Key Points
Question
Is being wait-listed for heart transplant associated with outcomes similar to those for receiving left ventricular assist device therapy alone among patients with advanced heart failure?
Findings
This cohort study used Interagency Registry for Mechanically Assisted Circulatory Support and United Network for Organ Sharing registry data to compare outcomes after matching for key clinical characteristics of 3411 patients with left ventricular assist device therapy alone and 3411 patients wait-listed for heart transplant. Wait-listing for heart transplant (with or without device therapy bridge) was associated with better 5-year survival rates than device therapy alone, and this was associated primarily with whether patients underwent heart transplant.
Meaning
Wait-listing for heart transplant appears to be associated with better midterm survival rates than receiving device therapy alone among patients with advanced heart failure.
Abstract
Importance
Given the shortage of donor hearts and improvement in outcomes with left ventricular assist device (LVAD) therapy, a relevant but, to date, unanswered question is whether select patients with advanced heart failure should receive LVAD destination therapy as an alternative to heart transplant.
Objective
To determine whether a strategy of LVAD destination therapy is associated with similar survival benefit as wait-listing for heart transplant with or without LVAD therapy among patients with advanced heart failure.
Design, Setting, and Participants
This retrospective propensity-matched cohort analysis used data on heart transplants from the United Network for Organ Sharing registry and LVAD implants from the Interagency Registry for Mechanically Assisted Circulatory Support from January 1, 2010, to December 31, 2014. The matched LVAD destination therapy cohort included 3411 patients. Data analysis for this study was conducted from December 22, 2017, to May 24, 2019.
Main Outcomes and Measures
Survival at 5 years was analyzed using Cox proportional hazards models.
Results
In total, 8281 patients had albumin level, creatinine level, and BMI data recorded and were included in the analysis. Despite propensity score matching, the 3411 patients receiving LVAD destination therapy still tended to be slightly older than the 3411 patients wait-listed for heart transplant (64.0 years [interquartile range, 55.0-70.0 years] vs 60.0 [interquartile range, 54.0-65.0 years]; P < .001), but there was no significant difference in sex (2701 men [79.2%] vs 2648 men [77.6%]; P = .13). After propensity score matching for age, sex, body mass index, renal function, and albumin level, 3411 patients were wait-listed for heart transplant. This included 1607 patients with bridge to transplant LVAD therapy and 1804 patients without LVAD. The strategy of wait-listing for heart transplant was associated with better 5-year survival than LVAD destination therapy (risk ratio, 0.42; 95% CI, 0.38-0.46) after matching and adjusting for key clinical factors. This survival advantage was associated with heart transplant (adjusted risk ratio for time-dependent transplant status, 0.27; 95% CI, 0.24-0.32).
Conclusions and Relevance
The present analysis suggests that heart transplant with or without bridge to transplant LVAD therapy was associated with superior 5-year survival compared with LVAD destination therapy among patients matched on several relevant clinical factors. Continued improvement in LVAD technology, along with prospective comparative research, appears to be needed to amend this strategy.
This national registry-based cohort study assesses whether left ventricular assist device destination therapy is associated with survival benefit comparable to wait-listing for heart transplant with or without left ventricular assist device therapy for bridge to transplant indication among patients with advanced heart failure.
Introduction
Although limited by donor organ availability, adult heart transplant (HT) remains the optimal treatment of patients with advanced heart failure (HF), with 1-year survival of more than 85% and median survival of 14 years.1 With such remarkable success, the profile of patients with advanced HF undergoing consideration for cardiac transplant has evolved such that older patients with more comorbid conditions are now being accepted as candidates. In fact, the waiting list of patients 65 years or older has increased more than that for any other age group.2 Despite satisfactory outcomes reported among these patients,3,4 there is increased risk of significant morbidity and mortality after transplant with advancing age and a greater number of comorbidities.5,6,7
Left ventricular assist device (LVAD) therapy was initially developed as a bridge to transplant (BTT) for patients, but with advances in technology and increasing durability, the long-term survival of patients receiving mechanical circulatory support has improved, extending indications for implantation to destination therapy (DT).8,9,10,11 The current literature suggests that continuous flow LVADs offer at least 1- to 2-year survival, comparable to cardiac transplant.12,13,14 With an ever-expanding HT waiting list,15 improving LVAD technology,16 and increasing complexity of patient comorbidities, a relevant but unanswered question is whether a strategy of proceeding to LVAD therapy for DT indication offers similar survival advantage compared with a strategy of wait-listing for HT with or without BTT LVAD therapy. We explored the answer to this question using data from the United Network for Organ Sharing (UNOS) registry and the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). The UNOS is a government-sponsored registry that includes all patients wait-listed for HT with or without LVAD support at the time of wait-listing; INTERMACS is a previously sponsored National Heart, Lung, and Blood Institute registry for the clinical outcomes of patients who receive US Food and Drug Administration–approved durable mechanical circulatory support devices in North America. A subset of registrants from each database was uniquely linked, permitting outcome analyses for patients with LVAD support who underwent HT.17
Methods
Study Design and Data Sources
A retrospective analysis of patients who underwent LVAD implant or HT or both between 2010 and 2014 was performed using data from the UNOS registry and INTERMACS. The latter registry includes patient characteristics at the time of implant, implant date, type of device, and significant postimplant events. Race and ethnicity are required data fields in both the UNOS registry and INTERMACS and are generally entered at the respective sites by coordinators. The present data analysis was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai. Written informed consent was obtained from study participants when they agreed to be listed in the UNOS registry or INTERMACS. No one received compensation or was offered any incentive for participating in these registries.
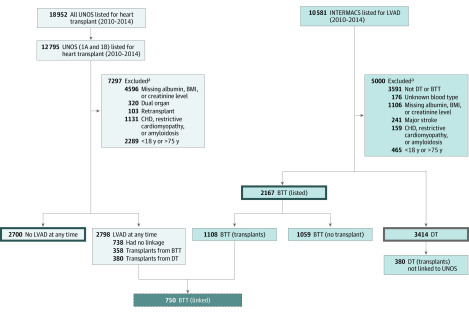
The INTERMACS data set from 2010 to 2014 was selected to coincide with the approval of the HeartMate II LVAD (Abbott) for the indication of DT and prior to the onset of the investigational trials of HeartMate 3 (Abbott). This data set included 10 581 patients supported by LVAD. The data coordinating center at the University of Alabama at Birmingham for INTERMACS provided a smaller deidentified data set that linked patient information between the UNOS registry and INTERMACS. This data set was obtained and then queried to investigate outcomes of patients implanted with a durable LVAD who subsequently underwent HT. In addition, a UNOS data set including all wait-listed HT candidates and outcomes from the same period was obtained. Figure 1 displays the sources of data used for comparative analyses.
Figure 1. Flow Diagram of Patients Included for Analysis From the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) and United Network for Organ Sharing (UNOS) Registry.
aSome patients had more than 1 exclusion. BMI represents body mass index; BTT, bridge to transplant; CHD, coronary heart disease; DT, destination therapy; and LVAD, left ventricular assist device.
Patient Population
Patients aged 18 to 75 years with end-stage HF wait-listed for HT in the UNOS registry or shown as implanted with an LVAD for treatment of advanced cardiomyopathy in INTERMACS were included for analysis. Patients with congenital heart disease, restrictive cardiomyopathy, or amyloidosis and patients who underwent retransplant were excluded, as were candidates for dual organ transplant, to permit comparisons of patients who would be both LVAD and HT candidates. Patients with a history of cerebrovascular events were also excluded. Analysis of comparable medical urgency between those receiving DT LVAD and those wait-listed for HT was established by restricting the UNOS data set to high-priority patients wait-listed in the previous allocation system as a status 1A or 1B. In a worst-case scenario analysis, patients who were delisted from transplant were counted as having died while on the waiting list. Reasons for delisting from transplant in UNOS included being too sick for transplant, receiving a transplant in another location, being too well for transplant, or having other nonspecified indications.
Statistical Analysis
The primary end point of interest was survival at 5 years. Patients were propensity score matched using a nearest neighbor matching algorithm allowing for discarding in both groups and going from highest to lowest score based on the following selected baseline factors known to be asssociated with outcomes after LVAD implant, after being wait-listed for HT, or after HT surgery and present in both data sets: age, sex, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), and creatinine and albumin levels. These baseline characteristics, both before and after matching, were summarized using descriptive statistics and compared between groups with χ2 tests for categorical variables and the t test or Wilcoxon rank sum test for continuous variables, as appropriate. Cox proportional hazards models were used to compare time from LVAD implant (for all patients in INTERMACS) or time of wait-listing (for patients registered in UNOS) with death between the matched groups of interest. For patients receiving a BTT LVAD, baseline covariates were obtained from INTERMACS, and time zero was considered as time of LVAD implant. All patients alive at 5 years of follow-up were censored at that time. In addition, patients receiving DT LVAD who also received HT and patients receiving BTT LVAD without posttransplant linked outcomes were censored at time of HT. Patients receiving DT LVAD and patients receiving BTT LVAD who achieved full recovery were censored at time of LVAD explantation. All models were adjusted for clinical factors that remained unbalanced after matching. Transplant status was treated as a time-dependent variable in all analyses. Kaplan-Meier curves were used to visualize overall survival. In a worst-case scenario sensitivity analysis, patients who were delisted from transplant were counted as having died while on the waiting list. All hypothesis testing was performed from December 22, 2017, to May 24, 2019, at a 2-sided statistical significance level of .05 using SAS, version 9.4 (SAS Institute Inc), or R, version 3.4.1 (The R Foundation).
Results
Baseline Characteristics
In total, 8281 patients had albumin level, creatinine level, and BMI data recorded and were included in the analysis. The reasons patients receiving DT LVAD were not considered eligible for HT wait-listing were provided in predefined descriptive fields in INTERMACS and were completed at the individual institution level. As per INTERMACS data, patients designated with DT were most commonly excluded from HT for the following reasons (which may be multiple): age (n = 959); high BMI (n = 530), chronic kidney disease (n = 555), current smoking history (n = 433), recent alcohol abuse (n = 119), recent illicit drug use (n = 118), limited social support (n = 149), pulmonary hypertension (n = 573), patient preference (ie, patient did not want transplant) (n = 154), and noncompliance (n = 126) (Figure 1).
Patient characteristics stratified by strategy of wait-listing for HT (with or without LVAD) vs DT LVAD for the 8281 patients are given in the Table. Propensity score matching was then performed for age, sex, BMI, and serum creatinine and albumin levels, yielding 3411 patients in each of the DT and HT groups. The initial overlap and then actual matching are shown in eFigure 1A and B in the Supplement, respectively. The Table also gives patient characteristics after propensity score matching. Patients receiving DT still tended to be slightly older than patients wait-listed for HT (64.0 years [interquartile range, 55.0-70.0 years] vs 60.0 years [interquartile range, 54.0-65.0 years]; P < .001), and there were significant differences across race/ethnicity and blood type but not sex (2701 men [79.2%] vs 2648 men [77.6%]; P = .13). Patients receiving DT had otherwise minor clinical differences in mean (SD) BMI (28.90 [6.72] vs 28.01 [5.28]; P < .001), serum creatinine level (1.45 [0.70] vs 1.37 [0.70] mg/dL; P < .001; to convert to μmol/L multiply by 88.4), and albumin level (3.35 [0.66] vs 3.48 [0.65] g/dL; P < .001; to convert to g/L, multiply by 10) compared with patients wait-listed for HT.
Table. Demographic and Clinical Characteristics of All Patients Before and After Propensity Score Matching.
| Characteristic | No. (%) of patients | P value | |
|---|---|---|---|
| BTT/UNOS | DT | ||
| Before matching | |||
| No. | 4867 | 3414 | |
| Age, median (IQR), y | 56.0 (47.0-63.0) | 64.0 (55.0-70.0) | <.001 |
| Race/ethnicity | |||
| Asian (non-Hispanic) | 145 (3.0) | 31 (0.9) | <.001 |
| Black (non-Hispanic) | 1147 (23.6) | 772 (22.6) | |
| Hispanic | 328 (6.7) | 90 (2.6) | |
| Other | 178 (3.7) | 189 (5.5) | |
| White (non-Hispanic) | 3069 (63.1) | 2332 (68.3) | |
| BMI, mean (SD) | 27.28 (5.29) | 28.94 (6.93) | <.001 |
| Male sex | 3631 (74.6) | 2704 (79.2) | <.001 |
| Blood type | |||
| A | 1868 (38.4) | 1315 (38.5) | <.001 |
| AB | 271 (5.6) | 129 (3.8) | |
| B | 705 (14.5) | 408 (12.0) | |
| O | 2023 (41.6) | 1562 (45.8) | |
| Creatinine, mean (SD), mg/dL | 1.30 (0.64) | 1.45 (0.70) | <.001 |
| Albumin, mean (SD), g/dL | 3.57 (0.66) | 3.36 (0.66) | <.001 |
| After matching | |||
| No. | 3411 | 3411 | |
| Age, median (IQR), y | 60.0 (54.0-65.0) | 64.0 (55.0-70.0) | <.001 |
| Race/ethnicity | |||
| Asian (non-Hispanic) | 89 (2.6) | 31 (0.9) | <.001 |
| Black (non-Hispanic) | 706 (20.7) | 772 (22.6) | |
| Hispanic | 220 (6.4) | 90 (2.6) | |
| Other | 113 (3.3) | 189 (5.5) | |
| White (non-Hispanic) | 2283 (66.9) | 2329 (68.3) | |
| BMI, mean (SD) | 28.01 (5.28) | 28.90 (6.72) | <.001 |
| Male sex | 2648 (77.6) | 2701 (79.2) | .13 |
| Blood type | |||
| A | 1324 (38.8) | 1314 (38.5) | <.001 |
| AB | 193 (5.7) | 129 (3.8) | |
| B | 484 (14.2) | 408 (12.0) | |
| O | 1410 (41.3) | 1560 (45.7) | |
| Creatinine, mean (SD), mg/dL | 1.37 (0.70) | 1.45 (0.70) | <.001 |
| Albumin, mean (SD), g/dL | 3.48 (0.65) | 3.35 (0.66) | <.001 |
Abbreviations: BMI, body mass index calculated as weight in kilograms divided by height in meters squared; BTT, bridge to transplant; DT, destination therapy; UNOS, United Network for Organ Sharing.
SI conversion factors: To convert serum creatinine level to micromoles per liter, multiply by 88.4; serum albumin to grams per liter, by 10.
Comparison of Strategies of Wait-listing for HT vs DT LVAD
Of the 3411 patients with DT, 380 ultimately received HT. Of the 3411 matched patients wait-listed to receive HT (with or without LVAD), 256 with BTT LVAD implant had data that were not linked to UNOS.
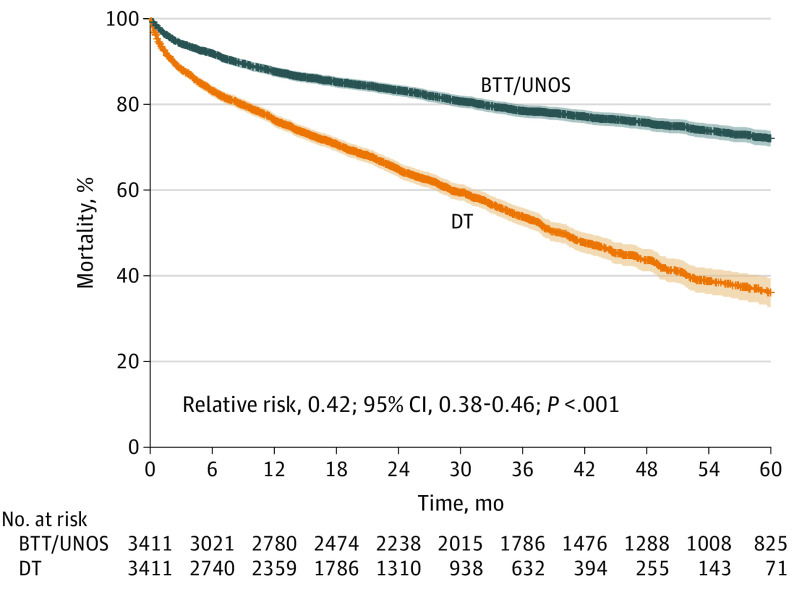
Figure 2 illustrates the outcome using the strategy of wait-listing for HT (with or without LVAD) vs DT LVAD. The strategy of wait-listing for HT was associated with a superior outcome at 5 years (relative risk [RR], 0.42; 95% CI, 0.38-0.46) after adjusting for select clinical factors. These survival curves accounted for waiting-list mortality with or without BTT LVAD. Overall 1-year survival was 87.7% in those wait-listed for HT (with or without BTT LVAD) compared with 76.4% in the DT LVAD group. The 5-year survival was 72.1% in those wait-listed for HT (with or without BTT LVAD) vs 36.1% in the DT LVAD group (P < .001). The difference in survival between the 2 strategies of wait-listing for HT vs proceeding directly to DT LVAD was associated primarily with whether patients underwent HT. After adjusting for time-dependent transplant status only, RR was 0.93 (95% CI, 0.83-1.05) for BTT in UNOS vs DT and 0.26 (95% CI, 0.22-0.30) for transplant status. This association remained after adjusting for the slight differences in clinical factors despite matching; RR was 0.97 (95% CI, 0.86-1.10) for BTT in UNOS vs DT and 0.27 (95% CI, 0.24-0.32) for transplant status.
Figure 2. Survival Curves Comparing Strategies of Wait-listing for Heart Transplant, With or Without Left Ventricular Assist Device (LVAD), With LVAD Destination Therapy (DT).
BTT represents bridge to transplant; UNOS, United Network for Organ Sharing.
Association With Age
To investigate whether there was an age threshold at which the strategy of pursuing LVAD support alone would be associated with comparable survival to HT, the cohorts were divided into 6 age strata (18-39, 40-49, 50-59, 60-64, 65-69, and >70 years). Superior survival associated with the strategy of wait-listing for HT across all age groups is shown in eFigure 2 in the Supplement. Survival across age strata of 18 to 64 years and of 65 years or older is given more frequent clinical equipoise in wait-listing older patients for transplant (eFigure 3 in the Supplement). Survival curves for patients younger than 65 and those 65 years or older wait-listed for transplant with or without LVAD were superimposed: 87.9% and 87.2% at 1 year, and 72.5% and 70.8% at 5 years. Older patients (≥65 years) with DT LVAD had worse survival (73.5% at 1 year) compared with younger matched patients, with 79.2% 1-year survival. There was no age up to 75 years of age identified at which device support was superior to transplant.
Comparison of BTT LVAD With DT LVAD
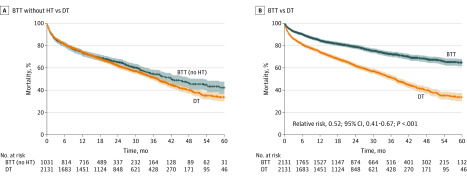
To explore differences in outcomes in LVAD-supported patients according to BTT vs DT implant, propensity score matching was performed to match these patients on age, sex, BMI, and creatinine and albumin levels. Characteristics after matching are given for these cohorts in eTable 1 in the Supplement. The patients with BTT LVAD were significantly younger (56 years [IQR, 47-63 years] vs 68 years [IQR, 64-72 years]; P < .001) and had slightly lower BMI (mean [SD], 28.17 [5.78] vs 29.15 [6.31]; P < .001), slightly lower creatinine level (mean [SD], 1.39 [0.81] mg/dL vs 1.49 [0.67] mg/dL; P < .001), and higher albumin level (mean [SD], 3.48 [0.67] g/dL vs 3.26 [0.66] g/dL; P < .001). Patients with BTT LVAD were also more frequently in INTERMACS profiles 1 and 2 at the time of implant compared with patients with DT LVAD. Despite these baseline differences after matching, when the patients with BTT LVAD who underwent transplant were excluded, the survival rate of patients with BTT LVAD who did not undergo HT was similar to that of patients with DT LVAD (Figure 3A). By contrast, Figure 3B shows significantly improved survival rate among patients with BTT LVAD when including those who underwent HT, suggesting that improved survival in these patients was associated with whether patients underwent HT (RR for time-dependent transplant status, 0.52; 95% CI, 0.41-0.67). Of note, there were no major differences in the clinical characteristics of the patients with BTT LVAD who did not undergo HT compared with patients with BTT LVAD who did undergo HT (eTable 2 in the Supplement). Baseline characteristics after matching for patients with BTT LVAD but without HT compared with patients with DT LVAD are provided in eTable 3 in the Supplement. As depicted in eTable 4 in the Supplement, 161 patients with DT LVAD who ultimately received HT were significantly older and had lower albumin levels compared with patients with BTT LVAD.
Figure 3. Patient Survival After Propensity Score Matching.
A, Survival after matching among patients with bridge to transplant (BTT) left ventricular assist device (LVAD) without heart transplant (HT) vs patients with LVAD destination therapy (DT). B, Survival after matching among all patients with BTT (accounting for posttransplant survival) vs patients with DT.
Posttransplant Survival of Patients Wait-listed for HT
The clinical characteristics of patients wait-listed for HT with BTT LVAD were similar to those wait-listed without BTT LVAD. The posttransplant survival rate for these patients was similar (RR, 1.01; 95% CI, 0.80-1.27).
Sensitivity Analyses
To account for residual confounding and to ensure the findings of our results, sensitivity analyses were performed. First, since approximately 22% of patients with missing serum albumin levels before matching were excluded, we compared the baseline characteristics of patients with albumin levels recorded and those without recorded albumin levels. The patients were not different with respect to age, sex, BMI, or creatinine levels (eTable 5 in the Supplement). Second, we conducted the same analyses including patients for whom the albumin level was missing and matched patients only on age, sex, BMI, and creatinine levels. In doing so, the primary results were similar; the strategy of wait-listing for HT with or without and a BTT LVAD was associated with superior 1- and 5-year survival rates compared with patients with DT LVAD (RR, 0.48; 95% CI, 0.45-0.53) contingent on patients receiving HT. Finally, a worst-case scenario analysis was conducted in which all patients delisted from HT were assumed dead within 1 week from delisting. The strategy of wait-listing for HT was again associated with superior midterm survival, more pronounced after 1 year (RR, 0.74; 95% CI, 0.68-0.80) (eFigure 4 in the Supplement).
Discussion
Heart transplant is dependent on the number of donor hearts available, yet the volume of patients experiencing advanced HF continues to increase.18 Given the improvements in LVAD technology, knowing whether HT and LVAD can provide comparable outcomes in selected populations has become increasingly relevant. Using national registry data, we compared similar cohorts of patients with advanced HF matched on the key factors of age, sex, BMI, and creatinine and albumin levels. We found that a strategy of pursuing HT, with or without BTT LVAD, was associated with superior 5-year survival rates compared with DT LVAD alone. This survival rate difference was associated with receiving HT because the survival of wait-listed patients who did not undergo HT was similar to that of patients receiving DT LVAD and persisted across all age groups.
Treatment of patients with advanced HF has seen outstanding advances in medical and device-based therapies during the last 20 years. Several studies have shown excellent survival rates conferred by both HT and mechanical circulatory support even among older, higher-risk individuals.2,3,16 A few studies have suggested similar short-term outcomes among patients undergoing HT vs LVAD.19,20 Sorabella et al,19 for example, showed no difference in the 2-year survival rate of patients aged 65 to 72 years undergoing DT LVAD, BTT LVAD, or HT. Such small single-center studies are limited, however, because most candidates for HT are younger and have fewer comorbid conditions compared with patients with DT, making between-group comparisons difficult. In studies using large registry data, posttransplant outcomes for patients undergoing LVAD as BTT are often unknown because INTERMACS and the UNOS registry are not linked.
By matching on key clinical factors known to be associated with surgical outcomes among patients wait-listed for HT in UNOS, patients in INTERMACS, and a unique data set linking the 2 registries, the present study found that the strategy of wait-listing for HT with or without bridging LVAD was associated with being superior to a strategy of LVAD therapy alone in similar patient cohorts.
Defining High-Risk Candidates
Given donor scarcity, defining candidates who will benefit most from HT therapy has been of paramount interest. As such, a number of retrospective studies have attempted to identify characteristics associated with increased risk to create prediction tools that might aid in clinical decision-making.5,6,21,22 One model derived from UNOS data found that age, diagnosis, type of mechanical support, ventilatory support, estimated glomerular filtration rate, and total serum bilirubin level allowed for a prediction of in-hospital mortality, with a C statistic of 0.7.21 Another study developed a score by integrating factors from pretransplant clinical characteristics to predict poor outcomes after HT.22 The constellation of factors included cardiac retransplant, low serum albumin level, reduced renal function, prior stroke, and more than 2 prior cardiothoracic surgical procedures. High risk has similarly been defined for LVAD recipients, with multiple studies identifying age, impaired renal function, elevated bilirubin level, poor right ventricular function, and high BMI as risk factors associated with poor outcomes following LVAD surgery.23,24,25,26,27 On the basis of these prior studies, we selected age, renal function, serum albumin level, and BMI as key variables on which patients were matched. In this analysis, despite matching on these factors, the strategy of pursuing HT in an era of the contemporary continuous flow device was associated with superior outcomes compared with LVAD alone.
Because age has consistently emerged as the most important risk factor in determining survival, we sought to examine its association with strategy and with outcome. Several single-center and registry-based studies have shown excellent survival rates with both HT and mechanical circulatory support among older, higher-risk individuals.2,3,16,28 Acceptable age thresholds for transplant candidacy vary among transplant centers, but age remains the most common reason given for patients given an DT LVAD.16 Using age strata from 18 to 75 years, we were unable to identify an age range that was associated with comparable midterm survival between transplant vs DT LVAD strategies. Even in the 70- to 75-year-old patients, a strategy of wait-listing for HT with or without an LVAD was associated with increased survival rates at 1 and 5 years.
Limitations
There are several limitations that warrant mention. The reasons patients were deemed candidates for DT as opposed to BTT are incompletely recorded in INTERMACS. Data on all types of durable LVADs were not available. For example, if a patient received an LVAD that was not approved by the US Food and Drug Administration, that patient would not have been included in INTERMACS. In addition, not all LVAD implanting centers participate in INTERMACS. Furthermore, the linkage of the UNOS and INTERMACS data sets was only 67.7% complete, leaving patients with BTT for whom posttransplant outcomes were unknown. The present analysis included a period that was contemporary but before the commercial availability of the newest durable LVAD, the HeartMate 3 (Abbott), which has shown select superior outcomes to its predecessor, the HeartMate II LVAD, at 2 years’ follow-up, with a decrease in pump thromboses and stroke but no difference in overall survival.12 Given the substantial difference observed in the present analysis, inclusion of such patients would be unlikely to alter our fundamental findings. Among patients receiving HT, we did not have information on donor characteristics that may be associated with outcomes in the study patients. These data also offered outcomes on a population of patients who were already selected for either LVAD or transplant (or both). Thus, data were available only on these preselected patients who had passed an “eyeball test” at their respective institutions for being acceptable candidates. The generalizability of these results, therefore, may be somewhat limited. There are likely other factors besides age, sex, BMI, and creatinine and albumin levels that are associated with outcomes; however, in the present analysis, patients were only matched on these factors to allow for comparable cohorts. Despite our efforts to match these populations, there were likely inherent differences in the populations studied, which may have been associated with the differences in outcome. We also conducted a number of sensitivity analyses, including a worst-case scenario, and the results were reassuringly similar. In this high-risk group of individuals, the outcome of quality of life is of importance. Unfortunately, we did not have information to conduct analyses on the association of strategy with this outcome. Finally, in October 2018, the UNOS Allocation system with expansion to 6 statuses was implemented, and how this change may affect our findings is unclear. As patients with device malfunction, complications, or both increase in priority in the new system, it is unlikely that the results of the present analysis would be significantly changed.
Conclusions
In the current era, HT, with or without BTT LVAD, was associated with superior 5-year survival rates compared with DT LVAD alone among patients matched on several relevant clinical factors. We believe our findings show that continued improvement in LVAD technology, along with prospective comparative research, is needed to amend this strategy.
eTable 1. BTT LVAD vs DT LVAD After Propensity Score Matching
eTable 2. BTT LVAD Not Transplanted vs BTT Transplanted From Matched Dataset
eTable 3. BTT LVAD Not Transplanted vs DT LVAD From Matched Dataset
eTable 4. BTT LVAD vs DT LVAD Patients Who Ultimately Were Transplanted
eTable 5. Patients From Analysis Data set Compared to Patients Who Were Excluded Solely 11 for Missing Albumin
eFigure 1. Propensity Matching
eFigure 2. Strategy of Listing for Transplant vs DT LVAD Broken Down by 6 Age Groups
eFigure 3. Survival Analysis by Younger (<65 Years) and Older (≥65 Years) Patients According to Strategy
eFigure 4. Worst-case Scenario Survival Curves Comparing Strategies of Listing of Transplant (With Or Without LVAD) to DT LVAD Where Delisted Patients Are Assumed Dead Within 1 Week
References
- 1.Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK. Honoring 50 years of clinical heart transplantation in Circulation: in-depth state-of-the-art review. Circulation. 2018;137(1):71-87. doi: 10.1161/CIRCULATIONAHA.117.029753 [DOI] [PubMed] [Google Scholar]
- 2.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 annual data report: heart. Am J Transplant. 2018;18(suppl 1):291-362. doi: 10.1111/ajt.14561 [DOI] [PubMed] [Google Scholar]
- 3.Cooper LB, Lu D, Mentz RJ, et al. Cardiac transplantation for older patients: characteristics and outcomes in the septuagenarian population. J Heart Lung Transplant. 2016;35(3):362-369. doi: 10.1016/j.healun.2015.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan JA, John R, Weinberg AD, et al. Long-term results of cardiac transplantation in patients 65 years of age and older: a comparative analysis. Ann Thorac Surg. 2003;76(6):1982-1987. doi: 10.1016/S0003-4975(03)01070-1 [DOI] [PubMed] [Google Scholar]
- 5.Kilic A, Weiss ES, Yuh DD, Shah AS, Conte JV. Factors associated with 5-year survival in older heart transplant recipients. J Thorac Cardiovasc Surg. 2012;143(2):468-474. doi: 10.1016/j.jtcvs.2011.10.036 [DOI] [PubMed] [Google Scholar]
- 6.Kilic A, Weiss ES, George TJ, et al. What predicts long-term survival after heart transplantation? an analysis of 9,400 ten-year survivors. Ann Thorac Surg. 2012;93(3):699-704. doi: 10.1016/j.athoracsur.2011.09.037 [DOI] [PubMed] [Google Scholar]
- 7.Kilic A, Conte JV, Shah AS, Yuh DD. Orthotopic heart transplantation in patients with metabolic risk factors. Ann Thorac Surg. 2012;93(3):718-724. doi: 10.1016/j.athoracsur.2011.11.054 [DOI] [PubMed] [Google Scholar]
- 8.Slaughter MS, Rogers JG, Milano CA, et al. ; HeartMate II Investigators . Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251. doi: 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Milano CA, Tatooles AJ, et al. ; HeartMate II Clinical Investigators . Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5(2):241-248. doi: 10.1161/CIRCHEARTFAILURE.111.963991 [DOI] [PubMed] [Google Scholar]
- 10.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451-460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 11.Gosev I, Kiernan MS, Eckman P, et al. ; Evolving Mechanical Support Research Group (EMERG) Investigators . Long-term survival in patients receiving a continuous-flow left ventricular assist device. Ann Thorac Surg. 2018;105(3):696-701. doi: 10.1016/j.athoracsur.2017.08.057 [DOI] [PubMed] [Google Scholar]
- 12.Mehra MR, Goldstein DJ, Uriel N, et al. ; MOMENTUM 3 Investigators . Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378(15):1386-1395. doi: 10.1056/NEJMoa1800866 [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail. 2017;19(5):595-602. doi: 10.1002/ejhf.779 [DOI] [PubMed] [Google Scholar]
- 14.Pinney SP, Anyanwu AC, Lala A, Teuteberg JJ, Uriel N, Mehra MR. Left ventricular assist devices for lifelong support. J Am Coll Cardiol. 2017;69(23):2845-2861. doi: 10.1016/j.jacc.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 15.Hsich EM. Matching the market for heart transplantation. Circ Heart Fail. 2016;9(4):e002679. doi: 10.1161/CIRCHEARTFAILURE.115.002679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38(2):114-126. doi: 10.1016/j.healun.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 17.Kirklin JK, Naftel DC. Mechanical circulatory support: registering a therapy in evolution. Circ Heart Fail. 2008;1(3):200-205. doi: 10.1161/CIRCHEARTFAILURE.108.782599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson LW. Crisis awaiting heart transplantation: sinking the lifeboat. JAMA Intern Med. 2015;175(8):1406-1409. doi: 10.1001/jamainternmed.2015.2203 [DOI] [PubMed] [Google Scholar]
- 19.Sorabella RA, Yerebakan H, Walters R, et al. Comparison of outcomes after heart replacement therapy in patients over 65 years old. Ann Thorac Surg. 2015;99(2):582-588. doi: 10.1016/j.athoracsur.2014.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144(3):584-603. doi: 10.1016/j.jtcvs.2012.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh TP, Almond CS, Semigran MJ, Piercey G, Gauvreau K. Risk prediction for early in-hospital mortality following heart transplantation in the United States. Circ Heart Fail. 2012;5(2):259-266. doi: 10.1161/CIRCHEARTFAILURE.111.965996 [DOI] [PubMed] [Google Scholar]
- 22.Schulze PC, Jiang J, Yang J, et al. Preoperative assessment of high-risk candidates to predict survival after heart transplantation. Circ Heart Fail. 2013;6(3):527-534. doi: 10.1161/CIRCHEARTFAILURE.112.000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birati EY, Hanff TC, Maldonado D, et al. Predicting long term outcome in patients treated with continuous flow left ventricular assist device: the Penn-Columbia Risk Score. J Am Heart Assoc. 2018;7(6):e006408. doi: 10.1161/JAHA.117.006408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lietz K, Miller LW. Patient selection for left-ventricular assist devices. Curr Opin Cardiol. 2009;24(3):246-251. doi: 10.1097/HCO.0b013e32832a0743 [DOI] [PubMed] [Google Scholar]
- 25.Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61(3):313-321. doi: 10.1016/j.jacc.2012.09.055 [DOI] [PubMed] [Google Scholar]
- 26.Menon AK, Mechelinck M, Unterkofler J, et al. Predictive value of EuroSCORE II in patients undergoing left ventricular assist device therapy. Thorac Cardiovasc Surg. 2016;64(6):475-482. [DOI] [PubMed] [Google Scholar]
- 27.Ketchum ES, Moorman AJ, Fishbein DP, et al. Predictive value of the Seattle Heart Failure Model in patients undergoing left ventricular assist device placement. J Heart Lung Transplant. 2010;29(9):1021-1025. doi: 10.1016/j.healun.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 28.Atluri P, Goldstone AB, Kobrin DM, et al. Ventricular assist device implant in the elderly is associated with increased, but respectable risk: a multi-institutional study. Ann Thorac Surg. 2013;96(1):141-147. doi: 10.1016/j.athoracsur.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. BTT LVAD vs DT LVAD After Propensity Score Matching
eTable 2. BTT LVAD Not Transplanted vs BTT Transplanted From Matched Dataset
eTable 3. BTT LVAD Not Transplanted vs DT LVAD From Matched Dataset
eTable 4. BTT LVAD vs DT LVAD Patients Who Ultimately Were Transplanted
eTable 5. Patients From Analysis Data set Compared to Patients Who Were Excluded Solely 11 for Missing Albumin
eFigure 1. Propensity Matching
eFigure 2. Strategy of Listing for Transplant vs DT LVAD Broken Down by 6 Age Groups
eFigure 3. Survival Analysis by Younger (<65 Years) and Older (≥65 Years) Patients According to Strategy
eFigure 4. Worst-case Scenario Survival Curves Comparing Strategies of Listing of Transplant (With Or Without LVAD) to DT LVAD Where Delisted Patients Are Assumed Dead Within 1 Week