Short abstract
In many Asian countries, herbs are used to treat disease. However, herbs also have adverse effects. Herb-induced liver injury has become a serious public health problem requiring urgent attention. The seeds of Swietenia macrophylla, a member of the family Polygonaceae, are often called skyfruit. We recently encountered a case of liver injury caused by skyfruit. The patient suffered from hepatocellular injury. We applied the updated Roussel Uclaf Causality Assessment Method (RUCAM) and the results indicated a highly probable relationship with skyfruit (total score 10). Moreover, we summarize another six cases of skyfruit-induced liver injury from the literature. The aim of our report is to help clinicians become more aware of the potential hepatotoxic effects of skyfruit and to accurately describe the clinical and laboratory characteristics of skyfruit-induced liver injury.
Keywords: Herb-induced liver injury (HILI), skyfruit, case report, Roussel Uclas Causality Assessment Method (RUCAM), herbal medicine, differential diagnosis
Introduction
Herb-induced liver injury (HILI) has become a serious public health problem requiring urgent attention.1,2 Skyfruit is used to treat hypertension, hyperlipidemia and diabetes worldwide.3,4 However, the cytotoxic effects of skyfruit on human hepatocytes should not be ignored. Here, we report a patient with skyfruit-induced liver injury. We used the updated Roussel Uclaf Causality Assessment Method (RUCAM) to judge causality.5 In addition, we searched the literature for studies relating to skyfruit-induced liver injury. We analyzed these studies to enable better diagnosis and treatment of skyfruit-associated HILI.
Case report
Ethics and consent
The study protocol was approved by the Ethical Committee of the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. Verbal informed consent was obtained from the patient.
Case description
A 63-year-old woman was admitted to hospital on August 30, 2019 with a 3-day history of epigastric pain, nausea and fever. Her epigastric pain and nausea were relieved after taking gastric drugs prior to admission. Her fever decreased 2 days following admission. She had a 10-year history of hypertension and had been taking amlodipine besylate tablets and irbesartan tablets for 2 years prior to admission. She had a >1-year history of diabetes and had been treated with metformin and glimepiride for 1 year prior to admission. She irregularly took Ganoderma lucidum spore powder 1 year prior to admission at her own initiative. Upon specific query, she reported a 3-day history of skyfruit use 1 week prior to admission (Figure 1), during which time she would eat three skyfruits once a day. She reported no history of alcohol ingestion. She had no tenderness or pain in the gallbladder area, and Murphy’s sign was negative on admission. Examination was unremarkable.
Figure 1.
A photograph of the skyfruit the patient consumed.
Investigations
As shown in Table 1, laboratory values prior to admission (before skyfruit consumption) were as follows: alanine aminotransferase (ALT), 35 U/L; aspartate transaminase (AST), 35 U/L; and total bilirubin (TBil), 15.9 µmol/L. Laboratory values on admission (following skyfruit consumption) were as follows: ALT, 466 U/L; AST, 298 U/L; and TBil, 23 µmol/L. Her laboratory values 1 week following admission, skyfruit withdrawal and administration of liver-protective treatments were as follows: ALT, 99 U/L; AST, 51 U/L; and TBil, 11.8 µmol/L. Over time, these values gradually regressed even further toward the normal range. Her ALT levels normalized most rapidly (Figure 2). Moreover, her IgE and γ-globulins were elevated (92.6 kIU/L and 26.9%, respectively) although her white cell counts and eosinophils were normal. Hepatotropic viral screens (hepatitis A, B, C, D, and E) and serum autoantibodies were negative. She reported no history of bile duct disease, and an upper abdominal ultrasound showed that she had fatty liver and gallbladder wall thickening.
Table 1.
Changes in liver enzymes of our patient over time.
| Time | ALT (7–40 U/L) | ALP (50–135 U/L) | R |
|---|---|---|---|
| Prior to admission | 35 | 104 | 1.1 |
| On admission | 466 | 104 | 15.1 |
| Day 4 post-admission | 185 | 104 | 6.0 |
| Day 7 post-admission | 99 | 86 | 3.9 |
| Day 11 post-admission | 64 | 86 | 2.5 |
| 1 month post-discharge | 20 | 80 | 0.8 |
R=(ALT/ULN)/(ALP/ULN).
ALT, alanine aminotransferase; ALP, alkaline phosphatase; ULN, upper limit of normal.
Figure 2.
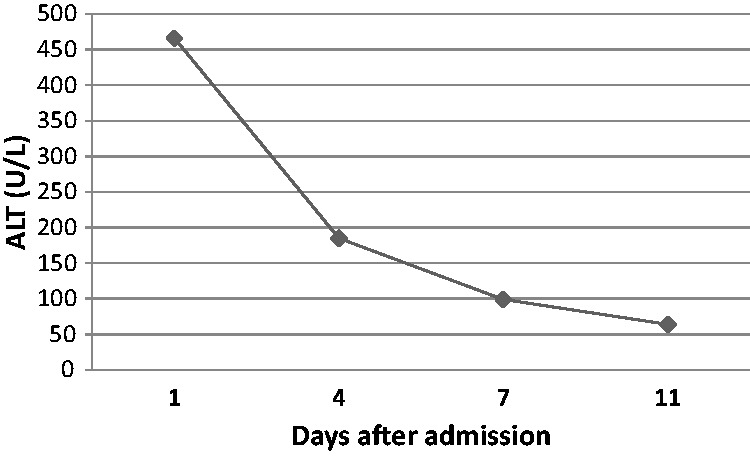
The patient’s alanine aminotransferase (ALT) levels for 11 days following admission.
To assess whether skyfruit was responsible for the patient’s acute liver injury, we used the updated RUCAM. The updated RUCAM is a specific causality assessment method relevant to liver injury.6 According to the total score, correlations can be classified into five categories: 0, relationship “excluded”; l–2: “unlikely”; 3–5: “possible”; 6–8: “probable”; and >8: “highly probable”. Clinical classifications were divided into three types:6 (1) hepatocellular injury, defined as ALT ≥ 5 upper limit of normal (ULN) and R ≥ 5; (2) cholestatic injury, defined as alkaline phosphatase (ALP) ≥ 2 ULN and R≤2; and (3) mixed injury, defined as ALT ≥ 5 ULN, ALP ≥ 2 ULN and 2 < R < 5. The R value was defined as the ratio of serum ALT (as a multiple of its ULN) to serum ALP (as a multiple of its ULN). Our patient suffered from hepatocellular injury with initial ALT 466 U/L and R 15.1. The results of RUCAM indicated a highly probable relationship with skyfruit and a total score 10 (Table 2). Considering these findings, we determined that skyfruit could not be re-administered in this patient.
Table 2.
Detailed updated RUCAM score for our patient (hepatocellular injury).
| Skyfruit | |
|---|---|
| R=(ALT/ULN)/ (ALP/ULN) | 15.1 |
| Time to onset | +3 |
| Course of the reaction | +3 |
| Risk factors | +1 |
| Concomitant drug use | 0 |
| Search for non-drug cases | +2 |
| Previous information on hepatotoxicity of the drug | +1 |
| Response to readministration | 0 |
| Total score | 10 |
RUCAM, Roussel Uclaf Causality Assessment Method; ALT, alanine aminotransferase; ALP, alkaline phosphatase; ULN, upper limit of normal.
Outcome and follow-up
Skyfruit consumption was stopped upon admission and supportive treatment was started. Magnesium isoglycyrrhizinate (200 mg/day by injection) and reduced glutathione (1.2 g/day) were administered for 3 days. Thereafter, magnesium isoglycyrrhizinate was decreased to 150 mg/day and reduced glutathione was stopped until the patient was discharged. Routine urine tests showed high levels of granulocytes and leukocytes. Mezlocillin and sulbactam were administered for 1 week although the patient showed no symptoms of low back pain, dysuria, or increased urinary frequency and urgency.
The patient was discharged from our hospital 10 days post-admission with telephone follow-up. Her liver biochemistry returned to normal upon examination 1 month following discharge.
Similar cases in the literature
To our knowledge, six cases of skyfruit-induced liver injury have been reported. Their clinical characteristics are summarized in Table 3. Patients were numbered from 1 to 6 arbitrarily. Their liver enzyme results are shown in Table 4.
Table 3.
General characteristics of reported cases of HILI associated with skyfruit.
| Patient number | Reference | Gender | Age | Medical history | Duration of skyfruit use |
|---|---|---|---|---|---|
| 1 | [7] | Male | 72 | Diabetes | 2 months |
| 2 | [7] | Male | 75 | Diabetes | 1.5 months |
| 3 | [7] | Male | 80 | Hypertension | 1.5 months |
| 4 | [8] | Male | 62 | Diabetes | 3 months |
| 5 | [8] | Female | 61 | Diabetes | 3 months |
| 6 | [9] | Female | 45 | Depression | 6 months |
HILI, herb-induced liver injury
Table 4.
Changes in liver enzymes over time and RUCAM scores of reported cases of HILI associated with skyfruit.
| Patient number | Reference | Time | ALT | ALP | RUCAM score |
|---|---|---|---|---|---|
| 1 | [7] | 0 | 678 | 254 | 7 |
| 1 week | 312 | 212 | |||
| 2 weeks | 182 | 166 | |||
| 6 weeks | N | N | |||
| 2 | [7] | 0 | 363 | 122 | 7 |
| 1 week | 115 | 86 | |||
| 4 weeks | N | N | |||
| 3 | [7] | 0 | 224 | 108 | 7 |
| 3 days | 113 | 98 | |||
| 2 weeks | N | N | |||
| 4 | [8] | 0 | 765.9 | 190.7 | 8 |
| 3 weeks | 9.5 | 187.4 | |||
| 1.3 years | 38 | 87 | |||
| 5 | [8] | 0 | 276 | 138 | 8 |
| 2 weeks | NA | NA | |||
| 7 weeks | N | N | |||
| 6 | [9] | 0 | 1267 | 124 | 7 |
| 75 days | N | N |
RUCAM, Roussel Uclaf Causality Assessment Method; HILI, herb-induced liver injury; ALT, alanine aminotransferase; ALP, alkaline phosphatase; N, normal; NA, not available.
Discussion
The seeds of Swietenia macrophylla, belonging to the family Polygonaceae, are usually called skyfruit. Skyfruit is used as a folk medicine to treat diabetes and hypertension in the Solomon Islands, Malaysia, India, and southern China.7 Skyfruit-induced liver injury is extremely rare; only seven cases, including the one described here, have been reported. HILI can be classified into two subtypes: idiosyncratic and intrinsic.8 Idiosyncratic HILI is characterized by its unpredictability9 and has a variable time of onset, significant inter-individual differences and no correlation with drug dosage. Intrinsic HILI is predictable and dose-dependent with a short latency period (hours). Thus, it is important to differentiate liver injury according to the average onset time. Information on reported cases of skyfruit-associated HILI is shown in Table 3, including the case described here of idiosyncratic HILI. The results of these seven reports clearly suggest that skyfruit can induce hepatocellular injury.
Skyfruit is increasingly used in China for the treatment of hypertension, hyperlipidemia and diabetes. Several cases of HILI potentially associated with skyfruit have been reported. Tan et al.10 reported one case who consumed of skyfruit; findings on liver biopsy corresponded with HILI. Zhao et al.11 reported another case who manifested skyfruit-induced liver injury with autoimmune characteristics. Yeap and colleagues reported a patient who developed liver failure related to consumption of mahogany seed extract.12 Fortunately, all skyfruit-induced cases of liver injury were conservatively treated and cured.
In our case, we suspected that epigastric pain, nausea and fever were the clinical manifestations of HILI. Upper abdominal ultrasound showed fatty liver and gallbladder wall thickening. The patient had a 10-year history of fatty liver. At irregular checkups, she reported that her liver function was normal. She was diagnosed with chronic cholecystitis because she had no tenderness or pain in the gallbladder area and ultrasound showed gallbladder wall thickening without gallbladder enlargement or increased tension. Asymptomatic urinary tract infection was diagnosed as the patient had no symptoms of low back pain, dysuria, or increased urinary frequency and urgency. As an older woman, the primary differential diagnoses based on the degrees of ALT, AST and TBil elevation were viral hepatitis, autoimmune liver disease and drug-induced liver injury. Hepatotropic viral screens (hepatitis A, B, C, D, and E) and serum autoantibodies were negative. She reported no history of bile duct diseases or alcohol ingestion. However, she was taking multiple drugs, and drug-induced liver injury became our primary focus.
For HILI subjects, it is important but not easy to identify the causative drug. First, one should exclude other types of liver diseases. Thereafter, a causality assessment method should be used to determine the relationship between liver injury and suspected drugs. In our study, the updated RUCAM was used to judge causality and this remains the most widely used method worldwide.13,14 The updated RUCAM score of 10 in our case demonstrated that her liver injuries were highly probably induced by skyfruit. G. lucidum spore powder was also stopped following admission; the patient had been sporadically taking it for 1 year at her own initiative. The patient had a regular checkup prior to admission on August 13 and her liver function was normal. Thus, it was unlikely that G. lucidum spore powder induced her liver injury. However, the patient took multiple drugs, which together could increase the burden on her liver.
According to the European Association for the Study of the Liver Guidelines,15 treatments for HILI consist of general measures and specific therapies. The most important general measure is to stop the suspicious drug. Studies have shown that spontaneous recovery can occur even without any other treatment. Specific therapies include cholestyramine for leflunomide, carnitine for valproate, and others. In our case, the patient took skyfruit into hospital with her. Her neighbor had told her that skyfruit could lower blood pressure and gave her some. Her neighbor had consumed skyfruit for several months and was asymptomatic. We stressed the importance of discontinuing skyfruit for the patient and explained the potential for inter-individual differences in reactions to this drug. Inter-individual differences are an important feature of skyfruit-induced liver injury. Moreover, liver-protective drugs were administered because of the patient’s high transaminase levels. There is currently no specific antidote for skyfruit.
Our study is the first to review the literature for studies on skyfruit-induced liver injury. clinicians should recognize the potential hepatotoxic effects of skyfruit when encountering cases of unexplained liver injury, especially in the Solomon Islands, Malaysia, India, and southern China.
Abbreviations
HILI: herb-induced liver injury; ALT: alanine aminotransferase; AST: aspartate transaminase; TBil: total bilirubin; RUCAM: Roussel Uclaf Causality Assessment Method; ALP: alkaline phosphatase; ULN: upper limit of normal; N: normal; NA: not available.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics and consent
The study protocol was approved by the Ethical Committee of the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. Verbal informed consent was obtained from the patient.
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province, China (Grant No. LQ18H070006).
ORCID iDs
Caixia Xia https://orcid.org/0000-0001-6917-5237
References
- 1.Jing J, Teschke R. Traditional Chinese medicine and herb-induced liver injury: comparison with drug-induced liver injury. J Clin Transl Hepatol 2018; 6: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, He X, Wang L, et al. Chinese herbal medicine hepatotoxicity: the evaluation and recognization based on large-scale evidence database. Curr Drug Metab 2019; 20: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ch’ng YS, Loh YC, Tan CS, et al. Vasodilation and antihypertensive activities of Swietenia macrophylla (mahogany) seed extract. J Med Food 2018; 21: 289–301. [DOI] [PubMed] [Google Scholar]
- 4.Dewanjee S, Maiti A, Das AK, et al. Swietenine: a potential oral hypoglycemic from Swietenia macrophylla seed. Fitoterapia 2009; 80: 249–251. [DOI] [PubMed] [Google Scholar]
- 5.Teschke R, Larrey D, Melchart D, et al. Traditional Chinese medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines (Basel) 2016; 3: 1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci 2015; 17: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun YP, Zhu LL, Liu JS, et al. Limonoids and triterpenoid from fruit of Swietenia macrophylla. Fitoterapia 2018; 125: 141–146. [DOI] [PubMed] [Google Scholar]
- 8.Andrade RJ, Chalasani N, Bjornsson ES, et al. Drug-induced liver injury. Nat Rev Dis Primers 2019; 5: 58. [DOI] [PubMed] [Google Scholar]
- 9.Teschke R. Idiosyncratic DILI: analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front Pharmacol 2019; 10: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan Y, Chen H, Zhou X, et al. RUCAM-based assessment of liver injury by xiang-tian-guo (Swietenia macrophylla) seeds, a plant used for treatment of hypertension and diabetes. Ann Hepatol 2019; 18: 406–407. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Qi Y, Cai Y. Fructus Swietenia macrophylla-induced liver injury: a report of 2 cases (in Chinese). J Clin Hepatol 2019; 35: 1086–1088. [Google Scholar]
- 12.Yeap V, Tan TJY, Loh T, et al. Liver failure associated with mahogany seed extract consumption. BMJ Case Rep 2018; 2018: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danan G, Teschke R. Roussel Uclaf Causality Assessment Method for drug-induced liver injury: present and future. Front Pharmacol 2019; 10: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danan G, Teschke R. Drug-induced liver injury: why is the Roussel Uclaf Causality Assessment Method (RUCAM) still used 25 years after its launch? Drug Saf 2018; 41: 735–743. [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver, Clinical Practice Guideline Panel, EASL Governing Board Representative. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol 2019; 70: 1222–1261. [DOI] [PubMed] [Google Scholar]



