results of comparisons between individual disease measurements, including lung CFU, spleen CFU, lung histopathology, and weight loss. *, P value considered significant.
We also compared the lung-associated traits of percent lung inflammation and lung CFU. Interestingly, we found no correlation between lung CFU and percent lung inflammation in the naive DO mice, while these two traits were modestly correlated in the BCG-vaccinated DO mice (Fig. 7C; see also Table 1). Despite this correlation across the population, some animals showed very low lung M. tuberculosis burden but extreme lung tissue destruction, while some animals with high lung CFU had a high proportion of healthy lung tissue. Not surprisingly, we found no relationship between lung inflammation and spleen burdens in either naive or BCG-vaccinated DO mice (data not shown). Taken together, these analyses suggest that all of these measurements of M. tuberculosis infection outcome (lung CFU, spleen CFU, weight loss, and lung inflammation) are informative and represent nonoverlapping pools of information. Thus, combining parameters may give a more comprehensive description of infection outcomes than using individual measurements and may enable better quantitation of the heterogeneous responses of DO mice to BCG vaccination and M. tuberculosis challenge.
DISCUSSION
Previous studies indicated that DO mice exhibited a wide range of outcomes after primary aerosol infection with M. tuberculosis (19). Here, we demonstrated the value of this novel mouse population for studies of vaccination against M. tuberculosis aerosol challenge. We have a particular interest in defining correlates of vaccine-induced protection (29–32), but we recognized that inbred mouse models may be limited by lack of genetic diversity; we therefore initiated studies in DO mice in part to extend that work. Using the only currently licensed tuberculosis vaccine, we found that the DO population readily controlled systemic BCG bacterial burdens (Fig. 2) and that BCG vaccination significantly improved survival upon challenge with M. tuberculosis (Fig. 3). Many individual BCG-vaccinated/M. tuberculosis-challenged DO mice exhibited low bacterial burdens, low or even no systemic dissemination, little weight loss, and only minor lung pathology (Fig. 4 to 6). Nonetheless, most of these disease parameters were at most modestly correlated with each other and were often discordant (Fig. 7). The wide heterogeneity of M. tuberculosis infection outcomes in DO mice after BCG vaccination stands in contrast to the relatively homogeneous outcomes in inbred B6 mice and other inbred strains. In contrast to inbred mice, humans are also variably protected against tuberculosis by BCG; in addition to factors such as the strain of BCG used or other environmental influences, the range of outcomes might also reflect genetic diversity. Thus, DO mice may represent a novel small-animal model of vaccination against tuberculosis that better reflects the spectrum of outcomes in people.
Improved animal models that recapitulate human tuberculosis infection and immunity are of interest to screen and compare new drugs, therapeutics, and vaccines, as well as to develop correlates that predict disease progression and vaccine efficacy. Both the present studies (Fig. 3) and previous studies (19) identified a DO population that succumbs rapidly to primary aerosol M. tuberculosis infection. Mice in this group may serve to represent susceptible humans who develop active tuberculosis within 1 to 2 years of exposure and/or the rare mice who have immunodeficiencies similar to syndromes representing Mendelian susceptibility to mycobacterial diseases (MSMD) (33). At the other extreme, some people appear to be inherently refractory to tuberculosis infection, often termed “resisters” (34), but no animals with this phenotype have been identified to date. Although we have not yet found an analogous group of DO mice, studies performed to date have used aerosol exposure to ∼20 CFU or more; future studies can evaluate lower doses, including an “ultralow dose” strategy (35, 36), to approach this issue.
In contrast to the results of primary M. tuberculosis infection, BCG-vaccinated DO mice readily controlled infection with this live attenuated vaccine (Fig. 2), similarly to healthy humans. Also of note, control of BCG vaccination declines in severely immunocompromised scid mice after about 4 to 5 weeks (37, 38), but all of our BCG-vaccinated male and female DO mice survived without visible symptoms until aerosol challenge with M. tuberculosis at 8 weeks after vaccination (data not shown). The survival of the BCG-vaccinated DO mice further indicates a lack of gross susceptibility to BCG infection. Similarly to inbred C57BL/6J mice (39) and humans (40), BCG may never be completely cleared from DO mice. The outbred and individual natures of DO mice obviate direct studies of relationships between residual BCG burden or clearance and protection against M. tuberculosis challenge, however.
Importantly, the wide range of outcomes seen in the BCG-vaccinated DO population challenged with M. tuberculosis (Fig. 3 to 6) also appears to reflect the range seen in humans. The levels of BCG efficacy are variable across different human populations (25), and recent human clinical trials of new tuberculosis vaccines (41–43) and BCG revaccination (44) showed efficacies ranging from none to ∼50%. The recent trial results provide fresh opportunities to further refine animal models to better align with outcomes in humans. Thus, future studies will seek to test tuberculosis vaccines currently in clinical trials in DO mice.
At the individual DO mouse level, this raises the important issue of how best to determine whether or not an individual is (or will be) “protected” from tuberculosis by BCG (or other) vaccination. In humans, the diagnostic criteria for tuberculosis rely on a constellation of signs such as cough, fever, weight loss, lung damage as detected by chest X-ray, the presence of M. tuberculosis bacteria in sputum samples, and M. tuberculosis-specific immunological responses as detected by a positive purified protein derivative (PPD) skin test or a positive interferon gamma release assay (IGRA) result (45). Not all clinical disease criteria can be applied directly to animals, in which typical measurements of tuberculosis disease usually include bacterial burdens in lungs, lung pathology, systemic bacterial burdens (spleens, lymph nodes, and livers), weight loss, and survival. Here, BCG-vaccinated and M. tuberculosis-challenged B6 mice had a relatively narrow range of bacterial burdens in both lungs and spleens at 14 weeks after challenge (Fig. 4). In contrast, the range of bacterial burdens and lung inflammation levels was much greater in BCG-vaccinated DO mice (Fig. 4 and 5). Further, lung bacterial burdens and lung inflammation in DO mice were correlated only modestly (Fig. 7C), and we readily found examples in which bacterial numbers and levels of lung inflammation in individual mice were not related. Similarly, symptoms such as weight loss did not readily correlate with otherwise-visible disease or predict mortality. Future work will therefore continue to evaluate quantitative measures for their independence or interdependence (Fig. 7) and use that information to explore a composite disease score that quantifies overall disease in mice and better approximates disease status in humans. Such tools would potentially help bridge animal and human studies and accelerate clinical trials, which are lengthy, logistically challenging, and expensive.
As noted above, previous studies by Beamer and colleagues (19) demonstrated populations of naive DO mice with different susceptibilities to primary aerosol M. tuberculosis infection, using a target infection dose of ∼100 CFU and a nose-only inhalation system. The results shown here for naive DO mice (Fig. 1; as comparators for BCG-vaccinated mice, Fig. 3 to 6) replicated earlier findings using an independent bacterial stock and a whole-body aerosol exposure system in a different facility, thus demonstrating reproducibility with this complex small-mammal population. Most importantly, we found that most (although not all) members of this supersusceptible population were protected by BCG vaccination in terms of extension of survival time (Fig. 3) and reduced systemic dissemination of M. tuberculosis (Fig. 4). In particular, M. tuberculosis was not detected in spleens of some mice, and we found a shift in the lung/spleen CFU ratio toward lung-centered disease in BCG-vaccinated DO mice (Fig. 4C). Alteration of M. tuberculosis dissemination is reminiscent of BCG’s value in protecting newborns against miliary tuberculosis (46), a phenotype not observed in inbred B6 mice and potentially supporting the idea of the relevance of DO mice to human vaccination studies.
Use of DO mice for tuberculosis vaccination studies is also supported by earlier studies that characterized the eight inbred strains that served as the founders for the DO population in terms of both primary M. tuberculosis respiratory infection and BCG vaccination (47). Those results, which also included two CC lines, documented a wide range of outcomes across the founder strain panel. Some strains, such as WSB, were protected by BCG vaccination as reflected by reduced lung and spleen bacterial burdens, whereas some (e.g., strain 129) were protected only against disseminated disease and others (e.g., strain PWK) were not protected at all. The outcomes were clearly heritable, indicating genetic control. Moreover, some strains that were well protected by BCG vaccination were highly susceptibility to primary M. tuberculosis infection, suggesting that different genes may contribute differentially to susceptibility or successful vaccination. Similarly to results shown here (Fig. 7), many of the traits measured in founder strains and CC mice were found not to readily correlate, but combinations of traits were associated with protection by BCG, further emphasizing the potential value of combining multiple metrics to describe and quantitate disease status.
Genetic mapping of quantitative traits represents a major advantage of DO and CC mouse populations (17), and mapping studies are likely to be informative since mouse DNA and human DNA share an estimated 90% to 95% homology and regulatory strategies (48). Further, much biological evidence supports the use of mice for evaluating genetic control of tuberculosis (11). Examples include similarities between mice and people in IFN-γ pathways that are important in human MSMD syndrome immunodeficiencies (33) and in the roles of type 1 interferons and neutrophils in disease progression (11, 49). Studies using DO mice have already advanced the discovery of genes involved in autoimmune silicosis (50), in a wide range of immunological features (51), and in viral infections (52). To date, this resource has not been much used in bacterial infections. The results reported here indicate that vaccine studies in outbred DO mice, complemented with studies using inbred but diverse CC mice, may represent an important new approach for discovering genes controlling vaccination against tuberculosis.
These results also reflect notable limitations. We chose to monitor mice for 14 weeks after M. tuberculosis challenge and then euthanize survivors for analyses. This approach was chosen not only due to our interest in monitoring survival for as long as possible but also in anticipation of future genetic studies. Further, control of M. tuberculosis disease that is induced by BCG vaccination typically begins to wane in B6 mice by this time point; as expected, this was indeed the case for B6 mice (Fig. 4A and B). This experimental design yielded important information about extension of survival conferred by vaccination and effects on long-term disease. However, we have not yet characterized the strength of BCG protection in DO mice at earlier time points. Future studies will determine whether the lack of significant reduction in lung bacterial burdens (Fig. 4A) and lung inflammation (Fig. 5A) in vaccinated DO mice at this late time point is due to waning responses, heterogeneity in responses, inherently poor responses, or some combination. Another important limitation is that, unlike the approach taken in studies performed with humans, we administered BCG to adult mice rather than to newborns. Nonetheless, it is tempting to speculate that the overall picture representing DO mice reflects the well-known decline over time of BCG-mediated protection against respiratory tuberculosis in humans, particularly in adolescents and adults (25).
The vaccination studies reported here largely used female DO mice, due to issues with the serious aggression of male DO mice, which necessitated individual housing. Other approaches will be needed to better study sex-related differences in responses to vaccination. Future studies may also include analyses of cytokine levels in tissues; previous studies detected cytokines associated with outcomes of primary M. tuberculosis infection in DO mice (19) and in founder strain inbred mice (26). Here, the late time point used for final tissue collection meant that it was unlikely that informative results would be found at the cytokine protein (or gene) level; thus, we have opted to store tissues for other future purposes, pending complete genotype and gene mapping results.
A notable strength of the outbred DO mouse population is also a weakness; similarly to humans, each DO mouse is a distinct individual that is unique, obviating the possibility of testing outcomes longitudinally or reproducing observations in individuals. This issue may be mitigated by the use of parallel studies performed with inbred CC mice (26). Further, we are optimistic that this inherent limitation will be balanced by the opportunities to better understand the genetic basis of vaccination responses and to leverage that information to improving tuberculosis vaccines. Thus, these results set the stage for such gene discovery efforts, which are in progress. Collectively, the results to date indicate that the heterogeneity of outcomes seen in DO mice after vaccination and M. tuberculosis challenge can productively be exploited to study infection and vaccination against tuberculosis in an outbred small-animal model that is relevant to human tuberculosis.
MATERIALS AND METHODS
Mice.
Male and female C57BL/6J and Diversity Outbred (DO) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were used when 6 to 10 weeks of age. Initial experiments were performed with male mice, but because some male DO mice were quite aggressive and had to be individually housed, subsequent experiments were performed with female mice. Within each experiment, all animals were age and sex matched. All mice were housed in microisolator cages and were given autoclaved food and water ad libitum. Studies were performed under protocols approved by the Animal Care and Use Committee (ACUC) of CBER/FDA. Animal protocols stressed practices and procedures designed to strictly minimize any suffering.
Bacteria and growth conditions.
Mycobacterium bovis BCG Pasteur (BCG) and M. tuberculosis Erdman were derived from the mycobacterial culture collection of the Trudeau Institute and propagated once to prepare the working seed stocks that were then used to prepare all infection stocks, without further passage. M. tuberculosis Erdman and BCG Pasteur were grown in 7H9 media supplemented with oleic acid-albumin-dextrose-catalase (OADC), glycerol, and 0.05% Tween 80 (Difco Laboratories, Detroit, MI) to mid-logarithmic phase as previously described, and then vials for single-infection use were frozen in 0.5-ml aliquots at –80°C until use (53). A sample from each batch of bacterial stock was subjected to quality control experiments to determine the number of CFU, to confirm typical colony morphologies, and to confirm vaccination efficacy or infection in mice.
Primary M. tuberculosis challenge and survival.
Groups of 5 to 10 B6 mice or 30 to 160 male DO mice were challenged by aerosol with M. tuberculosis and monitored for survival. For each experiment, a frozen vial of M. tuberculosis was thawed and diluted in PBS to the desired concentration and the suspension was added to the nebulizer. Mice were challenged over a 30-min exposure in a Middlebrook chamber (GlasCol, Terre Haute, IN). For initial studies in male mice, we targeted administration of a dose of 150 CFU for primary aerosol M. tuberculosis infection. For subsequent experiments in female mice involving aerosol M. tuberculosis challenge following vaccination, we targeted administration of a dose of 50 CFU. To determine the actual delivered dose, five B6 mice were euthanized 4 to 5 h after challenge, and the entire lung for each animal was homogenized and plated to determine uptake CFU; for these studies, the delivered dose was ∼150 CFU. After infection, mice were monitored and euthanized when clearly unable to reach food and water, following preestablished endpoint criteria.
BCG vaccination and M. tuberculosis challenge.
For each experiment, a single frozen vial of BCG was thawed and diluted to the desired concentration in sterile PBS. Groups of 5 to 10 B6 mice or 30 to 160 female DO mice were vaccinated subcutaneously with 105 CFU of BCG or were subjected to sham vaccination with phosphate-buffered saline (PBS [low endotoxin]) (Lonza, Walkersville, MD). At 8 weeks after vaccination, animals were challenged by aerosol with M. tuberculosis Erdman with either approximately 150 or ∼50 CFU delivered to the lungs as described above. Mice from five independent vaccination experiments similar in design were pooled to generate the data presented here, for a total of 35 naive B6 mice, 60 naive DO mice, 30 BCG-vaccinated B6 mice, and 240 BCG-vaccinated DO mice. The actual delivered dose was determined as described above; for vaccination studies, the delivered dose ranged from approximately 16 to 65 CFU, with an average of ∼45 CFU. In vaccination studies, animals were weighed prior to challenge and weekly after challenge. Mice that began to show signs of illness such as ruffled fur, weight loss, or cachexia were weighed daily. Mice were monitored and euthanized when clearly unable to reach food and water, following preestablished humane endpoint criteria.
Assessment of bacterial organ burdens and tissue pathology.
Bacterial burdens in organs were determined at the indicated time points after infection. Mice were euthanized, and organs were removed aseptically and transferred to sterile homogenizer bags containing 5 ml of sterile PBS per organ. Organs were disrupted using a stomacher (Seward, England), and the homogenates were serially diluted and plated for CFU enumeration on plates containing 10% OADC enrichment (Becton, Dickinson, Sparks, MD) medium, 10 μg/ml ampicillin, 50 μg/ml cycloheximide, and 2 μg/ml 2-thiophenecarboxylic acid hydrazide (TCH) (Sigma). Addition of TCH to agar plates inhibits BCG growth but has no effect on M. tuberculosis growth (54). Organ homogenates were also frozen and stored at –80°C to be used for later analyses. In some experiments, portions of each lung and spleen were removed and preserved in 10% formalin. Formalin-fixed samples were then sent to American Histolabs, Inc. (Gaithersburg, MD), where the tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Lungs and spleens were examined to characterize and score lung and spleen lesions for granulomas, necrosis, and cell types by a board-certified veterinary pathologist (G. L. Beamer) without knowledge of the groups. Image-Pro Plus software (Media Cybernetics, Rockville, MD) was utilized to assess the level of inflammation present in the densitometry scans of each H&E-stained image. Lung inflammation was quantitated by defining areas with dark pink and purple staining as inflamed (28). The percentage of dark pink and purple areas (compared to light pink and open areas) from a lung section of each mouse was determined by the software and reported as percent lung inflammation per sample.
Statistical analyses.
The statistical significance of differences within parameters was assessed using Student's t test, the Mann-Whitney sum rank test, Spearman correlation, or other tests as described in the text (GraphPad Prism, San Diego, CA).
ACKNOWLEDGMENTS
We are most grateful to our colleagues Chelsea Lehman and Hamda Khan for outstanding technical assistance and to Steven Derrick and Lara Mittereder, who provided thoughtful and comprehensive reviews of the manuscript.
REFERENCES
- 1.Salgame P, Geadas C, Collins L, Jones-López E, Ellner JJ. 2015. Latent tuberculosis infection–revisiting and revising concepts. Tuberculosis (Edinb) 95:373–384. doi: 10.1016/j.tube.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Orlova M, Schurr E. 2017. Human genomics of Mycobacterium tuberculosis infection and disease. Curr Genet Med Rep 5:125–131. doi: 10.1007/s40142-017-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein CM, Guwatudde D, Nakakeeto M, Peters P, Elston RC, Tiwari HK, Mugerwa R, Whalen CC. 2003. Heritability analysis of cytokines as intermediate phenotypes of tuberculosis. J Infect Dis 187:1679–1685. doi: 10.1086/375249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobat A, Gallant CJ, Simkin L, Black GF, Stanley K, Hughes J, Doherty TM, Hanekom WA, Eley B, Beyers N, Jais JP, van Helden P, Abel L, Hoal EG, Alcais A, Schurr E. 2010. High heritability of antimycobacterial immunity in an area of hyperendemicity for tuberculosis disease. J Infect Dis 201:15–19. doi: 10.1086/648611. [DOI] [PubMed] [Google Scholar]
- 5.Caporali R, Crepaldi G, Codullo V, Benaglio F, Monti S, Todoerti M, Montecucco C. 2018. 20 years of experience with tumour necrosis factor inhibitors: what have we learned? Rheumatology (Oxford) 57:vii5–vii10. doi: 10.1093/rheumatology/key059. [DOI] [PubMed] [Google Scholar]
- 6.Du Bruyn E, Peton N, Esmail H, Howlett PJ, Coussens AK, Wilkinson RJ. 2018. Recent progress in understanding immune activation in the pathogenesis in HIV-tuberculosis co-infection. Curr Opin HIV AIDS 13:455–461. doi: 10.1097/COH.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J, Triebold KJ, Dalton D, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 9.Orme IM. 1987. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol 138:293–298. [PubMed] [Google Scholar]
- 10.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med 193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orme IM, Ordway DJ. 2016. Mouse and guinea pig models of tuberculosis. Microbiol Spectr 4(4). doi: 10.1128/microbiolspec.TBTB2-0002-2015. [DOI] [PubMed] [Google Scholar]
- 12.Kramnik I, Demant P, Bloom BB. 1998. Susceptibility to tuberculosis as a complex genetic trait: analysis using recombinant congenic strains of mice. Novartis Found Symp 217:120–137. doi: 10.1002/0470846526.ch9. [DOI] [PubMed] [Google Scholar]
- 13.Pichugin AV, Yan BS, Sloutsky A, Kobzik L, Kramnik I. 2009. Dominant role of the sst1 locus in pathogenesis of necrotizing lung granulomas during chronic tuberculosis infection and reactivation in genetically resistant hosts. Am J Pathol 174:2190–2201. doi: 10.2353/ajpath.2009.081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature 434:767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chia R, Achilli F, Festing MF, Fisher EM. 2005. The origins and uses of mouse outbred stocks. Nat Genet 37:1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 16.McCune RM Jr, Tompsett R. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med 104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. 2012. High-resolution genetic mapping using the Mouse Diversity Outbred population. Genetics 190:437–447. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DE, Astle CM, Niazi MKK, Major S, Beamer GL. 2014. Genetically diverse mice are novel and valuable models of age-associated susceptibility to Mycobacterium tuberculosis. Immun Ageing 11:24. doi: 10.1186/s12979-014-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niazi MK, Dhulekar N, Schmidt D, Major S, Cooper R, Abeijon C, Gatti DM, Kramnik I, Yener B, Gurcan M, Beamer G. 2015. Lung necrosis and neutrophils reflect common pathways of susceptibility to Mycobacterium tuberculosis in genetically diverse, immune-competent mice. Dis Model Mech 8:1141–1153. doi: 10.1242/dmm.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna KC, Fallert Junecko BA, Reinhart TA, Kolls J, Báez-Saldaña R, Cruz-Lagunas A, Rodríguez-Reyna TS, Kumar NP, Tessier P, Roth J, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Cumming B, Kasprowicz VO, Steyn AJC, Babu S, Kaushal D, Zúñiga J, Vogl T, Rangel-Moreno J, Khader SA. 2013. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med 188:1137–1146. doi: 10.1164/rccm.201304-0803OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan AP, Fu C-P, Kao C-Y, Welsh CE, Didion JP, Yadgary L, Hyacinth L, Ferris MT, Bell TA, Miller DR, Giusti-Rodriguez P, Nonneman RJ, Cook KD, Whitmire JK, Gralinski LE, Keller M, Attie AD, Churchill GA, Petkov P, Sullivan PF, Brennan JR, McMillan L, Pardo-Manuel de Villena F. 2015. The mouse universal genotyping array: from substrains to subspecies. G3 (Bethesda) 6:263–279. doi: 10.1534/g3.115.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collaborative Cross Consortium. 2012. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics 190:389–401. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 24.Dockrell HM, Smith SG. 2017. What have we learnt about BCG vaccination in the last 20 years? Front Immunol 8:1134. doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zumla A, Raviglione M, Hafner R, von Reyn CF. 2013. Tuberculosis. N Engl J Med 368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 26.Smith CM, Proulx MK, Olive AJ, Laddy D, Mishra BB, Moss C, Gutierrez NM, Bellerose MM, Barreira-Silva P, Phuah JY, Baker RE, Behar SM, Kornfeld H, Evans TG, Beamer G, Sassetti CM. 2016. Tuberculosis susceptibility and vaccine protection are independently controlled by host genotype. mBio 7:e01516-16. doi: 10.1128/mBio.01516-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyser A, Troudt JM, Taylor JL, Izzo AA. 2011. BCG sub-strains induce variable protection against virulent pulmonary Mycobacterium tuberculosis infection, with the capacity to drive Th2 immunity. Vaccine 29:9308–9315. doi: 10.1016/j.vaccine.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derrick SC, Perera LP, Dheenadhayalan V, Yang A, Kolibab K, Morris SL. 2008. The safety of post-exposure vaccination of mice infected with Mycobacterium tuberculosis. Vaccine 26:6092–6098. doi: 10.1016/j.vaccine.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Kurtz SL, Elkins KL. 2015. Correlates of vaccine-induced protection against Mycobacterium tuberculosis revealed in comparative analyses of lymphocyte populations. Clin Vaccine Immunol 22:1096–1108. doi: 10.1128/CVI.00301-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Pascalis R, Mittereder L, Kennett NJ, Elkins KL. 2016. Activities of murine peripheral blood lymphocytes provide immune correlates that predict Francisella vaccine efficacy. Infect Immun 84:1054–1061. doi: 10.1128/IAI.01348-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Pascalis R, Chou AY, Bosio CM, Huang CY, Follmann DA, Elkins KL. 2012. Development of functional and molecular correlates of vaccine-induced protection for a model intracellular pathogen, F. tularensis LVS. PLoS Pathog 8:e1002494. doi: 10.1371/journal.ppat.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Pascalis R, Chou AY, Ryden P, Kennett NJ, Sjostedt A, Elkins KL. 2014. Models derived from in vitro analyses of spleen, liver, and lung leukocyte functions predict vaccine efficacy against the Francisella tularensis Live Vaccine Strain (LVS). mBio 5:e00936-13. doi: 10.1128/mBio.00936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosain J, Kong XF, Martinez-Barricarte R, Oleaga-Quintas C, Ramirez-Alejo N, Markle J, Okada S, Boisson-Dupuis S, Casanova JL, Bustamante J. 2019. Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol 97:360–367. doi: 10.1111/imcb.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons JD, Stein CM, Seshadri C, Campo M, Alter G, Fortune S, Schurr E, Wallis RS, Churchyard G, Mayanja-Kizza H, Boom WH, Hawn TR. 2018. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol 18:575–589. doi: 10.1038/s41577-018-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saini D, Hopkins GW, Seay SA, Chen CJ, Perley CC, Click EM, Frothingham R. 2012. Ultra-low dose of Mycobacterium tuberculosis aerosol creates partial infection in mice. Tuberculosis (Edinb) 92:160–165. doi: 10.1016/j.tube.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, Kocken CHM, Ottenhoff THM, Kondova I, Khayum MA, Haanstra KG, Vierboom MPM, Verreck F. 2019. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 25:255–262. doi: 10.1038/s41591-018-0319-9. [DOI] [PubMed] [Google Scholar]
- 37.Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, Soto CY, Clark SO, Hatch GJ, Aguilar D, Ausina V, Gicquel B. 2006. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine 24:3408–3419. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Tullius MV, Harth G, Maslesa-Galic S, Dillon BJ, Horwitz MA. 2008. A replication-limited recombinant Mycobacterium bovis BCG vaccine against tuberculosis designed for human immunodeficiency virus-positive persons is safer and more efficacious than BCG. Infect Immun 76:5200–5214. doi: 10.1128/IAI.00434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derrick SC, Yang A, Parra M, Kolibab K, Morris SL. 2015. Effect of cationic liposomes on BCG trafficking and vaccine-induced immune responses following a subcutaneous immunization in mice. Vaccine 33:126–132. doi: 10.1016/j.vaccine.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Talbot EA, Perkins MD, Silva SF, Frothingham R. 1997. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin Infect Dis 24:1139–1146. doi: 10.1086/513642. [DOI] [PubMed] [Google Scholar]
- 41.Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, Ayles HM, Henostroza G, Thienemann F, Scriba TJ, Diacon A, Blatner GL, Demoitie MA, Tameris M, Malahleha M, Innes JC, Hellstrom E, Martinson N, Singh T, Akite EJ, Khatoon Azam A, Bollaerts A, Ginsberg AM, Evans TG, Gillard P, Tait DR. 2018. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 379:1621–1634. doi: 10.1056/NEJMoa1803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, Scriba TJ, Akite EJ, Ayles HM, Bollaerts A, Demoitie MA, Diacon A, Evans TG, Gillard P, Hellstrom E, Innes JC, Lempicki M, Malahleha M, Martinson N, Mesia Vela D, Muyoyeta M, Nduba V, Pascal TG, Tameris M, Thienemann F, Wilkinson RJ, Roman F. 2019. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 381:2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 43.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H; MVA85A 020 Trial Study Team. 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, Mabwe S, Makhethe L, Erasmus M, Toefy A, Mulenga H, Hanekom WA, Self SG, Bekker LG, Ryall R, Gurunathan S, DiazGranados CA, Andersen P, Kromann I, Evans T, Ellis RD, Landry B, Hokey DA, Hopkins R, Ginsberg AM, Scriba TJ, Hatherill M; C-040-404 Study Team. 2018. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 46.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702. doi: 10.1001/jama.1994.03510330076038. [DOI] [PubMed] [Google Scholar]
- 47.Simonyan V, Chumakov K, Dingerdissen H, Faison W, Goldweber S, Golikov A, Gulzar N, Karagiannis K, Vinh Nguyen Lam P, Maudru T, Muravitskaja O, Osipova E, Pan Y, Pschenichnov A, Rostovtsev A, Santana-Quintero L, Smith K, Thompson EE, Tkachenko V, Torcivia-Rodriguez J, Voskanian A, Wan Q, Wang J, Wu TJ, Wilson C, Mazumder R. 2016. 2016. High-performance integrated virtual environment (HIVE): a robust infrastructure for next-generation sequence data analysis. Database (Oxford) 2016:baw022. doi: 10.1093/database/baw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See L-H, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu Y-C, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, et al. 2014. A comparative encyclopedia of DNA elements in the mouse genome. Nature 515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayeux JM, Escalante GM, Christy JM, Pawar RD, Kono DH, Pollard KM. 2018. Silicosis and silica-induced autoimmunity in the Diversity Outbred mouse. Front Immunol 9:874. doi: 10.3389/fimmu.2018.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillippi J, Xie Y, Miller DR, Bell TA, Zhang Z, Lenarcic AB, Aylor DL, Krovi SH, Threadgill DW, de Villena FP, Wang W, Valdar W, Frelinger JA. 2014. Using the emerging Collaborative Cross to probe the immune system. Genes Immun 15:38–46. doi: 10.1038/gene.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leist SR, Baric RS. 2018. Giving the genes a shuffle: using natural variation to understand host genetic contributions to viral infections. Trends Genet 34:777–789. doi: 10.1016/j.tig.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsen MH, Biermann K, Jacobs WR Jr.. 2007. Laboratory maintenance of Mycobacterium tuberculosis. Curr Protoc Microbiol Chapter 10:Unit 10A.1. [DOI] [PubMed] [Google Scholar]
- 54.Collins T, Levett PN. 1989. Radiometric studies on the use of selective inhibitors in the identification of Mycobacterium spp. J Med Microbiol 30:175–181. doi: 10.1099/00222615-30-3-175. [DOI] [PubMed] [Google Scholar]