Key Points
Question
Is pancreaticojejunostomy or pancreaticogastrostomy the best reconstruction method for patients at high risk for pancreatic fistula after pancreaticoduodenectomy?
Findings
In this randomized clinical trial of 72 patients at high risk for pancreatic fistula that combined the use of an externalized stent and prophylactic octreotide omission, no significant difference in the rate of pancreatic fistula was found between patients who underwent a pancreaticojejunostomy (38.9%) and those who underwent a pancreaticogastrostomy (50.0%). Pancreaticojejunostomy was associated with less severe morbidity and a reduced clinical burden in cases of pancreatic fistula.
Meaning
For patients at high risk for pancreatic fistula, pancreaticojejunostomy with the use of an externalized stent and prophylactic octreotide omission should be considered the most appropriate technical strategy.
Abstract
Importance
The operative scenarios with the highest postoperative pancreatic fistula (POPF) risk represent situations in which fistula prevention and mitigation strategies have the strongest potential to affect surgical outcomes after pancreaticoduodenectomy. Evidence from studies providing risk stratification is lacking.
Objective
To investigate whether pancreaticojejunostomy (PJ) or pancreaticogastrostomy (PG), both with externalized transanastomotic stent, is the best reconstruction method for patients at high risk of POPF after pancreaticoduodenectomy.
Design, Setting, and Participants
A single-center, phase 3, randomized clinical trial was conducted at the Department of General and Pancreatic Surgery, The Pancreas Institute, University of Verona Hospital Trust, Verona, Italy, from July 12, 2017, through March 15, 2019, among adults undergoing elective pancreaticoduodenectomy and considered at high risk for pancreatic fistula after intraoperative assessment of the fistula risk score, some of whom were randomized to undergo PG or PJ. All analyses were performed on an intention-to-treat basis.
Interventions
Intervention consisted of PJ or PG, both with externalized transanastomotic stent and octreotide omission.
Main Outcomes and Measures
The primary end point was POPF. The secondary end points were Clavien-Dindo grade 3 or higher morbidity, postpancreatectomy hemorrhage, delayed gastric emptying, and average complication burden.
Results
A total of 604 patients were screened for eligibility; 82 were at high risk for POPF (fistula risk score, 7-10), and 72 were randomized undergo PG (n = 36; 20 men and 16 women; median age, 65 years [interquartile range, 23-82]) or PJ (n = 36; 26 men and 10 women; median age, 63 years [interquartile range, 35-79]). There was no significant difference in the incidence of POPF between patients who underwent PG and patients who underwent PJ (18 [50.0%] vs 14 [38.9%]; P = .48), but for patients who developed a POPF, the mean (SD) average complication burden was lower for those who underwent PJ than for those who underwent PG (0.25 [0.13] vs 0.39 [0.17]; P = .04). The rates of postpancreatectomy hemorrhage (14 [38.9%] in the PG group vs 9 [25.0%] in the PJ group; P = .31) and delayed gastric emptying (16 [44.4%] in the PG group vs 18 [50.0%] in the PJ group; P = .81) were similar, but patients who underwent PG presented with a significantly higher incidence of Clavien-Dindo grade 3 or higher morbidity than those who underwent PJ (17 [47.2%] vs 8 [22.2%]; P = .047).
Conclusions and Relevance
Among patients at the highest risk for POPF, those who underwent PG or PJ experienced similar rates of POPF. However, PG was associated with an increased incidence of Clavien-Dindo grade 3 or higher morbidity and with an increased average complication burden for the patients who developed a POPF. For patients at high risk for pancreatic fistula, PJ with the use of externalized stent and octreotide omission should be considered the most appropriate technical strategy.
Trial Registration
ClinicalTrials.gov Identifier: NCT03212196
This randomized clinical trial investigates whether pancreaticojejunostomy or pancreaticogastrostomy, both with externalized transanastomotic stent, is the best reconstruction method for patients at high risk of postoperative pancreatic fistula after pancreaticoduodenectomy.
Introduction
Pancreatojejunostomy (PJ) and pancreaticogastrostomy (PG) are the 2 primary types of pancreatic remnant reconstruction after pancreatoduodenectomy (PD), but which method is superior in the prevention of postoperative pancreatic fistula (POPF) is still unclear. Although randomized clinical trials comparing the 2 techniques yielded conflicting results, pooled data slightly favor PG.1 However, the current evidence is inherently prone to bias owing to marked heterogeneity with respect to the surgical technique adopted and the individual patient’s fistula risk.
In recent years, procedure-specific composite metrics for risk stratification that feature an intraoperative assessment and that reveal associated clinical and economic significance have been developed and externally validated. The fistula risk score (FRS),2 which is based on the aggregate weight of endogenous and operative risk factors, defines discrete risk zones with escalating fistula rates. The operative scenarios with the highest fistula risk represent situations in which fistula prevention and mitigation strategies have the strongest potential to affect surgical outcomes. A large multicenter study3 explored the utility of such strategies for patients most vulnerable to the development of POPF and showed that risk mitigation was optimized by the combination of externalized stents and the omission of prophylactic octreotide, with the caveat that PJ reconstruction was used for all these patients.
To reappraise the effect of pancreatic stump management on patients at high risk of POPF, under the premise that an FRS-based risk adjustment facilitates an unbiased comparison, we designed a randomized clinical trial of PJ vs PG for patients with the highest risk profile (FRS, 7-10 [range, 0-10; 0 indicates no risk and 10 indicates highest possible risk]). The primary end point was the incidence of POPF. Because the current data support the use of externalized transanastomotic stents and the omission of prophylactic octreotide for patients at high risk of POPF, these 2 adjuncts were incorporated in the present trial design.
Methods
Study Design and Participants
This was a single-center, phase 3, randomized clinical trial conducted from July 12, 2017, through March 15, 2019 at the Unit of General and Pancreatic Surgery–The Pancreas Institute, University of Verona Hospital Trust, Verona, Italy. The study protocol (Supplement 1) was approved by the Ethics Committee of the provinces of Verona and Rovigo and registered at ClinicalTrial.gov (NCT03212196). We are responsible for the design and analysis of the study, the integrity and completeness of the data, the contents of this article, and the fidelity of this article to the trial protocol. The trial was performed in accordance with the good clinical practice guidelines, the principles of the Declaration of Helsinki,4 and the Consolidated Standards of Reporting Trials (CONSORT) guidelines. All eligible patients provided written informed consent at the time of hospital admission.
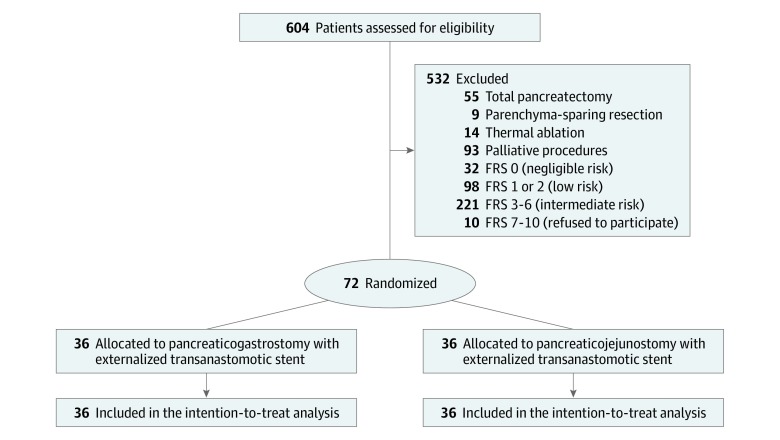
Seven specialized pancreatic surgeons (G. Marchegiani, G. Malleo, S.P., A.E., L.L., L.C., and M.T.) who completed the learning curve for PD and had a personal annual caseload exceeding 60 major pancreatic resections performed all the procedures. All surgeons were familiar with both of the anastomotic techniques used in this trial. Patients between the ages of 18 and 80 years with any indication for elective PD were eligible for inclusion. The CONSORT flowchart is reported in Figure 1.
Figure 1. CONSORT Study Flowchart.
FRS indicates fistula risk score.
Randomization and Masking
The FRS was assigned at the end of the resection phase based on the presence of 4 significant risk factors: presumptive pathologic characteristics, pancreatic texture, pancreatic duct size, and estimated blood loss. eTable 1 in Supplement 2 shows the quantitative calculation of the FRS. Gland texture was assessed by the first surgeon at the time of retropancreatic tunnel development, and responses were dichotomized into soft vs hard. Duct size was measured in the remnant pancreas from the outer dimensions of the main pancreatic duct using a disposable ruler. Estimated blood loss was calculated as follows: [(Total Weight of Surgical Swabs + Content of the Suction Canister) − Total Amount of Sterile Saline Used for Washing the Surgical Field]; 1 g was assumed to equal 1 mL. Patients at high risk (FRS, 7-10 points, which is considered in the high fistula risk zone) were enrolled in the trial and randomized by telephone in a 1:1 ratio using a computer-generated randomization list kept by independent data managers and concealed to the investigators.
Procedures
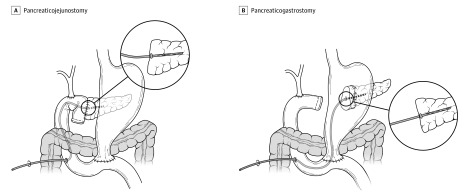
The surgical techniques for both PJ and PG are shown in Figure 2. Pancreatojejunostomy was performed according to the Cattel-Warren duct-to-mucosa technique using polyester-interrupted sutures (3/0 or 4/0) for the outer layer and polypropylene-interrupted sutures (5/0 or 6/0) for the inner layer. Pancreaticogastrostomy was performed according to the Bassi technique.5 After extensive mobilization, the pancreatic remnant was telescoped into the gastric cavity for at least 3 to 4 cm through a posterior gastrotomy and anastomosed via an anterior gastrotomy using polyester-interrupted sutures (3/0 or 4/0). Regardless of the randomization group, a transanastomotic stent consisting of a 5-, 6-, or 7.5-Fr PankreaPlus polyvinyl catheter (Peter Pflugbeil Gmbh Medizinische Instrumente) was placed. The stent was externalized through the pancreatobiliary limb beyond the hepaticojejunostomy employing the Wietzel tunnel technique. The largest stent that could traverse the anastomosis without generating tension was used. The distal end was connected to a sterile collection bag. A feeding jejunostomy was always placed beyond the digestive anastomosis (≥20 cm downstream). Two easy-flow drains were placed in the proximity of the pancreatic and biliary anastomoses. Postoperative management was standardized. All patients received preoperative antibiotic prophylaxis according to the results of preoperative rectal swab sample. No prophylactic octreotide was used. Proton pump inhibitors were routinely administered during the hospital stay. A solid diet was resumed gradually from postoperative day 3 in the absence of clinical concerns for delayed gastric emptying, pancreatic leakage, or other intraabdominal complications. Drain fluid amylase was measured on postoperative day 1, and a previously published protocol was used for drain management.6 Transanastomotic stents were removed in the clinics 4 weeks after the operation or after complete recovery from POPF, as appropriate.
Figure 2. Surgical Technique for Pancreaticojejunostomy and Pancreaticogastrostomy.
Outcomes
The primary end point of the study was the incidence of POPF as defined by the International Study Group of Pancreatic Surgery.7 Secondary end points were POPF severity; time to functional recovery; length of hospital stay; 90-day mortality; postpancreatectomy hemorrhage (PPH)8; delayed gastric emptying9; average complication burden for POPF, delayed gastric emptying, and PPH10; biliary fistula, gastrojejunostomy, duodenojejunostomy leakage, or chyle leak11; abdominal abscess; wound infection12; postoperative acute pancreatitis13,14; myocardial infarction; acute kidney failure; pulmonary embolism; pneumonia; need for mechanical ventilation; urinary tract infection; cerebrovascular events; reoperation; sepsis15; and 90-day readmission. The Clavien-Dindo16 and modified Accordion17 classifications were used to grade postoperative morbidity. Patients were followed up until 90 days after surgery using case-report forms. Discrepancies in reporting outcomes were resolved by consensus between the senior authors (R.S. and C.B.). Definitions of secondary end points are provided in eTable 2 in Supplement 2.
Statistical Analysis
The sample size calculation was based on previously published articles reporting the rate of POPF in high-risk cohorts (FRS, 7-10) after PJ and PG with externalized transanastomotic stents (17.0% in the PJ group18 and 47.0% in the PG group19). With the significance threshold set at .05 and power set at 80%, the sample size calculation suggested a study population of 72 patients (36 in each group). Adjustment for noncompliance or crossover was not made because the randomization occurred immediately before the anastomosis construction. All analyses were performed on an intention-to-treat basis. Continuous variables were expressed as mean and (SD) values or as median values with interquartile ranges and were compared using the independent samples t test or the Mann-Whitney test, as appropriate. Categorical variables were expressed as frequencies with percentages and compared using the χ2 test or the Fisher exact test in the case of small expected frequencies. All tests were 2-tailed. P < .05 was considered statistically significant. Statistical analyses were performed with SPSS software, version 20 for Mac (IBM Corp).
Results
The present trial was conducted from July 12, 2017, through March 15, 2019. A total of 604 patients affected by periampullary disease requiring surgical treatment were screened for eligibility. Of those, 433 underwent PD, and 82 were identified as high risk for POPF. Ten patients intraoperatively identified as high risk for POPF refused to sign the informed consent form the day before surgery and did not enter the trial. The CONSORT flowchart is reported in Figure 1. A total of 72 patients were randomized to undergo either PJ (n = 36) or PG (n = 36).
Baseline characteristics and intraoperative and pathologic details (Table 1) were comparable between groups. There was no significant difference in the incidence of POPF between the 2 groups (18 [50.0%] in PG group vs 14 [38.9% in PJ group]; P = .48; risk ratio, 0.778; 95% CI, 0.761-1.313). For patients who developed POPF, the mean (SD) average complication burden was significantly different when stratified by randomization group (0.39 [0.17] in the PG group vs 0.25 in the PJ group [0.13]; P = .04). Table 2 shows the surgical outcomes related to POPF in the 2 study groups. Transanastomotic stent malfunctioning occurred in 26 patients (36.1%): 18 developed stent dislocation and 8 stent occlusion. There was no difference between patients undergoing PG and patients undergoing PJ in the occurrence of stent malfunctioning (17 [47.2%] vs 9 [25.0%]; P = .09). Among 8 patients developing stent occlusion, there were 4 cases of POPF: 3 after PG and 1 after PJ. eTable 3 and eTable 4 in Supplement 2 show the analysis of surgical outcomes of patients who did not experience stent malfunctioning. eTable 4 in Supplement 2 shows surgical outcomes comparing patients with and patients without stent malfunctioning regardless of the type of anastomosis. Overall, the incidence of POPF was greater among patients with stent malfunctioning than patients without stent malfunctioning (17 of 26 [65.4%] vs 15 of 46 [32.6%]; P = .01).
Table 1. Baseline Characteristics and Surgical and Pathologic Findings.
| Characteristic | Overall (N = 72) | PJ (n = 36) | PG (n = 36) | P Value |
|---|---|---|---|---|
| Age, median (IQR), y | 64 (23-82) | 63 (35-79) | 65 (23-82) | .38 |
| Sex, No. (%) | ||||
| Male | 46 (63.9) | 26 (72.2) | 20 (55.6) | .22 |
| Female | 26 (36.1) | 10 (27.8) | 16 (44.4) | |
| BMI, median (IQR) | 25.1 (17.9-32.3) | 25.3 (17.9-31.3) | 25.3 (21.1-32.3) | .93 |
| Smoker, No. (%) | 18 (25) | 9 (25) | 9 (25) | >.99 |
| Alcohol abuse, No. (%) | 3 (4.2) | 2 (5.6) | 1 (2.8) | >.99 |
| Diabetes, No. (%) | 11 (15.3) | 4 (11.1) | 7 (19.4) | .51 |
| Weight loss, No. (%) | 36 (50) | 19 (52.8) | 17 (47.2) | .81 |
| Ischemic cardiac disease, No. (%) | 6 (8.3) | 2 (5.6) | 4 (11.1) | .67 |
| Peripheral arterial disease, No. (%) | 1 (1.4) | 0 | 1 (2.8) | >.99 |
| Hypertension, No. (%) | 30 (41.7) | 18 (50) | 12 (33.3) | .23 |
| COPD, No. (%) | 1 (1.4) | 0 | 1 (2.8) | >.99 |
| Chronic renal failure, No. (%) | 0 | 0 | 0 | NA |
| Charlson-Age comorbidity index, median (IQR) | 4 (0-9) | 4 (0-8) | 4 (2-9) | .18 |
| Previous laparotomy, No. (%) | 17 (23.6) | 9 (25) | 8 (22.2) | >.99 |
| ASA score, No. (%) | ||||
| 1 | 3 (4.2) | 2 (5.6) | 1 (2.8) | .50 |
| 2 | 51 (70.8) | 27 (75) | 24 (66.7) | |
| 3 | 18 (25) | 7 (19.4) | 11 (30.6) | |
| Preoperative bilirubin, mean (SD), mg/dL | 1.97 (3.5) | 1.91 (3.4) | 2.02 (3.6) | .90 |
| Jaundice, No. (%) | 26 (36.1) | 11 (30.6) | 15 (41.7) | .46 |
| Endoscopic stent, No. (%) | 25 (34.7) | 11 (30.6) | 14 (38.9) | .62 |
| Percutaneous drainage, No. (%) | 2 (2.7) | 1 (2.8) | 1 (2.85) | >.99 |
| Preoperative multidrug-resistant bacterial colonization, No. (%) | 7 (9.7) | 3 (8.3) | 4 (11.1) | >.99 |
| Neoadjuvant treatment, No. (%) | 11 (15.3) | 4 (11.1) | 7 (19.4) | .51 |
| Type of surgery, No. (%) | ||||
| Whipple | 18 (25) | 10 (27.8) | 8 (22.2) | .79 |
| Pylorus-preserving | 54 (75) | 26 (72.2) | 28 (77.8) | |
| Venous resection, No. (%) | 4 (5.6) | 2 (5.6) | 2 (5.6) | >.99 |
| Texture, No. (%) | ||||
| Hard | 0 | 0 | 0 | NA |
| Soft | 72 (100) | 36 (100) | 36 (100) | |
| Main duct diameter, median (range), mm | 2 (1) | 2 (1-4) | 2 (1-3) | .19 |
| Estimated blood loss, mean (SD), mL | 838 (696) | 907 (402) | 766 (322) | .84 |
| Fistula risk score, No. (%) | ||||
| 7 | 36 (50) | 19 (52.8) | 17 (47.2) | .84 |
| 8 | 21 (29.2) | 11 (30.6) | 10 (27.8) | |
| 9 | 12 (16.7) | 5 (13.9) | 7 (19.4) | |
| 10 | 3 (4.2) | 1 (2.8) | 2 (5.6) | |
| Lymphadenectomy, No. (%) | ||||
| Standard | 38 (52.8) | 20 (55.6) | 18 (50) | .81 |
| Extended | 34 (47.2) | 16 (44.4) | 18 (50) | |
| Intraoperative transfusions, No. (%) | 22 (30.6) | 10 (27.8) | 12 (33.3) | .80 |
| Intraoperative fluids, mean (SD), mL | 4355 (1544) | 4480 (1713) | 4225 (1362) | .36 |
| Time of surgery, mean (SD), min | 449 (88) | 441 (89) | 458 (88) | .38 |
| Tumor size, median (IQR), mm | 22 (3-90) | 27 (3-80) | 24 (10-90) | .49 |
| Resection margin, No. (%) | ||||
| R0 | 64 (88.8) | 32 (88.9) | 32 (88.9) | >.99 |
| R1 | 8 (11.1) | 4 (11.1) | 4 (11.1) | |
| Lymph nodes harvested, median (range) | 39 (12-64) | 40 (21-55) | 39 (12-64) | .46 |
| Positive lymph nodes, median (range) | 0 (0-44) | 0 (0-44) | 1 (0-17) | .82 |
| Pathologic diagnosis, No. (%) | ||||
| PDAC | 21 (29.1) | 9 (25.7) | 12 (34.3) | .37 |
| NET | 14 (19.4) | 7 (20) | 7 (20) | |
| Ampullary cancer | 11 (15.2) | 8 (22.9) | 3 (8.6) | |
| Duodenal cancer | 9 (12.5) | 6 (17.1) | 3 (8.6) | |
| Cystic | 4 (5.5) | 2 (5.7) | 2 (5.7) | |
| Cholangiocarcinoma | 9 (12.5) | 2 (5.7) | 7 (20) | |
| Other | 2 (2.7) | 1 (2.9) | 1 (2.9) |
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; IQR, interquartile range; NA, not applicable; NET, neuroendocrine tumor; PDAC, pancreatic ductal adenocarcinoma; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy.
SI conversion factor: To convert bilirubin to micromoles per liter, multiply by 17.104.
Table 2. Primary Outcome of Postoperative Pancreatic Fistula.
| Characteristic | PJ (n = 36) | PG (n = 36) | Risk Ratio (95% CI) | P Value |
|---|---|---|---|---|
| POPF, No. (%) | 14 (38.9) | 18 (50.0) | 0.778 (0.461-1.313) | .48 |
| BL, No. (%) | 5 (13.9) | 2 (5.6) | 2.535 (0.518-12.058) | .43 |
| Grade B, No. (%) | 14 (38.9) | 14 (38.9) | NA | .11 |
| Grade C, No. (%) | 0 | 4 (11.1) | ||
| ACB POPF, mean (SD) | 0.25 (0.13) | 0.39 (0.17) | NA | .04 |
| Drain amylase, median (range) | ||||
| POD 1 | 3299 (266-7500) | 1762 (92-7500) | NA | .31 |
| POD 5 | 148 (5-7500) | 50 (3-7500) | NA | .33 |
| Transanastomotic stent malfunction, No. (%) | 9 (25.0) | 17 (47.2) | 0.632 (0.405-1.985) | .09 |
| Jejunostomy displacement, No. (%) | 0 | 2 (5.6) | NA | .49 |
| Jejunostomy-related morbidity, No. (%) | 0 | 2 (5.6) | NA | .49 |
| Discharged with drain, No. (%) | 3 (8.8) | 7 (20.6) | 0.429 (0.121-1.403) | .31 |
| Drain removal, median PODs (range) | 7 (3-75) | 12 (3-71) | NA | .68 |
| Drain removal in patients with POPF, median PODs (range) | 28 (4-75) | 27 (4-71) | NA | .44 |
| Parenteral nutrition, median (range), d | 1 (0-80) | 7 (0-75) | NA | .18 |
| Parenteral nutrition in patients with POPF, median (range), d | 3 (0-80) | 16 (0-65) | NA | .22 |
| Enteral nutrition, median (range), d | 7 (0-75) | 11 (1-63) | NA | .27 |
| Enteral nutrition in patients with POPF, median (range), d | 11 (0-75) | 15 (1-63) | NA | >.99 |
Abbreviations: ACB, average complication burden; BL, biochemical leak; IQR, interquartile range; NA, not applicable; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy; POD, postoperative day; POPF, postoperative pancreatic fistula.
Table 3 summarizes the secondary end points. Pancreaticogastrostomy was associated with a significantly increased incidence of severe complications compared with PJ (Clavien-Dindo class ≥III: 17 [47.2%] in the PG group vs 8 [22.2%] in the PJ group; P = .047). There was also a different distribution of PPH severity grades (5 [13.9%] with grade A, 5 [13.9%] with grade B, and 4 [11.1%] with grade C in the PG group vs 0 with grade A, 8 [22.2%] with grade B, and 1 [2.8%] with grade C in the PJ group; P = .046). All other secondary end points were comparable between the two groups. Fourteen patients underwent relaparotomy. In the PJ group, 3 patients required relaparotomy for PPH (2 for bowel ischemia and 1 for bowel obstruction due to spigelian hernia). In the PG group, 4 patients required relaparotomy for PPH (3 for severe sepsis and 1 for bowel perforation). Eight patients were readmitted. In the PJ group, 2 patients were readmitted for organ space infection and 1 patient was readmitted for a late, massive intraluminal PPH caused by a superior mesenteric artery pseudoaneurysm. In the PG group, 3 patients were readmitted for organ space infection, 1 patient was readmitted owing to an intraluminal PPH caused by a superior mesenteric artery pseudoaneurysm, and 1 patient was readmitted owing to the rupture of the distal end of the transanastomotic stent, which occurred during its removal and was resolved endoscopically. This was the only stent-related complication.
Table 3. Postoperative Complications.
| Characteristic | No. (%) | Risk Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| PJ (n = 36) | PG (n = 36) | |||
| Clavien-Dindo score ≥III | 8 (22.2) | 17 (47.2) | 0.471 (0.233-0.949) | .047 |
| Clavien-Dindo score | .20 | |||
| I | 3 (8.3) | 4 (11.1) | NA | NA |
| II | 25 (69.4) | 15 (41.7) | ||
| IIIa | 2 (5.6) | 6 (16.7) | ||
| IIIb | 2 (5.6) | 1 (2.8) | ||
| IVa | 1 (2.8) | 4 (11.1) | ||
| IVb | 1 (2.8) | 4 (11.1) | ||
| V | 2 (5.6) | 2 (5.6) | ||
| ACB overall, mean (SD) | 0.33 (0.21) | 0.42 (0.25) | NA | .13 |
| Abscess | 15 (41.7) | 15 (41.7) | 1.000 (0.579-1.727) | >.99 |
| Biliary fistula | 2 (5.6) | 3 (8.3) | 0.667 (0.118-3.755) | >.99 |
| Gastrojejunostomy or duodenojejunostomy fistula | 2 (5.6) | 1 (2.8) | 2.522 (0.190-21.089) | >.99 |
| Chyle leak | 3 (8.3) | 6 (16.7) | 0.501 (0.135-1.847) | .48 |
| Postoperative acute pancreatitis | 18 (50) | 21 (58.3) | 0.857 (0.559-1.315) | .64 |
| PPH | 9 (25) | 14 (38.9) | 0.643 (0.320-1.293) | .31 |
| Grade A | 0 | 5 (13.9) | NA | .046 |
| Grade B | 8 (22.2) | 5 (13.9) | ||
| Grade C | 1 (2.8) | 4 (11.1) | ||
| ACB PPH, mean (SD) | 0.46 (0.25) | 0.43 (0.22) | NA | .77 |
| DGE | 18 (50) | 16 (44.4) | 1.125 (0.689-1.836) | .81 |
| Grade A | 5 (13.9) | 3 (8.3) | NA | .69 |
| Grade B | 11 (30.6) | 9 (25) | ||
| Grade C | 2 (5.6) | 4 (11.1) | ||
| ACB DGE, mean (SD) | 0.27 (0.03) | 0.26 (0.07) | NA | .87 |
| Occlusion | 0 | 1 (2.8) | NA | >.99 |
| Sepsis | 9 (25) | 11 (30.6) | 0.818 (0.386-1.732) | .79 |
| Postoperative multidrug resistant bacterial colonization | 3 (8.3) | 4 (11.1) | 0.751 (0.181-3.115) | >.99 |
| Pleural effusion | 16 (44.4) | 22 (61.1) | 0.727 (0.464-1.139) | .24 |
| Pneumonia | 6 (16.7) | 12 (33.3) | 0.501 (0.211-1.187) | .17 |
| Reintubation | 5 (13.9) | 10 (27.8) | 0.501 (0.190-1.318) | .25 |
| Severe arrhythmias | 2 (5.6) | 6 (16.7) | 0.333 (0.072-1.543) | .26 |
| Myocardial infarction | 0 | 2 (5.6) | NA | .49 |
| Cardiac arrest | 2 (5.6) | 2 (5.6) | 1.000 (0.149-6.718) | >.99 |
| Myocardial disfunction requiring inotropes | 2 (5.6) | 8 (22.2) | 0.251 (0.057-1.097) | .09 |
| Urinary tract infection | 6 (16.7) | 3 (8.3) | 2.511 (0.541-7.388) | .48 |
| Acute kidney failure | 3 (8.3) | 3 (8.3) | 1.000 (0.216-4.628) | >.99 |
| Stroke | 1 (2.8) | 0 | NA | >.99 |
| Surgical site infection | 10 (27.8) | 13 (36.1) | 0.769 (0.389-1.523) | .61 |
| Transfusion | 16 (44.4) | 22 (61.1) | 0.727 (0.464-1.139) | .24 |
| Relaparotomy | 6 (16.7) | 8 (22.2) | 0.751 (0.289-1.944) | .77 |
| Unplanned ICU admission | 7 (19.4) | 13 (36.1) | 0.538 (0.243-1.192) | .19 |
| Length of ICU admission, median (range), d | 1 (1-40) | 9 (1-59) | NA | .49 |
| Mortality | 2 (5.6) | 2 (5.6) | 1.000 (0.149-6.718) | >.99 |
| 30-d Readmission | 3 (8.8) | 5 (14.7) | 0.601 (0.156-2.315) | .71 |
| Length of hospital stay, median (range), d | 23 (8-80) | 45 (11-94) | NA | .31 |
| Time to functional recovery, median (range), d | 19 (6-80) | 22 (7-88) | NA | .27 |
Abbreviations: ACB, average complication burden; DGE, delayed gastric emptying; ICU, intensive care unit; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy; PPH, postpancreatectomy hemorrhage.
Four patients (5.5%) died within 90 days of the index operation. In the PJ group, 1 patient died from severe sepsis due to carbapenemase-resistant Klebsiella pneumoniae (found at the preoperative rectal swab), and 1 patient died of severe PPH with massive bowel ischemia. In the PG group, 2 patients died: the first death was due to severe sepsis caused by carbapenemase-resistant K pneumoniae after grade C POPF, and the second death was due to massive bowel ischemia determined by severe sepsis associated with vancomycin-resistant Enterococcus faecium.
Discussion
The present randomized clinical trial of PJ vs PG after PD for high-risk patients, where both the risk and the outcomes are defined by the most current international standards, demonstrated no significant difference in POPF rates. The analysis of secondary outcomes revealed a greater overall POPF average complication burden, a greater rate of severe morbidity, and more severe cases of PPH in patients undergoing PG.
The present trial has considered only patients at the highest risk for pancreatic fistula. These patients usually experience an extremely complex postoperative course characterized by a higher incidence of life-threatening morbidity, including POPF, PPH, sepsis, the need for relaparotomy, and increased mortality. In this scenario, fistula prevention and mitigation strategies have the strongest potential to affect surgical outcomes.
Regardless of the randomization group, prophylactic octreotide was omitted, and a transanastomotic stent was used. In a large study on the characterization and management of high-risk pancreatic anastomoses,3 these 2 adjuncts were associated with a significant improvement in POPF rates, which exceeded the benefit of any individual approach or combination of other strategies outside of patient, surgeon, and institutional risk factors. Moreover, at least 2 International Study Group for Pancreatic Fistula20 (ISGPF)–era randomized clinical trials demonstrated that external stents can reduce the rates of POPF in high-risk cases (soft pancreas and/or small pancreatic duct).21,22 However, both this optimal mitigation strategy and the results of external stent trials were contingent on the use of PJ. Whether different outcomes could have been obtained after PG reconstruction remained unclear and constituted the conceptual backbone for the present trial. The only 3 positive studies of the 7 ISGPF-era randomized clinical trials23,24,25,26,27,28,29 comparing PJ vs PG were in favor of the latter technique, although none of these studies applied a risk stratification system. With the absence of risk stratification, the anastomotic technique applied to the group containing fewer patients at high risk for POPF always showed better results.
Only in the trial by Topal et al23 was a subanalysis performed whereby the beneficial effects of PG were even more pronounced for patients with a main pancreatic duct less than 3 mm. Pooled data of these 7 randomized clinical trials23,24,25,26,27,28,29 (1184 participants) confirmed that the incidence of POPF was lower in the PG group than in the PJ group (12.8% vs 19.3%), with an estimated risk ratio of 1.51 for PJ.1 However, 6 of the 7 studies were at high risk of bias in at least 1 domain. A major issue affecting the quality of evidence was the precision of the outcomes. Therefore, the overall results were considered unreliable to support the use of PG over PJ. The only study analyzing PG reconstruction in an FRS-based high-risk cohort, although retrospective, reported a POPF rate as high as 47.0%.19 Another potential issue with PG is the degree of heterogeneity with respect to the surgical technique applied, even in randomized clinical trials (PG with gastric partition, double-layer invaginating PG, and telescoped PG with number of suture layers based on the surgeon’s judgment).
In addition to the risk-stratification process, the omission of prophylactic octreotide, and the use of external stents, surgeons participating in the present trial were familiar with PG and used a standardized technique for both types of pancreatic stump reconstruction; each surgeon individually performed more than 60 pancreatic resections annually. According to an international survey,30 PG was selected as the preferred reconstruction method by less than one-tenth of respondents, and the single-layer, invaginating anastomosis, reappraised by Bassi et al5 in 2006, was the most commonly used PG variant worldwide.
The present trial confirms that the highest fistula risk zone (FRS, 7-10) was associated with an overall POPF rate of 44.4% and a relatively high 90-day mortality rate of 5.5%. Although there was no significant difference in the primary outcome measure between the 2 study groups, the incidence of POPF among patients undergoing PG reconstruction was 50.0%. Furthermore, the average complication burden of the overall POPF, a quantitative measure offering insight into the average morbidity associated with each type of POPF, was greater in the PG group. No grade C POPF was observed after PJ. In keeping with previous literature,23,24,25,26,27,28,29 there was a greater incidence of overall PPH and PPH-related interventional procedures after PG. All these findings translated to a greater incidence of major complications (Clavien-Dindo grade ≥III) in patients who underwent PG.
In addition, the POPF rate was increased 2-fold among patients with transanastomotic stent malfunctioning compared with patients without (65.4% vs 32.6%; eTable 5 in Supplement 2). Although the PankreaPlus stent (Peter Pflugbeil Gmbh Medizinische Instrumente) has been specifically designed with a bulge to ensure fixation in the pancreatic duct, malfunctioning occurred in 36.1% of the overall study population. Stent malfunctioning (displacement or occlusion) was more common in patients who underwent PG than in patients who underwent PJ (47.5% vs 25.0%), although this difference was not statistically significant. In PJ, the pancreas is in apposition with the bowel, and the stent can be further secured to the jejunal limb with a rapidly absorbable stitch at the anastomosis line; in PG, it is easier for the stent to slip out from the free surface of the pancreatic stump into the gastric cavity. Conversely, mechanical damage of the anastomotic site due to stent displacement would theoretically have a greater effect on patients who had undergone PJ. Whatever the mechanism of stent malfunctioning, one would argue whether the major determinant of POPF occurrence and clinical severity in this high-risk cohort is stent malfunctioning itself, the reconstruction method, or a combination of both.
Strengths and Limitations
This study has some strengths. The main strength is the use of an FRS-based risk adjustment, allowing for an unbiased comparison between groups, for the first time, to our knowledge, in a randomized clinical trial of PJ vs PG. Furthermore, the scenarios identified by the high-risk FRS zone, which are associated with elevated rates of POPF, represented situations wherein the method of pancreatic stump reconstruction has the strongest potential to affect postoperative outcomes.
This study also has some limitations that may be associated with the small sample size, which was calculated based on the only studies reporting POPF rates after PJ or PG in high-risk FRS cohorts at the time of study design. Subgroup analyses were also performed; however, their results should be considered with caution because they were outside the original study design.
Conclusions
This randomized clinical trial of PJ vs PG in the context of an optimal fistula mitigation strategy for patients with a high-risk FRS, defined by the use of a transanastomotic externalized stent and the omission of prophylactic octreotide, did not show significant differences in the rate of POPF between the 2 groups. However, among patients who developed a POPF, the average complication burden, severity of PPH, and rate of major complications were greater among patients who had undergone PG, indicating that PJ is probably the best pancreatic stump reconstruction method after PD in high-stakes cases. The trial is still ongoing with respect to long-term end points, including pancreatic remnant volume, exocrine and endocrine insufficiency, and quality of life.
Trial Protocol
eTable 1. Fistula Risk Score Calculation
eTable 2. Definition of Outcomes
eTable 3. Postoperative Pancreatic Fistula (Primary Outcome) in Patients Without Transanastomotic Stent Malfunction
eTable 4. Postoperative Complications in Patients Without Transanastomotic Stent Malfunction
eTable 5. Postoperative Complications Stratified for Transanastomotic Stent Malfunction
Data Sharing Statement
References
- 1.Lyu Y, Li T, Cheng Y, Wang B, Chen L, Zhao S. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: an up-to-date meta-analysis of RCTs applying the ISGPS (2016) criteria. Surg Laparosc Endosc Percutan Tech. 2018;28(3):139-146. doi: 10.1097/SLE.0000000000000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216(1):1-14. doi: 10.1016/j.jamcollsurg.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Ecker BL, McMillan MT, Asbun HJ, et al. Characterization and optimal management of high-risk pancreatic anastomoses during pancreatoduodenectomy. Ann Surg. 2018;267(4):608-616. doi: 10.1097/SLA.0000000000002327 [DOI] [PubMed] [Google Scholar]
- 4.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 5.Bassi C, Butturini G, Salvia R, Crippa S, Falconi M, Pederzoli P. Open pancreaticogastrostomy after pancreaticoduodenectomy: a pilot study. J Gastrointest Surg. 2006;10(7):1072-1080. doi: 10.1016/j.gassur.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Bassi C, Molinari E, Malleo G, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252(2):207-214. doi: 10.1097/SLA.0b013e3181e61e88 [DOI] [PubMed] [Google Scholar]
- 7.Bassi C, Marchegiani G, Dervenis C, et al. ; International Study Group on Pancreatic Surgery (ISGPS) . The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584-591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 8.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20-25. doi: 10.1016/j.surg.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761-768. doi: 10.1016/j.surg.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 10.Marchegiani G, Andrianello S, Nessi C, et al. Neoadjuvant therapy versus upfront resection for pancreatic cancer: the actual spectrum and clinical burden of postoperative complications. Ann Surg Oncol. 2018;25(3):626-637. doi: 10.1245/s10434-017-6281-9 [DOI] [PubMed] [Google Scholar]
- 11.Besselink MG, van Rijssen LB, Bassi C, et al. ; International Study Group on Pancreatic Surgery . Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365-372. doi: 10.1016/j.surg.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606-608. doi: 10.2307/30148464 [DOI] [PubMed] [Google Scholar]
- 13.Bannone E, Andrianello S, Marchegiani G, et al. Postoperative acute pancreatitis following pancreaticoduodenectomy: a determinant of fistula potentially driven by the intraoperative fluid management. Ann Surg. 2018;268(5):815-822. doi: 10.1097/SLA.0000000000002900 [DOI] [PubMed] [Google Scholar]
- 14.Connor S. Defining post-operative pancreatitis as a new pancreatic specific complication following pancreatic resection. HPB (Oxford). 2016;18(8):642-651. doi: 10.1016/j.hpb.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollmer CM Jr, Lewis RS, Hall BL, et al. Establishing a quantitative benchmark for morbidity in pancreatoduodenectomy using ACS-NSQIP, the Accordion Severity Grading System, and the Postoperative Morbidity Index. Ann Surg. 2015;261(3):527-536. doi: 10.1097/SLA.0000000000000843 [DOI] [PubMed] [Google Scholar]
- 18.McMillan MT, Ecker BL, Behrman SW, et al. Externalized stents for pancreatoduodenectomy provide value only in high-risk scenarios. J Gastrointest Surg. 2016;20(12):2052-2062. doi: 10.1007/s11605-016-3289-6 [DOI] [PubMed] [Google Scholar]
- 19.Wang S-E, Chen S-C, Shyr B-U, Shyr Y-M. Comparison of modified Blumgart pancreaticojejunostomy and pancreaticogastrostomy after pancreaticoduodenectomy. HPB (Oxford). 2016;18(3):229-235. doi: 10.1016/j.hpb.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi C, Dervenis C, Butturini G, et al. ; International Study Group on Pancreatic Fistula Definition . Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery. 2005;138(1):8-13. doi: 10.1016/j.surg.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 21.Pessaux P, Sauvanet A, Mariette C, et al. ; Fédération de Recherche en Chirurgie (French) . External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg. 2011;253(5):879-885. doi: 10.1097/SLA.0b013e31821219af [DOI] [PubMed] [Google Scholar]
- 22.Motoi F, Egawa S, Rikiyama T, Katayose Y, Unno M. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg. 2012;99(4):524-531. doi: 10.1002/bjs.8654 [DOI] [PubMed] [Google Scholar]
- 23.Topal B, Fieuws S, Aerts R, et al. ; Belgian Section of Hepatobiliary and Pancreatic Surgery . Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol. 2013;14(7):655-662. doi: 10.1016/S1470-2045(13)70126-8 [DOI] [PubMed] [Google Scholar]
- 24.Figueras J, Sabater L, Planellas P, et al. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013;100(12):1597-1605. doi: 10.1002/bjs.9252 [DOI] [PubMed] [Google Scholar]
- 25.El Nakeeb A, Hamdy E, Sultan AM, et al. Isolated Roux loop pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a prospective randomized study. HPB (Oxford). 2014;16(8):713-722. doi: 10.1111/hpb.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Cruz L, Cosa R, Blanco L, López-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. 2008;248(6):930-938. doi: 10.1097/SLA.0b013e31818fefc7 [DOI] [PubMed] [Google Scholar]
- 27.Grendar J, Ouellet JF, Sutherland FR, Bathe OF, Ball CG, Dixon E. In search of the best reconstructive technique after pancreaticoduodenectomy: pancreaticojejunostomy versus pancreaticogastrostomy. Can J Surg. 2015;58(3):154-159. doi: 10.1503/cjs.010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy versus pancreatojejunostomy for reconstruction after pancreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg. 2016;263(3):440-449. doi: 10.1097/SLA.0000000000001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wellner UF, Sick O, Olschewski M, Adam U, Hopt UT, Keck T. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 2012;16(9):1686-1695. doi: 10.1007/s11605-012-1940-4 [DOI] [PubMed] [Google Scholar]
- 30.McMillan MT, Malleo G, Bassi C, Sprys MH, Vollmer CM Jr. Defining the practice of pancreatoduodenectomy around the world. HPB (Oxford). 2015;17(12):1145-1154. doi: 10.1111/hpb.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Fistula Risk Score Calculation
eTable 2. Definition of Outcomes
eTable 3. Postoperative Pancreatic Fistula (Primary Outcome) in Patients Without Transanastomotic Stent Malfunction
eTable 4. Postoperative Complications in Patients Without Transanastomotic Stent Malfunction
eTable 5. Postoperative Complications Stratified for Transanastomotic Stent Malfunction
Data Sharing Statement